MicroRNA-183 Family Members Regulate Sensorineural Fates in the Inner Ear (original) (raw)
Abstract
Members of the microRNA (miRNA) 183 family (miR-183, miR-96, and miR-182) are expressed abundantly in specific sensory cell types in the eye, nose, and inner ear. In the inner ear, expression is robust in the mechanosensory hair cells and weak in the associated statoacoustic ganglion (SAG) neurons; both cell types can share a common lineage during development. Recently, dominant-progressive hearing loss in humans and mice was linked to mutations in the seed region of miR-96, with associated defects in both development and maintenance of hair cells in the mutant mice. To understand how the entire triplet functions in the development of mechanosensory hair cells and neurons of the inner ear, we manipulated the levels of these miRNAs in zebrafish embryos using synthesized miRNAs and antisense morpholino oligonucleotides (MOs). Overexpression of miR-96 or miR-182 induces duplicated otocysts, ectopic or expanded sensory patches, and extra hair cells, whereas morphogenesis of the SAG is adversely affected to different degrees. In contrast, knockdown of miR-183, miR-96, and miR-182 causes reduced numbers of hair cells in the inner ear, smaller SAGs, defects in semicircular canals, and abnormal neuromasts on the posterior lateral line. However, the prosensory region of the posterior macula, where the number of hair cells is reduced by ∼50%, is not significantly impaired. Our findings suggest both distinct and common roles for the three miRNAs in cell-fate determination in the inner ear, and these principles might apply to development of other sensory organs.
Introduction
Seven hair cell-bearing mechanosensory organs are present in the zebrafish inner ear to subserve hearing and balance. These organs arise from prosensory patches that give rise to both sensory hair cells and supporting cells (Whitfield et al., 2002). Otic afferent neurons also originate in close proximity to prosensory domains before delaminating into the underlying mesenchyme (Haddon and Lewis, 1996). Specification of the neurosensory cells of the inner ear involves several transcription factors and the Notch signaling pathway. Key genes include Neurogenin1, Sox3, Fgf10, Delta, and Hes5 for the neuronal fate (Alsina et al., 2004; Abello et al., 2007); Sox2 for prosensory patches (Kiernan et al., 2005; Dabdoub et al., 2008); and Atoh1 for hair cells (Bermingham et al., 1999; Millimaki et al., 2007). Although Delta-Notch signaling appears unnecessary for specifying the proneural domain (Abello et al., 2007), it negatively regulates both neuronal and hair cell numbers via lateral inhibition (Haddon et al., 1998), and it has a positive effect on prosensory specification (Kelley, 2006).
Hair cells and otic neurons express a conserved set of three microRNAs (miRNAs) in mice and zebrafish, called the miR-183 family (Wienholds et al., 2005; Weston et al., 2006). This includes miR-183, miR-96, and miR-182. These miRNAs are also abundant in the neurosensory organs of invertebrates and in several other primary sensory cell types in zebrafish (Wienholds et al., 2005; Pierce et al., 2008). Identical expression patterns and close genomic clustering suggests that the entire triplet probably arises from a common primary transcript (Weston et al., 2006).
Primary miRNA transcripts are processed into single-stranded mature miRNAs of 21–23 nucleotides in length that bind to the 3′ untranslated region of target mRNAs to suppress their translation (Kloosterman and Plasterk, 2006). Essential to target recognition is the seed region, located at nucleotides 2-7 of the mature single-stranded miRNA (Lewis et al., 2005). With highly similar sequences in their seed regions, miR-183, miR-96, and miR-182 are expected to share some, but not all, of their targets in common (Xu et al., 2007), but this has yet to be proven.
The function of miRNAs in hearing and deafness is beginning to be revealed. Disruption of miRNA processing through conditional knock-out of Dicer under the control of the Pax2 promoter supports a role for miRNAs in inner ear morphogenesis and innervation (Soukup et al., 2009). Dicer knock-out during hair cell differentiation using a _Brn3c:Cre_-driver reveals a requirement for miRNAs in the maintenance of hair cell fate (Friedman et al., 2009). Two separate mutations in the miR-96 seed region were linked to progressive human deafness (Mencia et al., 2009). Diminuendo mice with a point mutation in the miR-96 seed region show progressive loss of hearing and hair cells (Lewis et al., 2009). Interaction of the mutant miR-96 with novel targets (Lewis et al., 2009) might explain why the remaining two members of the miR-183 family failed to compensate for the miR-96 mutation despite their similar (albeit nonidentical) seed regions. Alternatively, either the absolute level of all miR-183 family members or the unique functions of the individual miRNAs could explain why the gene functions are not completely redundant. Here we used miRNA knockdown and overexpression, alone and in combinations, to distinguish among these possibilities in zebrafish embryos.
Materials and Methods
Zebrafish rearing and husbandry.
Adult wild-type (AB and TLAB; a mixed genotype bred from TL and AB crosses), ET4, and ET20 transgenic fish (Parinov et al., 2004) were kept and bred, and one-cell stage embryos were collected as described previously (Westerfield, 1994). Embryos were incubated at 28°C in E3 medium (Brand et al., 2002).
Morpholino oligonucleotide and miRNA injections.
Morpholino oligonucleotide (MO) and miRNA injections were performed following a published protocol (Kloosterman et al., 2007). Pulled-glass capillary micropipettes with tips broken to 10–20 μm were used to deliver 1 nl of MO or miRNA into one-celled zebrafish embryos. MOs, including standard control MOs (GeneTools), were dissolved in water to the desired concentrations (0.33–1.0 mm) and injected into the yolk or the embryo cytoplasm. Unless stated otherwise, MOs were injected at the following concentrations: miR-96 MO (96 MO), 0.5 mm; miR-182/183 MO (182/183 MO), 1 mm each; all three miRNA MO (all3 MO), 0.5 mm each. Synthesized double-stranded miRNAs (Sigma) were stored as 100 μm stocks and diluted to 5–40 μm in DNase-/RNase-free water for injection into the cytoplasm. Sequences of MOs against miRNAs (5′ to 3′) were as follows: miR-182 MO, TGTGAGTTCTACCATTGCCAAA; pre-miR-182 MO, CTACCTCACCAGTGTGAGTTCTAC; miR-183 MO, CAGTGAATTCTACCAGTGCCATA; pre-miR-183 MO, GGTAATTCACTGATAGTGTGC; miR-96 MO, TGCTAGTGCCAAAACAGGCAAAGA. We also tested another miR-96 MO (AGCAAAAATGTGCTAGTGCCAAA) and pre-miR-96 MO (GTGCCAAAACAGGCAAAGAAGAG), and they were toxic to embryos. Sequences of synthesized miRNAs (sense/antisense strand, 5′ to 3′) were as follows: miR-182, UUUGGCAAUGGUAGAACUCACA/UGAGUUCUACCAUUGCCAUUUU; miR-183, UAUGGCACUGGUAGAAUUCACUG/GUGAAUUCUACCAGUGCCAAUUU; miR-96, UUUGGCACUAGCACAUUUUUGCU/CAAAAAUGUGCUAGUGCCAUUUU. All experimental procedures were approved by the Purdue University Animal Care and Use Committee.
Whole-mount immunostaining.
At 24–48 h postfertilization (hpf), zebrafish embryos were anesthetized with 0.08 mg/ml Tricaine-S (Western Chemical), fixed in 4% paraformaldehyde in PBS (0.137 m sodium chloride, 2.7 mm potassium chloride, 9.9 mm sodium phosphate, 1.8 mm potassium phosphate) at 4°C overnight, and immersed in 0.1 or 1% Triton X-100 in PBS until otoliths completely dissolved. Specimens were incubated in blocking solution [10% calf serum, 2% bovine serum albumin (BSA), and 0.1% Triton X-100 in PBS] at room temperature for 1 h and in primary and secondary antibodies at 4°C overnight. Primary antibodies were as follows: anti-hair cell soma-1 (HCS-1; 1:250; provided by Dr. J. Corwin, University of Virginia, Charlottesville, VA), (anti-human neuronal protein HuC/HuD HuC; 1:50; Invitrogen), anti-Sox2 (1:1000; Millipore), anti-islet1 (39.4D5, 1:100; Developmental Studies Hybridoma Bank), anti-Prox1 (1:2000; Millipore), anti-phosphorylated histone 3 (1:100; EMD Biosciences), and anti-Caspase3 active (1:100; R & D Systems). Secondary antibodies (1:500; Invitrogen) were as follows: Alexa-488 or Alexa-568 goat anti-mouse IgG2a, goat anti-mouse IgG2b, and goat anti-rabbit IgG.
