Requirement for Neo1p in Retrograde Transport from the Golgi Complex to the Endoplasmic Reticulum (original) (raw)
Abstract
Neo1p from Saccharomyces cerevisiae is an essential P-type ATPase and potential aminophospholipid translocase (flippase) in the Drs2p family. We have previously implicated Drs2p in protein transport steps in the late secretory pathway requiring ADP-ribosylation factor (ARF) and clathrin. Here, we present evidence that epitope-tagged Neo1p localizes to the endoplasmic reticulum (ER) and Golgi complex and is required for a retrograde transport pathway between these organelles. Using conditional alleles of NEO1, we find that loss of Neo1p function causes cargo-specific defects in anterograde protein transport early in the secretory pathway and perturbs glycosylation in the Golgi complex. Rer1-GFP, a protein that cycles between the ER and Golgi complex in COPI and COPII vesicles, is mislocalized to the vacuole in neo1-ts at the nonpermissive temperature. These phenotypes suggest that the anterograde protein transport defect is a secondary consequence of a defect in a COPI-dependent retrograde pathway. We propose that loss of lipid asymmetry in the cis Golgi perturbs retrograde protein transport to the ER.
INTRODUCTION
Yeast Saccharomyces cerevisiae contains 16 P-type ATPases that can be phylogenetically grouped into five subfamilies: heavy metal ion transporters, Na+ transporters, Ca2+ transporters, H+ transporters, P4 ATPases with unknown substrate, and potential aminophospholipid translocases (APTs; Catty et al., 1997). The APT subfamily contains five genes, DRS2, DNF1, DNF2, DNF3, and NEO1, that share 40-83% amino acid sequence similarity with each other (Catty et al., 1997; Hua et al., 2002). APTs, or flippases, couple ATP hydrolysis to the translocation of phosphatidylserine (PS) and/or phosphatidylethanolamine (PE) from the external leaflet of a membrane bilayer to the cytosolic leaflet and appear to be responsible for concentrating PS and PE in the cytosolic leaflet of biological membranes (Balasubramanian and Schroit, 2003). This activity has been primarily defined in the plasma membrane of blood cells (Seigneuret and Devaux, 1984; Auland et al., 1994) and in bovine chromaffin granule membranes (Zachowski et al., 1989). The latter APT appears to be equivalent to ATPase II, which is 47% identical to Drs2p from yeast (Tang et al., 1996). The human genome encodes multiple members of the APT subfamily including genes implicated in genetic disorders. FIC1 mutations cause a defect in bile secretion and ATP10C is in an imprinted region of chromosome 18 linked to Angelman syndrome and potentially autism (Thompson and Jansen, 2000 Herzing et al., 2001; Meguro et al., 2001). In addition, the mouse ATP10C gene is linked to an increased body fat phenotype (Dhar et al., 2000).
The localization and cellular requirements for the yeast APTs are starting to be defined. Drs2p and Dnf3p localize to late Golgi and perhaps endosomal membranes, whereas Dnf1p and Dnf2p localize primarily to the plasma membrane (Chen et al., 1999; Hua et al., 2002; Pomorski et al., 2003). Deletion of all four of these genes is lethal in yeast, indicating that DRS2 and the _DNF_s form an essential gene group (Hua et al., 2002). Strains harboring disruptions of DNF1,2 or DRS2 exhibit a defect in lipid translocation across the plasma membrane and expose more PE on the external leaflet than wild-type cells (Tang et al., 1996; Gomes et al., 2000; Pomorski et al., 2003). These mutant phenotypes are consistent with the proposed translocase activity for the Drs2/Neo1 family ATPases, although this conclusion has been controversial (Siegmund et al., 1998; Marx et al., 1999) and direct evidence that these proteins catalyze lipid translocation is still lacking.
Drs2 and Dnf proteins are also required for protein trafficking between the Golgi complex, plasma membrane, and endosomal/vacuolar system (Chen et al., 1999; Gall et al., 2002; Hua et al., 2002; Pomorski et al., 2003). DRS2 interacts genetically with clathrin and ARF, and _drs2_Δ exhibits defects in late Golgi function that are similar to clathrin mutants (Chen et al., 1999). In addition, drs2 mutants also exhibit a defect in generating a specific class of exocytic vesicles carrying invertase and acid phosphatase (Gall et al., 2002). These vesicles require clathrin for their formation and at least a portion of them are clathrin-coated. The _drs2_Δ _dnf1_Δ double mutant exhibits a significantly stronger defect in the alkaline phosphatase and carboxypeptidase Y (CPY) vacuolar transport pathways compared with the single mutants. This suggests that Drs2p and Dnf1p are functionally redundant in their ability to support vacuolar protein transport, whereas only Drs2p can support the generation of exocytic vesicles. Moreover, the _dnf1_Δ _dnf2_Δ _dnf3_Δ triple deletion mutant exhibits a defect in an endosome to TGN recycling pathway traveled by a v-SNARE protein (Hua et al., 2002). ARF, clathrin, and/or adaptins (AP-1, AP-3 and GGAs) have been implicated in each of these pathways that require Drs2/Dnf proteins for normal function.
Among the five potential APTs in yeast, NEO1 is unique in that deletion of this gene alone is lethal (Prezant et al., 1996), and so none of the other APT subfamily members can perform the essential function of Neo1p. In addition, overexpression of NEO1 cannot suppress the _drs2_Δ cold-sensitive growth defect, nor can overexpression of DRS2 rescue _neo1_Δ lethality (Hua et al., 2002). NEO1 was first identified in a screen for genes that confer resistance to the aminoglycoside neomycin upon overexpression (Prezant et al., 1996). Neo1p overexpression did not confer resistance to other drugs tested including ethidium bromide, cycloheximide, and chloramphenicol, indicating that Neo1p is not a multidrug resistance protein. The Neo1p ATPase activity is required to support cell viability and to confer neomycin resistance (Prezant et al., 1996). It is not known if Neo1p can directly pump neomycin out of the cell or if it confers the drug resistance through another mechanism. Nothing is known about the essential cellular functions of Neo1p.
Because Drs2p and Dnf proteins are involved in protein transport in the late secretory and endosomal pathways, we hypothesized that Neo1p might also be required for protein transport in the secretory pathway. To test this possibility, conditional alleles of neo1 were generated and their effect on protein transport was determined. We found that Neo1p is indeed required for efficient protein transport in the early secretory pathway and Golgi-dependent glycosylation. Protein transport between the ER and Golgi is mediated by small transport vesicles, with COPII-coated vesicles carrying cargo in the anterograde direction and COPI-coated vesicles carrying cargo in the retrograde direction (Kirchhausen, 2000). Temperature-sensitive mutations in COPII subunits (e.g., sec12 or sec23) cause a block in the ER-to-Golgi transport and the accumulation of all cargo proteins in the ER at the nonpermissive temperature (Barlowe, 2002). COPI mutations (e.g., sec21) perturb retrograde transport and can also cause a defect in ER-to-Golgi transport, although not all proteins are subject to this block. For example, Hsp150 and invertase are secreted efficiently from sec21 cells, whereas carboxypeptidase Y (CPY) and pro–α-factor are blocked in the ER (Gaynor and Emr, 1997). Mutations in early Golgi ARF GAPs (Glo3 and Gcs1) also perturbs CPY transport and invertase glycosylation but not invertase secretion (Poon et al., 1999). Rer1p can be used to distinguish a primary defect in anterograde or retrograde transport, because this protein is trapped in the ER in COPII mutants but mislocalized to the vacuole in COPI mutants (Sato et al., 2001). The neo1 mutants also exhibit cargo-selective defects in anterograde protein transport and mislocalize Rer1p to the vacuole. These phenotypes suggest that Neo1p is required for COPI-dependent retrograde transport from the Golgi to the ER.
MATERIALS AND METHODS
Media, Strains, and Plasmids
Yeast strains were grown in standard rich medium (YP) or synthetic minimal (SM) media containing required supplements, with 2% glucose (YPD) or 2% galactose as indicated. YPD sorbitol plates were made by autoclaving YPD agar supplemented with the indicated amount of sorbitol. YPD, pH 8.0, plates were made by adding 10 mM Tris base to YPD and adjusting to pH 8.0 before autoclaving. Neomycin, cation, ethanol, calcofluor white (CW), and NaF plates were made by adding the indicated concentration of the chemicals after autoclaving. Sporulation was done by growing cells in YP 2% KOAc presporulation media to 1.0 OD/ml before shifting to SPM media (0.3% KOAc, 0.02% raffinose, and 0.25× supplements) for 3-7 d.
Yeast strains used in this study are listed in Table 1. The yeast knockout strain collection was originally purchased from Research Genetics, which is now Resgen, Invitrogen Corporation (Carlsbad, CA). ZHY9075E was generated by sporulation of BY4743 _neo1_Δ pBM743-NEO1 (Hua et al., 2002). ZHY219RR and all ZHY628 strains were generated by replacing pBM743-NEO1 in ZHY9075E by either p413-NEO1 (carrying wild-type NEO1) or p413-neo1-1 through 6 (carrying neo1 ts alleles).
