κ-Opioid receptors control the metabolic response to a high-energy diet in mice (original) (raw)
Abstract
General opioid receptor antagonists reduce food intake and body weight in rodents, but the contributions of specific receptor subtypes are unknown. We examined whether genetic deletion of the κ-opioid receptor (KOR) in mice alters metabolic physiology. KOR-knockout (KO) and wild-type (WT) mice were fed a high-energy diet (HED) for 16 wk. KO mice had 28% lower body weight and 45% lower fat mass when compared to WT mice fed an HED. No differences in caloric intake were found. An HED reduced energy expenditure in WT mice, but not in KO mice. KOR deficiency led to an attenuation of triglyceride synthesis in the liver. Malonyl CoA levels were also reduced in response to an HED, thereby promoting hepatic β-oxidation. Glycemic control was also found to be improved in KO mice. These data suggest a key role for KORs in the central nervous system regulation of the metabolic adaptation to an HED, as we were unable to detect expression of KOR in liver, white adipose tissue, or skeletal muscle in WT mice. This study provides the first evidence that KORs play an essential physiological role in the control of hepatic lipid metabolism, and KOR activation is a permissive signal toward fat storage.—Czyzyk, T. A., Nogueiras, R., Lockwood, J. F., McKinzie, J. H., Coskun, T., Pintar, J. E., Hammond, C., Tschöp, M. H., Statnick, M. A. κ-Opioid receptors control the metabolic response to a high-energy diet in mice.
Keywords: malonyl CoA, corticosterone, hepatocytes, energy expenditure, nonexercise activity thermogenesis
Rates of overweight and obesity have increased dramatically over the past 35 yr, yet few pharmacological treatments with widespread efficacy have been developed. In 2004, the estimated prevalence of overweight (BMI≥25 kg/m2) in the United States was 66% (1). The number of overweight adults worldwide is expected to increase 1.5 times by the year 2015 to 2.3 billion (World Health Organization, 2006; http://www.int/mediacentre/factsheets/ fs311/en/index.html). It is expected that as obesity rates rise, so will the costs associated with treating comorbid conditions, including diabetes and nonalcoholic fatty liver disease (2). Currently, there are only two FDA-approved drugs on the market in the United States, both of which have side effects that make them a relatively unpopular choice for weight-loss management. There are also a very small number of compounds in phase III clinical studies being developed to treat obesity (3). A better understanding of body weight regulation is necessary for development of effective therapies for weight loss and weight management.
The classic opioid receptor system includes three Gi-coupled receptors, μ-, δ-, and κ-opiod receptor (MOR, DOR, and KOR), as well as their endogenous ligands. Several nonclinical studies have demonstrated a role for opioid receptors in energy balance. Both systemic and intracerebroventricular administration of general opioid receptor antagonists reduce food intake and body weight in rodent models, including genetically obese Zucker and diet-induced obese rats (4,5,6,7,8). Pharmacological studies have demonstrated that both μ- and κ-specific antagonists can reduce both spontaneous and deprivation-induced feeding in rodents (9, 10). In particular, the KOR antagonist nor-binaltorphimine showed robust reductions in the intake of palatable diets high in fat or sucrose (11, 12). However, the expression patterns of specific opioid receptors overlap in many regions of the brain. Therefore, the possibility exists that the in vivo contributions of each receptor subtype to regulating food intake and body weight might be distinct from pharmacological studies (13). To what extent endogenous opioid receptors may directly regulate lipid metabolism, beyond effects on body weight and energy balance, is unknown.
Knockout (KO) mice lacking MOR, DOR, or KOR have been generated (14). To date, body weight and metabolic studies have been limited to MOR-KO mice, which appear to be somewhat resistant to weight gain on a high-fat diet (15, 16). The purpose of our experiments was to determine whether genetic ablation of KOR in mice alters energy balance and glucose or lipid metabolism in response to a calorically dense, high-energy diet (HED). We found that KOR-KO mice were resistant to weight gain even after prolonged exposure to an HED and that this was driven by maintenance of energy expenditure and locomotor activity levels and an increase in fatty acid β-oxidation in the liver. We propose that KORs are acting centrally since we failed to detect expression of KOR or the precursor to its endogenous ligand, prodynorphin, in metabolically relevant peripheral tissues. Collectively, our data suggest that the KOR signaling pathway might normally be permissive to weight gain on consumption of calorically dense food. Moreover, we provide evidence suggestive of KOR signaling in the brain as modulating lipid metabolism in peripheral tissues.
MATERIALS AND METHODS
Animals
All procedures were approved by the Institutional Animal Care and Use Committees of Eli Lilly and Company and the University of Cincinnati and were in accordance with the U.S. National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals. Age-matched wild-type (WT) and KOR-KO male mice lacking exon 3 of the KOR gene (Oprk1) (17, 18) were bred from homozygous mating pairs maintained on the 129S6 background at Taconic Farms (Germantown, NY, USA). Mice were assigned to a high-fat HED (RD12451; 45% fat, 4.73 kcal/g, Research Diets, New Brunswick, NJ, USA) at 7–8 wk of age. Age-matched controls were maintained on a standard rodent chow diet (NIH-31M; 5% fat, 4.02 kcal/g, Taconic Farms). Mice were group housed (3 or 4 mice/cage) throughout the study. All mice were maintained on a 12-h light-dark cycle (lights on 7 AM, lights off 7 PM).
Plasma collection and hormonal analysis
Mice were sacrificed 4 h after the start of the light cycle. Whole-trunk blood samples were collected in tubes containing 20 μl 0.5 M EDTA and were spun for 15 min at 3000 g at 4°C. Plasma was stored at −80°C until being shipped to Millipore (St. Charles, MI, USA) for radioimmunoassay analysis of T4, IGF-1, leptin, and corticosterone.
