Origin and formation of the first two distinct cell types of the inner cell mass in the mouse embryo (original) (raw)
Abstract
A crucial question in mammalian development is how cells of the early embryo differentiate into distinct cell types. The first decision is taken when cells undertake waves of asymmetric division that generate one daughter on the inside and one on the outside of the embryo. After this division, some cells on the inside remain pluripotent and give rise to the epiblast, and hence the future body, whereas others develop into the primitive endoderm, an extraembryonic tissue. How the fate of these inside cells is decided is unknown: Is the process random, or is it related to their developmental origins? To address this question, we traced all cells by live-cell imaging in intact, unmanipulated embryos until the epiblast and primitive endoderm became distinct. This analysis revealed that inner cell mass (ICM) cells have unrestricted developmental potential. However, cells internalized by the first wave of asymmetric divisions are biased toward forming pluripotent epiblast, whereas cells internalized in the next two waves of divisions are strongly biased toward forming primitive endoderm. Moreover, we show that cells internalized by the second wave up-regulate expression of Gata6 and Sox17, and changing the expression of these genes determines whether the cells become primitive endoderm. Finally, with our ability to determine the origin of cells, we find that inside cells that are mispositioned when they are born can sort into the correct layer. In conclusion, we propose a model in which the timing of cell internalization, cell position, and cell sorting combine to determine distinct lineages of the preimplantation mouse embryo.
Keywords: cell fate, epiblast, primitive endoderm, Gata6, Sox17
The first decision determining cell fate in the mouse embryo is taken when two populations of cells are physically partitioned by successive waves of asymmetric divisions commencing at the eight-cell stage (1–5). Cells positioned inside the embryo develop into the inner cell mass (ICM), whereas outside cells develop into the first extraembryonic tissue, the trophectoderm, that will give rise to the placenta. The second decision determining cell fate distinguishes two ICM cell types: the pluripotent epiblast (EPI) that generates cells of the future body and the second extraembryonic tissue, primitive endoderm (PE) (6). It has not yet been established how this second cell fate decision is made. Two possibilities have been considered. In the positional/induction hypothesis, cell fate is determined by position, based on the observation that surface cells adjacent to the blastocyst cavity become PE, whereas deeper cells become EPI. Whether this is the underlying mechanism in the developing embryo is unknown. Moreover, later it was observed that cells expressing PE and EPI markers, Gata6 and Nanog, respectively, are distributed initially in a salt-and-pepper pattern (7, 8) and that there is actin-dependent cell movement between deep and surface layers of the ICM before the lineages become distinct (9, 10). These observations seemed consistent with the alternative cell-sorting hypothesis, which proposes that EPI and PE lineages are specified at random and then are sorted into composite layers (7). However, lineage-tracing studies carried out to date have not been able to resolve how the initial salt-and-pepper pattern arises, either because the studies stopped following cells before the EPI and PE lineages were distinct (11, 12) or because the studies commenced only after their progenitor cells already had been internalized (9, 10). Thus, several important questions remain unanswered. First, do PE and EPI progenitors truly arise in the ICM at random, as is generally thought? Second, is there a connection between the developmental origin of an ICM cell and its subsequent movement between layers? Finally, how might the transcription factors associated with PE formation regulate processes that influence the cell-fate decisions?
Results
Tracking the Origins, Pedigree, and Behavioral History of Every Cell in the EPI and PE of the Blastocyst.
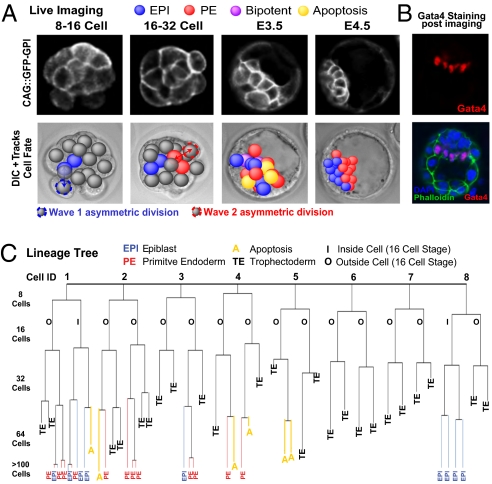
To record the origins, cell divisions, positions, and movements of all progenitor cells contributing to the EPI and PE, we filmed embryos in 4D, on 15 focal planes for ≈55 h from the time when cells are first internalized to the point at which their final fate is known in the late blastocyst. Using Simi Biocell software (12), we tracked and analyzed a total of 505 ICM cells in 20 embryos derived from a transgenic line expressing a GPI-tagged GFP cell-surface marker (GFP-GPI) (13) (Fig.1_A_ and Movie S1). At the end of imaging, embryos had an average of 19 ICM cells: 11 in the surface PE layer and 8 in EPI. This increase in cell number reflected the balance between cell division and apoptosis and was consistent with earlier studies (14, 15) and with cell numbers in freshly collected embryos (Fig. S1_A_). During imaging, embryos developed a surface ICM layer expressing the PE marker Gata4 (Fig.1_B_). Finally, when imaged embryos were transplanted into foster mothers, they developed to term (Fig. S1_B_). These multiple lines of evidence demonstrate that our imaging conditions permitted normal development. Tracking birth and behavior of all inside cells allowed us to determine the wave of asymmetric division in which all EPI and PE progenitors are internalized, which cells change positions between surface and deeper ICM layers, when and under what circumstances this change of position happens, and which sublineage is terminated by apoptosis (a representative lineage tree is shown in Fig. 1_C_; all lineages are shown in Fig. S2).