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| BY4742 | MATα his3 leu2 ura3 lys2 | Invitrogen |
| BY4743 _neo1_Δ | MATa/α his3/his3 leu2/leu2 ura3/ura3 lys2/LYS2 met15/MET15 neo1Δ::KanMX/NEO1 | Invitrogen |
| ZHYNEO1-MYC | MATα his3 leu2 ura3 lys2 NEO1::13XMYC | Hua et al. (2002) |
| ZHY9075E | MATα his3 leu2 ura3 neo1Δ pBM743-NEO1 | This study |
| ZHY219RR | MATα his3 leu2 ura3 neo1Δ p413-NEO1 | This study |
| ZHY628-15B | MATα his3 leu2 ura3 neo1Δ p413-neo1-1 | This study |
| ZHY628-34A | MATα his3 leu2 ura3 neo1Δ p413-neo1-2 | This study |
| ZHY628-12F | MATα his3 leu2 ura3 neo1Δ p413-neo1-3 | This study |
| ZHY628-22D | MATα his3 leu2 ura3 neo1Δ p413-neo1-4 | This study |
| ZHY628-26H | MATα his3 leu2 ura3 neo1Δ p413-neo1-5 | This study |
| ZHY628-36A | MATα his3 leu2 ura3 neo1Δ p413-neo1-6 | This study |
| ZHY129-15B28A | MATα his3 leu2 ura3 lys2 neo1-1::HIS3-KanMX | This study |
| ZHY129-34A2C | MATα his3 leu2 ura3 lys2 neo1-2::HIS3-KanMX | This study |
| ZHY124-15B1B | MATα his3 leu2 ura3 ade2 trp1 suc2 neo1-1::HIS3-KanMX | This study |
| ZHY124-15B1C | MATa his3 leu2 ura3 ade2 trp1 suc2 neo1-1::HIS3-KanMX | This study |
| ZHY124-34A2A | MATa his3 leu2 ura3 ade2 trp1 suc2 neo1-2::HIS3-KanMX | This study |
| ZHY124-34A2B | MATα his3 leu2 ura3 ade2 trp1 suc2 neo1-2::HIS3-KanMX | This study |
| BY4741 _arf1_Δ | _MATa his3 leu2 ura3 met15 arf1_Δ | Invitrogen |
| BY4741 _glo3_Δ | _MATa his3 leu2 ura3 met15 glo3_Δ | Invitrogen |
| BY4741 _gcs1_Δ | _MATa his3 leu2 ura3 met15 gcs1_Δ | Invitrogen |
| BY4741 _gda1_Δ | _MATa his3 leu2 ura3 met15 gda1_Δ | Invitrogen |
| BY4741 _mnn9_Δ | _MATa his3 leu2 ura3 met15 mnn9_Δ | Invitrogen |
| BY4741 _dnf1_Δ | _MATa his3 leu2 ura3 met15 dnf1_Δ | Invitrogen |
| BY4741 _dnf2_Δ | _MATa his3 leu2 ura3 met15 dnf2_Δ | Invitrogen |
| ZHY615D1C | _MATa his3 leu2 ura3 lys2 drs2_Δ | Hua et al. (2002) |
| EGY1211-6B | MATa leu2 ura3 his3 trp1 suc2 sec21-1 | Gaynor et al. (1998) |
| EGY101-16D | MATa leu2 ura3 his3 trp1 suc2 ret1-1 | Gaynor et al. (1998) |
| TBY102 | MATα ura3 leu2 his3 trp1 lys2 suc2 sec18-1 | Brigance et al. (2000) |
| TBY103 | MATa leu2 ura3 his3 trp1 sec23-1 | Chen et al. (1999) |
| TGY144 | MATα leu2 ura3 his3 trp1 lys2 sec1-1 | Chen et al. (1999) |
| TBY120 | MATα leu2 ura3 his3 trp1 lys2 sec12-4 | This study |
| CTY1-1A | MATa leu2 his3 lys2 sec14-1 | Bankaitis et al. (1989) |
| AFM69-1A | MATα leu2 ura3 his3 sec7-4 | Franzusoff and Schekman (1989) |
The plasmid p413-NEO1PM was generated by replacing the ADH promoter from p413-ADH with the PCR-amplified NEO1 promoter region (518 bp) on a _Sac_I/_Xba_I fragment. The NEO1 coding sequence from pYGW1-NEO1 (Hua et al., 2002) was cloned into the _Eco_RI/_Sal_I site of p413-NEO1PM to generate p413-NEO1. Sequencing of p413-NEO1 revealed two nucleotide changes compared with published sequence and causes two amino acid changes (V186M and T558A). This plasmid fully rescues the _neo1_Δ lethality phenotype. Other plasmids used in this study are pSKY5/RER1-0 (a cen-based URA3 plasmid carrying Rer1-GFP; Sato et al., 2001), pOH-URA3 (a 2-μm plasmid with OCH1-HA; Harris and Waters, 1996), and pRS426-MNN1 (Graham et al., 1994).
Isolation of neo1-ts Alleles
Random PCR mutagenesis (with 3.5 mM MgCl2, 0.5 mM MnCl2, 0.45 mM dATP, 0.72 mM dCTP, 0.16 mM dGTP, and 1.12 mM dTTP in the PCR reaction for 35 cycles) was used to generate mutations in the NEO1 gene. Initial attempts at mutagenesis using higher concentrations of dNTPs and fewer cycle numbers failed to yield a neo1-ts allele. Primers used for PCR were 5′-TTTGTGCCAACCCTATTATATGAA, and 5′-GCATTGTTTTATTCCATGTGTAGA. Plasmid p413-NEO1 was gapped using _Nco_I and _Stu_I and was cotransformed with the mutagenized PCR products into ZHY9075E. The resulting transformants (∼10,000) were then replicated onto 5-fluoroorotic acid (5-FOA) plates to select against the original wild-type NEO1 plasmid (pBM743-NEO1 harboring URA3). Approximately 1000 colonies grew on the 5-FOA plates, which were then tested for growth at 37°C. Six colonies exhibited a tight ts growth defect at 37°C. Plasmids were rescued from these six strains and named p413-neo1-1 to p413-neo1-6. The following mutations are found in the six neo1 ts alleles: neo1-1, D356V, I381V, A420V, E528D, T529P, Q538L, D568V, T604N, Q727R, R805K, E807K, T1043S; neo1-2, H346L, A431V, V457I, L465I, P491L, S502C, S542C, P581L, S618T, K672E, L719W, D725V, I732N, L752S, Q812R, A854V; neo1-3, H279Q, T337I, A370V, A375S, V402A, V430L, N437I, R496H, M569K; neo1-4, M234I, S297T, C319S, N366I, E528K, R565H, E624D, V674A, D716E, S729N, M872K; neo1-5, A370V, P491Q, S542R, G698S, S908T, A940V; neo1-6, C380Y, F661S, C855S, A951T, Y957N, V986I, H993L, T1000S.
Integration of neo1-ts Alleles into the Yeast Genome
To facilitate genetic crosses of neo1-ts mutants with other mutants, neo1-ts alleles were integrated into the NEO1 locus. The integrating neo1-ts plasmids (pZH1125-15B and pZH1125-34A for neo1-1 and neo1-2, respectively) were made by first inserting the _Sac_I/_Sal_I fragment from p413-neo1-1 or p413-neo1-2 into pRS303, and then a 600-bp PCR-amplified KanMX fragment was inserted into the _Apa_I/Xho_I site of the resulting plasmid. pZH1125-15B and pZH1125-34A were then cut with Nru_I, a unique restriction enzyme site located within the KanMX fragment, and then transformed into BY4743 neo1_Δ::KanMX, to generate the BY4743 neo1_Δ::neo1-1 (or neo1-2) HIS3 strain. These strains were then subjected to sporulation, and the progeny were tested for ts growth to identify the integrated neo1-ts mutants (ZHY129-15B28A and ZHY129-34A2A). To integrate neo1 ts alleles into the SEY6210 background, a PCR-amplified KanMX-NEO1 knockout module (http://www-sequence.s-tanford.edu/group/yeast_deletion_project/deletions3.html) was first transformed into a SEY6210.5 diploid (Robinson et al., 1988). Then the neo1 ts integration plasmids pZH1125-15B and pZH1125-34A were transformed into the resulting strain. The transformants were then subjected to sporulation and tested for a ts growth phenotype to identify neo1-ts integrated mutants (All ZHY124 strains are in SEY6210 background).
Genetic Analysis of neo1 ts Mutants
Genetic crosses between neo1-ts (ZHY129-15B28A and ZHY129-34A2C) and arf1_Δ, glo3_Δ_, gcs1_Δ_, gda1_Δ_, mnn9_Δ_, dnf1_Δ_, dnf2_Δ, and _dnf2_Δ strains were done in the BY4741/BY4742 strain background. Genetic crosses between neo1-ts (ZHY124-15B1B, ZHY124-15B1C, ZHY124-34A2A, and ZHY124-34A2B) and sec21-1, ret1-1, sec18-1, sec23-1, sec1-1, sec12-1, sec14-1, and sec7-4 were done in the SEY6210 strain background. Diploids from the above crosses were subjected to sporulation and tetrad dissection, and the double mutants were collected if available.
Immunological and Imaging Methods
Cell labeling, immunoprecipitation (Gaynor and Emr, 1997), and immunoblotting (Chen et al., 1999) were performed as described previously. Anti-α-factor (Graham and Emr, 1991) and anti-carboxypeptidase Y (CPY) serum (Klionsky et al., 1988) were used for immunoprecipitation. The 9E10 mouse monoclonal c-Myc antibody (Oncogene Research Products, Darmstadt, Germany) (1:100), polyclonal affinity-purified rabbit anti-HA antibody (Zymed Laboratories, South San Francisco, CA; 1:100), and anti-Mnn1p antibody (Graham et al., 1994; 1:50) were used for immunofluorescence microscopy. Immunofluorescence and green fluorescent protein (GFP) fluorescence were observed using an Axioplan microscope (Carl Zeiss, Thornwood, NY), and fluorescent images were processed using MetaMorph 4.5 software (Universal Imaging, Downingtown, PA). Samples for electron microscopy were prepared as described previously (Rieder et al., 1996) and sections (50-60 nm) were viewed on a CM12 electron microscope (Philips, Eindhoven, Netherlands).