Determination of energy balance
Whole-body composition was measured using nuclear magnetic resonance (NMR) imaging (Whole Body Composition Analyzer; EchoMRI, Houston, TX, USA). Animals were monitored in a custom 32-cage indirect calorimetry, food intake, and locomotor activity monitoring system (TSE LabMaster; TSE Systems, Bad Homburg, Germany), as described previously (19, 20). See also Supplemental Methods. Mice were acclimated for 48 h to the test chambers and then monitored for an additional 48 h. Data collected from the last 48 h were used to calculate all parameters for which results are reported.
Western blot analysis of UCP-1
Western blot analyses were performed following standard procedures. UCP-1 was detected with a rabbit polyclonal antibody (662045; Calbiochem, San Diego, CA, USA) at a dilution of 1:25,000. Calreticulin was detected with a rabbit polyclonal antibody (ab2907; Abcam, Cambridge, MA, USA) at a dilution of 1:5000 and served as an internal control for protein loading in each well.
Quantitative real-time PCR analysis
Total RNA was extracted from frozen tissue samples homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and purified with the MagAttract RNA Universal Tissue M48 kit (Qiagen, Valencia, CA, USA) with DNase treatment. cDNAs were synthesized using 1 μg of total RNA (high-capacity cDNA reverse transcription kit; Applied Biosystems, Foster City, CA, USA). Quantitative PCR (qPCR) was performed using Taqman Probe and primer set assay kits obtained from Applied Biosystems (Supplemental Table 4). Reactions were cycled in an ABI Prism 7900HT Sequence Detection System (Applied Biosystems), and data were analyzed with Sequence Detection Software (SDS), version 2.2.2. All individual samples were measured in triplicate. Data were normalized to acidic ribosomal binding protein (ARBP). ARBP cDNA was detected with the following primers: forward primer 5-GGCCCGAGAAGACCTCCTT and reverse primer 5-TCAATGGTGCCTCTGGAGATT.
Glucose and insulin tolerance tests
Blood glucose levels were measured with an Accucheck glucometer (Roche, Indianapolis, IN, USA) after an intraperitoneal injection of either 2 mg/g d-glucose or 0.075 U/kg insulin (Humalin; Eli Lilly, Indianapolis, IN, USA). Area under the curve (AUC) values were determined, and data were analyzed with 2-way ANOVA and post hoc analysis.
Measurement of hepatic malonyl CoA and triglyceride (TAG) levels
Hepatic malonyl CoA levels were measured by LC-MS/MS using a modified method of Minkler et al. (21). See Supplemental Methods for details of procedure. Total TAG content was measured with Infinity Triglycerides reagent (Thermo Fisher Scientific, Middletown, VA, USA).
Lipogenesis assays
The rate of TAG synthesis was estimated using a 14C-labeled acetate incorporation assay. Briefly, 6-wk-old WT and KOR-KO mice were deprived of food for 4 h and then given an oral dose of glucose (0.25 mg/g). Thirty minutes later, mice were given an i.p. injection of 100 μCi 14C-labeled acetate (Amersham, Pittsburgh, PA, USA). Hepatic and epididymal white adipose tissues were collected 2 h after injection. Lipids were extracted with a 2:1 mixture of chloroform:methanol in Lysing Matrix D tubes (MP Biomedicals, Solon, OH, USA) using a FastPrep FP120 (Bio101; Thermo Fisher Scientific). A 500-μl sample of the extraction was counted in liquid scintillation fluid. Another 50-μl sample was run on thin-layer chromatography plates (Whatman, Piscataway, NJ, USA) using a hexane:ethyl ether:acetic acid (80:20:1) organic mobile phase for 30 min, then exposed to a phosphorimaging screen (Molecular Dynamics, Sunnyvale, CA, USA) overnight. 14C-triolene (American Radiochemical Company, St. Louis, MO, USA) was run as a standard. Data were analyzed with Image Quant software (Molecular Dynamics) and expressed as relative density.
Analysis of nutrient absorption
A separate cohort of KOR-KO and WT mice fed either standard chow or the HED were individually housed in metabolic cages and allowed 24 h to acclimate to the environment. Feces were collected and weighed after 48 h. The caloric contents of the feces samples (calories/gram of feces) were measured using standard bomb calorimetry methods. The amount of calories excreted in the feces was subtracted by the number of calories consumed, and the percentage efficiency of absorption was compared for each genotype and diet. Mice were 5 mo old when feces were collected. KOR-KO and WT mice had a 2-wk exposure to the HED prior to bomb calorimetry measurements.
Data analysis and statistics
Values are plotted as means ± se for each genotype. Statistical analyses were conducted with GraphPad Prism 4 (GraphPad Software, San Diego, CA, USA). Comparisons among genotype and diet were performed with 2-way ANOVA and Bonferroni post hoc tests. Unpaired t tests were also used to compare two groups as indicated. Post hoc analysis results are indicated in the corresponding figure legends. Growth curves were analyzed with 2-way repeated-measures ANOVA.
RESULTS
KOR-KO mice fed an HED have reduced body weight and adiposity that are independent of reductions in food intake or nutrient absorption
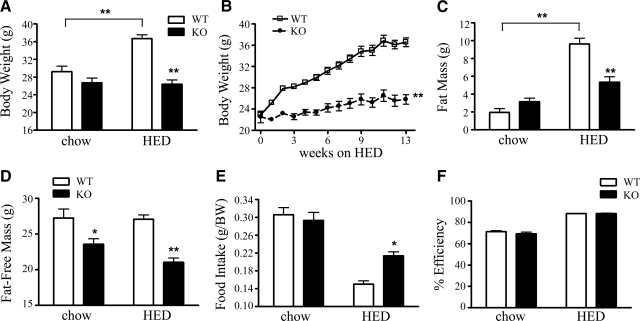
Age-matched male WT and KOR-KO (17, 18) 129S6 mice were maintained on an HED from 8 wk of age (45% kcal fat, 4.73 kcal/g) for 16 wk to assess their metabolic phenotypes. No initial body weight differences were found among the genotypes (21.5 vs. 21.7 g for WT and KO, respectively; n =7 or 8). While WT mice gained significant body weight when fed the HED, KOR-KO mice did not weigh more than their chow-fed controls (Fig. 1_A_). Weekly body weight measurements demonstrated that WT mice gained 15.2 g during HED exposure, compared to KOR-KO mice, which gained only 4.6 g (Fig. 1_B_). Body composition analysis with quantitative NMR (qNMR) revealed that KOR-KO mice fed the HED accrued 45% less fat mass compared to WT mice after 16 wk of an HED (Fig. 1_C_). KOR-KO mice were also found to have significantly reduced fat-free mass that was independent of diet (Fig. 1_D_). Changes in body weight and composition were not likely due to daily reductions in total caloric intake. When normalized to body weight, cumulative HED intakes in KOR-KO mice were slightly increased relative to WT mice during a 48-h period (Fig. 1_E_). Furthermore, no differences in nutrient absorption were found in KOR-KO mice on either standard chow or HED compared to WTs (Fig. 1_F_).
