Sorafenib induces growth arrest and apoptosis of human glioblastoma cells via dephosphorylation of STAT3 (original) (raw)
. Author manuscript; available in PMC: 2011 Apr 6.
Abstract
Glioblastoma is the most common type of primary brain tumor and is rapidly progressive with few treatment options. Here, we report that sorafenib (≤ 10 μM) inhibited cell proliferation and induced apoptosis in two established cell lines (U87, U251) and two primary cultures (PBT015, PBT022) from human glioblastomas. Effects of sorafenib on these tumor cells were associated with inhibiting phosphorylated STAT3 (Tyr705). Expression of a constitutively activated STAT3 mutant partially blocked the effects of sorafenib, consistent with a role for STAT3 inhibition in the response to sorafenib. Phosphorylated JAK1 was inhibited in U87 and U251 cells, while phosphorylated JAK2 was inhibited in primary cultures. Sodium vanadate, a general inhibitor of protein tyrosine phosphatases, blocked the inhibition of phosphorylation of STAT3 (Tyr705) induced by sorafenib. These data indicate that the inhibition of STAT3 activity by sorafenib involves both inhibition of upstream kinases (JAK1 and JAK2) of STAT3 and increased phosphatase activity. Phosphorylation of AKT was also reduced by sorafenib. In contrast, MAPK were not consistently inhibited by sorafenib in these cells. Two key cyclins (D and E) and the anti-apoptotic protein Mcl-1 were down-regulated by sorafenib in both cell lines and primary cultures. Our data suggest that inhibition of STAT3 signaling by sorafenib contributes to growth arrest and induction of apoptosis in glioblastoma cells. These findings provide a rationale for potential treatment of malignant gliomas with sorafenib.
Keywords: Sorafenib, glioblastoma, STAT3, apoptosis, proliferation
Introduction
Glioblastoma multiforme (GBM), a high-grade glioma (WHO grade IV), is the most common and lethal primary malignant brain tumor, and its prognosis remains very poor with median survival time not exceeding 15 months (1, 2). The majority of glioblastomas develop very rapidly without clinical, radiological, or morphologic evidence from a less malignant precursor lesion (primary glioblastomas). Due to the dismal prognosis of glioblastomas with currently available therapies, there is an urgent need for new treatments based on a better understanding of the molecular basis of malignant progression in this tumor.
Despite the genetic heterogeneity of malignant gliomas (3), common molecular alterations are often found in signal transduction pathways. Such pathways include growth factors, PI3K-AKT-mTOR, and Raf-MEK-MAPK/ERK (4-6). However, the large heterogeneity and low prevalence of each molecular abnormality have decreased the statistical power of studies seeking to establish their prognostic implications (7). The activity of STAT (Signal Transducer and Activator of Transcription) proteins, particularly STAT3, is frequently elevated in a wide variety of solid tumors and hematological malignancies, and is associated with proliferation and maintenance of tumors (8, 9). Thus, STAT3 has emerged as a promising molecular target for cancer therapy (10). Activated STAT3 is expressed in many types of brain tumors, including both low-grade and high-grade gliomas (11). Inhibition of the STAT3 signaling pathway suppressed proliferation and induced apoptosis in glioblastomas (12, 13). Thus, STAT3 may have an important role in the formation and maintenance of glioblastomas.
Sorafenib (BAY43-9006, Nexavar) is an oral multi-kinase inhibitor that was originally developed for its inhibitory effect on Raf and receptor tyrosine kinase (RTK) signaling (14). Recent findings showed that sorafenib inhibited tumor growth and angiogenesis, and induced apoptosis, through either Raf-MEK-MAPK dependent or independent pathways, depending on the type of tumors being investigated (15, 16). Sorafenib induces apoptosis in imatinib mesylate-resistant Bcr/Abl human leukemia cells in association with STAT5 inhibition (17). We previously reported that sorafenib induces apoptosis and inhibits cell proliferation associated with the inhibition of STAT3 signaling in medulloblastomas (18). Evaluation of sorafenib from Phase I and II clinical trials on several forms of advanced solid tumors showed favorable tolerability and promising clinical antitumor activity (19-21).