Fig. 1.
Live cell imaging and tracking. (A) GFP-GPI expression from E.2.5 to E4.5. Deconvolved fluorescence and differential interference contrast time-lapse images overlaid with Simi BioCell cell-tracking spheres. (B) Embryos stained to reveal Gata4-positive cells adjacent to mature blastocyst cavity confirming normal development during each imaging session. Gata4-positive cells were present in a one-cell-thick surface layer. (C) Lineage tree from representative embryo. All cells were traced to the early 32-cell blastocyst; then inside cells were traced to late blastocyst. A cell was defined as occupying an inside position by the orientation of the cell division that generated it, by its enclosure from the outside environment by neighboring cells, and by continued analysis of its position as development progressed. Allocation to trophectoderm (TE), EPI, or PE and apoptosis (A) are indicated.
Successive Waves of Divisions Generate Inside Cells with Progressively Restricted Fate.
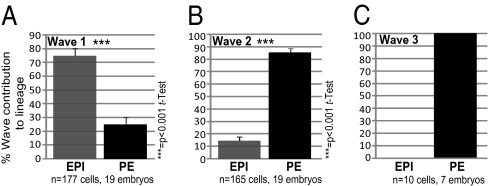
We identified three waves of asymmetric divisions giving rise to inside cells. The first and second waves (the fourth and fifth rounds of cleavage, Movie S2) generate the majority of the ICM cells in all embryos, and the third wave (the sixth round of cleavage), giving fewer inside cells, occurs in one-third of all embryos (7/20) (Movie S3). On average, 50% (n = 177) of ICM cells originated from cells internalized by the first wave, 47% (n = 165) from the second wave, and 3% (n = 10) from the third wave (Figs. S2 and S3 A and B). To determine the origins of all PE and EPI cells, we traced the cells back to the emergence of their progenitors inside the embryo (Fig. 1_C_). At the 16-cell stage, when the first wave of asymmetric divisions is completed, the mean number of inside cells was 2.84 (range 1–5) (Fig. S3_D_). Strikingly, we found that a significant majority of inside cells (75%, n = 177) from this wave were EPI progenitors, and only 25% were PE progenitors (Fig. 2_A_ and Fig. S3_C_, Movie S4). From another perspective, on average nearly 80% (79.5%) of the EPI was contributed by the first wave, and in nearly half (8/19) of all blastocysts 100% of the EPI was derived from wave 1 cells (Fig. S3_D_). When the entire EPI was built from cells generated by the first wave, the “surplus” wave 1 cells contributed to PE (Fig. S3 C and D). This finding indicates that inside cells generated by wave 1 are pluripotent (i.e., are not restricted to form only EPI). Upon completion of the second wave, at the 32-cell stage, the number of ICM cells had increased to 10.9 on average (range 7–14). In striking contrast, most inside cells generated by wave 2 (85%, n = 165 cells) gave rise to PE, and only 15% gave rise to EPI (Fig. 2_B_, Movie S5). In nearly half of all embryos (8/19), 100% of the inner cells generated by the second wave contributed to PE (Fig. S3_C_). Finally, in embryos (n = 10) in which a third wave of asymmetric division occurred (at the transition from the 32-cell stage to the 64-cell stage), this wave contributed exclusively to PE (Fig. 2_C_). Together, these results identified an unexpected relationship between the “wave of origin” and eventual fate of ICM cells.
Fig. 2.
Proportion of the first (A), second (B), and third (C) waves of asymmetric division contributing to EPI and PE.
Cell Movement Unites Cells from Same Wave with a Common Fate.