Figure 1.
Diet-dependent alterations in body weight, adiposity, and food intake in KOR-KO mice. A) Body weight after 16 wk of free access to HED. Mice were ∼6 mo of age at time of measurement. B) Weekly body weight of KO and WT mice fed an HED. **P < 0.001 by wk 2. C) Fat mass. D) Fat-free mass was calculated as body weight minus fat mass. E) Cumulative food intake over 48 h normalized to body weight. Food intake was measured at time of indirect calorimetry. F) Analysis of fecal caloric content. Percentage efficiency of nutrient absorption over a 48-h period was calculated in 5-mo-old KOR-KO and WT mice fed chow or HED. *P < 0.01, **P < 0.001; n = 7–8/group. Open bars and squares, WT; solid bars and circles, KOR KO. Error bars show means ± se.
Deletion of KOR increases energy expenditure and locomotor activity without altering nutrient partitioning
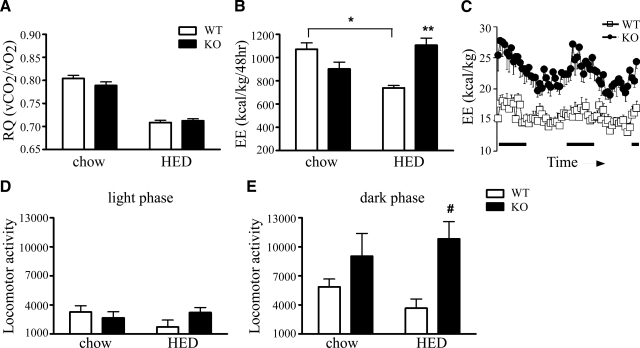
Indirect calorimetry was used to determine both respiratory quotient (RQ) and energy expenditure. Increases in lipid as metabolic substrate choice can be reflected by a reduction in RQ values. However, we found no differences in the 24-h RQ of KOR-KO mice when compared to WT mice (Fig. 2_A_). Our data suggest that during the satiated state, deletion of KOR did not directly alter fuel partitioning in metabolically active tissues. While total energy expenditure of WT mice decreased after 16 wk of an HED as expected, energy expenditure in KOR-KO mice remained unchanged (Fig. 2_B_). Moreover, energy expenditure levels remained at levels observed with standard chow in both the light and dark phases of the circadian cycle (Fig. 2_C_). Consistent with increased nonexercise activity thermogenesis (NEAT)-induced energy expenditure, spontaneous locomotor activity levels were higher in KOR-KO mice fed the HED compared to WT mice (Fig. 2_D_, E). Interestingly, we found that at 2 mo of age, KOR-KO mice already had 23% higher energy expenditure levels (_P_=0.03, unpaired t test), but identical body weight and locomotor activity levels as WT controls, suggesting that there are other mechanisms besides increased locomotor activity that are driving increased energy expenditure in KOR-KO mice.
Figure 2.
Energy expenditure and locomotor activity measurements. A) Average RQ over a 48-h period was similar in KO and WT mice. B) 48-h total energy expenditure (EE) determined by indirect calorimetry after 16 wk of HED. C) Real-time measurements of EE in mice after 16 wk of HED. Measurements were taken every 45 min. Black bars on x axis indicate lights-off. D) Locomotor activity during lights-on. E) Locomotor activity during lights-off. Data are expressed as total EE per kilogram body weight and total locomotor activity during 48 h of recording. *P < 0.01, **P < 0.001; #P = 0.05; n = 7–8/group. Open bars and squares, WT; solid bars and circles, KOR KO. Error bars show means ± se.
To investigate potential peripheral mechanisms for increased energy expenditure in KOR-KO mice, we measured core body temperature and the expression of UCP-1 and UCP-3 levels in brown adipose tissue (BAT) and skeletal muscle tissue, respectively. There were no differences in basal body temperatures between KOR-KO and WT control mice fed an HED (Table 1), nor did we observe differences during a 5-h cold exposure challenge at 4°C (WT vs. KOR KO: chow 35.1±0.3 vs. 35.7±0.1°C, HED 35.7±0.3 vs. 35.5±0.3°C; _n_=6 or 7). Consistent with that observation, BAT UCP-1 protein levels of KOR-KO mice did not differ from those of WT controls (0.83±0.09 vs. 1.2±0.1 for WT and KO; _P_=0.06; _n_=4 WT, 5 KO). However, we found UCP-3 levels in skeletal muscle to be lower in KOR-KO mice (Supplemental Table 1). Therefore, it is unlikely that increased thermogenic activities in BAT or skeletal muscle were contributing significantly to the preservation of energy expenditure levels in KOR-KO mice fed the HED.
TABLE 1.