Molecularly-targeted therapies such as sorafenib, which can disrupt molecular defects in signaling pathways, may provide clinical benefits in treatment of glioblastomas. Our present results show that sorafenib inhibits cell proliferation and induces apoptosis in two established cell lines (U87, U251) and two primary cultures (PBT015, PBT022) of human glioblastomas. The biological effects of sorafenib on glioblastomas are associated with the inhibition of STAT3 signaling as well as the down-regulation of cyclin D, cyclin E, and Mcl-1 proteins. Our findings suggest that sorafenib is a promising agent for the treatment of human malignant gliomas.
Materials and Methods
Reagents and antibodies
Sorafenib was kindly provided by Onyx and Bayer Pharmaceuticals. Anti-cyclin D1, and anti-cyclin D3 were obtained from Calbiochem. Anti-cyclin E was obtained from BD Biosciences. Anti-cyclin D2 and anti-Mcl-1 were obtained from Santa Cruz. Horseradish peroxidase-labeled anti-mouse and anti-rabbit secondary antibodies were from GE Healthcare. All other antibodies were purchased from Cell Signaling.
Cell lines and primary culture
Established human glioblastoma cell line, U87, was obtained from the American Type Culture Collection (ATCC), and a tumorgenic clone of U251 was generously provided by Dr. Walter Debinski. Both U87 and U251 cells were maintained in DMEM (with L-glutamine) supplemented with 10% fetal bovine serum (FBS), and 1% Antibiotic-Antimycotic (AA). The primary cultures (PBT015 and PBT022) were derived from glioblastoma (WHO grade IV) specimens obtained from patients undergoing surgical treatment. Collection of tissue was in accordance with City of Hope Institutional Review Board-approved protocols. Tumors were graded by the attending neuropathologist in accordance with WHO established guidelines. Freshly obtained tumor specimens were finely minced, and enzymatically dissociated into single cells using 400 u/mL Collagenase III in DMEM:F12 media. Red blood cells were lysed using ACK Lyse according to the manufacturer's instructions. Adherent cells were grown in media consisting of DMEM:F12, 2 mM L-glutamine, 25 mM HEPES buffer, 7% heat-inactivated fetal calf serum, 100 U/mL penicillin and 100 μg/mL streptomycin. PBT015 and PBT022 cells were subsequently maintained in DMEM (with L-glutamine) supplemented with 10% FBS and 1% AA. All cultured cells were grown in a humidified atmosphere of 5% CO2 at 37°C.
Proliferation assays
Cell proliferation assays were performed with CellTiter 96 Aqueous One Solution Cell proliferation Assay from Promega which contains 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS). Each well of 96-well plate was seeded with 5000 cells in culture medium. After overnight culture (16 h) the cells were treated with different concentrations of sorafenib and controls were treated with vehicle (DMSO). After 24 h or 48 h treatment, MTS was added to the cells according to the supplier's protocol and absorbance was measured at 490 nm using an automated ELISA plate reader.
Apoptosis assay
U87, U251, PBT015 and PBT022 cells (2 × 105) were seeded in 60 mm culture dishes in culture medium. The following day the cells were treated with indicated concentrations of sorafenib for 48 h. After treatment, all cells including both detached and attached cells were collected, and the apoptotic cells were detected by Annexin V-FITC Apoptosis Detection Kit (BD Biosciences). The cells were stained with Annexin V-FITC and propidium iodide (PI) according to the supplier's instructions. Viable and apoptotic cells were detected by flow cytometry in the Analytical Cytometry Core at City of Hope National Medical Center. Apoptotic cells include both the early apoptotic portion (Annexin V-positive) and the late apoptotic portion (Annexin V- and PI-positive).
Immunoblotting analysis
Twenty μg total proteins were resolved in a 4-15% gradient Tris-HCl gel from BIO-RAD. After gel electrophoresis, the proteins were transferred to Hybond-C membranes (Amersham). The membranes were blocked for 1 h at room temperature (RT) in 10% non-fat dry milk in PBST (1 × PBS with 0.1% Tween-20), followed by an overnight incubation at 4 ° C with primary antibodies in PBST with 2% non-fat dry milk. Horseradish peroxidase labeled anti-mouse or anti-rabbit secondary antibodies were incubated 1 h at RT. Immunoreactivity was detected with SuperSignal West Pico substrate (Pierce).