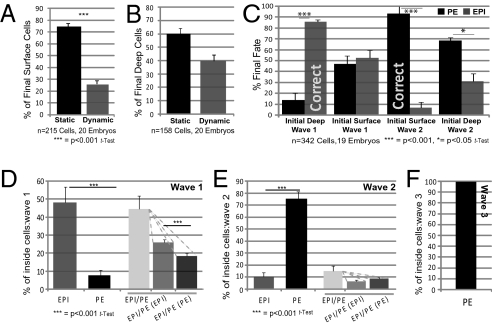
The differential contributions of inner cells from successive division waves to either EPI or PE led us to consider whether cells already are positioned according to their predominant fate when the blastocyst cavity forms, with PE progenitors on the surface and EPI progenitors in the deep ICM. Alternatively, are EPI and PE progenitors distributed randomly and then segregate? Although our studies and those of others identified some cell movement within the ICM, they could not address this question, because the wave of asymmetric divisions giving rise to cells that changed their position was unknown (9, 10). Cell movement becomes apparent from ≈7 h after completion of the fifth cleavage and continues until the seventh cleavage (Fig. S4_A_). Tracking of all cells revealed that a significant majority of cells at the ICM surface (alongside the nascent cavity) maintained their position: from 115 mother cells at the surface, 75% of progeny (161/215 cells) maintained a surface position, and 25% moved deep (Fig. 3_A_ and Fig. S4B). However, deep ICM cells showed greater mobility: from the progeny of 103 deep mother cells, 40% (63/158 cells) moved to the ICM surface, and 60% stayed deep (Fig. 3_B_ and Fig. S4_C_). Thus, the significant majority (68.6%) of the total final ICM population (256/373) remained in their originally allocated compartment; the remainder changed position (Fig. S4_E_).
Fig. 3.
Cell movement and fate in the ICM. ICM surface (A) and deep (B) cell dynamics from early to late blastocyst. (C) Relationship of final cell fate to cell origin and behavior. Cells are grouped according to final fate and are classified according to originating asymmetric division and initial ICM position. Cells from wave 2 initially at the ICM surface are highly likely to become PE (>90%, P < 0.001, t test). Cells from wave 1 initially deep contribute significantly to EPI (almost 90%, P < 0.001, t test). “Correct” refers to allocation of wave 1 cells to EPI and wave 2 cells to PE before cell movement; n = 19, because one embryo was analyzed from the 32-cell stage. (D_–_F) Bipotency in wave 1 (D), in wave 2 (E), and in wave 3 (F) showing mean values of proportions of cells per embryo with indicated final fates.
Further analysis of cell movement revealed that 86% of the cells from the first wave that initially were positioned in deep rather than surface layers of the ICM did not move and formed EPI (Fig.3_C_). However, as many as half (53%) of the cells from the first wave that originally were at the surface moved deep. Similarly 93% of the cells from the second wave that originally were at the surface did not move and formed PE. However, a significant majority (69%) of cells originating from wave 2 that initially were deep moved to join other PE progenitors originating in wave 2 at the ICM surface. We also found that 86% of cells (18/21) moving to the ICM surface were first associated with two to three transient cavities that formed in one-third of embryos before a single major cavity came to predominate (Fig. S4_F_). Together, cell movement to the ICM surface was more common than movement in the opposite direction, and a significant majority (83%) of such cells (n = 60) were generated in the second wave (Fig. S4_D_). Reciprocally, 89% of cells (n = 54) moving from the ICM surface to a deep layer were generated in the first wave. By following cell outlines, we were able to determine that the repositioning of cells from surface to deep layers occurred either through direct cell movement (75.5%, n = 54 cells) or in divisions in which one daughter cell remained on the surface and the other segregated to a deep layer (Fig. S4_G_ and Movie S6).
The finding that ICM cells on the surface can produce both PE and EPI progeny was consistent with our previous studies (16, 17, 9). However now we were able to address how, where, and how frequently within the ICM this possibility arises. We found that although a significant majority of the final population of inner cells (79%, 295/373) was contributed by unipotent mothers, 21% originated from mother cells, mainly at the surface, that were able to generate both EPI and PE (Fig. S4 H and I). Such bipotent mother cells were generated mainly in the first wave: Only 14% of cells arising from wave 2 and none from wave 3 contributed to both lineages (Fig. 3 D_–_F). Thus, more cells contributing to both ICM lineages arise during the first wave than during the second, further suggesting that the later the division that gives rise to an inside cell, the greater is the likelihood of that cell becoming PE.
We noted that at the time of cell sorting, some cells began to be eliminated by apoptosis (Movie S7 and refs. 9 and 10). Apoptosis occurred mainly in deep ICM (Fig. S4_J_). To determine whether a cell's death might relate to its cell origin and/or positioning, we classified all cells depending upon whether they were “appropriately” positioned according to their wave of origin and asked whether this positioning influenced their survival. For example, cells internalized in the first wave were considered appropriately positioned if they ended up in the deep ICM, regardless of whether they were positioned deep originally or moved deep later. This analysis revealed that three groups of cells showing particularly high proportions of apoptotic cells were all positioned inappropriately with respect to their wave of origin (Fig. S4_K_): 41% of wave 1 deep cells that inappropriately repositioned to the ICM surface; 57% of wave 2 cells were inappropriately positioned deep and remained deep; and 58% of wave 2 ICM surface cells that were inappropriately repositioned deep. This result suggests a possible relationship between cell origin and position, on one hand, and cell death on the other. However, a similar proportion of wave 1 cells that remained on the surface underwent apoptosis, as did appropriately positioned cells.