Metabolic and hormonal analysis of KOR-KO mice after 16 wk of HED
| Diet and genotype | BW (g) | Glucose (mg/dl) | AUC (×103) | Basal temp. (°C) | T4 (ng/ml) | IGF-1 (ng/ml) | Leptin (ng/ml) | Cort (ng/ml) | |
|---|---|---|---|---|---|---|---|---|---|
| GTT | ITT | ||||||||
| Chow | |||||||||
| WT | 29.2 ± 1.2 | 68 ± 4.3 | 21.5 ± 1.5 | 5.3 ± 0.6 | 37.8 ± 0.1 | 167 ± 6 | 174 ± 15 | 1.2 ± 0.3 | 114 ± 24 |
| KO | 26.7 ± 1.1 | 66 ± 3.0 | 23.8 ± 0.8 | 6.1 ± 0.5 | 37.2 ± 0.1* | 146 ± 11 | 133 ± 20 | 1.7 ± 0.3 | 252 ± 41* |
| HED | |||||||||
| WT | 36.7 ± 0.87 | 71 ± 5.5 | 25.4 ± 1.1 | 6.4 ± 0.6 | 37.7 ± 0.1 | 108 ± 8 | 181 ± 13 | 16.7 ± 3.9 | 76 ± 13 |
| KO | 26.4 ± 0.99** | 53 ± 1.4* | 21.3 ± 1.4# | 5.4 ± 0.3 | 37.4 ± 0.1 | 121 ± 5 | 142 ± 16 | 6.5 ± 1.7* | 149 ± 19^ |
KOR-KO mice fed an HED have reduced hepatic fat storage due to a reduction in TAG formation and an increase in fatty acid β-oxidation
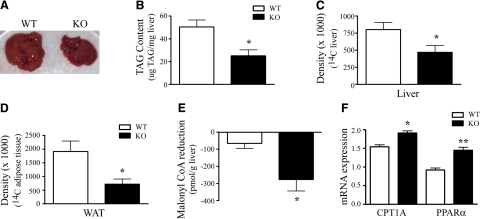
Visual inspection of hepatic tissue collected after 16 wk of an HED revealed that KOR-KO mice had smaller livers that were darker in color compared to WT mice (Fig. 3_A_). We measured total hepatic TAG content to determine whether the morphological changes seen were due to a reduction in lipid accumulation in this tissue. KOR-KO mice had lower levels of total hepatic TAGs on both standard chow (1.68 vs. 1.28 μg TAG/mg liver; _P_=0.03, unpaired t test; _n_=5 WT, 8 KO) and after 16 wk of an HED (Fig. 3_B_). Lower liver TAGs could be due to either a reduction in TAG synthesis or an increase in hepatic fatty acid oxidation. We measured the amount of 14C-acetate-incorporated TAGs in the liver and found that there was a 41% reduction in 14C-acetate incorporated in KOR-KO mice (Fig. 3_C_). Similar reductions were found in epididymal white adipose tissue (Fig. 3_D_), consistent with reduced lipid storage and lower total fat mass in KOR-KO mice fed an HED. Since malonyl CoA is the primary negative regulator of fatty acid β-oxidation, we measured malonyl CoA in the liver using liquid chromatography mass spectrometry (LC-MS/MS) methods. While malonyl CoA levels were reduced by 9% after 16 wk of HED exposure in WT mice, they were reduced by 34% in KOR-KO mice (Fig. 3_E_). Consistent with a reduction of malonyl CoA, there was an up-regulation of CPT-1A mRNA expression in the liver (Fig. 3_F_). Moreover, we found increased expression of the prolipolytic nuclear transcription factor PPARα in KOR-KO mice on HED exposure (Fig. 3_F_). Taken together, these data suggest that KOR-KO mice react to HED exposure with both reduced TAG production and increased lipid oxidation in the liver.
Figure 3.
Reductions in TAGs and increased lipid oxidation contribute to reduced fat mass in KOR-KO mice. A) Livers from KOR-KO mice were visibly smaller compared to WT after 16 wk of HED. B) Total liver TAG content after HED exposure (_P_=0.01; unpaired t test; _n_=6). C) Densiometric analysis of 14C incorporation into hepatic TAGs (_P_=0.04; unpaired t test). Tissue was harvested 2 h after i.p. injection of 14C-acetate from chow-fed WT and KOR-KO mice. D) Densiometric analysis of 14C incorporation into TAGs in epididymal white adipose tissue (WAT) from chow-fed WT and KOR-KO mice (_P_=0.02; unpaired t test; _n_=6). E) Changes in liver malonyl CoA levels in WT and KOR-KO mice after 16 wk of HED (_P_=0.03; unpaired t test; _n_=4). F) qPCR analysis of liver tissue after 16 wk of HED. Data are mRNA expression levels normalized to ARBP. *P = 0.01, **P < 0.001; unpaired t tests; n = 7–8. Open bars, WT; solid bars, KOR KO. Error bars show means ± se.
qPCR demonstrates that KOR mRNA is absent from peripheral tissues with key roles in energy and lipid metabolism
We performed qPCR analysis to determine whether KORs could be signaling directly in peripheral tissues to alter lipid metabolism. Previous studies have reported conflicting results as to whether KORs are present in liver tissue. Furthermore, there have been no studies examining KOR expression in either adipose or skeletal muscle. We found no evidence of KOR mRNA transcripts or its endogenous agonist ligand prodynorphin in liver, muscle, or white adipose tissue, supporting the hypothesis that genetic ablation of KOR is most likely driving changes in body weight, energy expenditure, and peripheral lipid metabolism due to receptor deficiency in the central nervous system (CNS).