RESULTS
Neo1-myc Localizes to the ER and Golgi Complex
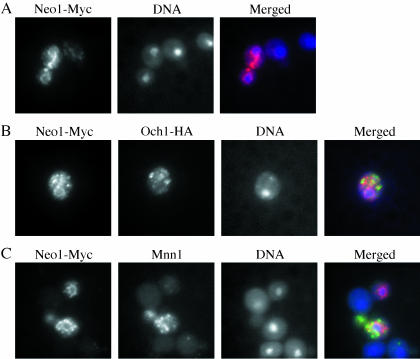
To better understand the essential function of Neo1p, we first examined the intracellular localization of this protein by immunofluorescence. A 13-myc epitope tag was integrated into the genome at the 3′ end of NEO1. This strain grew as well as wild-type, indicating that Neo1-myc was fully functional. Immunofluorescence localization of myc-tagged Neo1p suggested a role for this protein in the early secretory pathway. Neo1-Myc was found in a continuous membrane structure that surrounds the nucleus and extends to the periphery of the cell body (Figure 1A), which is typical for ER localization in yeast cells. Neo1-Myc was also found in punctate structures where it partially colocalized with both the early Golgi marker Och1p (Figure 1B) and the late Golgi marker Mnn1p (Figure 1C). Therefore, Neo1-Myc localizes to both ER and Golgi membranes. This conclusion is consistent with the distribution of Neo1-HA in subcellular fractions examined by the Holthuis group, although they did not observe ER staining by immunofluorescence (Pomorski et al., 2003). Thus, it is possible that the c-myc epitope tag induced an artificially high concentration of Neo1-myc in the ER of our strain.
Figure 1.
Neo1-Myc localizes to the ER and Golgi complex. (A) Strain ZHYNEO1-MYC was labeled with a mouse monoclonal anti-myc antibody. (B) The Neo1-Myc strain (ZHYNEO1-MYC) expressing HA-tagged OCH1 (from pOH-URA3) was labeled with rabbit anti-HA antibody and mouse anti-myc to detect Och1-HA and Neo1-Myc. (C) Strain ZHYNEO1-MYC overexpressing Mnn1p (pRS426 MNN1) was stained with affinity-purified polyclonal rabbit anti-Mnn1p and mouse anti-myc. DNA was stained with DAPI to identify the position of the nucleus in all samples. For the overlay images, Neo1-Myc localization is shown in red, Och1-HA or Mnn1p is in green, and DNA is in blue.
Neo1p Depletion Causes Defects in Protein Transport and Golgi Glycosylation
A conditional allele of NEO1 was produced by cloning this gene under transcriptional control of the GAL1 promoter (Hua et al., 2002) so that its expression could be controlled by carbon source (Johnston and Davis, 1984). The _neo1_Δ GAL::NEO1 strain grew well on inducing galactose media, but did not form visible colonies on glucose media where Neo1p expression was repressed. After initial growth in the presence of galactose, ∼18 h were required for cell growth to slow down in YP glucose medium and ∼24 h in synthetic minimal glucose medium. This represented ∼10 generations for both media, which is typical for proteins that do not turn over rapidly.
To determine if Neo1p is involved in protein trafficking, cells depleted for Neo1p were pulse-labeled and chased to examine the kinetics of pro–α-factor processing and transport (Figure 2). The yeast α-factor mating pheromone is synthesized in the ER as a high-molecular-weight precursor and is further modified in the Golgi complex to produce the heterogeneously glycosylated pro–α-factor form that migrates as a high-molecular-mass smear by SDS-PAGE. This precursor is processed in the TGN through a series of proteolytic events, initiated by Kex2p, to produce the mature α-factor peptide, which is secreted (Fuller et al., 1988).
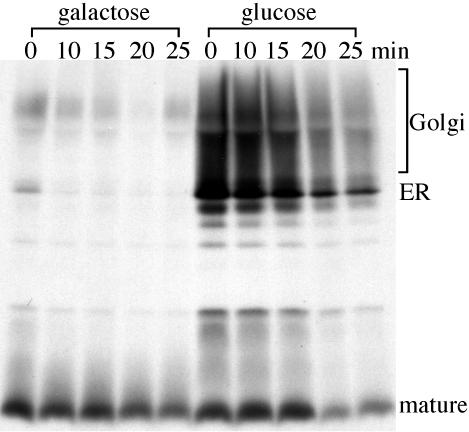
Figure 2.
Neo1p depletion causes protein transport and glycosylation defects. ZHY9075E (_neo1_Δ GAL::NEO1) was grown in galactose medium and subcultured in media containing either galactose or glucose media for 24 h. Galactose induces high-level expression of Neo1p and glucose shuts off expression from the GAL::NEO1 construct. Cells were labeled with [35S]methionine/cysteine for 15 min and then chased for the times indicated. α-Factor was recovered from each sample by immunoprecipitation and subjected to SDS-PAGE.
The _neo1_Δ GAL::NEO1 strain was grown in SM galactose and shifted to SM glucose for 24 h to deplete Neo1p. Cells were then labeled with [35S]methionine/cysteine for 15 min and chased for the time points indicated. In cells maintained in galactose, pro–α-factor was processed to the mature form very quickly (Figure 2, galactose), indicating that protein transport from the ER through the Golgi complex was efficient. However, for cells grown in glucose media, both the ER and Golgi forms of pro–α-factor were present throughout the chase. In addition, the Golgi pro–α-factor forms appeared to be substantially underglycosylated because most of this precursor migrated just above the ER form (Figure 2, glucose). This result indicates that depletion of Neo1p causes defects in protein transport from the ER to the Golgi and potentially through the Golgi complex. Depletion of Neo1p also appeared to perturb Golgi-dependent glycosylation.
Isolation of neo1-ts Mutants
To facilitate studies of its essential function, we screened for temperature sensitive (ts) alleles of NEO1. The middle three fourths of the NEO1 gene, including the ATPase domain and six flanking transmembrane domains, was targeted for mutagenesis (Figure 3 and MATERIALS AND METHODS). Among nearly 10,000 transformants screened, we found six colonies that grew well at 27°C but exhibited a tight ts growth defect. Three mutants could not form visible colonies at 34°C, and three could grow at 34°C but not 37°C (Figure 4, YPD). All six mutant alleles have been sequenced and each mutant contains from 6 to 16 missense mutations, with 10 mutations on average, spanning the targeted 810 amino acids (Figure 3). It does not appear that the P-type ATPase conserved motifs were more mutated than the other regions. Instead, it seems that the mutations clustered at the predicted transmembrane motifs, especially in transmembrane domain 3 (TMD3) where five of the six alleles carried mutations. Other clustered mutations included four residues that were mutated in two different alleles, and five sites where adjacent amino acids were mutated in the same or different alleles. The neo1-5 allele only contains six mutations, and three of them overlap with mutations from other alleles, suggesting that mutations of A370, P491, and S542 likely contribute to the ts phenotype (Figure 3). Another interesting phenomenon is that the mutations tend to cluster even within one allele. For example, five of eight mutations from neo1-6 reside within 50 of the total of 810 amino acids targeted. Eight of nine mutations in neo1-3 reside within the first third of this region (Figure 3). This phenomenon might reflect a destabilization of local secondary structures in the ts mutant proteins.
Figure 3.
Mutations present in the six neo1-ts alleles. The NEO1 wild-type sequence from amino acid 234-1043 subjected to mutagenesis is shown with corresponding residue numbers on top. Amino acids substitutions found in the six neo1 ts alleles are aligned underneath the corresponding wild-type residue. The superscript for each amino acid substitution indicates the neo1 allele number from which it is derived (neo1-1 to neo1-6). P-type ATPase conserved motifs are marked with asterisks. Predicted transmembrane domains three through seven are labeled as TMD3-7.
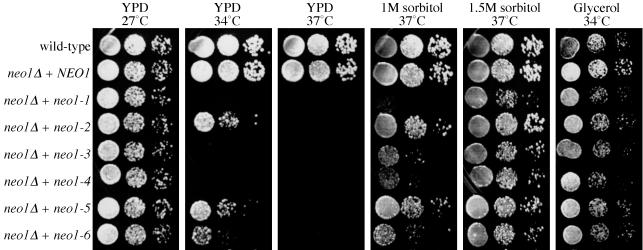
Figure 4.
The growth defect of neo1-ts mutants is suppressed by osmotic support or nonfermentable carbon sources. Serial dilutions of the wild-type strain (BY4742) or _neo1_Δ carrying indicated alleles (expressing from plasmids) were spotted onto YPD plates, YPD supplemented with 1 or 1.5 M sorbitol, or YP with 3% glycerol as the carbon source. Plates were incubated at the indicated temperatures for two or three days before photographing.