Whole-mount in situ hybridization.
Whole-mount in situ hybridization (WMISH) was performed as described previously (Kloosterman et al., 2006). Embryos older than 22 hpf were treated with phenylthiourea (0.2 mm) to inhibit pigmentation. Embryos were fixed in 4% paraformaldehyde in PBS at 4°C overnight or at room temperature for 2 h, followed by dehydration in methanol at −20°C for at least 2 h. After rehydration through graded methanols and treatment with proteinase K (2.8–22 μg/ml), embryos were preincubated in hybridization buffer for 2–3 h and then were hybridized with antisense RNA probes (1 μg/ml) at 65°C or locked nucleic acid (LNA)-modified DNA probes for miRNAs (20 nm; Exiqon) at 51°C overnight. The 3′ ends of LNA-modified probes were labeled with digoxygenin (DIG; Roche Applied Science) according to the manufacturer's recommendation before hybridization or were ordered prelabeled from Exiqon. After hybridization and high-stringency washes, embryos were blocked in 2% goat serum and 2 mg/ml BSA in 0.1% Tween 20 in PBS at room temperature for 1 h and incubated in anti-DIG conjugated to alkaline phosphatase antibody (Roche Applied Science) at 4°C overnight. Embryos were incubated in BCIP (5-bromo-4-chloro-3′-indolyl phosphate p-toluidine salt; 175 μg/ml) and NBT (nitro-blue tetrazolium chloride; 225 μg/ml) (Roche Applied Science) at room temperature for 2 h or at 4°C overnight for purple color reaction.
Imaging and data analysis.
Live embryos and WMISH embryos were imaged under a Nikon E800 microscope equipped with differential interference contrast optics. Immunostained embryos were imaged under a Nikon E800 fluorescence microscope or a Bio-Rad 1024 confocal microscope. For quantitative data, only one side of each embryo was included. Cell counting and area measurements were performed on reconstructed confocal stacks using NIH ImageJ 1.4 software. Student's t test, ANOVA and Tukey's Studentized Range test were used to compare control and experimental groups and returned a p value. *p < 0.05 was interpreted as significant.
Results
miR-182 and miR-96 are sufficient to induce ectopic hair cells
To test whether the miR-183 family is able to promote hair cell formation in the inner ear, we overexpressed individual members of the miR-183 family in zebrafish embryos via injection of synthetic double-stranded miRNAs. Seven sensory organs are recognized in the adult zebrafish: the utricular or anterior macula (AM), the saccular or posterior macula (PM), the lagena macula, the macula neglecta, the anterior crista (AC), the lateral crista (LC), and the posterior crista (PC) (Platt, 1993; Bang et al., 2001). Embryos were initially analyzed at 26 hpf, shortly after hair cells begin to differentiate in the first two sensory organs, the AM and PM. miRNA overexpression was confirmed by in situ hybridization, with injected embryos showing widespread, diffuse expression in addition to the normal strong signals in mechanosensory organs. Overexpression of miR-182 (5–20 μm) or miR-96 (40 μm) resulted in malformations such as curled bodies, enlarged heart chambers, and abnormal eyes at 24 hpf. Despite these defects, some miR-182- or miR-96-injected embryos had duplicated otic vesicles on one or both sides (Fig. 1A–C), whereas nonduplicated otic vesicles looked normal.
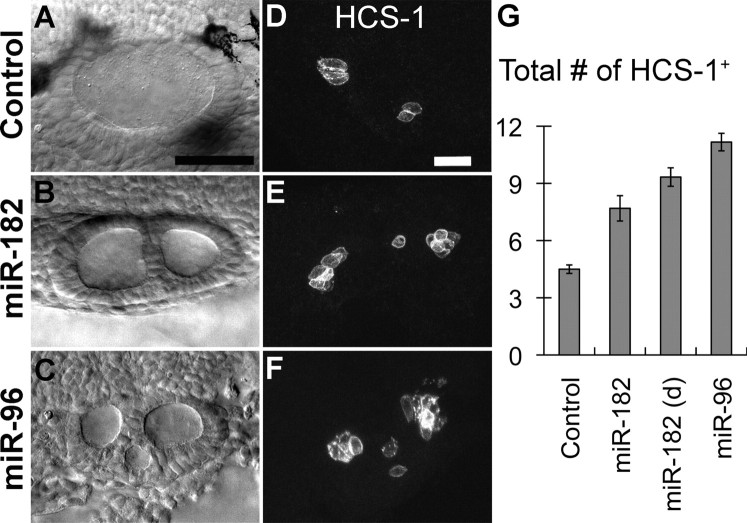
Figure 1.
Overexpression of double-stranded miR-182 or miR-96 induces duplicated otic vesicles and ectopic hair cells at 26 hpf. A–C, Nomarski images of a normal otic vesicle in a control or duplicated otic vesicle in morphants. D–F, Immunostaining with HCS-1 reveals more hair cells in miR-182- and miR-96-injected embryos than controls. Lateral views are shown; anterior is left, and dorsal is up. G, Quantification of hair cell numbers (mean ± SEM) at 26 hpf after injection of water (controls, n = 10), miR-182 (20 μm) yielding normal otocysts (n = 13), miR-182 (20 μm) yielding duplicated otocysts (d; n = 15), or miR-96 (40 μm, n = 12). See supplemental Table S1 (available at www.jneurosci.org as supplemental material) for further details. Scale bars: A–C, 50 μm; D–F, 25 μm.
The onset of normal expression of the miR-183 family is concurrent with formation of the first two pairs of hair cells, called tether cells (Riley et al., 1997), at 20 hpf (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). The genes continue to be expressed robustly in tether cells as they mature into hair cells by 24 hpf. After 24 hpf, additional hair cells accumulate around the tether cell pairs to form the definitive AM and PM (Haddon and Lewis, 1996). Expression of the miR-183 family is also strong in these later-added hair cells (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). The number and onset of hair cell differentiation detected by expression of the miRNAs is similar to that detected with a mouse monoclonal antibody, HCS-1, initially raised against bullfrog sensory organs (Gale et al., 2000). Duplicated otic vesicles appeared as two or more otic vesicles fused together, in which an ectopic third or fourth macula was often, but not always, present between the AM and PM (Fig. 1D–F). Because macular identity could be difficult in treated embryos, total hair cell numbers in the inner ear were counted unilaterally at 26 hpf. Uninjected embryos or embryos injected with water had three to six total hair cells per otocyst (mean ± SEM, 4.3 ± 0.1; n = 53). Only three control embryos (6%) had more than five hair cells. In contrast, many embryos injected with miR-182 or miR-96 developed excessive numbers of hair cells. Injection of miR-182 (5, 7.5, or 10 μm) led to more than five hair cells per ear in 8, 54, or 32% of embryos, respectively. On average, there were 71–107% and 148% more hair cells in embryos injected with miR-182 (20 μm) and miR-96 (40 μm), respectively, than in matched controls from the same batch (Fig. 1G). Whereas miR-182-induced duplicated ears are more likely to produce ectopic sensory domains than nonduplicated ones, this duplication is not required to induce more hair cells (Fig. 1G). In contrast, embryos injected with miR-183 (40 μm) showed overall normal body extension and normal hair cell numbers at 26 hpf (mean ± SEM, 4.4 ± 0.3; n = 8). Therefore, miR-96 and miR-182, but not miR-183, promote hair cell production and also induce ectopic sensory domains at early stages of otocyst development.