In conclusion, our results provide evidence that assignment to EPI or PE cell types occurs largely, but not exclusively, in specific waves of asymmetric division. Moreover, we observe that many cells are positioned according to their prospective fate when the blastocyst cavity forms, and the remainder tend to sort and join their appropriately positioned cousins from the same wave (Fig. 3_C_). Such cell sorting resolves the initial salt-and-pepper distribution and brings like cells together. However, we also observe plasticity within the ICM, because a proportion of cells produce daughters that follow a different fate, in agreement with our earlier observations (9). Finally, our results indicate that cells not correcting their position tend to undergo apoptosis, although not all cell death is caused by cells being mispositioned.
Gata6 Is Important, but Not Sufficient to Drive Cells to PE.
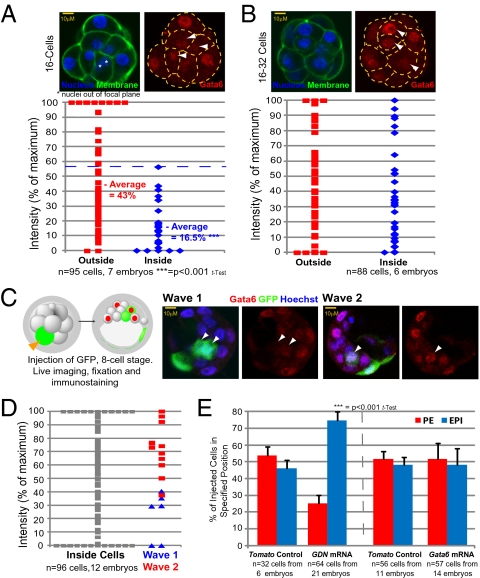
Because Gata6 is implicated in the decision to form PE (8, 9), we wished to examine its expression pattern to determine whether it correlates with waves of asymmetric division. We found that at the 16-cell stage, Gata6 levels are higher in outside cells than in inside cells, but after the transition from the 16-cell stage to the 32-cell stage Gata6 levels become much higher in some inside cells (Fig. 4 A and B). To determine whether this heterogeneity in Gata6 expression depends on a cell's origin, we traced back through the history of cells with different Gata6 levels. This investigation required injecting a random eight-cell blastomere with mRNA for GFP as a lineage marker and filming embryos to determine whether such cells divided symmetrically or asymmetrically, and in which wave of division. We then correlated the level of Gata6 expression, revealed by immunostaining, with cell origin (Fig. 4_C_). We found that cells with the highest Gata6 levels are generated in the second wave, whereas cells with no Gata6 or in which its expression is low are generated in the first wave (Fig. 4_D_). This result is consistent with the lineage analysis indicating cells from wave 2 contribute most of the PE.
Fig. 4.
Gata6 expression and PE formation. Immunostaining of Gata6 in (A) 16-cell and (B) 16- to 32-cell embryos. Gata6 staining intensities are expressed as a percentage of maximum. White arrowheads indicate inside cells. (C) Correlation of Gata6 expression with wave of origin. Shown is an eight-cell blastomere injected with GFP mRNA, monitored for wave 1 or wave 2 division and immunostained for Gata6 at E3.5. White arrowheads indicate inside cells expressing GFP. (D) Gata6 levels in inside cells, relative to cells from a specific wave. Intensities are expressed as a percentage of maximum. (E) Cells injected with dominant negative Gata6 (GDN) localized deep in the ICM in 74.8% of cases (P < 0.001, t test, n = 64 cells from 21 embryos). Overexpression of Gata6 in eight-cell blastomeres does not alter cell fate.
To determine whether such heterogeneity in Gata6 could affect cell fate, we generated mosaic embryos in which distinguishable clones of cells had either higher or lower levels of Gata6. This approach revealed that up-regulating Gata6 by injecting Gata6 mRNA into an eight-cell blastomere does not result in the cell's progeny contributing more to the PE than to the EPI (Fig. 4_E_ and Fig. S5 A and B). However, down-regulation of Gata6 function, by expression of dominant-negative Gata6 (9), causes cells to contribute significantly more to the EPI (74.8% of injected cells vs. 46% of _Tomato_-only control cells; Fig. 4_E_ and Fig. S5_B_) than to the PE. Together, these results provide evidence that Gata6 is present at higher levels in cells internalized in the second wave and that its expression is important but is not sufficient to drive cells to PE.
Expression of the PE Marker Sox17 Increases the Chances of Cells Acquiring PE Identity.