The neo1-ts mutants we isolated can still divide a few times after shifting to the nonpermissive temperature. The neo1-ts mutants formed microcolonies containing a few to several dozens of cells when grown at 37°C and this varied depending on the allele. In addition, the growth rate of neo1-ts mutants in liquid media continued unperturbed for ∼6 h when cells were shifted from 27 to 37°C. These data indicate that either all of the neo1-ts alleles are slowly inactivated after temperature shift, that they never lose complete function at 37°C, or that cells can divide a few times in the absence of Neo1p function.
The ts Growth Defect of neo1-ts Mutants Is Suppressed by High Osmotic Support
The glycosylation defect observed by depleting Neo1p suggested that cell wall biosynthesis might be perturbed in neo1 mutants. Mutants that grow poorly because of a defective cell wall can be remedied by sorbitol or other osmotic support and all six neo1-ts mutants can be rescued by osmotic support at the nonpermissive temperature. The growth of most mutants improved significantly on 1 M sorbitol, and they grew nearly as well as wild-type on 1.5 M sorbitol (Figure 4 and Table 2). In addition, 1 M NaCl also suppressed the neo1 ts growth defect (Table 2). The yeast cell wall consists of glucans, mannans, and chitin (Klis et al., 2002). Many cell wall biosynthesis mutants are hypersensitive to the chitin-binding compound calcofluor white (CW). The neo1-ts mutants were also hypersensitive to CW (Table 2), further suggesting a cell wall defect.
Table 2.
Growth profile of six neo1-ts mutants
| WT | neo1-1 | neo1-2 | neo1-3 | neo1-4 | neo1-5 | neo1-6 | _neo1_Δ | |
|---|---|---|---|---|---|---|---|---|
| 27°C Glucose | +++ | +++ | +++ | +++ | +++ | +++ | +++ | — |
| 34°C Glucose | +++ | — | ++ | — | — | ++ | + | — |
| 37°C Glucose | +++ | — | — | — | — | — | — | — |
| 27°C Galactose | +++ | +++ | +++ | +++ | +++ | +++ | +++ | — |
| 34°C Galactose | +++ | + | +++ | + | + | +++ | ++ | — |
| 37°C Galactose | +++ | — | + | — | — | + | — | — |
| 37°C 1 M sorbitol | +++ | + | +++ | ++ | ++ | +++ | ++ | — |
| 37°C 1.5 M sorbitol | +++ | +++ | +++ | +++ | +++ | +++ | +++ | — |
| 37°C 1 M NaCl | +++ | + | +++ | + | + | +++ | ++ | — |
| 27°C pH 8.0 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | nd |
| 500 μg/ml neomycin | +++ | — | — | + | — | — | +++ | nd |
| 200 mM Ca2+ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | nd |
| 500 mM Mg2+ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | nd |
| 5 mM Zn2+ | +++ | ++ | ++ | ++ | ++ | ++ | +++ | nd |
| 5 mM Mn2+ | +++ | +++ | ++ | +++ | +++ | +++ | +++ | nd |
| 100 μM Co2+ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | nd |
| 34°C Glycerol | +++ | +++ | +++ | +++ | +++ | +++ | +++ | — |
| 37°C Glycerol | ++ | ++ | +++ | ++ | ++ | +++ | ++ | — |
| 34°C Ethanol | ++ | + | ++ | + | + | ++ | + | — |
| 37°C Ethanol | + | + | ++ | + | + | ++ | + | — |
| 20 μg/ml CW | +++ | — | — | — | — | — | — | nd |
| 60 mM NaF | +++ | ++ | + | ++ | ++ | +++ | +++ | nd |
Interestingly, nonfermentable carbon sources like glycerol or ethanol also supported neo1-ts growth at the nonpermissive temperature (Figure 4 and Table 2), and even galactose partially supported neo1-ts growth (Table 2). One possible explanation for these results is that cells grow more slowly on the nonglucose carbon source, and this might allow for more time to assemble a functional cell wall. However, it is also possible that in nonglucose carbon source media, other pathways are activated to suppress the neo1-ts mutant phenotype. It is noteworthy that neither the high osmotic support, nor the nonglucose carbon sources were able to suppress _neo1_Δ lethality (Table 2), indicating that Neo1p is not solely involved in cell wall synthesis. This also indicates that despite the heavy mutagenesis, all of the neo1-ts alleles retain some function at 37°C; otherwise these mutants would fail to grow on sorbitol.
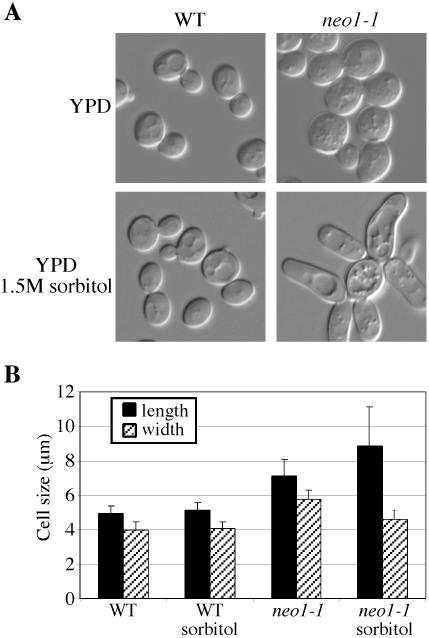
Because NEO1 confers resistance to neomycin when overexpressed, we tested the neo1-ts mutants for neomycin hypersensitivity. As predicted, most neo1-ts mutants are hypersensitive to neomycin (Table 2). Interestingly, neo1-6 is not nearly as sensitive to neomycin as the other mutants. This suggests that the different neo1 ts alleles might affect different functions of Neo1p. Neo1p is homologous to Drs2p, and drs2 has been shown to be hypersensitive to Mn2+, Ca2+, and other cations. Therefore, the neo1-ts mutants were also tested on the plates containing different cations at permissive temperature. The neo1-ts mutants are not significantly hypersensitive to any cation tested (Table 2). However, a few neo1 ts alleles did exhibit an increased sensitivity to NaF, an inhibitor of P-type ATPases (Table 2). Hypersensitivity to calcofluor white, neomycin, and NaF at the permissive growth temperature indicates that all of the neo1-ts alleles have partially lost function at 27°C. Consistent with this, we find that the neo1-ts cells grown at permissive temperature are enlarged and a small percentage of the cells are also elongated (Figure 5, A and B). This distinctive phenotype is not significantly aggravated when cells are shifted to the nonpermissive temperature. Interestingly, growth in 1.5 M sorbitol appeared to have partially suppressed the increased width of the cell, but severely exacerbated the hyperelongated morphology (Figure 5, A and B).
Figure 5.
neo1-ts cells are large. (A) Wild-type (BY4742) and neo1-1 (ZHY628-15B) strains were grown at 27°C in YPD media or YPD media supplemented with 1.5 M sorbitol and imaged. (B) Fifty cells of wild-type and neo1-1 strains grown at 27°C in YPD media or YPD sorbitol media were measured for length and width. A Student's t test indicated that the mutant cell sizes are significantly different from the wild-type (p < 0.0001).
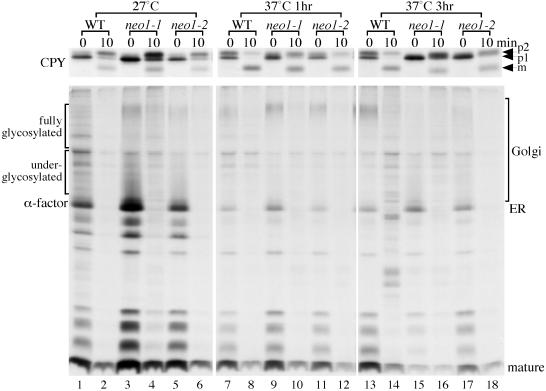
Protein Transport and Glycosylation Defects Exhibited by neo1-ts
Strains harboring a strong (neo1-1) or weak (neo1-2) ts allele of NEO1 were examined for defects in protein transport and modification at the permissive and nonpermissive temperatures. Cells growing at 27°C, or shifted to 37°C for 1 or 3 h, were subjected to pulse-chase analysis of CPY and α-factor transport. CPY is synthesized in the ER as the p1 precursor form and is modified on N-linked oligosaccharides by Golgi mannosyltransferases to form the p2 precursor. p2 CPY is sorted from secreted proteins in the late Golgi and is transported through a prevacuolar endosome to the vacuole where it is processed to the mature form (mCPY; Stevens et al., 1982; Vida et al., 1993).
At 27°C, the kinetics of transport and modification of CPY and α-factor in the neo1 mutants was similar to wild-type cells, but a defect became apparent after incubation at 37°C (Figure 6). In wild-type cells preincubated at 37°C for 1 h, about half of the labeled CPY was in the p2 form at the beginning of the chase period (0 min), and most of the protein was converted to the mature form 10 min later. However, most of CPY was still in the ER form at 0 min in the neo1 mutants and the p2 form of CPY was underglycosylated, causing a faster migration in the gel so that it could not be clearly separated from the p1 form (Figure 6). For the “tighter” neo1-1 mutant, only half of CPY was converted to the mature form in 10 min. The transport and glycosylation defects became more apparent for both mutants after 3 h at 37°C.
Figure 6.
neo1-ts exhibits Golgi glycosylation and protein transport defects at the nonpermissive temperature. Wild-type (BY4742) and two neo1-ts strains (ZHY628-15B and ZHY628-34A) were grown at 27°C, and then shifted to 37°C for 1 or 3 h. Cells were then labeled with [35S]methionine/cysteine for 6 min, and chased for 0 and 10 min. CPY and α-factor were immunoprecipitated from cells collected at each chase time and subjected to SDS-PAGE.