The inhibition of target genes by miR-182 or miR-96 in tissues where they are normally not expressed might contribute to the defects seen in overexpression embryos. The developmental abnormalities observed after miRNA delivery included increased cell death along the body. We were able to reduce the cell death by coinjecting morpholinos against p53 (p53 MO), a protein that participates in programmed cell death, as described previously (Robu et al., 2007). The overall development of the embryos was improved slightly, whereas the production of excess hair cells after injection of miR-182 or miR-96 was similar with or without coinjection of p53 MO (at 26 hpf; mean ± SEM; uninjected: 4.6 ± 0.2, n = 12; miR-182/p53 MO: 6.8 ± 0.5, n = 16; miR-96/p53 MO: 11.2 ± 0.7, n = 22). It is notable that either toxicity or general developmental delay might be expected to reduce hair cell numbers, and yet we observed an increase in response to miRNA delivery, arguing for a direct and relatively specific effect on hair cells.
miR-182 induces expanded sensory domains and defects in the SAG
At 48 hpf, the AM and PM of normal embryos have enlarged to include ∼20 hair cells each, and the initiation of semicircular canal formation is apparent as a set of outgrowths (or canal pillars) projecting into the lumen of the otocyst. In contrast to the 26 hpf time point, at 48 hpf, miR-182- or miR-96-injected embryos no longer displayed duplicated otic vesicles. Rather, otic vesicles were often larger and more rounded than controls through expansion along the dorsoventral axis. The otocysts contained visible, but irregular, outgrowth of the canal pillars.
Hair cells will not survive >1–2 d in the absence of supporting cells, as shown in the case of the mindbomb (mib) mutant, where the 10-fold excess of hair cells seen at 36 hpf is 85% reduced by 48 hpf, and hair cells are gone by 60 hpf (Haddon et al., 1999). With this in mind, we looked for evidence of the continued survival of miRNA-induced ectopic hair cells. Indeed, when compared with control embryos injected with water, the total number of hair cells in the AM and PM was increased by 14% in miR-182-injected embryos (Fig. 2A,B,D). In detail, it was increased by 14% in the AM (control: 21 ± 0.7, n = 30; miR-182: 24 ± 0.6, n = 30; p < 0.005, Student's t test) and by 13% in the PM (mean ± SEM; control: 18.9 ± 0.5, n = 30; miR-182: 21.4 ± 0.6, n = 29; p < 0.005, Student's t test). The spatial distribution of the extra hair cells no longer suggested the presence of an ectopic macula, as observed at 26 hpf. Instead, both the AM and PM were well defined. In a few embryos, a single ectopic hair cell was located between the two maculae. With those few exceptions, we presume that the ectopic organs had either fused with the AM or the PM during the second day of development or were eliminated by cell death. Two other abnormalities were noted in the AM of miR-182 embryos: in 25% of samples, some hair cells were positioned ectopically in the supporting cell layer, and in one sample, Sox2 was also expressed ectopically beneath the supporting cell layer (i.e., below the epithelium).
Figure 2.
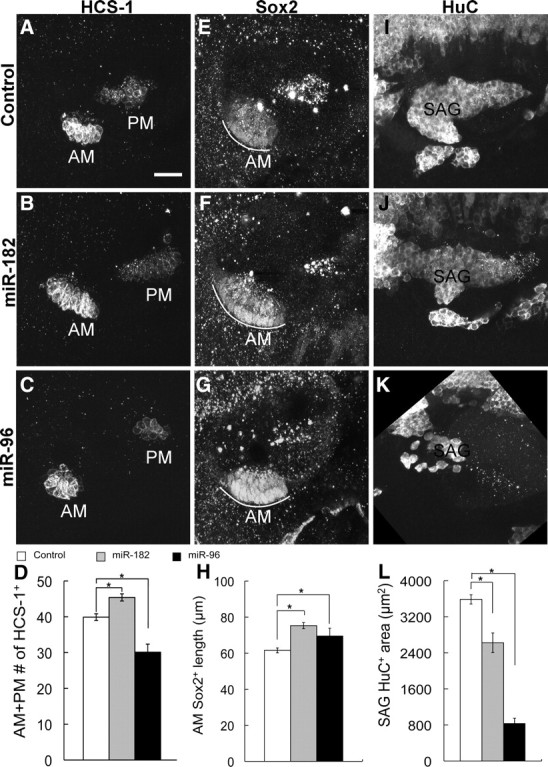
Overexpression of double-stranded miR-182 and miR-96 affect hair cells and the prosensory region differently at 48 hpf. Immunostaining of water-injected controls and miR-182- or miR-96-injected embryos with the indicated antibodies is shown at 48 hpf. Lateral views are shown; anterior is left, and dorsal is up. A–D, The total number of hair cells in the AM and PM was increased by miR-182 injection (20 μm, n = 29) and decreased by miR-96 injection (40 μm, n = 26) compared with water-injected controls (n = 30). E–H, The length of the Sox2+ domain of the AM (white curves) was expanded in miR-182 (20 μm, n = 15) and a subset of miR-96-injected (40 μm, n = 5) embryos compared with controls (n = 15). I–L, miR-182 (20 μm, n = 15) generated smaller, truncated SAG compared with controls (n = 15), whereas the SAG in a subset of miR-96-injected embryos (40 μm, n = 7) was nearly ablated. Results are presented as mean ± SEM. *p < 0.05, Tukey's Studentized Range test. See supplemental Table S1 (available at www.jneurosci.org as supplemental material) for further details. Scale bar, 25 μm.
Sox2 is a transcription factor expressed in prosensory regions of the chicken otocyst and mouse cochlea and is later downregulated in hair cells but remains in supporting cells (Neves et al., 2007). In zebrafish, Sox2 is first expressed in a broad domain across the otocyst, then in separate patches overlapping with the five sensory organs, and finally is restricted to the supporting cell layer once hair cells are differentiated. In miR-182-injected embryos, the length of the AM, as measured by Sox2 expression, was increased by 22% (Fig. 2E,F,H), implying an expansion of the prosensory region rather than a simple switch from supporting to hair cell fates.
By 48 hpf, delamination of neuronal precursors is finished in normal embryos (Haddon and Lewis, 1996), and the SAG assumes an elongated comma shape when visualized with a neuronal marker, HuC. In contrast to the hair cell effect, overexpression of miR-182 reduced SAG size in approximately two-thirds of the embryos. On average, the area occupied by the SAG in _Z_-projections of confocal image stacks was reduced by 27% in miR-182-injected embryos (Fig. 2I,J,L). Since the miR-183 family is only weakly expressed in the SAG compared with its presence in hair cells (supplemental Fig. S1, available at www.jneurosci.org as supplemental material), excess miR-182 in the SAG might lead to repression of factors required for neurogenesis. In short, these data suggest that overexpression of miR-182 can promote hair cell fates and prosensory regions within certain sensory domains, possibly at the expense of neuronal fates.
miR-96 disrupts prosensory and SAG organization
In contrast to miR-182-injected embryos, overexpression of miR-96 disrupted the cohesion of sensory maculae and their segregation into two defined patches. In about one-third of miR-96-injected embryos, the AM and PM were no longer distinguishable, because they had either merged into one continuous patch or an additional macula(e) formed between them. Furthermore, in 27% of embryos, hair cells were dispersed instead of being in a compact patch. Ectopic hair cells were seen in supporting cell layers of the maculae. At 48 hpf, the total number of hair cells in the AM and PM was reduced by 24% in miR-96-injected embryos (Fig. 2C,D). Although miR-96 treatment generated extra hair cells at 26 hpf, the subsequent reduction at 48 hpf suggests that hair cells might be eliminated by some unknown mechanisms, such as cell death or expulsion into the underlying mesenchyme, as seen extensively in mib mutants (Haddon et al., 1999).
Misplaced hair cells in the otocyst are often apoptotic as they are ejected from the normal epithelia (Kwak et al., 2006). To test whether hair cells in the supporting cell layer in miR-182- or miR-96-injected embryos are dying, we looked at expression of activated Caspase3 as an indicator of apoptosis (Bricaud and Collazo, 2006). In 12 of 26 miR-182- or miR-96-injected embryos at 48 hpf, individual misplaced hair cells were found in the supporting cell layer, but none of them were Caspase3+ (supplemental Fig. S2, available at www.jneurosci.org as supplemental material).