KOR-KO mice have normal thyroid hormone levels, but increased plasma corticosterone levels
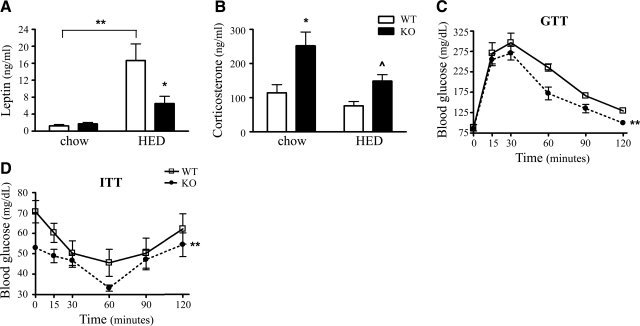
We measured plasma leptin, corticosterone, insulin-like growth factor-1 (IGF-1) as a surrogate marker for growth hormone, and thyroxine T4 levels in WT and KOR-KO mice maintained on either chow or HED, since these hormones could profoundly alter metabolism in both mice and humans. Plasma leptin levels were 61% lower in KOR-KO mice compared to WT controls fed the HED (Fig. 4_A_) and were consistent with reduced adiposity in KOR-KO mice. IGF-1 and T4 levels were not significantly different in KOR-KO mice compared to WTs fed either diet (Table 1). Unexpectedly, we found that KOR-KO mice had a 54 and 49% increase in corticosterone levels on both the standard chow and HED, respectively (Fig. 4_B_). Consistent with that observation, there was an up-regulation in KOR-KO mice of several lipogenic and gluconeogenic genes in liver and to a lesser extent in white adipose tissue that are known to be regulated by corticosterone (Supplemental Tables 2 and 3). As expected, given their protection from HED-induced obesity, glycemic control was improved in KOR-KO mice fed the HED after both glucose and insulin challenges (Fig. 4_C_, D and Table 1).
Figure 4.
Analysis of plasma leptin, corticosterone, and glucose levels in KOR-KO mice. A) Plasma leptin levels in WT and KOR-KO mice were measured by radioimmunoassay. B) KO mice in both chow and HED groups had significantly elevated plasma corticosterone levels. *P < 0.01, **P < 0.001, P̂ = 0.01 unpaired t test. C) Glucose tolerance test (GTT) after 13 wk of HED. D) Insulin tolerance test (ITT) after 14 wk of HED. n = 7–8/group. Open bars and squares, WT; solid bars and circles, KOR KO. Error bars show means ± se.
DISCUSSION
Two independent KOR-KO strains have been reported in which either exon 1 (22) or exon 3 (17, 18) have been deleted. However, there have been no studies identifying the metabolic phenotypes in these mice. Here, we report for the first time that KOR-KO mice are resistant to weight gain when fed a high-fat, energy-dense diet. Central administration of the highly selective KOR antagonist nor-binaltorphimine robustly reduced the intake of palatable diets high in fat or sucrose but had more modest effects on spontaneous feeding (11, 12). Our results, however, suggest that deletion of KOR did not modify food intake. The maintenance of energy expenditure and locomotor activity levels and a reduction in hepatic TAGs are consistent with reduced body weight and fat mass in KOR-KO mice after an HED. Our findings are also consistent with studies in mice deficient for the opioid receptor ligand dynorphin, which exhibit lower body fat mass (23). In addition, KOR-KO mice showed improved glycemic control, suggesting that deletion of KOR might offer a global protection from the deleterious effects of calorically dense high-fat diets in mice.
While defects in gastrointestinal transit or nutrient absorption could contribute to the lean phenotype of KOR-KO mice, it is unlikely that it is the sole contributor. If this were the case, one might expect to have observed a reduction in fat mass in KOR-KO mice, even with standard chow. We did not see a reduction in fat mass, only in fat-free mass, in 6-mo-old KOR-KO mice fed standard chow. We performed an analysis of fecal caloric content in KOR-KO and WT mice fed either standard chow or HED. While the absorption efficiencies were higher for mice fed HED, there were no differences between WT and KOR-KO mice fed either diet. Furthermore, no differences were found in 48-h fecal output. Our data are in agreement with previous studies showing that the highly selective KOR agonist U50,488H given i.c.v. or peripherally was not able to inhibit GI transit in MOR KO animals, suggesting that the dominant effects of opioids on GI transit are mediated through MOR and not KOR (24).
Several studies have demonstrated that KOR signaling is inhibitory to dopaminergic transmission in the striatum and other regions (18, 25, 26), providing a plausible mechanism whereby signaling through KORs could modulate the interaction between diet, energy expenditure, and locomotor activity (Fig. 5). Indeed, administration of the KOR agonist U50,488H reduces locomotor activity (25). Previous studies have not reported changes in spontaneous locomotor activity levels in KOR-KO mice on the C57BL6 background strain (18, 22), but these studies only measured activity for short periods of time. We performed real-time monitoring of locomotor activity over 24 h and found that KOR-KO mice had increased nocturnal activity regardless of whether they were fed a standard chow or an HED (Fig. 2_E_). Mice lacking melanin-concentrating hormone bred on a 129S6 background were found to have greater locomotor activity and energy expenditure levels when fed an HED compared to when the mutation was on the C57BL6 strain (27). It is possible that there are strain and diet-dependent interactions between locomotor activity and energy homeostasis in KOR-KO mice.
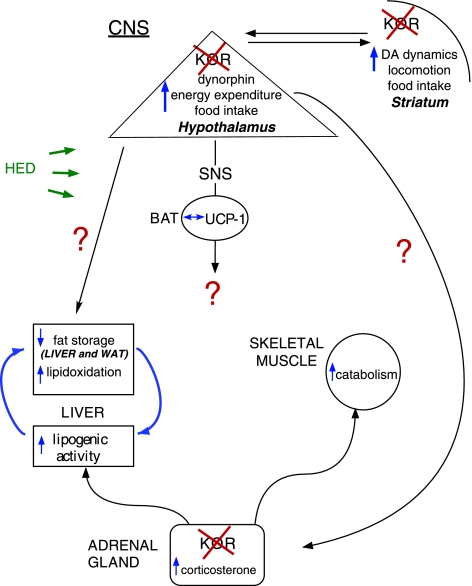
Figure 5.
Model of food intake and body weight regulation in KOR-KO mice. KOR signaling pathway is permissive to fat storage when a calorically dense, high-fat diet is consumed. It is likely that changes in energy expenditure and fatty acid metabolism in KOR-KO mice are mediated by the CNS since KOR was absent from key peripheral tissues, including the liver, skeletal muscle, and white adipose tissue. Documented changes in the dopamine signaling pathway in KOR-KO mice (18) provide a potential common mechanism by which dietary intake, locomotor activity, and energy expenditure may be simultaneously altered. Loss of KORs in the adrenal glands may contribute to the compensatory up-regulation of lipogenic genes observed in KOR-deficient mice.