After 1 h of incubation at the nonpermissive temperature, the transport and modification of pro–α-factor (immunoprecipitated from the same cell extracts as CPY) was surprisingly normal (Figure 6). A defect became apparent after a 3-h shift to the nonpermissive temperature in the neo1 mutants, with more ER form accumulating and less mature form present at the beginning of the chase. The fully glycosylated form of pro–α-factor, which can be seen in the 37°C 1-h panel (Figure 6, lanes 9 and 11), was replaced by underglycosylated precursor in the 3 h neo1-ts samples (Figure 6, lanes 15 and 17). These results are consistent with what was observed in the Neo1p depletion experiments and suggest that Neo1p is required for efficient protein transport from the ER to the Golgi and for synthesis of the outer chain on N-linked oligosaccharides in the Golgi complex.
neo1-ts Exhibits COPI Mutant Phenotypes
Proteins are transported from the ER to the Golgi complex in COPII-coated transport vesicles. Temperature-sensitive COPII mutants (e.g., sec12 or sec23) exhibit a block in the ER-to-Golgi transport of all cargo proteins tested (Hicke and Schekman, 1989; Barlowe and Schekman, 1993). The differential effect of neo1-ts on CPY and α-factor transport noted above is more consistent with a defect in Golgi-to-ER retrograde transport mediated by COPI. COPI mutants are known to exhibit cargo-specific defects in anterograde transport that is presumably a secondary effect of perturbing the recycling of cargo receptors (Gaynor and Emr, 1997).
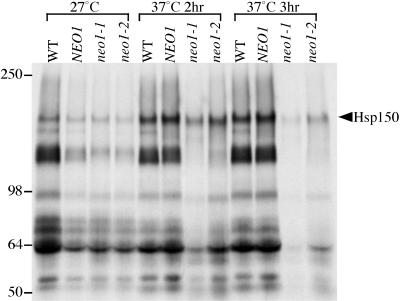
To test if neo1 mutants exhibit a cargo-specific defect in protein transport, general secretion was examined in the neo1 ts mutants (Figure 7). Cells maintained at 27°C or shifted to 37°C for 2 or 3 h were labeled with [35S]methionine/cysteine. Cells and medium were separated by centrifugation, and proteins secreted into the medium were precipitated with TCA and subjected to SDS-PAGE. At 27°C, the general secretion of proteins into the media was about the same between the wild-type strains (WT and NEO1) and neo1 mutants. When cells were shifted to 37°C for 2 h, Hsp150, the top band on the gel migrating at about 150 kDa, was still secreted from both neo1 mutants, whereas lower molecular weight proteins were missing (Figure 7, 2 h). This pattern of secreted proteins is similar to the COPI mutant phenotype, in which only a subset of secreted proteins were affected at the nonpermissive temperature (Gaynor and Emr, 1997). When cells were shift to 37°C for 3 h, Hsp150 secretion also decreased in neo1-1 (Figure 7, 3 h), although neo1-2 still exhibited the cargo-specific defect in secretion. We also noticed that Hsp150 from neo1 ts mutants labeled at the nonpermissive temperature migrated slightly faster in gels than that from the wild-type cells. This suggests that Golgi O-glycosylation is partially defective in neo1, because Hsp150 is extensively O-glycosylated with no N-linked oligosaccharides.
Figure 7.
General secretion defect in neo1-ts. Wild-type (BY4742) and neo1_Δ harboring either NEO1 (ZHY219RR), neo1-1_ (ZHY628–15B), or neo1-2 (ZHY628–34A) were grown at 27°C, and part of each culture was shifted to 37°C for 2 or 3 h. Cells were then labeled with [35S]methionine/cysteine for 10 min and then chased for 30 min. Cells and media were separated by centrifugation, and the proteins in the media were precipitated with TCA and subjected to SDS-PAGE.
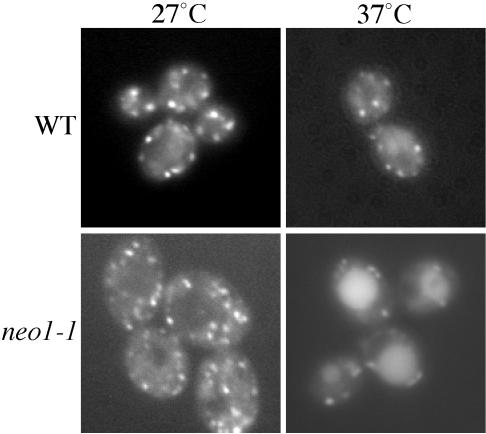
Rer1p is a protein that continuously cycles between the early Golgi complex and the ER, and is a sensitive marker to distinguish whether a mutant exhibits a primary defect in anterograde or retrograde transport (Sato et al., 2001). Sato et al. (2001) found that Rer1-GFP is primarily localized to the Golgi in wild-type cells, but is trapped in the ER in COP II mutants and mislocalized to the vacuole in COPI mutants. To test if Neo1p is involved in anterograde or retrograde transport between the ER and Golgi complex, Rer1-GFP was expressed in neo1-ts cells. At the permissive temperature, Rer1-GFP was mainly localized in punctate structures in both wild-type and neo1-1 cells, although a slightly more diffuse localization was noted for the neo1-1 cells (Figure 8, 27°C). After cells were shifted to nonpermissive temperature for 2 h, a significant amount of Rer1-GFP localized to vacuoles in neo1-1 cells (Figure 8, 37°C). Wild-type cells also exhibited more Rer1-GFP in the vacuole at 37°C, but not to the extent observed in neo1-1 cells. No ER accumulation of Rer1-GFP was found in the neo1-1 mutant. This result suggests that neo1 does not directly perturb the COPII-dependent anterograde pathway but does perturb a COPI-dependent retrograde transport pathway. Consistent with these results, we also find that neo1-ts cells secrete more Kar2p (an ER resident protein bearing the HDEL retrieval signal) than wild-type yeast (our unpublished results).
Figure 8.
Rer1-GFP is mislocalized to the vacuole of neo1-1 cells. Wild-type (BY4742) and neo1-1 (ZHY628-15B) harboring an Rer1-GFP plasmid were grown at 27°C, and half of each culture was shifted to 37°C for 2 h. Fluorescent images were captured and representative cells are shown. Cells with large vacuoles were chosen to better distinguish vacuolar fluorescence from the Golgi signal. No ER localization was observed in any of the cells.
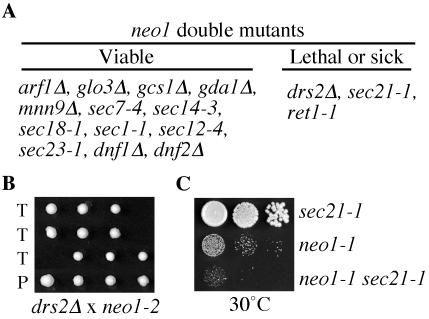
Mutations affecting the same pathway will often show genetic interactions and so double mutants were constructed by crossing neo1-1 with several different mutants with defects in protein transport or glycosylation (Figure 9A). The viable double mutants were then tested for growth at several different temperatures. No interaction was observed between neo1-1 and the COPII alleles (sec12-4, sec23-1), but the neo1 COPI (sec21-1, ret1-1) double mutants grew slower on the tetrad dissection plates and showed an increase in temperature sensitivity relative to the single mutants (Figure 9C and our unpublished results). The strongest genetic interaction was observed between neo1-1 and _drs2_Δ. Most of the double mutants produced from this cross failed to grow (Figure 9B), and the few that did survive grew extremely slowly. Although the drs2 neo1 synthetic lethality may be caused by the combined defects in clathrin and COPI pathways, it more likely reflects a common biochemical function for Neo1p and Drs2p in the Golgi complex. These proteins are 30% identical in amino acid sequence and are both in the APT family of P-type ATPases. No significant genetic interaction was observed between neo1-1 and arf1, ARF-GAPs (gcs1_Δ, glo3_Δ), or an ARF-GEF (sec7-4), which was surprising considering that both drs2 arf1 and drs2 neo1-ts are synthetically lethal. In addition, no genetic interaction was observed with mutations that perturb later steps in protein transport (sec14-3, sec1-1) or glycosylation (gda1_Δ, mnn9_Δ), or deletions of other APT family members (dnf1_Δ, dnf2_Δ; Figure 9A). Thus, although the genetic interaction found between neo1-1 and COPI alleles was modest, it was specific and supported a role for Neo1p in COPI function.
Figure 9.
Genetic interactions between neo1-1 and drs2 or COPI mutations. (A) Summary of double mutant phenotypes. Strains harboring the alleles indicated were crossed with integrated neo1-1 or neo1-2 mutant, sporulated, and subjected to tetrad dissection. Double mutants harboring neo1-1 and the alleles listed in the viable column were recovered at the expected frequency and showed a ts growth profile comparable to one of the two parents. In contrast, most _neo1-1 drs2_Δ or _neo1-2 drs2_Δ double mutants were inviable, and the neo1-1 sec21-1 and neo1-1 ret1-1 strains were more temperature sensitive than either parent (Lethal or sick column). (B) Synthetic lethality between neo1-2 and _drs2_Δ. Dissected tetrads from the _neo1-2/drs2_Δ cross are shown and the missing colonies in rows labeled “T” (tetratype) were predicted to be double mutants. “P” is a parental ditype with two pairs of single mutant progeny. (C) Growth profile of neo1-1 sec21-1 compared with both parental strains at 30°C, a semipermissive temperature for neo1-1.