Electrophoretic mobility shift assay (EMSA)
For the detection of DNA-binding activity of STAT3 by EMSA, nuclear protein extracts were prepared using high-salt extraction as previously described (22). Ten μg of nuclear protein from control cells or cells treated with 10 μM sorafenib was incubated with 32P-radiolabeled double-stranded DNA oligonucleotides using a high-affinity variant of the sis-inducible element (hSIE; sense strand, 5′-AGC-TTC-ATT-TCC-CTG-AAA-TCC-CTA-3′) derived from the c-fos gene promoter, which binds activated Stat3 and Stat1 proteins (23). Anti-STAT3 polyclonal antibody was used to identify STAT3 in “super-shift” assays. For use in super-shift assays, 1 μL of the concentrated STAT3 antibodies was pre-incubated with nuclear protein for 20 min at room temperature prior to the addition of radiolabeled probe (30 min, 30°C) and separation by non-denaturating polyacrylamide gel-electrophoresis and autoradiographic detection.
Plasmids transfections
The constitutively-activated STAT3 mutant plasmid (pSTAT3-C) was murine STAT3 which was cloned into pRc/CMV vector with a FLAG epitope (24). pSTAT3-C was transfected into VC312 cells by Lipofectamine™ 2000 (Invitrogen). Stable cell line was selected by G418 and confirmed by immunoblotting analysis.
Statistical Analysis
The comparisons between vehicle control (DMSO) and sorafenib-treated groups were done by using Student's t test. _P_-values less than 0.05 are considered as significant.
Results
Sorafenib inhibits proliferation and induces apoptosis in U87 and U251 cells
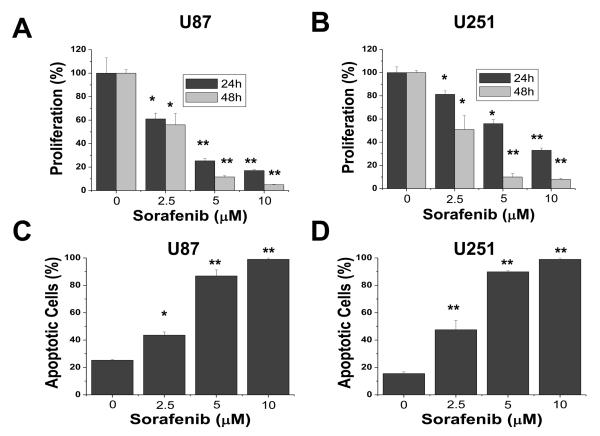
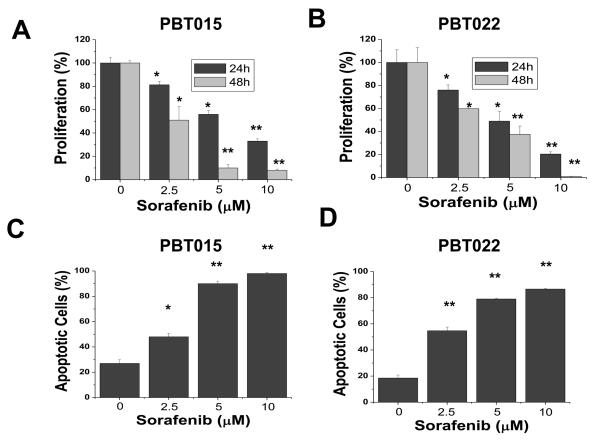
To characterize the effects of sorafenib on cell proliferation in glioblastomas, we performed dose-response and time-course studies in two established cell lines, U87 (Fig. 1A) and U251 (Fig. 1B). Cells were treated with increasing concentrations of sorafenib (2.5, 5, 10 μM) for 24 h and 48 h. Control cells were treated with the vehicle (DMSO) only. Because previous studies suggest that sorafenib binds to serum proteins (25), all treatments with sorafenib were performed in 1% serum to reduce the effect of serum. Sorafenib markedly inhibited proliferation of both U87 and U251 cells in a dose- and time-dependent manner. We next investigated whether sorafenib could induce apoptosis in U87 and U251 cells. After treatment with increasing concentrations of sorafenib (2.5, 5, 10 μM) for 48 h, cells were analyzed by Annexin V-FITC/PI staining and flow cytometry. Apoptotic cells shown in Figure 1C and 1D included both early apoptotic cells (Annexin V-positive) and late apoptotic cells (Annexin V- and PI-positive). Sorafenib inhibited survival of these tumor cells in a dose-dependent manner. These results show that proliferation and survival was greatly reduced for U87 and U251 cells exposed to 10 μM sorafenib, which has been shown to be a therapeutically achievable concentration in clinical trials with doses of 400 mg sorafenib twice daily (26).