Sox2 expression after miR-96 injections mirrored the results with hair cell markers in showing disorganization of prosensory domains. In about one-third of embryos, expression of Sox2 was continuous instead of being separated into two maculae. Others with two distinct domains nonetheless had irregularities in the shape of the Sox2+ patches. In about half of miR-96-injected embryos, Sox2 was significantly downregulated and could not be measured accurately. However, in 5 of 15 embryos where expression of Sox2 in the AM and PM were clear and well separated, the length of Sox2 expression in the AM was increased by 13% (Fig. 2G,H). In addition, the SAG no longer appeared as an obvious patch in about half of the embryos, and in the others, the size of the SAG was reduced by 77% (Fig. 2K,L). Increasing the level of miR-96 not only alters the number of neurons in the SAG but also its organization, possibly by reducing the number or dispersal of delaminating neuroblasts.
Knockdown of the miR-183 family
Using complementary MOs, we knocked down the miR-183 family individually and in various combinations. This approach should permit an evaluation of the necessity of the miR-183 family in otic development in the absence of confounding gain-of-function effects that may be associated with miRNA mutants in mice and humans (Lewis et al., 2009; Mencia et al., 2009). Knockdown of the individual miRNAs in zebrafish embryos was confirmed by in situ hybridization (Kloosterman et al., 2006), where signals of the targeted miRNAs were absent after MO injection (Fig. 3).
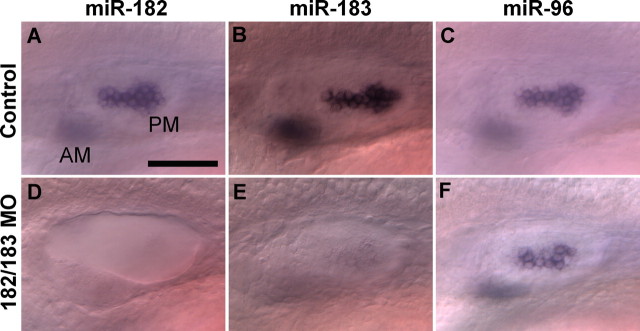
Figure 3.
In situ hybridization confirms the specificity of MOs for miRNA knockdown. Expression of miR-182 (A, D) and miR-183 (B, E), but not miR-96 (C, F), is reduced in both the AM (out of focus) and the PM after injection of miR-182/183 MOs (1 mm each; D–F) compared with controls injected with standard MOs (1 mm; A–C). Lateral views are shown; anterior is left, and dorsal is up. Scale bar, 50 μm.
Processing of miRNAs involves cleavage of the original transcripts into primary miRNAs and precursor miRNAs (pre-miRNAs) (Kloosterman and Plasterk, 2006). Since miR-183, miR-96, and miR-182 are clustered within 1.17 kb in zebrafish, and are likely derived from the same primary transcript (Chen et al., 2005; Weston et al., 2006), it was important to ask whether binding of MOs against one miRNA could indirectly interfere with production of the other two. We were able to rule out this possibility. For example, simultaneous delivery of miR-182 and miR-183 MOs (1 mm each) reduced expression of both targeted miRNAs in hair cells, whereas miR-96 expression was unchanged (Fig. 3). A similar protocol confirmed the target specificity of MO knockdown for each of the individual miRNAs (data not shown).
The miR-183 family is required for normal numbers of hair cells and neurons in the inner ear
Since we failed to detect expression of the miR-183 family at 16 hpf, with the exception of miR-182 signal in one embryo (supplemental Fig. S1, available at www.jneurosci.org as supplemental material), we initially examined the effects of gene knockdown on the appearance of tether cells at 24 hpf. Tether cells were normal in the miR-183 family morphants. However, by 48 hpf, knockdown of members of the miR-183 family consistently yielded fewer hair cells (Fig. 4A–E and supplemental Fig. S3, available at www.jneurosci.org as supplemental material). In miR-182/183 double morphants (182/183 MO; 1 mm each), the number of hair cells was reduced by 22% (mean ± SEM; control: 20.2 ± 0.7, n = 17; 182/183 MO: 15.6 ± 0.8, n = 32) in the AM and by 42% in the PM (Fig. 4C,E). Also, we observed hair cell reductions in miR-96 morphants (96 MO; 0.5 mm) and when all three miRNAs were knocked down simultaneously (all3 MO, 0.5 mm each) (Fig. 4B,D,E). Moreover, we knocked down miR-183 (pre-miR-183 MO, 1 mm) in mib embryos. At 30 hpf, miR-183 mib morphants had fewer hair cells in the PM than uninjected mib embryos (mean ± SEM; uninjected: 16.8 ± 0.8, n = 14; pre183 MO: 14 ± 0.9, n = 14) (supplemental Fig. S4, available at www.jneurosci.org as supplemental material), although both sets overproduced hair cells compared with wild-type embryos. Altogether, results from MO treatments implicate the miR-183 family in regulating the continued addition of hair cells in the maculae, but not the initial appearance of tether cells. Tether cells and later-added hair cells of the maculae are believed to form through different mechanisms under the influence of Atoh1b and Atoh1a, respectively (Millimaki et al., 2007).
Figure 4.
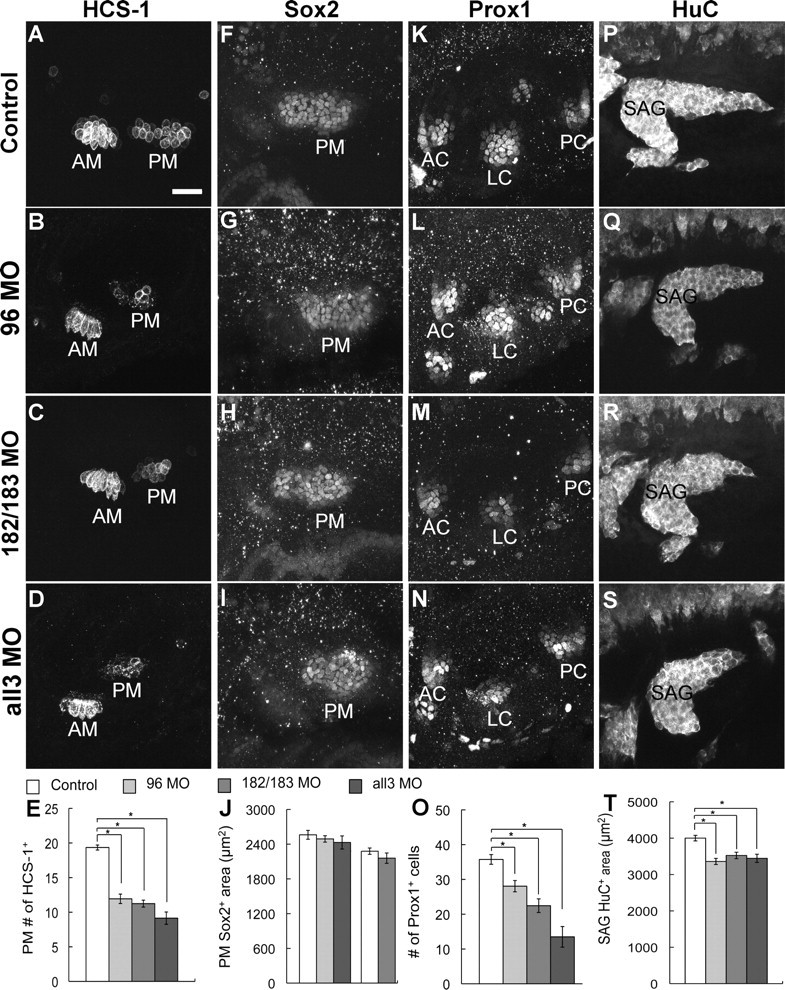
Knockdown of the miR-183 family affects formation of hair cells and neurons but not the prosensory macula. Immunostaining of uninjected controls and morphants with the indicated antibodies is shown at 48 hpf. Lateral views are shown; anterior is left, and dorsal is up. A–E, Fewer hair cells are present in the AM and PM of morphants. Quantification shows the number of hair cells in the PM. F–J, The area (in square micrometers) occupied by Sox2+ cells of the PM was not significantly changed in morphants compared with their controls (quantification graph: column 1, control for 96 MO and all3 MO; column 4, control for 182/183 MO). K–O, There were fewer Prox1+ cells in the LC of morphants, whereas the numbers in the AC and PC were unchanged. Quantification only shows the number of Prox1+ cells in the LC. P–T, The area (in square micrometers) occupied by HuC+ cells of the SAG was reduced in morphants. Results are presented as mean ± SEM. *p < 0.05, Tukey's Studentized Range test. See supplemental Table S1 (available at www.jneurosci.org as supplemental material) for further details. Scale bar, 25 μm.