The anterograde protein transport defect appeared more severe for cells depleted for Neo1p compared with the experiments using neo1-ts mutants. We suspected that the different temperatures used to assay protein transport might have contributed to these differences. Therefore, we labeled Neo1-depleted cells at 37°C to examine CPY and α-factor transport at the higher temperature. CPY transport was approximately twofold faster for both wild-type and Neo1p-depleted cells at 37°C (compared with 30°C) but the transport defect was still apparent in the depleted cells (Figure 10, CPY). In contrast to CPY, the protein transport defect was significantly alleviated at 37°C for pro–α-factor (Figure 10, 37°C). More ER form of pro–α-factor was present at the beginning of the chase (time 0) in the Neo1-depleted cells than in wild-type, but this quickly chased to the mature form. These results are very similar to those obtained with the neo1-ts strains at 37°C and suggest that there is a greater demand for Neo1p function in recycling cargo receptors at lower temperatures.
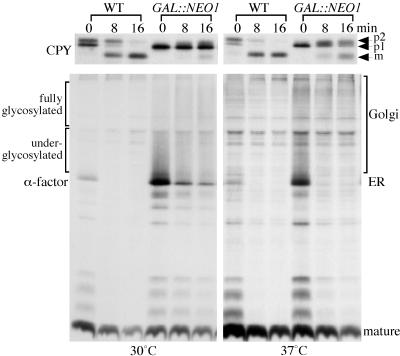
Figure 10.
Neo1p depletion causes a more severe protein transport defect at lower temperature. Wild-type and _neo1_Δ GAL::NEO1 strains were grown in galactose and then shifted to glucose media for 24 h at 30°C. For labeling at 37°C, half of the cultures cells were shifted to 37°C for the last 2.5 h before labeling. Cells were then labeled with [35S]methionine/cysteine for 8 min and chased for 0, 8, and 16 min. CPY and α-factor was immunoprecipitated from cells collected at each chase time and subjected to SDS-PAGE.
The neo1-1 Mutant Accumulates Abnormal Membrane Structures
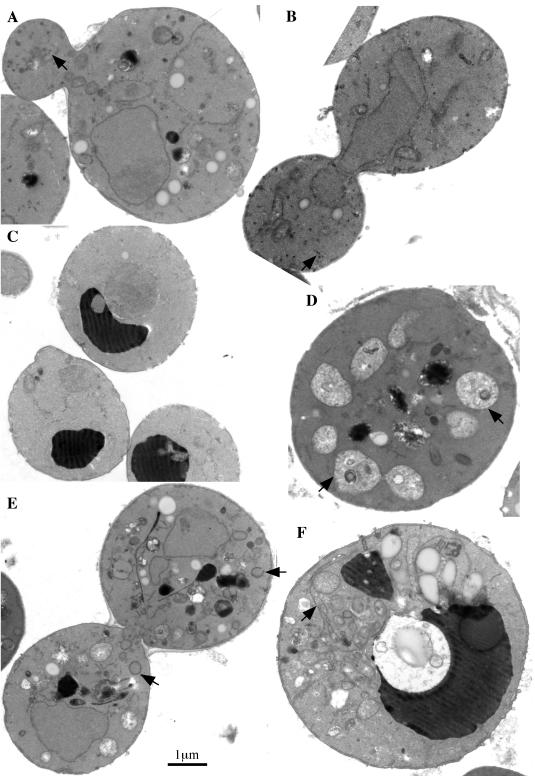
Because the neo1 mutants exhibited significant defects in the secretory pathway, we examined these cells by electron microscopy (EM) to see if any organelle membrane or intermediate transport vesicles accumulate in this mutant. The appearance of neo1-1 cells grown at 27 and 37°C were quite different (Figure 11). At the permissive temperature, the neo1-1 mutant accumulated small vesicles (40.9 ± 9.2 nm in diameter) and lipid droplets (Figure 11, A and B) compared with wild-type cells. Vacuoles were also fragmented, which was confirmed by staining living cells with FM4–64 or CDC-FDA (our unpublished results). The fragmented vacuole phenotype was not completely penetrant, and cells with normal vacuoles could easily be found (see Figure 8). When neo1 was shifted to the nonpermissive temperature for 3 h, the number of small vesicles was substantially reduced, and several kinds of abnormal membrane structures were found to accumulate. Some cell sections contained multiple vacuole-like structures, although they did not stain darkly as do vacuoles in wild-type cells (Figure 11D, arrows). Other sections showed an accumulation of abnormal membrane structures that are likely enlarged Golgi cisternae (Figure 11E, arrows), and continuously looped membrane sheets, which are likely an expanded ER (Figure 11F, arrow). The accumulation of different abnormal membrane structures in neo1 indicates a defect in membrane trafficking through the secretory pathway.
Figure 11.
neo1-ts cells accumulate abnormal membrane structures. (A and B) neo1-1 (ZHY628–15B) was grown at the permissive temperature of 27°C and processed for electron microscopy. (C) Wild-type cells (BY4742) grown at 37°C for 3 h. Wild-type cells grown at 27°C were indistinguishable and are not shown. (D-F) neo1-1 grown at 37°C for 3 h. Arrows indicate small vesicles in A and B, poorly stained fragmented vacuoles in D, abnormal Golgi membranes in E, and enlarged ER membrane in F.
DISCUSSION
Neo1p is an integral membrane P-type ATPase and potential APT. Other members of the yeast APT family (Drs2p and Dnf1, 2, and 3) have been implicated in protein transport in the late secretory and endosomal/vacuolar pathways. In this study, we present several lines of evidence implicating Neo1p in protein transport and modification in the early secretory pathway. 1) Neo1-myc localizes to both ER and Golgi compartments. 2) Neo1p depletion at 30°C causes a strong defect in CPY and pro–α-factor transport from the ER to the Golgi and potentially through the Golgi complex. 3) Golgi-dependent modification of N-linked oligosaccharides on CPY and pro–α-factor is perturbed upon Neo1p depletion. 4) The neo1-ts mutations cause these same protein transport and glycosylation defects at the nonpermissive temperature, albeit less severely, and seem to preferentially perturb CPY exit from the ER. 5) The neo1-ts mutants exhibit a block in the secretion of a subset of proteins, whereas others, such as Hsp150, are secreted efficiently. 6) Rer1-GFP, a protein that continuously cycles between the ER and Golgi, is mislocalized to the vacuole in neo1-ts. 7) The neo1-ts mutants accumulate small vesicles at the permissive temperature and accumulate ER and Golgi membranes at the non-permissive temperature.
The neo1 mutants exhibit several phenotypes in common with COPI mutants. These include a cargo-specific defect in secretion, aberrant glycosylation of cargo in the Golgi, and mislocalization of Rer1-GFP to the vacuole. Rer1p is an integral membrane protein required for retrograde transport of other membrane proteins to the ER. It has a C-terminal dilysine-like motif that binds COPI and links other cargo (Sec12p or Sec71p) to COPI via transmembrane domain interactions (Sato et al., 1997, 2001). Thus, Rer1p appears to be a cargo receptor for proteins retrieved from the Golgi to the ER in COPI vesicles and must continuously shuttle between the ER and Golgi to perform this task. COPII mutants accumulate Rer1-GFP in the ER and COPI mutants mislocalize Rer1-GFP to the vacuole (Sato et al., 2001). The mislocalization of Rer1-GFP to the vacuole of neo1-ts suggests that these cells have a primary defect in retrograde rather than anterograde transport. In addition, the sec21 (γ-COPI) mutant perturbs anterograde transport of a subset of secreted proteins and the spectrum of affected and unaffected proteins is similar in the neo1 mutants (Gaynor and Emr, 1997). This is likely caused by a failure to recycle cargo receptors needed for anterograde transport of some proteins.
Although all COPI mutants appear to exhibit a defect in retrograde transport, not all display a defect in anterograde protein transport (Cosson et al., 1996; Duden et al., 1998). Thus, the modest anterograde transport defect observed in neo1-ts at 37°C does not indicate that the retrograde defect is marginal. In fact, the Neo1p requirement for anterograde transport appears less critical at higher temperatures because depletion of Neo1p at 30°C produced a stronger pro–α-factor block than depletion at 37°C. Moreover, it is clear that none of the neo1-ts alleles cause a complete loss of function at the nonpermissive temperature. The ts growth defect of all of the neo1-ts mutants can be suppressed by osmotic support, but this treatment does not allow _neo1_Δ cells to grow at any temperature tested. Therefore, the protein trafficking defects observed in neo1-ts cells was caused by a partial loss of Neo1p function. Neo1p depletion caused a stronger defect in anterograde protein transport and even these experiments may not represent a complete loss of function phenotype. These cells were labeled at a time of depletion where a growth defect first became apparent, rather than when growth stopped. This was done in an attempt to define the immediate consequences of diminished Neo1p function. Likewise, neo1-ts cells were examined 1–3 h after temperature shift instead of the 6 h required to completely inhibit growth.