Figure 1.
Sorafenib inhibits proliferation and survival of U87 and U251 cells. (A) and (B) Effect of sorafenib on proliferation of U87 and U251 cells. Cells were treated with 0, 2.5, 5, or 10 μM sorafenib for 24 h and 48 h and cell proliferation was evaluated by MTS assay as described in Methods. Sorafenib induced apoptosis (C and D) in U87 and U251 cells after 48 h treatment. Apoptotic cells represented Annexin V-FITC positive or PI and Annexin V-FITC double-positive cells as determined by flow cytometry. Each experiment was performed in triplicate or duplicate and repeated twice independently. Each bar graph represents the mean, and the error bars represent ± SD. *, p<0.01; **, p<0.001.
Sorafenib inhibits STAT3 phosphorylation at Tyr705 in U87 and U251 cells
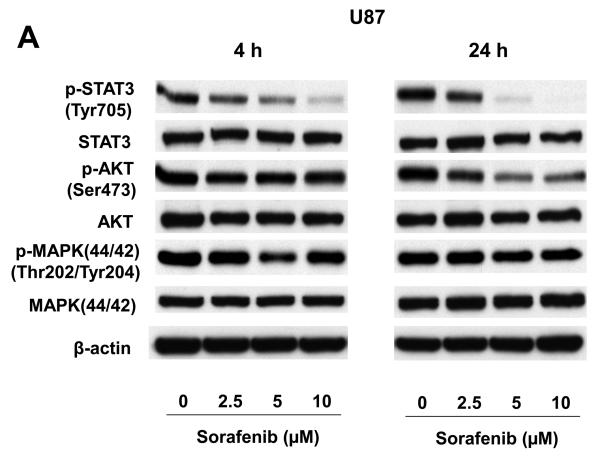
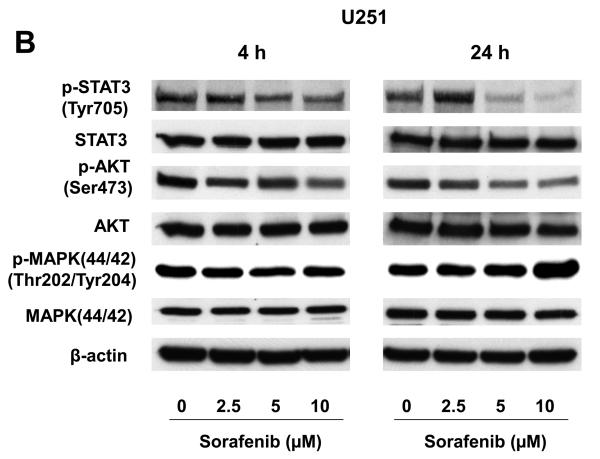
We investigated the levels of total and phosphorylated STAT3, AKT and MAPK (p44/42) proteins in U87 and U251 cells after sorafenib treatment. Total protein levels of STAT3, AKT, and MAPK were not significantly changed after 4 h or 24 h sorafenib treatment (Fig. 2A and 2B). By contrast, phosphorylation of STAT3 at Tyr705 was reduced at both an early time point (4 h) and a late time point (24 h) following sorafenib treatment (Fig. 2A and 2B). The inhibition of p-STAT3 (Tyr705) was dose and time-dependent. Phosphorylation of AKT was decreased after 24 h treatments in both U87 and U251 cells. However, phosphorylation of MAPK did not change substantially at either time points. These results indicate that inhibition of STAT3 signaling is an early response to sorafenib treatment, and a common response to sorafenib in both U87 and U251 cells.
Figure 2.