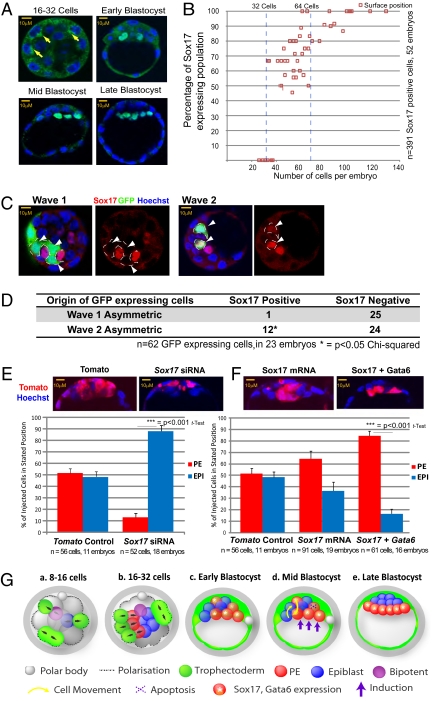
To assess the potential involvement of other transcription factors in PE formation, we reexamined our previous microarray study of preimplantation development (18). This analysis drew our attention to Sox17, which is dramatically up-regulated from the transition from the 16-cell stage to the 32-cell stage, correlating with the expression of genes functioning in PE development (Fig. S6_A_) (19, 20). Indeed, Sox17 has been implicated in PE differentiation and development in vitro (21, 22), but its expression pattern and function in the preimplantation embryo remained unknown. We found that nuclear localization of Sox17 is clearly detectable at about the 32-cell stage (Fig. 5 A and B). When embryos had up to around 50 cells, 34% of Sox17-positive cells are deep, and 66% are on the ICM surface. As development progresses, all Sox17-expressing cells become restricted to the ICM surface, consistent with formation of the PE layer. Moreover, we found that Sox17 is coexpressed in cells with PE markers such as Laminin and Gata4 (Fig. S6 B and C), in accord with an involvement in PE specification.
Fig. 5.
Sox17 expression and PE formation. (A) Staining to reveal Sox17 (yellow arrows indicate cytoplasmic staining) from 16- to 32-cell embryos to E4.5. (B) Proportion of cells in surface ICM with nuclear Sox17 as development proceeds. (C) Correlation of Sox17 expression with wave of origin. An eight-cell blastomere was injected with GFP mRNA and associated with a particular division wave before staining for Sox17 at the 32-cell stage. White arrowheads indicate inside cells expressing GFP. (D) Origins of Sox17-positive and -negative cells in relation to division waves from C. Sox17 expression was seen in 92% of GFP-expressing cells from wave 2, compared with 12% of such cells from wave 1 (P > 0.05, χ2 test). (E) Sox17 RNAi directs cellular descendents to EPI rather than PE. (F) Cells overexpressing Sox17 and Gata6 are directed preferentially to PE. (G) Working model. (a and b) Cells generated in the first wave of asymmetric division are biased to generate EPI and bipotent precursors, whereas cells generated in the subsequent waves are biased to generate PE over EPI. Black arrows indicate orientation of cell division. (c) PE progenitors express Sox17 and higher levels of Gata6. Most inner cells are positioned according to their fate when the embryo cavitates in deep or surface ICM, but some are not. (d) Cells positioned within an inappropriate layer tend to relocate according to their wave of origin and fate: PE-destined cells relocate to the surface, and EPI-destined cells relocate deep. Some cells, particularly in deep ICM, apoptose. Hypothetically, induction from the cavity might further enhance PE fate in surface cells. (e) In the mature blastocyst, PE is fully segregated from EPI.
To determine whether the detected heterogeneity in Sox17 expression correlates with the wave of cell origin, we traced the origins of Sox17-expressing cells in experiments analogous to those described above for Gata6 (Fig.4_C_). A cell line expressing PDGFRα-GFP as a PE marker could not be used for this purpose, because PDGFRα-GFP expression is too broad at this stage (Fig. S6_D_) (10). We found that as much as 92% of Sox17-expressing cells originated in wave 2 (Fig. 5 C and D), indicating that levels of both Sox17 and Gata6 expression are biased by cell origin. The correlation between the expression of Sox17 and wave 2 origin is more tightly restricted than for Gata6, a finding confirmed by coimmunostaining of Gata6 and Sox17 (Fig. S6_E_). Interestingly the inside cells that showed some expression of the trophectoderm marker Cdx2 also were derived preferentially from wave 2 (Fig. S7). Together these data suggest that cells internalized at the later stage, and so most likely to form PE, are the ones with the highest levels of differentiation-associated Gata6, Cdx2, and Sox17.
Because we found that Sox17 is strongly up-regulated in PE-destined cells, we next examined whether the presence or absence of Sox17 in a clone of cells would predispose them to contribute to PE or EPI. We found that reducing Sox17 expression, by microinjecting a random eight-cell blastomere with Sox17 siRNA (Fig. 5_E_ and Fig. S8 A and G), gives cells a strong bias to contribute to EPI (87.4%, n = 52 cells) (Fig. 5_E_), whereas cells in which Sox17’s level remained unaffected contribute to PE (Fig. S8_B_). Of the control-injected cells (n = 56) 51.6% formed PE, and 48.4% formed EPI. When we elevated Sox17 in random eight-cell blastomeres (n = 91), the outcome was reversed: 64% contributed to PE, and 36% formed EPI (Fig. 5_F_ and Fig. S8 C and H). Because, as with Sox17, Gata6 expression is stronger in cells internalized in the second wave, we then generated embryos with marked clones of cells with higher levels of both these genes. This experiment revealed that elevation of Sox17 and Gata6 together had a much stronger effect and drove cells toward PE (84% PE versus 16% EPI, n = 61; Fig. 5_F_ and Fig. S8_F_) without affecting cell-cycle length or apoptosis (Fig. S8 D and E). Tracking cells in such embryos showed that cells with elevated Sox17 and Gata6 that were not at the ICM surface when the blastocyst cavity formed tended to sort to become PE, and those remaining deep apoptosed (Fig. S8 I and J). Together, these results indicate that expression of Sox17 and Gata6 together can reinforce PE identity in wave 2 generated cells.