Surprisingly, neo1-ts cells accumulated a large number of small vesicles (30–50 nm) at the permissive temperature, where it is clear from the cell morphology and hypersensitivity to various chemicals that Neo1p is partially defective. This same vesicle accumulation phenotype is observed when sec21 is incubated at a semipermissive temperature (Rambourg et al., 1994). These vesicles are likely in transit between the ER and Golgi, but whether they are COPI or COPII vesicles is not known. Most of these vesicles disappear when neo1-ts is shifted to 37°C and ER and Golgi membranes accumulate. COPI mutants also accumulate ER at the nonpermissive temperature but the Golgi accumulation appears to be unique to neo1. This difference may reflect the hypomorphic nature of the neo1-ts alleles or that neo1 perturbs additional transport steps through, or from, the Golgi complex. However, it is important to note that neo1 does not appear to perturb COPII function and at least some proteins (Hsp150) are secreted normally. In addition, the CPY that escapes the ER-to-Golgi block in neo1 is sorted to the vacuole. Thus, a number of transport steps are occurring normally in neo1 and the defect appears rather specific to the Golgi-to-ER retrograde pathway.
Both COPI and neo1 mutations perturb glycosylation in the Golgi complex. For neo1-ts strains, the glycosylation defect appears to be the primary cause of the 37°C growth defect. This growth defect is sorbitol-remedial and the neo1-ts mutants are hypersensitive to calcofluor white. These phenotypes suggest an abnormal cell wall and are consistent with the Golgi-dependent glycosylation defects observed for CPY and α-factor. How neo1 mutants perturb the function of Golgi glycosyltransferases is not known. However, localization of an α-1,6-mannosyltransferase (Och1p) that initiates outer chain elongation is perturbed in sec21. Rather than being mislocalized to the vacuole, Och1p changes from a punctate distribution by immunofluorescence to a dispersed granular appearance that might reflect residence in small vesicles or Golgi fragments (Gaynor and Emr, 1997). In addition, two different α-1,6-mannosyltransferase complexes containing Mnn9p, which elongate the outer chain on N-linked oligosaccharides, have been shown to cycle between the ER and Golgi complex. Like Rer1p, these proteins are mislocalized to the vacuole when retrograde transport is perturbed (Todorow et al., 2000). Therefore, it is possible that a reduced ability to form COPI vesicles in neo1 causes the glycosylation defect by mislocalizing α-1,6-mannosyltransferases. The fact that _neo1_Δ lethality cannot be rescued by osmotic support indicates that Neo1p has an essential function independent of its role in glycosylation and cell wall synthesis. This essential Neo1p function appears to be in COPI-dependent Golgi-to-ER retrograde protein transport, an essential process in yeast.
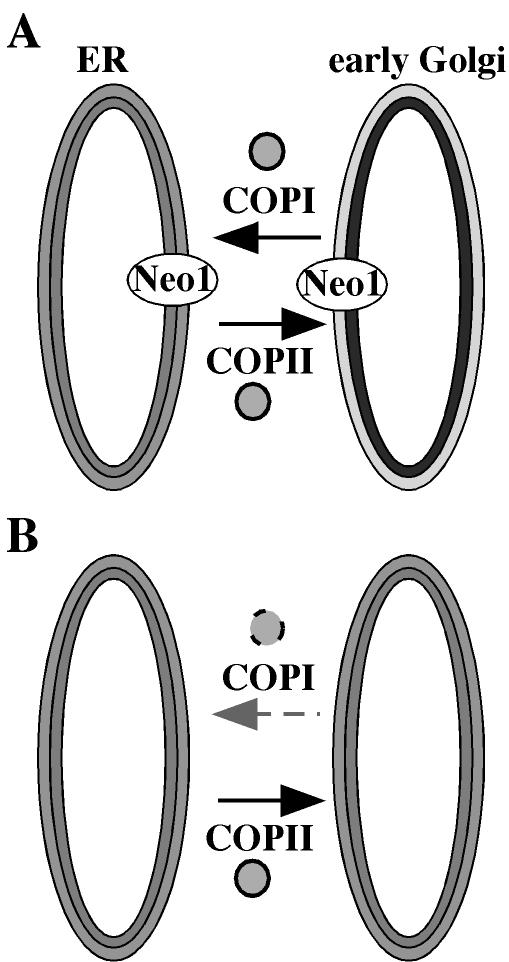
How do potential APTs influence protein transport in the secretory pathway? Drs2p appears to play a direct role in forming secretory vesicles from Golgi membranes because inactivation of Drs2 or clathrin ts proteins causes a rapid loss of a specific class of exocytic vesicles (Gall et al., 2002). Drs2p also interacts directly with Gea2p, an ARF guanine nucleotide exchange factor, physically coupling Drs2p to vesicle budding machinery (Chantalat et al., 2004). The data presented in this work implicate Neo1p in COPI vesicle budding from early Golgi membranes, but whether this is a direct or indirect requirement remains to be determined. The onset of defects in transport is rather slow after shifting the neo1-ts mutants to the nonpermissive temperature. This could be explained by the hypomorphic nature of the neo1-ts alleles, but it could also indicate an indirect requirement for Neo1p in COPI vesicle formation. One model to explain the requirement for Neo1p is that an asymmetric distribution of phospholipids in the _cis_-Golgi is required for optimal COPI vesicle formation. ER and Golgi membranes in mammalian cells have been suggested to be asymmetric (Bollen and Higgins, 1980; Higgins, 1984). Neo1p might establish this phospholipid asymmetry, but it slowly decays after shifting the neo1-ts mutants to the nonpermissive temperature. As the membrane becomes more symmetric, the ability of ARF and COPI to form retrograde vesicles would diminish (Figure 12). It is also possible that Neo1p plays a more direct role in this process by interacting with components of the vesicle budding machinery, or by facilitating outward bending of the membrane during vesicle formation.
Figure 12.
Model for the role of Neo1p in retrograde transport. (A) In wild-type cells, Neo1p probably establishes phospholipid asymmetry in early Golgi membranes (exhibited as light and dark shades of the bilayer membrane), and COPI vesicles form normally. (B) When Neo1p is not functional, lipid asymmetry is lost and the efficiency of COPI vesicle formation is greatly reduced.
We have recently found that late Golgi membranes purified from a drs2-ts mutant display a ts defect in translocating NBD-PS from the luminal to the cytosolic leaflet. The drs2 mutants also expose more PS on the outer leaflet of the plasma membrane than do wild-type cells (our unpublished results). These data strongly support the proposed APT activity for Drs2p. It is possible that both Neo1p and Drs2p control lipid asymmetry in the Golgi complex, with Neo1p acting in the cis compartments and Drs2p in the trans. In this case, loss of both proteins would severely perturb the transbilayer lipid distribution in the entire Golgi complex, perhaps explaining the synthetic lethality between _drs2_Δ and neo1-1.
COPI and clathrin-coated vesicles have been generated from synthetic liposomes using only cytosolic coat components (Spang and Schekman, 1998; Takei et al., 1998), suggesting that coat components alone are sufficient to drive vesicle formation without contribution from membrane proteins. However, these studies could not determine if vesicle formation occurred in vitro with the same rate and efficacy required in vivo. We have found that Neo1p, an essential P-type ATPase and integral membrane protein, is required for efficient Golgi-to-ER protein transport. All five members of the Drs2/Neo1 family proteins have now been implicated in protein trafficking at different steps in the secretory or endocytic pathways (Chen et al., 1999; Gall et al., 2002; Hua et al., 2002; Pomorski et al., 2003). The Drs2/Neo1 proteins appear to be APTs (Tang et al., 1996; Gomes et al., 2000; Pomorski et al., 2003), suggesting that the regulation of membrane phospholipid asymmetry plays an important role in the formation of coated transport vesicles.
Acknowledgments
We thank Ken Sato, Akihiko Nakano, Gerald Waters, and Scott Emr for plasmids, strains, and antibodies. We also thank Sophie Chen from the Graham laboratory for help in strain and media preparation during the course of these experiments. This work was supported by National Institutes of Health Grant GM-62367 (to T.R.G.).