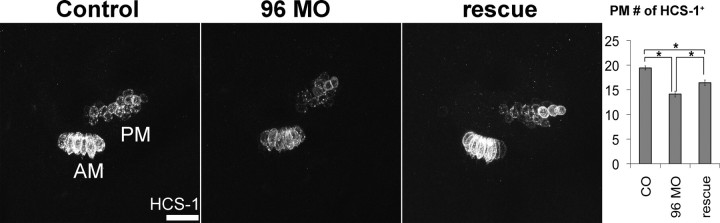
One test of functional redundancy among the miR-183 family members is to determine whether overexpression of one miRNA can compensate for knockdown of another to rescue the loss of hair cells. Indeed, coinjection of miR-182 (10 μm) and 96 MO (0.5 mm) into one-cell-stage embryos showed evidence of partial rescue (Fig. 5). The number of hair cells in the PM was increased by 16% in coinjected embryos compared with injection of miR-96 MO alone. However, hair cell numbers in coinjected embryos failed to reach the levels present in uninjected controls: they were reduced by 15%.
Figure 5.
Overexpression of miR-182 rescues loss of hair cells in miR-96 morphants. Rescue embryos were coinjected with 96 MO (0.5 mm) and miR-182 (10 μm). Immunostaining of HCS-1 shows the AM and PM at 48 hpf. Lateral views are shown; anterior is left, and dorsal is up. Quantification shows the number of hair cells in the PM in rescue embryos was larger than in miR-96 morphants (96 MO), but smaller than in control (CO). See supplemental Table S1 (available at www.jneurosci.org as supplemental material) for further details. Scale bar, 25 μm.
To confirm the target specificity of miRNA MOs, we compared the morphant phenotypes using MOs against mature miRNAs versus pre-miRNAs. MOs against miR-182, miR-183, pre-miR-182, or pre-miR-183 each reduced hair cell numbers in the PM (supplemental Table S2, available at www.jneurosci.org as supplemental material). The magnitude of hair cell reductions was dose dependent. MOs against miR-182 at 0.33, 0.5, and 1 mm yielded progressively fewer hair cells in the PM (supplemental Table S2, available at www.jneurosci.org as supplemental material), suggesting that the absolute levels of miR-183 family members can influence hair cell development. Overall, knockdown of the three miRNAs together seemed more effective than knockdown of two miRNAs or individual ones (supplemental Table S2, available at www.jneurosci.org as supplemental material).
Next, we asked whether loss of hair cells in the miR-183 family morphants might be a consequence of an earlier defect in prosensory development. In lateral views of 48 hpf embryos, the boundaries of the PM, where hair cell loss is most profound, are well defined by antibodies against Sox2. Yet, only a few morphants exhibited smaller Sox2+ domains. Overall, the average areas occupied by Sox2+ cells of the PM in control embryos and miR-182/183 double morphants were not significantly different (Fig. 4F,H,J). Likewise, PM size was unaltered in morphants treated with miR-96 MO or all3 miRNA MOs (Fig. 4G,I,J). By counting the number of Sox2+ cells in the PM as an alternative analysis, we saw a 10% reduction that did not reach statistical significance after injection of MOs against pre-miR-182 and pre-miR-183 (0.5 mm each; mean ± SEM; control: 77.5 ± 5.1, n = 13; pre182/pre183 MO: 70.8 ± 3.6, n = 13; p = 0.29). Similar results were obtained when Islet1 was used to measure the area of the PM domain (mean ± SEM; control: 2560.6 ± 92.1, n = 20; 182/183 MO: 2323.2 ± 77.2, n = 22; p = 0.06). These results suggest that macular hair cell reduction is more likely to be a direct effect on hair cell specification, differentiation, or maintenance, rather than a secondary consequence of the loss (or failed expansion) of macular prosensory territories.
The three cristae are specified later than the two maculae, with thickened sensory epithelia displaying a couple of hair cells in each crista at 48 hpf (Whitfield et al., 2002). Like the macular organs, miR-183 family members are expressed abundantly in early developing hair cells of cristae (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). Cristae responded somewhat differently than maculae when miRNA levels were reduced: there was a detectable delay in the onset of hair cell production. At 48 hpf, the number of cristae with hair cells was reduced by 46–80% in different morphants (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). Moreover, the number of hair cells in the AC, LC, and PC of miR-182/183 double morphants was reduced by 54, 82, and 53%, respectively (mean ± SEM; AC: control, 1.9 ± 0.1, n = 40; 182/183 MO, 0.9 ± 0.1, n = 52; LC: control, 1.3 ± 0.2, n = 40; 182/183 MO, 0.2 ± 0.1, n = 52; PC: control, 1.6 ± 0.1, n = 40; 182/183 MO, 0.8 ± 0.1, n = 52). Therefore, both the onset of differentiation and the number of hair cells were disrupted in the cristae in the miR-183 family morphants.
We next examined expression of Prox1 as a marker of the prosensory cristae. Prox1 is expressed in supporting cells in the mouse cochlea and is induced by Sox2 (Dabdoub et al., 2008). In the zebrafish inner ear, Prox1 was first expressed in a broad domain, and then at ∼48 hpf, it was restricted to three discrete patches beneath and beyond the hair cells of the cristae (Fig. 4K). The number of Prox1-immunopositive cells in the AC and PC primordia was unchanged in morphants compared with controls (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). However, the LC, which is specified slightly later than the other two cristae, had a 22–62% reduction in the number of Prox1+ cells in different morphants (Fig. 4K–O).
Loss of function for the miR-183 family could affect formation of the SAG, where the miRNAs are also expressed. At 48 hpf, the SAG in miR-182/183 double morphants was in the correct position but appeared truncated, with the posterior wing most severely affected (Fig. 4R). The number of HuC+ cells was significantly reduced by 15% (mean ± SEM; control: 135.6 ± 7.6, n = 10; 182/183 MO: 114.7 ± 4.2, n = 11), and the area occupied by HuC+ cells was decreased by 12% (Fig. 4P,R,T). Likewise, a slight but significant reduction (14–16%) in the size of the SAG was also observed in miR-96 and miRNA triple morphants (Fig. 4Q,S,T). Overall, the loss of neurons in the SAG was less severe than loss of hair cells in the two maculae, although both sensory and ganglion tissues showed a tendency toward a stronger effect on the posterior components compared with the anterior components of the inner ear.
The miR-183 family contributes to morphogenesis of the semicircular canals and the lateral line neuromasts
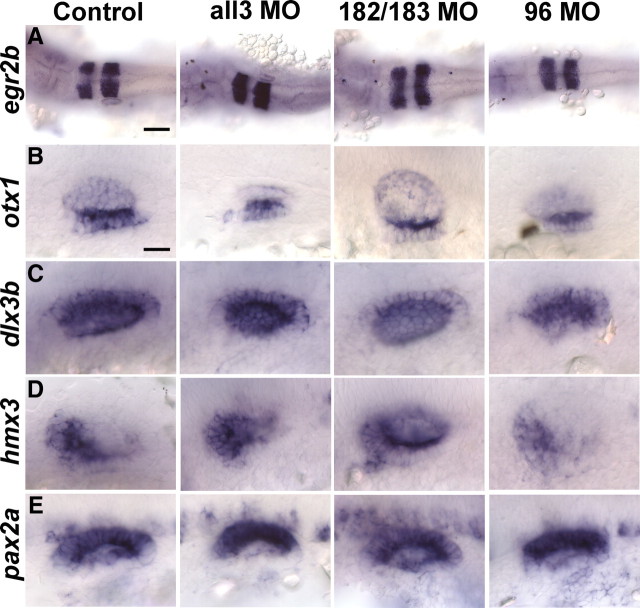
While knockdowns of the miR-183 family members individually or together did not affect survival rate or gross development, they all yielded similar defects in otocyst morphogenesis, albeit to different degrees (supplemental Fig. S5, available at www.jneurosci.org as supplemental material). At 48 hpf, when canal pillars are obviously protruding into the otocyst lumen in controls, these structures are missing in the morphants (Fig. 6A,B and supplemental Fig. S5, available at www.jneurosci.org as supplemental material). By 4 d after fertilization, each of the miR-96 morphants and 90% of morphants injected with all3 miRNA MOs had recovered their canals. Actually, a canal morphant phenotype was unexpected because the miR-183 family is not expressed in canals (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). To determine whether there could be an earlier problem with prepatterning the otic vesicle of morphants, we tested 24 hpf embryos for expression of the hindbrain marker egr2b; a ventral otic marker, otx1; a dorsal otic marker, dlx3b; an anterior otic marker, hmx3; and a medial otic marker, pax2a. Nearly normal spatial expression of these genes in the miR-183 family morphants indicated that general patterning of the hindbrain and otic vesicle was not changed (Fig. 7).