Discussion
Our study has allowed us to address the long-standing questions of how the EPI and PE progenitor cells first arise, randomly or otherwise, and how EPI and PE progenitor cells then sort into correct layers. Our findings indicate these questions are interrelated and that both a cell's fate and its potential sorting depend largely on the wave of asymmetric cell division from which it was born. We found that the first cells to arrive inside form significantly different numbers of PE and EPI cells when compared with the second and third sets of cells to be internalized (Fig. 5_G_). Specifically, the later the division giving rise to an inside cell, the greater the likelihood of the cell becoming PE. Thus, our results provide evidence that the origin of PE and EPI progenitor cells in the ICM is not random but depends greatly on the wave of cell division that generates them. We also observed some plasticity in cell fate at this stage, in agreement with some earlier studies indicating that not all ICM cells are progenitors for only one lineage (9, 17) but in contrast to others (7). Importantly, we found this plasticity is greater in cells internalized first, most of which retain pluripotency. Thus, approximately 50% of the cells internalized in wave 1 are progenitors of EPI exclusively; the remainder are bipotent but predominantly generate EPI rather than PE. The net outcome is that 75% of the first wave of inside cells give rise to EPI, whereas 85% and 100% of inside cells generated in waves 2 and 3, respectively, give rise to PE. Our results show that most cells occupy a position appropriate for their subsequent fate when the blastocyst cavity forms (i.e., PE progenitors at the ICM surface and EPI progenitors deep), suggesting that the formation of the blastocyst cavity is not a totally random process. Of the remaining cells positioned inappropriately in relation to their wave of origin, a considerable majority sort, and some die. This process unites cells of similar origin and fate. Thus, our studies indicate that a cell's destiny is set largely by the wave of division generating it and that cell sorting is the major mechanism that allows correction of inappropriate cell positioning.
The increasingly stronger bias of successive waves of asymmetric division to contribute to PE suggests that developmental progression may influence the cell-fate decision. Could this bias relate to the timing of cell polarization and differentiation at the eight-cell stage and ensuing changes in gene expression? This process would accord with wave 2-derived inside cells showing higher levels of Cdx2, a protein whose expression is fortified in outer cells as over time they become specified to trophectoderm fate (23, 24). Indeed cells arising from the second wave of division, and even more cells arising from the third wave, are generated by mother cells that are more advanced in their differentiation process. An alternative view is that the passage of time allows the embryo to adopt a specific spatial configuration that can influence development (e.g., because of cell–cell interactions). These interactions influence the Hippo signaling pathway to down-regulate genes responsive to Tead4/Yap activation, such as Cdx2 in inner cells (24). It also is possible that cells internalized first might form an internal population that then induces a change in outside cell properties.
The repositioning of inner cells originally lying adjacent to transient cavities alongside presumptive, similar cells that line the final cavity suggests a relationship between blastocyst cavity formation and PE specification. These cells might have acquired PE characteristics while associated with the transient cavities, but the cause-and-effect relationship between cavity and PE fate cannot be established with certainty, because cavities tend to form in the vicinity of wave 2-generated cells whose fate is strongly biased toward PE. However, our results suggest that the cavity may have an inductive effect, because we observe that some wave 1 cells remain on the surface and contribute to PE.
Although following each and every cell in intact embryos, as we do here, indicates that the wave of asymmetric division strongly influences the fate of the inside cells it generates, this influence is not absolute. On average, 15% of cells internalized by divisions in wave 2 contribute to EPI. Moreover, although both PE and EPI lineages are largely segregated in the mature blastocyst, the end point of our studies, a small fraction of PE cells still might change fate subsequently. Indeed some PE-cell descendants eventually can become embryonic endoderm either by direct incorporation into that layer (25) or via the EPI first and then via the primitive streak after implantation. Thus, although cells internalized later show a strong bias to contribute to PE rather than EPI, these cells can contribute cells to the future body, and therefore their developmental potential is unrestricted.
In addition to the previously identified role of a Grb2-dependent pathway (8), our study identifies a role for Sox17, in addition to Gata6, for PE development. The role of Sox17 in PE genesis in the embryo has not been addressed previously. However, our results are consistent with its importance for PE development in vitro (21, 22) and its role in definitive endoderm (26) and suggest that Sox17 can participate in differing stages of endoderm development. Our study allows us to identify competitive interactions between clones of cells that express Sox17 side by side with those that do not, as is the case in the developing embryo. It reveals that cells with elevated Sox17 and Gata6 levels have a clear competitive advantage, over their neighbors in driving PE formation.