References
- Auland, M.E., Roufogalis, B.D., Devaux, P.F., and Zachowski, A. (1994). Reconstitution of ATP-dependent aminophospholipid translocation in proteoliposomes. Proc. Natl. Acad. Sci. USA 91, 10938-10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian, K., and Schroit, A.J. (2003). Aminophospholipid asymmetry: a matter of life and death. Annu. Rev. Physiol. 65, 701-734. [DOI] [PubMed] [Google Scholar]
- Bankaitis, V.A., Malehorn, D.E., Emr, S.D., and Greene, R. (1989). The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J. Cell Biol. 108, 1271-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe, C. (2002). COPII-dependent transport from the endoplasmic reticulum. Curr. Opin. Cell Biol. 14, 417-422. [DOI] [PubMed] [Google Scholar]
- Barlowe, C., and Schekman, R. (1993). SEC12 encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature 365, 347-349. [DOI] [PubMed] [Google Scholar]
- Bollen, I.C., and Higgins, J.A. (1980). Phospholipid asymmetry in rough- and smooth-endoplasmic-reticulum membranes of untreated and phenobarbital-treated rat liver. Biochem. J. 189, 475-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigance, W.T., Barlowe, C., and Graham, T.R. (2000). Organization of the yeast Golgi complex into at least four functionally distinct compartments. Mol. Biol. Cell 11, 171-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catty, P., de Kerchove d'Exaerde, A., and Goffeau, A. (1997). The complete inventory of the yeast Saccharomyces cerevisiae P-type transport ATPases. FEBS Lett. 409, 325-332. [DOI] [PubMed] [Google Scholar]
- Chantalat, S., Park, S.-K., Hua, Z., Liu, K., Gobin, R., Peyroche, A., Rambourg, A., Graham, T.R., and Jackson, C.L. (2004). The Arf activator Gea2p and the P-type ATPase Drs2p interact at the Golgi in Saccharomyces cerevisiae. J. Cell Sci. (in press). [DOI] [PubMed]
- Chen, C.Y., Ingram, M.F., Rosal, P.H., and Graham, T.R. (1999). Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function. J. Cell Biol. 147, 1223-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson, P., Demolliere, C., Hennecke, S., Duden, R., and Letourneur, F. (1996). Delta- and zeta-COP, two coatomer subunits homologous to clathrin-associated proteins, are involved in ER retrieval. EMBO J. 15, 1792-1798. [PMC free article] [PubMed] [Google Scholar]
- Dhar, M., Webb, L.S., Smith, L., Hauser, L., Johnson, D., and West, D.B. (2000). A novel ATPase on mouse chromosome 7 is a candidate gene for increased body fat. Physiol. Genomics 4, 93-100. [DOI] [PubMed] [Google Scholar]
- Duden, R., Kajikawa, L., Wuestehube, L., and Schekman, R. (1998). epsilon-COP is a structural component of coatomer that functions to stabilize alpha-COP. EMBO J. 17, 985-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzusoff, A., and Schekman, R. (1989). Functional compartments of the yeast Golgi apparatus are defined by the sec7 mutation. EMBO J. 8, 2695-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller, R.S., Sterne, R.E., and Thorner, J. (1988). Enzymes required for yeast prohormone processing. Annu. Rev. Physiol. 50, 345-362. [DOI] [PubMed] [Google Scholar]
- Gall, W.E., Geething, N.C., Hua, Z., Ingram, M.F., Liu, K., Chen, S.I., and Graham, T.R. (2002). Drs2p-dependent formation of exocytic clathrin-coated vesicles in vivo. Curr. Biol. 12, 1623-1627. [DOI] [PubMed] [Google Scholar]
- Gaynor, E.C., Chen, C.Y., Emr, S.D., and Graham, T.R. (1998). ARF is required for maintenance of yeast Golgi and endosome structure and function. Mol. Biol. Cell 9, 653-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor, E.C., and Emr, S.D. (1997). COPI-independent anterograde transport: cargo-selective ER to Golgi protein transport in yeast COPI mutants. J. Cell Biol. 136, 789-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, E., Jakobsen, M.K., Axelsen, K.B., Geisler, M., and Palmgren, M.G. (2000). Chilling tolerance in Arabidopsis involves ALA1, a member of a new family of putative aminophospholipid translocases. Plant Cell 12, 2441-2454. [PMC free article] [PubMed] [Google Scholar]
- Graham, T.R., and Emr, S.D. (1991). Compartmental organization of Golgi-specific protein modification and vacuolar protein sorting events defined in a yeast sec18 (NSF) mutant. J. Cell Biol. 114, 207-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, T.R., Seeger, M., Payne, G.S., MacKay, V.L., and Emr, S.D. (1994). Clathrin-dependent localization of alpha 1, 3 mannosyltransferase to the Golgi complex of Saccharomyces cerevisiae. J. Cell Biol. 127, 667-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, S.L., and Waters, M.G. (1996). Localization of a yeast early Golgi mannosyltransferase, Och1p, involves retrograde transport. J. Cell Biol. 132, 985-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzing, L.B., Kim, S.J., Cook, E.H., Jr., and Ledbetter, D.H. (2001). The human aminophospholipid-transporting ATPase gene ATP10C maps adjacent to UBE3A and exhibits similar imprinted expression. Am. J. Hum. Genet. 68, 1501-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke, L., and Schekman, R. (1989). Yeast Sec23p acts in the cytoplasm to promote protein transport from the endoplasmic reticulum to the Golgi complex in vivo and in vitro. EMBO J. 8, 1677-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J.A. (1984). The transverse distribution of phospholipids in the membranes of Golgi subfractions of rat hepatocytes. Biochem. J. 219, 261-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, Z., Fatheddin, P., and Graham, T.R. (2002). An essential subfamily of Drs2p-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol. Biol. Cell 13, 3162-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, M., and Davis, R.W. (1984). Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol. Cell. Biol. 4, 1440-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen, T. (2000). Three ways to make a vesicle. Nat. Rev. Mol. Cell. Biol. 1, 187-198. [DOI] [PubMed] [Google Scholar]
- Klionsky, D.J., Banta, L.M., and Emr, S.D. (1988). Intracellular sorting and processing of a yeast vacuolar hydrolase: proteinase A propeptide contains vacuolar targeting information. Mol. Cell. Biol. 8, 2105-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis, F.M., Mol, P., Hellingwerf, K., and Brul, S. (2002). Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 26, 239-256. [DOI] [PubMed] [Google Scholar]
- Marx, U., Polakowski, T., Pomorski, T., Lang, C., Nelson, H., Nelson, N., and Herrmann, A. (1999). Rapid transbilayer movement of fluorescent phospholipid analogues in the plasma membrane of endocytosis-deficient yeast cells does not require the Drs2 protein. Eur. J. Biochem 263, 254-263. [DOI] [PubMed] [Google Scholar]
- Meguro, M., Kashiwagi, A., Mitsuya, K., Nakao, M., Kondo, I., Saitoh, S., and Oshimura, M. (2001). A novel maternally expressed gene, ATP10C, encodes a putative aminophospholipid translocase associated with Angelman syndrome. Nat. Genet. 28, 19-20. [DOI] [PubMed] [Google Scholar]
- Pomorski, T., Lombardi, R., Riezman, H., Devaux, P.F., Van Meer, G., and Holthuis, J.C. (2003). Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol. Biol. Cell 14, 1240-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon, P.P., Cassel, D., Spang, A., Rotman, M., Pick, E., Singer, R.A., and Johnston, G.C. (1999). Retrograde transport from the yeast Golgi is mediated by two ARF GAP proteins with overlapping function. EMBO J. 18, 555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezant, T.R., Chaltraw, W.E., Jr., and Fischel-Ghodsian, N. (1996). Identification of an overexpressed yeast gene which prevents aminoglycoside toxicity. Microbiology 142, 3407-3414. [DOI] [PubMed] [Google Scholar]
- Rambourg, A., Clermont, Y., Jackson, C.L., and Kepes, F. (1994). Ultrastructural modifications of vesicular and Golgi elements in the Saccharomyces cerevisiae sec21 mutant at permissive and non-permissive temperatures. Anat. Rec. 240, 32-41. [DOI] [PubMed] [Google Scholar]
- Rieder, S.E., Banta, L.M., Kohrer, K., McCaffery, J.M., and Emr, S.D. (1996). Multilamellar endosome-like compartment accumulates in the yeast vps28 vacuolar protein sorting mutant. Mol. Biol. Cell 7, 985-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, J.S., Klionsky, D.J., Banta, L.M., and Emr, S.D. (1988). Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol. Cell. Biol. 8, 4936-4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, K., Sato, M., and Nakano, A. (1997). Rer1p as common machinery for the endoplasmic reticulum localization of membrane proteins. Proc. Natl. Acad. Sci. USA 94, 9693-9698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, K., Sato, M., and Nakano, A. (2001). Rer1p, a retrieval receptor for endoplasmic reticulum membrane proteins, is dynamically localized to the Golgi apparatus by coatomer. J. Cell Biol. 152, 935-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneuret, M., and Devaux, P.F. (1984). ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc. Natl. Acad. Sci. USA. 81, 3751-3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund, A., Grant, A., Angeletti, C., Malone, L., Nichols, J.W., and Rudolph, H.K. (1998). Loss of Drs2p does not abolish transfer of fluorescence-labeled phospholipids across the plasma membrane of Saccharomyces cerevisiae. J. Biol. Chem. 273, 34399-34405. [DOI] [PubMed] [Google Scholar]
- Spang, A., and Schekman, R. (1998). Reconstitution of retrograde transport from the Golgi to the ER in vitro. J. Cell Biol. 143, 589-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, T., Esmon, B., and Schekman, R. (1982). Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell 30, 439-448. [DOI] [PubMed] [Google Scholar]
- Takei, K., Haucke, V., Slepnev, V., Farsad, K., Salazar, M., Chen, H., and De Camilli, P. (1998). Generation of coated intermediates of clathrin-mediated endocytosis on protein-free liposomes. Cell 94, 131-141. [DOI] [PubMed] [Google Scholar]
- Tang, X., Halleck, M.S., Schlegel, R.A., and Williamson, P. (1996). A subfamily of P-type ATPases with aminophospholipid transporting activity [published erratum appears in Science 1996 Dec 6;274(5293):1597]. Science 272, 1495-1497. [DOI] [PubMed] [Google Scholar]
- Thompson, R., and Jansen, P.L. (2000). Genetic defects in hepatocanalicular transport. Semin. Liver Dis. 20, 365-372. [DOI] [PubMed] [Google Scholar]
- Todorow, Z., Spang, A., Carmack, E., Yates, J., and Schekman, R. (2000). Active recycling of yeast Golgi mannosyltransferase complexes through the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 97, 13643-13648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida, T.A., Huyer, G., and Emr, S.D. (1993). Yeast vacuolar proenzymes are sorted in the late Golgi complex and transported to the vacuole via a prevacuolar endosome-like compartment. J. Cell Biol. 121, 1245-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachowski, A., Henry, J.P., and Devaux, P.F. (1989). Control of transmembrane lipid asymmetry in chromaffin granules by an ATP-dependent protein. Nature 340, 75-76. [DOI] [PubMed] [Google Scholar]