Figure 6.
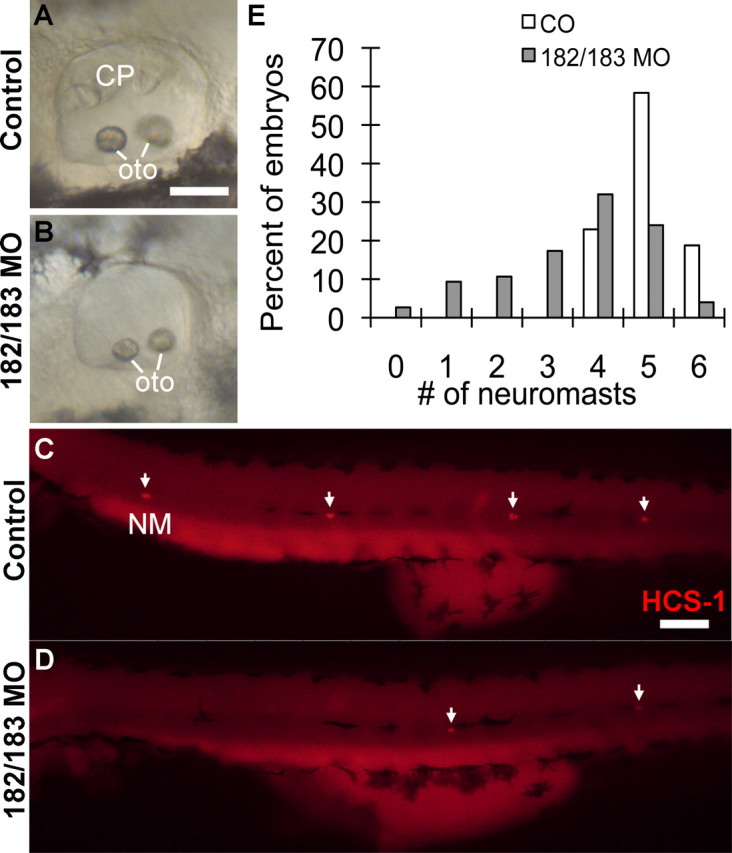
Knockdown of miR-182 and miR-183 affects morphogenesis of the semicircular canals and formation of neuromasts on the posterior lateral line at 48 hpf. A, B, Differential interference contrast images of semicircular canal pillars (CP) in the inner ear of uninjected controls (A) and 182/183 morphants (B). oto, Otoliths. C, D, Immunostaining of HCS-1 shows the distribution of hair cells in neuromasts (NM) along the body. White arrows indicate individual NMs. Lateral views are shown; anterior is left, and dorsal is up. E, Histogram of the number of neuromasts on the posterior lateral line (quantification: mean ± SEM; control: 5.0 ± 0.1, n = 48; 182/183 MO: 3.6 ± 0.2, n = 75) indicates a reduced number of neuromasts in 182/183 morphants than in controls (uninjected or injected with 1 mm standard MOs). Scale bars: A, B, 50 μm; C, D, 100 μm.
Figure 7.
Early patterning of the otocyst is not affected in the miR-183 family morphants. In situ hybridization and bright-field images of embryos at 24 hpf are shown. Uninjected controls, >20 embryos for each probe; 182/183 MO, 7–12 embryos for each probe; 96 MO, 16–25 embryos for each probe; all3 MO, 3–8 embryos for each probe. Purple signals show expression of egr2b (A) in the hindbrain and otx1 (B), dlx3b (C), hmx3 (D), and pax2a (E) in the otocyst. A, Dorsal view is shown, and anterior is left. B–D, Anterior is left, and dorsal is up. E, Anterior is left, and medial is up. Scale bars: A, 100 μm; B–E, 25 μm.
Besides the sensory organs of the inner ear, the neuromasts of the head and lateral line also contain hair cells that robustly express the miR-183 family (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). In the miR-183 family morphants, the number and distribution of neuromasts on the posterior lateral line were significantly altered (Fig. 6C–E and supplemental Fig. S6, available at www.jneurosci.org as supplemental material). Also, the average number of hair cells in each existing neuromast was reduced by 32% in miR-182/183 double morphants (mean ± SEM; control: 3.7 ± 0.1, n = 48; 182/183 MO: 2.5 ± 0.1, n = 73). Transgenic zebrafish of the ET20 enhancer-trap line express enhanced green fluorescent protein along the migration route of the neuromast primordium as it travels toward the tail, as well as in the supporting mantle cells of the differentiating neuromasts (Parinov et al., 2004). We stained for hair cells in the ET20 line and found that despite the presence of fewer neuromasts, a nearly complete lateral line migration trace was evident in most miR-182/183 double morphants. In fact, the defect seemed to be in the deposition of neuromasts, with unusually large gaps observed between some neuromasts.
Cell proliferation and cell death in the miR-183 family morphants
Loss of function for the miR-183 family resulted in morphological defects and reduced numbers of hair cells and neurons in the inner ear. To understand whether these phenotypes were caused by changes in cell fates or by abnormal levels of proliferation or survival, we used antibodies against phospho-histone H3 (pH3) to mark mitotic cells and antibodies against activated Caspase3 to show apoptosis (Bricaud and Collazo, 2006). The apparent increase in Caspase3+ cells in the morphants at 32 hpf (supplemental Fig. S7, available at www.jneurosci.org as supplemental material) is not statistically significant and in any case is mitigated by very small numbers of positive cells present in the inner ear (usually fewer than three per side). At the same time point, there was no significant difference in the number of pH3+ cells in the otocyst in miR-182/183 double morphants or in miR-96 morphants compared with controls injected with standard MOs (supplemental Fig. S7, available at www.jneurosci.org as supplemental material).
Discussion
Despite the presence of hundreds of miRNAs in zebrafish and other vertebrate embryos, our knowledge about the functions of individual miRNAs during development is limited to a few examples. Here we present evidence from gain and loss of function that members of the miR-183 family have both conserved and varying roles during development of hair cells and neurons in the inner ear and lateral line.
We show, for the first time, that in addition to transcription factors such as Atoh1 and disruption of Delta-Notch signaling, misexpression of two related miRNAs can induce excessive hair cells. As might be expected, knockdown of these miRNAs has the opposite effect to their overexpression in modifying hair cell numbers, causing significant reductions. These results reveal the necessity of miRNAs in ensuring the proper development of sensory epithelia, particularly in fine tuning the precise number of sensory cells. Our results for hair cells reinforce a general appreciation of miRNA function that operates not as a switch in choosing between different cell fates, but more as a rheostat for modulating the precise levels of target genes needed to either acquire or maintain a particular cell fate.