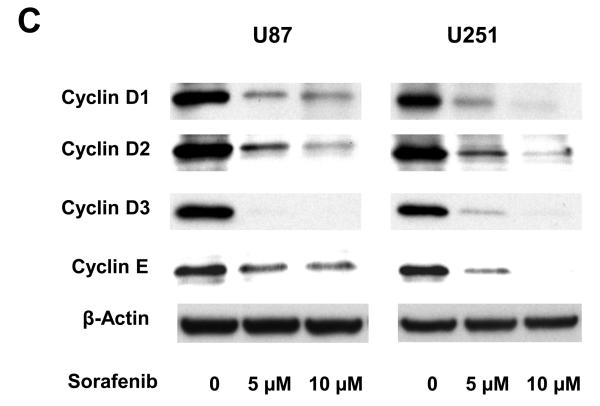
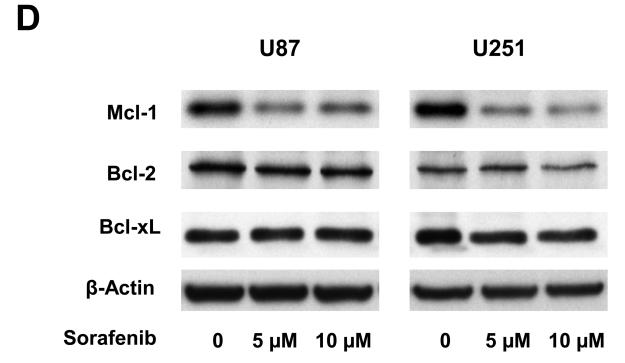
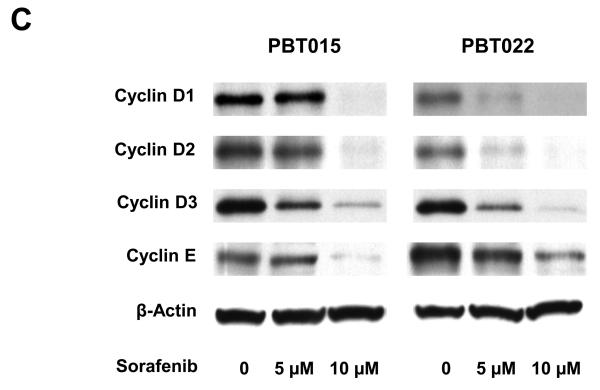
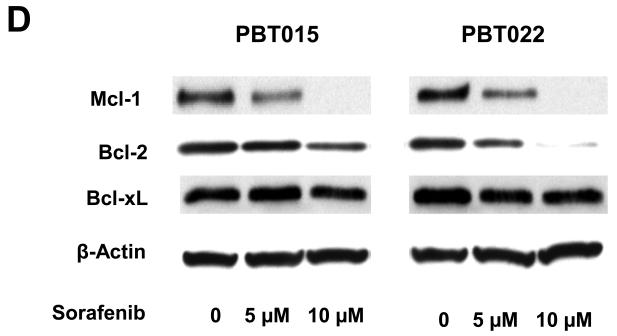
Effects of sorafenib on major signaling pathways and expression of regulatory proteins for cell cycle and apoptosis in U87 and U251 cells. (A) and (B) Immunoblotting analyses for STAT3, AKT and MAPK were performed with specific antibodies as described in Methods. Total protein was isolated from U87 or U251 cells incubated with 0, 2.5, 5, or 10 μM sorafenib for 4 h and 24 h. (C) Sorafenib inhibited expression of cyclin D1, D2, D3 and E after 24 h treatment. (D) Effect of sorafenib on anti-apoptotic proteins, Mcl-1, Bcl-2 and Bcl-xL after 24 h treatment. Anti-β-actin monoclonal antibody was used as a loading control.
Expression of cyclin D, E and Mcl-1 is reduced by sorafenib in U87 and U251 cells
Because sorafenib strongly inhibits proliferation of U87 and U251 cells (Fig. 1), we investigated the effect of sorafenib on key cell-cycle regulators, including D-type and E-type cyclins. Immunoblot analyses were performed to determine the expression of cyclin D1/D2/D3 and cyclin E in U87 and U251 cells after 24 h sorafenib treatment. Figure 2C shows that sorafenib decreased the expression of cyclin D1/D2/D3 and cyclin E in U87 and U251 cells. These results are consistent with the inhibition of cell proliferation in these tumor cells. The Bcl-2 family of proteins has a key role in survival of normal and tumor cells (27). The expression of three anti-apoptotic proteins in this family, Mcl-1, Bcl-xL and Bcl-2, was investigated after sorafenib treatment. Mcl-1 was decreased in both U87 and U251 cells after sorafenib treatment (Fig. 2D), while Bcl-2 and Bcl-xL levels were not inhibited in these cells. These results are consistent with the induction of apoptosis by sorafenib (Fig. 1), and implicate the importance of Mcl-1 in this response.