We found several alterations in hepatic function in KOR-KO mice that were exposed to an HED. Our model (Fig. 5) incorporates an increase in β-oxidation of fatty acids in KOR-KO mice. Malonyl-CoA is an intermediate in the pathways of de novo fatty acid biosynthesis and fatty acid elongation. Moreover, malonyl-CoA functions as an allosteric inhibitor of carnitine palmitoyltransferase 1 (CPT-1), the rate-limiting enzyme regulating mitochondrial β-oxidation of fatty acids (28). A reduction in malonyl CoA would be expected to remove inhibition on CPT-1 and increase fatty acid β-oxidation (29). Indeed, we found reductions in liver malonyl CoA levels and elevated CPT-1A mRNA expression in KOR-KO mice on an HED, consistent with increased lipid oxidation and a significant reduction of TAGs in this tissue. We also found that there was an up-regulation of lipogenic and gluconeogenic gene programs in the liver, including substantial changes of SCD-1 and PEPCK (Supplemental Table 2). However, these changes were insufficient to cause weight gain in KOR-KO mice. Collectively, our data provide compelling evidence to suggest that futile cycling in hepatocytes might be a major contributor to increased energy expenditure and decreased body weight in KOR-KO mice on a calorically dense diet. Because UCP-3 levels were low (Supplemental Table 1), it is unlikely that an increase in mitochondrial density or thermogenic activity in skeletal muscle in response to KOR deficiency was contributing to the preservation of increased energy expenditure on the HED.
To identify the tissues whereby deletion of KOR might produce changes in lipid oxidation and utilization, we assessed KOR and preprodynorphin gene expression in numerous peripheral tissues. However, we did not detect expression of KOR or its ligand in the liver, white adipose tissue, or skeletal muscle. Therefore, it is reasonable to conclude that the effects of KOR deletion on peripheral lipid metabolism following exposure to a HED are mediated by the CNS (Fig. 5). We propose that one or more brain regions may mediate the energy expenditure and locomotor activity effects in KOR-KO mice. One such region is the hypothalamus that serves as a critical mediator of energy balance and contains several nuclei expressing high levels of KOR, including the paraventricular nucleus (PVN) and lateral hypothalamus (LH) (30, 31). Furthermore, mRNAs encoding the endogenous KOR ligand prodynorphin colocalize in almost all orexin-containing neurons in the LH (32), as well as in proopiomelanocortin-containing neurons within the arcuate nucleus (33). KOR is also expressed in several nonhypothalamic brain regions, including the amygdala, striatum, prefrontal cortex, and ventral tegmental area, all of which contain neural circuits that could alter energy expenditure directly, for example, via changes in basal locomotor activity levels, or indirectly through projections to and from the hypothalamus (34,35,36). The presynaptic localization of KORs (37, 38) suggests that there is a disinhibition of regions involved in regulating energy expenditure and nocturnal locomotor activity in KOR-KO mice.
We found an increase in basal corticosterone levels in KOR-KO mice that was independent of diet. Expression of KOR has been localized to the adrenal cortex (39), and removal of this inhibitory Gi-coupled receptor from this tissue might be expected to stimulate corticosterone release. Another likely possibility is that removal of KOR-mediated signaling from corticotrophin-releasing hormone neurons in the hypothalamic PVN leads to activation of the hypothalamic-pituitary-adrenal axis (40). Although acute glucocorticoid treatment increases the mobilization of energy stores, prolonged exposure increases adipose tissue mass in rodents (41). Therefore, it is unlikely that this phenomenon is responsible for the reduced fat mass in KOR-KO mice. It seems tempting to speculate, however, that the increased corticosterone levels may at least in part be responsible for the lower lean mass since elevated glucocorticoids are known to increase catabolism of skeletal muscle (42).
Our studies and others have demonstrated that gross changes in body weight are absent in both single opioid receptor KO mice and also in combinatorial mutant mice lacking all three opioid receptors MOR, DOR, and KOR when fed a standard low-fat chow diet (43). The data presented here support the hypothesis that overactivation of KORs contributes to the development of obesity specifically during prolonged consumption of high-fat calorically dense diets (44, 45). KOR antagonists might be useful in reducing the peripheral metabolic damage of high-fat, high-calorie diets by suppressing body weight gain, maintaining energy expenditure levels, and favoring the oxidation of fatty acids.
Supplementary Material
Supplemental Data
Acknowledgments
The authors thank Aaron Showalter, Jim Baker, and Kelly Credile for their outstanding technical support and helpful advice. The authors also thank David Peake, Lawrence Goodwin, Thomas Seng, David Yurek, Rita Davidson, and Ming-Shang Kuo for development of LC-MS/MS methods for malonyl CoA measurements. Research at the University of Cincinnati was funded by National Institutes of Health grants NIDDK59630, NIDDK69987, and NIDDK56863 (to M.H.T.). Portions of this work were presented in abstract form for the 2009 Keystone Symposia, Obesity: Novel Aspects of the Regulation of Body Weight (Banff, AB, Canada).