We do not know how miRNA-182 and -96 promote a hair cell fate. They may act indirectly to activate hair cell genes or to repress supporting cell genes. Alternatively, or in addition, they may downregulate translation of proteins required for proliferation or repress proteins that promote a prosensory or proneural progenitor state. How, then, can we account for observed increases in prosensory patch size after miR-182 overexpression when it is normally restricted to the hair cells? One possibility is that miR-182 is interacting with the target(s) expressed in prosensory cells that normally would not be confronted with its repressive activity. We propose an alternative hypothesis: that expansion of prosensory domains seen when miR-182 is overexpressed may be induced indirectly, by a signal emanating from the extra differentiating hair cells. Perhaps the gradual addition of hair cells is balanced by a gradual expansion of the surrounding pool of progenitors that is dependent on those newborn hair cells. In this way, the progenitor pool is not exhausted by hair cell specification, even in normal ears. This would serve to buffer the system from natural fluctuations in the timing or levels of regulatory factors that influence rates of proliferation or cell-fate specification, thereby ensuring that the sensory organs grow slowly and consistently over the life of the animal. The expansion of prosensory regions at 48 hpf was not seen in response to miR-96 overexpression, perhaps because the early increase in hair cell numbers at 26 hpf did not persist.
The zebrafish inner ear is further complicated by having two different cell types, the neurons and the hair cells, that express different levels of the miR-183 family after differentiation. Our results suggest that the roles of the miR-183 family in inner ear sensory organs and the SAG are distinct. Neurons, which normally express lower levels than hair cells, are negatively affected by miRNA overexpression, whereas hair cells are promoted by the same treatment. This finding suggests that some of the miRNA target transcripts that must be strongly repressed in incipient hair cells may nevertheless be required, at least at a moderate level, in neurons. To date, we know of no predicted binding sites for miR-183 family members on the transcripts for neurogenic genes known to be involved in specifying SAG neurons.
Curiously, miRNA knockdown does not yield results that are fully complementary to results from miRNA overexpression (i.e., more neurons coupled with fewer hair cells). Instead, both neuron and hair cell numbers are reduced in miRNA morphants, even though the prosensory domain is normal in size. In this case, perhaps the lack of target gene repression affects both cell types similarly, prolonging the precursor state and delaying both neuronal and hair cell specification. We also note that loss of cells is more severe in the PM and posterior SAG of miRNA morphants, whereas overproduction of hair cells is more significant in the AM after miRNA overexpression, for unknown reasons.
It is unclear whether we ought to expect a reciprocal relationship in the numbers of neurons and sensory cells in zebrafish. This was observed after manipulation of Six1 (Bricaud and Collazo, 2006) and might be predicted if the two cell types can share common progenitors as in the chicken (Satoh and Fekete, 2005). Although the progenitor domains defined by neurogenin1 for neurons and atoh1 for hair cells appear to be adjacent rather than overlapping at 18 hpf in zebrafish (Andermann et al., 2002; Millimaki et al., 2007), their proximity might offer an opportunity for reciprocal interactions. Furthermore, in mouse, the progenitors are initially intermingled but then separate over time as the epithelium transitions from neurogenic to sensory (Raft et al., 2007). If an increase in one pool of progenitors occurs at the expense of the other, then reducing the number of emigrating neuroblasts with miRNA knockdowns might be predicted to increase the number of Sox2+ cells remaining behind in the prosensory domain, which was not observed. However, we note that the continued expansion of the prosensory region might be delayed as a consequence of the differentiation of fewer hair cells, assuming a feed-forward effect on prosensory growth as described earlier.
Because miRNAs function through inhibition of their target genes at the posttranscriptional level, the presence of both miRNAs and the pool of targets is critical. Targets of the miR-183 family predicted by miRBase (http://microrna.sanger.ac.uk/targets/v5/), which uses the miRanda algorithm, include Sox2, Islet-1, and Prox1. However, indirect immunofluorescence showed that the expression of all three proteins was relatively unchanged in miRNA morphants. Nonetheless, loss-of-function results indicate that the three miRNAs overlap functionally in terms of hair cells and neurons and therefore probably share at least some common targets. This idea is also supported by the ability of excess miR-182 to partially mitigate the loss of hair cells caused by miR-96 knockdown. On the other hand, results from overexpression of individual miR-183 family members show different abilities to increase hair cell numbers and prosensory size, suggesting that they each bind some distinct targets. This conclusion is supported by bioinformatics-based target predictions (Xu et al., 2007). It is surprising then, that knock-out of miR-182 alone failed to produce a phenotype in mouse retina (Jin et al., 2009), where the gene is abundantly expressed (Xu et al., 2007). This contrasts with our results for inner ear in which knockdown of miR-182 alone has a phenotype.
The knockdown of miR-183 family members causes two morphogenetic defects, delays in semicircular canal formation and irregularities in lateral line deposition, that are difficult to reconcile with the expression patterns of these genes. Neither the canal anlagen nor the migrating lateral line primordia express the miR-183 family at levels that can be detected by WMISH. In both cases, direct effects could involve morpholino-mediated reductions in the targeted miRNAs that are below the level of detection. Alternatively, both events are highly orchestrated processes that may be particularly vulnerable to the nonspecific effects of morpholino treatments. The discovery of known target genes for the miRNA-183 family members should assist in revealing the underlying molecular basis of these morphogenetic perturbations.
Regeneration of hair cells remains a challenge in the mammalian inner ear, whereas it happens naturally in birds and fish (Haddon and Lewis, 1996). Although misexpression of Atoh1 successfully induces ectopic cochlear hair cells in postnatal rat cochlea in vitro and adult guinea pigs in vivo, some of these hair cells can be immature or can have morphological defects (Kelley, 2006). Therefore, a more complete regeneration of hair cells from the underlying supporting cells may require additional positive or negative regulators of cell differentiation, including perhaps miRNAs. The abundant expression of the miR-183 family in hair cells, combined with the ability of miR-182 to induce extra hair cells, argues for a potential therapeutic role for this family in promoting hair cell regeneration, perhaps by either promoting expression of hair cell genes or inhibiting prosensory and supporting cell genes.
In summary, our data support a hypothesis that the precise levels of miR-183 family members in hair cells and SAG neurons helps to regulate their respective cell fates. When these miRNAs are knocked down in different combinations, we observe a gradation in the strength of the hair cell phenotype. This suggests that the three miRNAs functionally overlap in hair cells, where the total level of the three genes must be important. This conclusion is consistent with their highly similar seed regions. Meanwhile, unique phenotypes after overexpression indicate that the three miRNAs also have distinct targets. Although it remains a challenge to determine their interactions with target genes, our results suggest that miR-182, at least, could be considered as a potential candidate for therapies designed to promote hair cell regeneration.
Footnotes
This work was supported by National Institutes of Health Grant R21 DC008997 (D.M.F.). We thank Deb Biesemeier and Katie Byrum for technical assistance, Bruce Riley for plasmids, Daniel Szeto for technical guidance and critical reading of this manuscript, and members of our laboratory for helpful discussions.