Sorafenib inhibits primary cultures of human glioblastomas
PBT015 and PBT022 cells were isolated from glioblastoma (WHO grade IV) patients and cultured as described in Materials and Methods. To evaluate whether sorafenib has the same effect on low-passage primary cultures as on established cell lines, PBT015 and PBT022 cells were treated with sorafenib in the same manner as U87 and U251 cells. Proliferation of PBT015 (Fig. 3A) and PBT022 (Fig. 3B) cells was inhibited by sorafenib in a dose- and time-dependent manner. Sorafenib also induced apoptosis of PBT015 (Fig. 3C) and PBT022 (Fig. 3D) cells in a dose-dependent manner after 48 h treatment. This inhibitory effect on the proliferation and survival of PBT015 and PBT022 cells is similar to that of U87 and U251 cells (Fig. 1).
Figure 3.
Sorafenib inhibits proliferation and survival in two primary cultures from human glioblastomas. (A) and (B) Effect of sorafenib on proliferation of PBT015 and PBT022 cells. Cells were treated with 0, 2.5, 5, or 10 μM sorafenib for 24 h and 48 h and cell proliferation was evaluated by MTS assay as described in Methods. (C) and (D) showed induction of apoptosis by sorafenib in PBT015 and PBT022 cells. Apoptotic cells represented Annexin V-FITC positive and PI and Annexin V-FITC double-positive cells as determined by flow cytometry. Each experiment was performed in triplicate or duplicate and repeated twice independently. Each bar graph represents the mean, and the error bars represent ± SD. *, p<0.01; **, p<0.001.
Sorafenib inhibits signaling pathways in primary cultures of glioblastomas
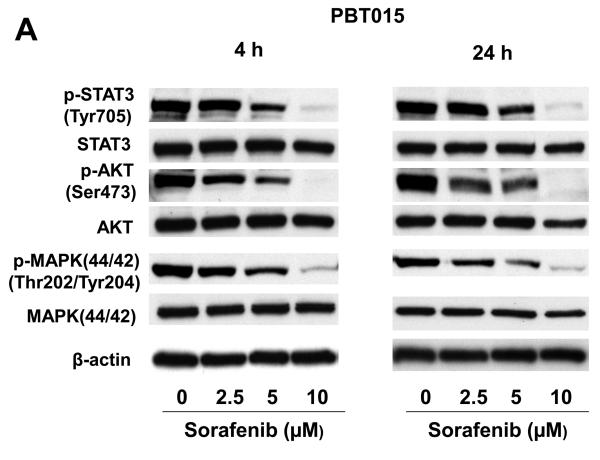
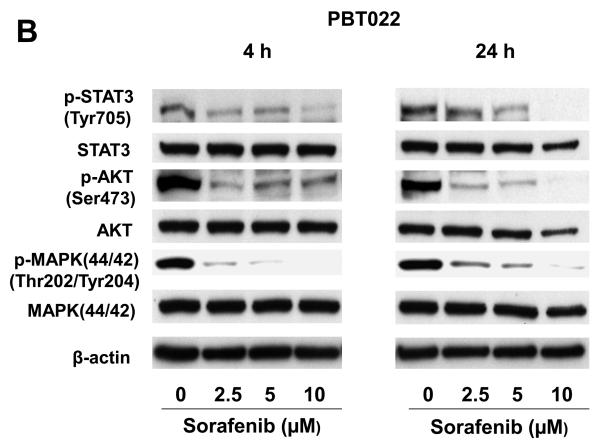
We examined the STAT3, AKT, and MAPK (p44/42) signaling pathways after sorafenib treatments in PBT015 and PBT022 cells. Though total protein levels of STAT3, AKT, and MAPK were not significantly changed, phosphorylated STAT3 (Tyr705), AKT (Ser473), and MAPK (Thr202/Tyr204) were dramatically decreased by sorafenib after 4 h and 24 h treatment (Fig. 4A and 4B). While sorafenib did not affect phosphorylated MAPK in the two established cell lines (U87 and U251) examined, sorafenib substantially reduced the phosphorylation of MAPK in the two low-passage primary cultures. These results indicate that there are different properties between primary cultures and established cell lines from human glioblastomas. Importantly, inhibition of STAT3 signaling is a common response in both cell lines and primary cultures.