In conclusion, our noninvasive lineage-tracing studies of un-manipulated embryos together with gene expression analysis lead us to propose a model in which trophectoderm precursors are separated from pluripotent cells (biased to become EPI) in the first cell-fate decision (wave 1) and from cells that show a strong bias to become PE in the second cell-fate decision (waves 2 and 3) (Fig. 5_G_). The emerging picture suggests the outer cells might serve as a transitory population of stem cells that seed the inside of the embryo with two cell types. In the first wave of asymmetric division the relatively short engagement of outer cells in their own differentiative program might make them more able to generate pluripotent EPI. The advance of outer cells toward differentiation, evidenced, for example, by their expressing greater levels of Gata6 and Cdx2, could bias them to generate PE in the second and third division waves. Thus, it appears that the stage of development of the outside mother cell can affect the developmental properties of her inside daughter. Moreover, the identity of a particular generation of inside cells is largely preserved, no matter whether it is correctly positioned at first, because cells move to occupy the same layers of the ICM as their cousins of the same age group.
Materials and Methods
Live Cell Imaging.
Eight-cell embryos were collected in M2 medium containing BSA from spontaneously ovulating F1 (C57BL/6xCBA) females mated with transgenic CAG::GFP-GPI (13) males, were cultured in KSOM medium (Millipore), and were observed on an inverted epifluorescent Zeiss Axiovert 200M microscope with a 20×/0.75NA objective. Two-channel (green fluorescence and transmitted light) multisection images were acquired every 15 min with a Hamamatsu ORCA ER CCD camera on 15 focal planes every 4 μm, with an exposure of 4 ms for transmitted light and 200 ms for fluorescence. Imaged embryos were immunostained for Gata4 to confirm sorting of the PE and EPI during imaging or were transferred to foster mothers as previously described (16) to assess their development. Simi Biocell software was used for cell tracking, as previously described (12).
Immunostaining.
Primary antibodies used were goat anti-Sox17 (R&D Systems), goat anti-Gata4 (C20; Santa Cruz Biotechnology), rabbit anti-laminin (Sigma), rabbit anti-Nanog (Abcam), and mouse anti-Gata6 (R&D Systems). Immunostaining was carried out as described previously (9, 23). Multichannel images were acquired on multiple sections using an Olympus FV-1000 confocal microscope. Immunostaining intensities were measured with ImageJ and normalized relative to nuclear staining intensity.
Injection of mRNA and siRNA.
Full-length ORF Sox17 DNA was cloned into pBluescript RN3P. To overexpress Sox17, one blastomere was injected with Sox17 mRNA (100 ng/μL) and Tomato-FP mRNA (400 ng/μL) or, for controls, with Tomato-FP mRNA alone. The same procedure was followed for Gata6 and GDN (100 ng/μL). To down-regulate Sox17, 8 μM Sox17 siRNA was injected together with Tomato-FP mRNA. Nontargeting siRNA was used as a control. Embryos were cultured to E4.5 and subsequently immunostained for Sox17. Statistical analyses: as indicated. Fig. S9 provides a summary of statistics based on total cell numbers.
Note Added in Proof.
While this paper was under review, complementary findings describing the role of Sox17 in directing cells towards the PE lineage have been reported (27).
Supplementary Material
Supporting Information
Acknowledgments
We are grateful to Kat Hadjantonakis and Bill Richardson for sharing the transgenic lines, to Marko Hyvönen, Jonathon Pines, and to members of the Zernicka-Goetz laboratory for discussions. This work was supported by the Wellcome Trust.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Johnson MH, Ziomek CA. The foundation of two distinct cell lineages within the mouse morula. Cell. 1981;24:71–80. doi: 10.1016/0092-8674(81)90502-x. [DOI] [PubMed] [Google Scholar]
- 2.Barlow P, Owen DAJ, Graham CF. DNA synthesis in the preimplantation mouse embryo. J Embryol Exp Morphol. 1972;27:431–445. [PubMed] [Google Scholar]
- 3.Pedersen RA, Wu K, Bałakier H. Origin of the inner cell mass in mouse embryos: Cell lineage analysis by microinjection. Dev Biol. 1986;117:581–595. doi: 10.1016/0012-1606(86)90327-1. [DOI] [PubMed] [Google Scholar]
- 4.Fleming TP. A quantitative analysis of cell allocation to trophectoderm and inner cell mass in the mouse blastocyst. Dev Biol. 1987;119:520–531. doi: 10.1016/0012-1606(87)90055-8. [DOI] [PubMed] [Google Scholar]
- 5.Dyce J, George M, Goodall H, Fleming TP. Do trophectoderm and inner cell mass cells in the mouse blastocyst maintain discrete lineages? Development. 1987;100:685–698. doi: 10.1242/dev.100.4.685. [DOI] [PubMed] [Google Scholar]