References
- Abello G, Khatri S, Giraldez F, Alsina B. Early regionalization of the otic placode and its regulation by the Notch signaling pathway. Mech Dev. 2007;124:631–645. doi: 10.1016/j.mod.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Alsina B, Abello G, Ulloa E, Henrique D, Pujades C, Giraldez F. FGF signaling is required for determination of otic neuroblasts in the chick embryo. Dev Biol. 2004;267:119–134. doi: 10.1016/j.ydbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Andermann P, Ungos J, Raible DW. Neurogenin1 defines zebrafish cranial sensory ganglia precursors. Dev Biol. 2002;251:45–58. doi: 10.1006/dbio.2002.0820. [DOI] [PubMed] [Google Scholar]
- Bang PI, Sewell WF, Malicki JJ. Morphology and cell type heterogeneities of the inner ear epithelia in adult and juvenile zebrafish (Danio rerio) J Comp Neurol. 2001;438:173–190. doi: 10.1002/cne.1308. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Brand M, Granato M, Nüsslein-Volhard C. Keeping and raising zebrafish. In: Nüsslein-Volhard C, Dahm R, editors. Zebrafish: A practical approach. Oxford: Oxford UP; 2002. pp. 7–37. [Google Scholar]
- Bricaud O, Collazo A. The transcription factor six1 inhibits neuronal and promotes hair cell fate in the developing zebrafish (Danio rerio) inner ear. J Neurosci. 2006;26:10438–10451. doi: 10.1523/JNEUROSCI.1025-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PY, Manninga H, Slanchev K, Chien M, Russo JJ, Ju J, Sheridan R, John B, Marks DS, Gaidatzis D, Sander C, Zavolan M, Tuschl T. The developmental miRNA profiles of zebrafish as determined by small RNA cloning. Genes Dev. 2005;19:1288–1293. doi: 10.1101/gad.1310605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, Pevny LH, Kelley MW. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci U S A. 2008;105:18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman LM, Dror AA, Mor E, Tenne T, Toren G, Satoh T, Biesemeier DJ, Shomron N, Fekete DM, Hornstein E, Avraham KB. MicroRNAs are essential for development and function of inner ear hair cells in vertebrates. Proc Natl Acad Sci U S A. 2009;106:7915–7920. doi: 10.1073/pnas.0812446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JE, Meyers JR, Corwin JT. Solitary hair cells are distributed throughout the extramacular epithelium in the bullfrog's saccule. J Assoc Res Otolaryngol. 2000;1:172–182. doi: 10.1007/s101620010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon C, Lewis J. Early ear development in the embryo of the zebrafish, Danio rerio. J Comp Neurol. 1996;365:113–128. doi: 10.1002/(SICI)1096-9861(19960129)365:1<113::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Haddon C, Jiang YJ, Smithers L, Lewis J. Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development. 1998;125:4637–4644. doi: 10.1242/dev.125.23.4637. [DOI] [PubMed] [Google Scholar]
- Haddon C, Mowbray C, Whitfield T, Jones D, Gschmeissner S, Lewis J. Hair cells without supporting cells: further studies in the ear of the zebrafish mind bomb mutant. J Neurocytol. 1999;28:837–850. doi: 10.1023/a:1007013904913. [DOI] [PubMed] [Google Scholar]
- Jin ZB, Hirokawa G, Gui L, Takahashi R, Osakada F, Hiura Y, Takahashi M, Yasuhara O, Iwai N. Targeted deletion of miR-182, an abundant retinal microRNA. Mol Vis. 2009;15:523–533. [PMC free article] [PubMed] [Google Scholar]
- Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci. 2006;7:837–849. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Pelling AL, Leung KK, Tang AS, Bell DM, Tease C, Lovell-Badge R, Steel KP, Cheah KS. Sox2 is required for sensory organ development in the mammalian inner ear. Nature. 2005;434:1031–1035. doi: 10.1038/nature03487. [DOI] [PubMed] [Google Scholar]
- Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–450. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Kloosterman WP, Wienholds E, de Bruijn E, Kauppinen S, Plasterk RH. In situ detection of miRNAs in animal embryos using LNA-modified oligonucleotide probes. Nat Methods. 2006;3:27–29. doi: 10.1038/nmeth843. [DOI] [PubMed] [Google Scholar]
- Kloosterman WP, Lagendijk AK, Ketting RF, Moulton JD, Plasterk RH. Targeted inhibition of miRNA maturation with morpholinos reveals a role for miR-375 in pancreatic islet development. PLoS Biol. 2007;5:e203. doi: 10.1371/journal.pbio.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak SJ, Vemaraju S, Moorman SJ, Zeddies D, Popper AN, Riley BB. Zebrafish pax5 regulates development of the utricular macula and vestibular function. Dev Dyn. 2006;235:3026–3038. doi: 10.1002/dvdy.20961. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lewis MA, Quint E, Glazier AM, Fuchs H, De Angelis MH, Langford C, van Dongen S, Abreu-Goodger C, Piipari M, Redshaw N, Dalmay T, Moreno-Pelayo MA, Enright AJ, Steel KP. An ENU-induced mutation of miR-96 associated with progressive hearing loss in mice. Nat Genet. 2009;41:614–618. doi: 10.1038/ng.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencia A, Modamio-Hoybjor S, Redshaw N, Morin M, Mayo-Merino F, Olavarrieta L, Aguirre LA, del Castillo I, Steel KP, Dalmay T, Moreno F, Moreno-Pelayo MA. Mutations in the seed region of human miR-96 are responsible for nonsyndromic progressive hearing loss. Nat Genet. 2009;41:609–613. doi: 10.1038/ng.355. [DOI] [PubMed] [Google Scholar]
- Millimaki BB, Sweet EM, Dhason MS, Riley BB. Zebrafish atoh1 genes: classic proneural activity in the inner ear and regulation by Fgf and Notch. Development. 2007;134:295–305. doi: 10.1242/dev.02734. [DOI] [PubMed] [Google Scholar]
- Neves J, Kamaid A, Alsina B, Giraldez F. Differential expression of Sox2 and Sox3 in neuronal and sensory progenitors of the developing inner ear of the chick. J Comp Neurol. 2007;503:487–500. doi: 10.1002/cne.21299. [DOI] [PubMed] [Google Scholar]
- Parinov S, Kondrichin I, Korzh V, Emelyanov A. Tol2 transposon-mediated enhancer trap to identify developmentally regulated zebrafish genes in vivo. Dev Dyn. 2004;231:449–459. doi: 10.1002/dvdy.20157. [DOI] [PubMed] [Google Scholar]
- Pierce ML, Weston MD, Fritzsch B, Gabel HW, Ruvkun G, Soukup GA. MicroRNA-183 family conservation and ciliated neurosensory organ expression. Evol Dev. 2008;10:106–113. doi: 10.1111/j.1525-142X.2007.00217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt C. Zebrafish inner ear sensory surfaces are similar to those in goldfish. Hear Res. 1993;65:133–140. doi: 10.1016/0378-5955(93)90208-i. [DOI] [PubMed] [Google Scholar]
- Raft S, Koundakjian EJ, Quinones H, Jayasena CS, Goodrich LV, Johnson JE, Segil N, Groves AK. Cross-regulation of Ngn1 and Math1 coordinates the production of neurons and sensory hair cells during inner ear development. Development. 2007;134:4405–4415. doi: 10.1242/dev.009118. [DOI] [PubMed] [Google Scholar]
- Riley BB, Zhu C, Janetopoulos C, Aufderheide KJ. A critical period of ear development controlled by distinct populations of ciliated cells in the zebrafish. Dev Biol. 1997;191:191–201. doi: 10.1006/dbio.1997.8736. [DOI] [PubMed] [Google Scholar]
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, Ekker SC. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Fekete DM. Clonal analysis of the relationships between mechanosensory cells and the neurons that innervate them in the chicken ear. Development. 2005;132:1687–1697. doi: 10.1242/dev.01730. [DOI] [PubMed] [Google Scholar]
- Soukup GA, Fritzsch B, Pierce ML, Weston MD, Jahan I, McManus MT, Harfe BD. Residual microRNA expression dictates the extent of inner ear development in conditional Dicer knockout mice. Dev Biol. 2009;328:328–341. doi: 10.1016/j.ydbio.2009.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M. Ed 2.1. Eugene, OR: University of Oregon; 1994. The zebrafish book: A guide for the laboratory use of zebrafish (Brachydanio rerio) [Google Scholar]
- Weston MD, Pierce ML, Rocha-Sanchez S, Beisel KW, Soukup GA. MicroRNA gene expression in the mouse inner ear. Brain Res. 2006;1111:95–104. doi: 10.1016/j.brainres.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Whitfield TT, Riley BB, Chiang MY, Phillips B. Development of the zebrafish inner ear. Dev Dyn. 2002;223:427–458. doi: 10.1002/dvdy.10073. [DOI] [PubMed] [Google Scholar]
- Wienholds E, Kloosterman WP, Miska E, Alvarez-Saavedra E, Berezikov E, de Bruijn E, Horvitz HR, Kauppinen S, Plasterk RH. MicroRNA expression in zebrafish embryonic development. Science. 2005;309:310–311. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- Xu S, Witmer PD, Lumayag S, Kovacs B, Valle D. MicroRNA (miRNA) transcriptome of mouse retina and identification of a sensory organ-specific miRNA cluster. J Biol Chem. 2007;282:25053–25066. doi: 10.1074/jbc.M700501200. [DOI] [PubMed] [Google Scholar]