Figure 4.
Effects of sorafenib on STAT3, AKT and MAPK signalings and expression of regulatory proteins for cell cycle and apoptosis in primary cultures of glioblastoma. Immunobloting assays were performed with specific antibodies. Sorafenib inhibits phosphorylation of STAT3 (Tyr705), AKT (Ser473), MAPK (Thr202/Tyr204) after 4 h and 24 h treatment in PBT015 (A) and PBT022 (B) cells. (C) Expression of cyclin D1/D2/D3 and cyclin E was inhibited by sorafenib after 24 h treatment. (D) Effect of sorafenib on Mcl-1, Bcl-2 and Bcl-xL proteins after 24 h treatment.
Sorafenib also inhibited the expression of cyclin D1/D2/D3 and cyclin E in PBT015 and PBT022 cells (Fig. 4C). Anti-apoptotic proteins, Mcl-1 and Bcl-2, were decreased in PBT015 and PBT022 after sorafenib treatment (Fig. 4D), while Bcl-xL level was not changed in any of the glioblastoma cell lines tested. However, inhibition of Mcl-1 is a common response in both established cell lines and primary cultures of glioblastomas.
Sorafenib rapidly inhibits STAT3 phosphorylation at Tyr705 and DNA-binding activity
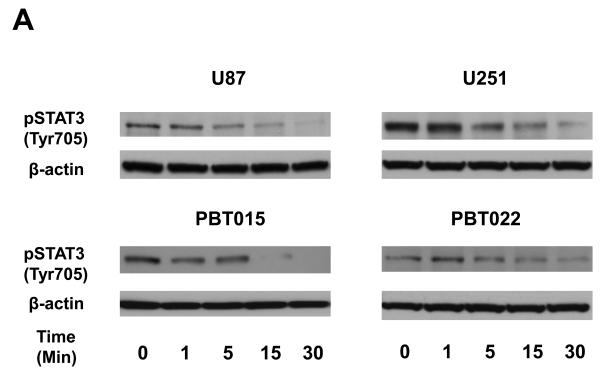
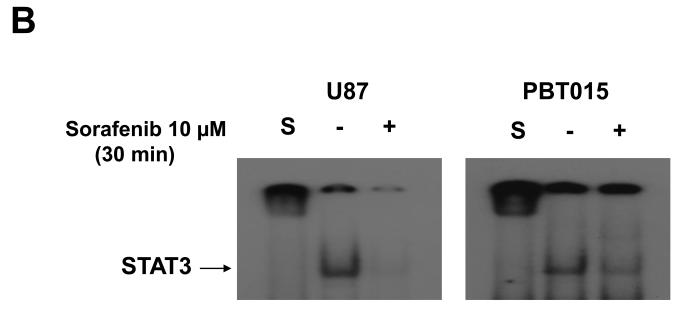
To determine whether inhibition of STAT3 phosphorylation at Tyr705 is an early event, we treated two established cell lines (U87 and U251), and two primary cultures (PBT015 and PBT022) of human glioblastomas with 10 μM sorafenib for 0, 1, 5, 15 and 30 min. Immunoblotting analysis (Fig. 5A) showed that the inhibition of phosphorylation of STAT3 at Tyr705 by sorafenib was detected at 5 min to 15 min in these tumor cells. Phosphorylation of STAT3 on Tyr705 is important for STAT3 dimerization, translocation and DNA binding (8). Therefore, we evaluated whether sorafenib inhibited the formation of STAT3/DNA complexes in nuclei of tumor cells from glioblastomas. Electrophoretic mobility shift assay (EMSA) was employed to detect the effect of sorafenib on DNA-binding activity of STAT3. Nuclear extracts were prepared from cells treated with 10 μM sorafenib for 30 min. Figure 5B shows that sorafenib inhibited the formation of STAT3/DNA complex in nuclei of U87 and PBT015.