- 6.Gardner RL. Investigation of cell lineage and differentiation in the extraembryonic endoderm of the mouse embryo. J Embryol Exp Morphol. 1982;68:175–198. [PubMed] [Google Scholar]
- 7.Rossant J, Chazaud C, Yamanaka Y. Lineage allocation and asymmetries in the early mouse embryo. Philos Trans R Soc Lond B Biol Sci. 2003;358(1436):1341–1348. doi: 10.1098/rstb.2003.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell. 2006;10:615–624. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 9.Meilhac SM, et al. Active cell movements coupled to positional induction are involved in lineage segregation in the mouse blastocyst. Dev Biol. 2009;331(2):210–221. doi: 10.1016/j.ydbio.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plusa B, Piliszek A, Frankenberg S, Artus J, Hadjantonakis AK. Distinct sequential cell behaviours direct primitive endoderm formation in the mouse blastocyst. Development. 2008;135:3081–3091. doi: 10.1242/dev.021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurotaki Y, Hatta K, Nakao K, Nabeshima Y, Fujimori T. Blastocyst axis is specified independently of early cell lineage but aligns with the ZP shape. Science. 2007;316:719–723. doi: 10.1126/science.1138591. [DOI] [PubMed] [Google Scholar]
- 12.Bischoff M, Parfitt DE, Zernicka-Goetz M. Formation of the embryonic-abembryonic axis of the mouse blastocyst: Relationships between orientation of early cleavage divisions and pattern of symmetric/asymmetric divisions. Deelopmentv. 2008;135:953–962. doi: 10.1242/dev.014316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhee JM, et al. In vivo imaging and differential localization of lipid-modified GFP-variant fusions in embryonic stem cells and mice. Genesis. 2006;44:202–218. doi: 10.1002/dvg.20203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Copp AJ. Interaction between inner cell mass and trophectoderm of the mouse blastocyst. I. A study of cellular proliferation. J Embryol Exp Morphol. 1978;48:109–125. [PubMed] [Google Scholar]
- 15.Handyside AH, Hunter S. Cell division and death in the mouse blastocyst before implantation. Rouxs Arch Dev Biol. 1986;195:519–526. doi: 10.1007/BF00375893. [DOI] [PubMed] [Google Scholar]
- 16.Weber RJ, Pedersen RA, Wianny F, Evans MJ, Zernicka-Goetz M. Polarity of the mouse embryo is anticipated before implantation. Development. 1999;126:5591–5598. doi: 10.1242/dev.126.24.5591. [DOI] [PubMed] [Google Scholar]
- 17.Perea-Gomez A, et al. Regionalization of the mouse visceral endoderm as the blastocyst transforms into the egg cylinder. BMC Dev Biol. 2007;16;7:96. doi: 10.1186/1471-213X-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang QT, et al. A genome-wide study of gene activity reveals developmental signaling pathways in the preimplantation mouse embryo. Dev Cell. 2004;6:133–144. doi: 10.1016/s1534-5807(03)00404-0. [DOI] [PubMed] [Google Scholar]
- 19.Yang DH, et al. Disabled-2 is essential for endodermal cell positioning and structure formation during mouse embryogenesis. Dev Biol. 2002;251:27–44. doi: 10.1006/dbio.2002.0810. [DOI] [PubMed] [Google Scholar]
- 20.Gerbe F, Cox B, Rossant J, Chazaud C. Dynamic expression of Lrp2 pathway members reveals progressive epithelial differentiation of primitive endoderm in mouse blastocyst. Dev Biol. 2008;313:594–602. doi: 10.1016/j.ydbio.2007.10.048. [DOI] [PubMed] [Google Scholar]
- 21.Shimoda M, et al. Sox17 plays a substantial role in late-stage differentiation of the extraembryonic endoderm in vitro. J Cell Sci. 2007;120:3859–3869. doi: 10.1242/jcs.007856. [DOI] [PubMed] [Google Scholar]
- 22.Qu XB, Pan J, Zhang C, Huang SY. Sox17 facilitates the differentiation of mouse embryonic stem cells into primitive and definitive endoderm in vitro. Dev Growth Differ. 2008;50:585–593. doi: 10.1111/j.1440-169x.2008.01056.x. [DOI] [PubMed] [Google Scholar]
- 23.Jedrusik A, et al. Role of Cdx2 and cell polarity in cell allocation and specification of trophectoderm and inner cell mass in the mouse embryo. Genes Dev. 2008;22:2692–2706. doi: 10.1101/gad.486108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishioka N, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Kwon GS, Viotti M, Hadjantonakis AK. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev Cell. 2008;15:509–520. doi: 10.1016/j.devcel.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanai-Azuma M, et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- 27.Niakan KK, et al. Sox17 promotes differentiation in mouse embryonic stem cells by directly regulating extraembryonic gene expression and indirectly antagonizing self-renewal. Genes Dev. 2010;24:312–326. doi: 10.1101/gad.1833510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information