References
- Ogden C L, Carroll M D, Curtin L R, McDowell M A, Tabak C J, Flegal K M. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Lean M E. Pathophysiology of obesity. Proc Nutr Soc. 2000;59:331–336. doi: 10.1017/s0029665100000379. [DOI] [PubMed] [Google Scholar]
- Cooke D, Bloom S. The obesity pipeline: current strategies in the development of anti-obesity drugs. Nat Rev. 2006;5:919–931. doi: 10.1038/nrd2136. [DOI] [PubMed] [Google Scholar]
- Levine A S, Grace M, Billington C J, Zimmerman D M. Central administration of the opioid antagonist, LY255582, decreases short- and long-term food intake in rats. Brain Res. 1991;566:193–197. doi: 10.1016/0006-8993(91)91698-z. [DOI] [PubMed] [Google Scholar]
- Shaw W N, Mitch C H, Leander J D, Mendelsohn L G, Zimmerman D M. The effect of the opioid antagonist LY255582 on body weight of the obese Zucker rat. Int J Obes. 1991;15:387–395. [PubMed] [Google Scholar]
- Shaw W N. Long-term treatment of obese Zucker rats with LY255582 and other appetite suppressants. Pharmacol Biochem Behav. 1993;46:653–659. doi: 10.1016/0091-3057(93)90557-a. [DOI] [PubMed] [Google Scholar]
- Statnick M A, Tinsley F C, Eastwood B J, Suter T M, Mitch C H, Heiman M L. Peptides that regulate food intake: antagonism of opioid receptors reduces body fat in obese rats by decreasing food intake and stimulating lipid utilization. Am J Physiol. 2003;284:R1399–R1408. doi: 10.1152/ajpregu.00632.2002. [DOI] [PubMed] [Google Scholar]
- Sahr A E, Sindelar D K, Alexander-Chacko J T, Eastwood B J, Mitch C H, Statnick M A. Activation of mesolimbic dopamine neurons during novel and daily limited access to palatable food is blocked by the opioid antagonist LY255582. Am J Physiol. 2008;295:R463–R471. doi: 10.1152/ajpregu.00390.2007. [DOI] [PubMed] [Google Scholar]
- Morley J E, Levine A S. Involvement of dynorphin and the kappa opioid receptor in feeding. Peptides. 1983;4:797–800. doi: 10.1016/0196-9781(83)90069-4. [DOI] [PubMed] [Google Scholar]
- Morley J E, Levine A S, Kneip J, Grace M, Zeugner H, Shearman G T. The kappa opioid receptor and food intake. Eur J Pharmacol. 1985;112:17–25. doi: 10.1016/0014-2999(85)90234-1. [DOI] [PubMed] [Google Scholar]
- Koch J E, Glass M J, Cooper M L, Bodnar R J. Alterations in deprivation, glucoprivic and sucrose intake following general, mu and kappa opioid antagonists in the hypothalamic paraventricular nucleus of rats. Neuroscience. 1995;66:951–957. doi: 10.1016/0306-4522(95)00001-y. [DOI] [PubMed] [Google Scholar]
- Brugman S, Clegg D J, Woods S C, Seeley R J. Combined blockade of both micro - and kappa-opioid receptors prevents the acute orexigenic action of Agouti-related protein. Endocrinology. 2002;143:4265–4270. doi: 10.1210/en.2002-220230. [DOI] [PubMed] [Google Scholar]
- Bodnar R J. Endogenous opioids and feeding behavior: a 30-year historical perspective. Peptides. 2004;25:697–725. doi: 10.1016/j.peptides.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Kieffer B L, Gaveriaux-Ruff C. Exploring the opioid system by gene knockout. Prog Neurobiol. 2002;66:285–306. doi: 10.1016/s0301-0082(02)00008-4. [DOI] [PubMed] [Google Scholar]
- Tabarin A, Diz-Chaves Y, Carmona Mdel C, Catargi B, Zorrilla E P, Roberts A J, Coscina D V, Rousset S, Redonnet A, Parker G C, Inoue K, Ricquier D, Penicaud L, Kieffer B L, Koob G F. Resistance to diet-induced obesity in mu-opioid receptor-deficient mice: evidence for a “thrifty gene.”. Diabetes. 2005;54:3510–3516. doi: 10.2337/diabetes.54.12.3510. [DOI] [PubMed] [Google Scholar]
- Zuberi A R, Townsend L, Patterson L, Zheng H, Berthoud H R. Increased adiposity on normal diet, but decreased susceptibility to diet-induced obesity in mu-opioid receptor-deficient mice. Eur J Pharmacol. 2008;585:14–23. doi: 10.1016/j.ejphar.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansonoff M A, Zhang J, Czyzyk T, Rothman R B, Stewart J, Xu H, Zjwiony J, Siebert D J, Yang F, Roth B L, Pintar J E. Antinociceptive and hypothermic effects of salvinorin A are abolished in a novel strain of κ-opioid receptor-1 knockout mice. J Pharmacol Exp Therap. 2006;318:641–648. doi: 10.1124/jpet.106.101998. [DOI] [PubMed] [Google Scholar]
- Chefer V I, Czyzyk T, Bolan E A, Moron J, Pintar J E, Shippenberg T S. Endogenous κ-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J Neurosci. 2005;25:5029–5037. doi: 10.1523/JNEUROSCI.0854-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger P T, Kirchner H, Gunnel S, Schrott B, Perez-Tilve D, Fu S, Benoit S C, Horvath T, Joost H G, Wortley K E, Sleeman M W, Tschop M H. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. Am J Physiol Gastrointest Liver Physiol. 2008;294:G610–G618. doi: 10.1152/ajpgi.00321.2007. [DOI] [PubMed] [Google Scholar]
- Pfluger P T, Herranz D, Velasco-Miguel S, Serrano M, Tschop M H. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkler P E, Kerner J, Kasumov T, Parland W, Hoppel C L. Quantification of malonyl-coenzyme A in tissue specimens by high-performance liquid chromatography/mass spectrometry. Anal Biochem. 2006;352:24–32. doi: 10.1016/j.ab.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le Meur M, Roques B P, Maldonado R, Kieffer B L. Disruption of the κ-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective κ-agonist U-50,488H, and attenuates morphine withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainsbury A, Lin S, McNamara K, Slack K, Enriquez R, Lee N J, Boey D, Smythe G A, Schwarzer C, Baldock P, Karl T, Lin E J, Couzens M, Herzog H. Dynorphin knockout reduces fat mass and increases weight loss during fasting in mice. Mol Endocrinol. 2007;21:1722–1735. doi: 10.1210/me.2006-0367. [DOI] [PubMed] [Google Scholar]
- Roy S, Liu H C, Loh H H. mu-Opioid receptor-knockout mice: the role of mu-opioid receptor in gastrointestinal transit. Brain Res Mol Brain Res. 1998;56:281–283. doi: 10.1016/s0169-328x(98)00051-5. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Therap. 1988;244:1067–1080. [PubMed] [Google Scholar]
- Margolis E B, Lock H, Chefer V I, Shippenberg T S, Hjelmstad G O, Fields H L. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkotou E, Jeon J Y, Wang X, Marino F E, Carlson M, Trombly D J, Maratos-Flier E. Mice with MCH ablation resist diet-induced obesity through strain-specific mechanisms. Am J Physiol Regul Integr Comp Physiol. 2005;289:R117–R124. doi: 10.1152/ajpregu.00861.2004. [DOI] [PubMed] [Google Scholar]
- Ruderman N B, Saha A K, Kraegen E W. Minireview: malonyl CoA, AMP-activated protein kinase, and adiposity. Endocrinology. 2003;144:5166–5171. doi: 10.1210/en.2003-0849. [DOI] [PubMed] [Google Scholar]
- Chien D, Dean D, Saha A K, Flatt J P, Ruderman N B. Malonyl-CoA content and fatty acid oxidation in rat muscle and liver in vivo. Am J Physiol Endocrinol Metab. 2000;279:E259–E265. doi: 10.1152/ajpendo.2000.279.2.E259. [DOI] [PubMed] [Google Scholar]
- Shuster S J, Riedl M, Li X, Vulchanova L, Elde R. The κ opioid receptor and dynorphin colocalize in vasopressin magnocellular neurosecretory neurons in guinea-pig hypothalamus. Neuroscience. 2000;96:373–383. doi: 10.1016/s0306-4522(99)00472-8. [DOI] [PubMed] [Google Scholar]
- Smith M J, Wise P M. Localization of kappa opioid receptors in oxytocin magnocellular neurons in the paraventricular and supraoptic nuclei. Brain Res. 2001;898:162–165. doi: 10.1016/s0006-8993(01)02154-0. [DOI] [PubMed] [Google Scholar]
- Chou T C, Lee C E, Lu J, Elmquist J K, Hara J, Willie J T, Beuckmann C T, Chemelli R M, Sakurai T, Yanagisawa M, Saper C B, Scammell T E. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maolood N, Meister B. Dynorphin in pro-opiomelanocortin neurons of the hypothalamic arcuate nucleus. Neuroscience. 2008;154:1121–1131. doi: 10.1016/j.neuroscience.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Raynor K, Kong H, Breder C D, Takeda J, Reisine T, Bell G I. Cloning and functional comparison of κ and δ opioid receptors from mouse brain. Proc Natl Acad Sci U S A. 1993;90:6736–6740. doi: 10.1073/pnas.90.14.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePaoli A M, Hurley K M, Yasada K, Reisine T, Bell G. Distribution of κ opioid receptor mRNA in adult mouse brain: an in situ hybridization histochemistry study. Molec Cell Neurosci. 1994;5:327–335. doi: 10.1006/mcne.1994.1039. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox C A, Meng F, Akil H, Watson S J. Kappa 1 receptor mRNA distribution in the rat CNS: comparison to kappa receptor binding and prodynorphin mRNA. Mol Cell Neurosci. 1994;5:124–144. doi: 10.1006/mcne.1994.1015. [DOI] [PubMed] [Google Scholar]
- Svingos A L, Colago E E, Pickel V M. Cellular sites for dynorphin activation of κ-opioid receptors in the rat nucleus accumbens shell. J Neurosci. 1999;19:1804–1813. doi: 10.1523/JNEUROSCI.19-05-01804.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos A L, Colago E E. κ-Opioid and NMDA glutamate receptors are differentially targeted within rat medial prefrontal cortex. Brain Res. 2002;946:262–271. doi: 10.1016/s0006-8993(02)02894-9. [DOI] [PubMed] [Google Scholar]
- Kapas S, Purbrick A, Hinson J P. Action of opioid peptides on the rat adrenal cortex: stimulation of steroid secretion through a specific mu opioid receptor. J Endocrinol. 1995;144:503–510. doi: 10.1677/joe.0.1440503. [DOI] [PubMed] [Google Scholar]
- Wittmann W, Schunk E, Rosskothen I, Gaburro S, Singewald N, Herzog H, Schwarzer C. Prodynorphin-derived peptides are critical modulators of anxiety and regulate neurochemistry and corticosterone. Neuropsychopharmacology. 2009;34:775–785. doi: 10.1038/npp.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt J P. Dietary fat, carbohydrate balance, and weight maintenance. Ann N Y Acad Sci. 1993;683:122–140. doi: 10.1111/j.1749-6632.1993.tb35699.x. [DOI] [PubMed] [Google Scholar]
- Tataranni P A, Larson D E, Snitker S, Young J B, Flatt J P, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996;271:E317–E325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- Czyzyk T A, King M A, Zhang J, Schuller A G, Pasternak G W, Pintar J E. Production of combinatorial opioid receptor knockout mice. 29th Annu Soc Neurosci Meet. 1999;25:179. [Google Scholar]
- Welch C C, Kim E M, Grace M K, Billington C J, Levine A S. Palatability-induced hyperphagia increases hypothalamic dynorphin peptide and mRNA levels. Brain Res. 1996;721:126–131. doi: 10.1016/0006-8993(96)00151-5. [DOI] [PubMed] [Google Scholar]
- Chang G Q, Karatayev O, Ahsan R, Gaysinskaya V, Marwil Z, Leibowitz S F. Dietary fat stimulates endogenous enkephalin and dynorphin in the paraventricular nucleus: role of circulating triglycerides. Am J Physiol Endocrinol Metab. 2007;292:E561–E570. doi: 10.1152/ajpendo.00087.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data