Figure 5.
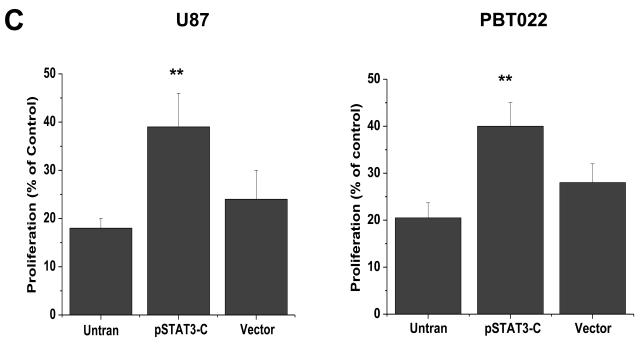
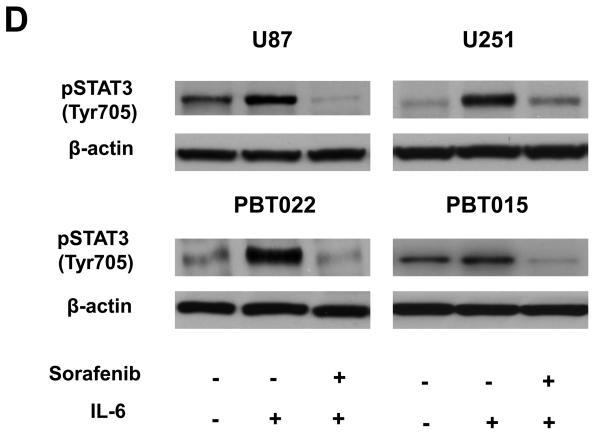
Sorafenib inhibits formation of STAT3/DNA complex and expression of constitutively activated STAT3 partially reverses effects of sorafenib. (A) Immunoblotting analyses showed that the inhibition of STAT3 (Tyr705) phosphorylation occurred at 5 to 15 min after 10μM sorafenib treatments. (B) EMSA was performed to detect the effects of sorafenib on the formation of STAT3/DNA complex in nuclear extracts of U87 and PBT015 cells. P, positive control; S, supershift with STAT3 antibody. (C) Expression of constitutively activated STAT3 mutant (pSTAT3-C) partially blocked effects of sorafenib on proliferation of U87 and PBT022 cells after 24 h treatment with 10 μM sorafenib. **, p<0.001. (D) Sorafenib inhibits the phosphorylation of STAT3 (Tyr705) induced by IL-6 in these tumor cells by immunoblotting analyses. Cells were treated with 10 ng/ml IL-6 for 10 min after 25 min incubation with 10 μM sorafenib.
Expression of constitutively activated STAT3 mutant partially rescues the effects of sorafenib
To further confirm that inhibiting STAT3 activity is critical for the biological effects of sorafenib on glioblastoma cells, we transfected a constitutively activated STAT3 mutant (pSTAT3-C) (24) into U87 and PBT022 cells. Stable cell lines were established by antibiotic selection (G418) and pRC was used as control vector. Cells containing either pSTAT3-C or control vector were treated with 10 μM sorafenib for 24 h, and proliferation assays were performed. Expression of constitutively activated STAT3 increased the resistance of U87 and PBT022 cells to sorafenib compared to untransfected cells or transfected cells with control vector (Fig. 5C).
Phosphorylation of STAT3 at Tyr705 induced by IL-6 is inhibited by sorafenib
The ability of IL-6 to directly activate the STAT3 via JAK family kinases produces serious unintended consequences in the progression of neoplasia (28). The expression of IL-6 in glioblastoma patients shortened their survival (29). To confirm whether sorafenib also inhibits the phosphorylation of STAT3 at Tyr705 induced by IL-6, tumor cells were treated with 10 μM sorafenib for 20 min, and then IL-6 (10 ng/ml) was added to cells for 10 min. Immunoblotting assays showed that sorafenib greatly inhibited the phosphorylation of STAT3 induced by IL-6 in both cell lines and primary cultures (Fig. 5D).
Effects of sorafenib on the activities of JAK1, JAK2 and Src in glioblastoma cells
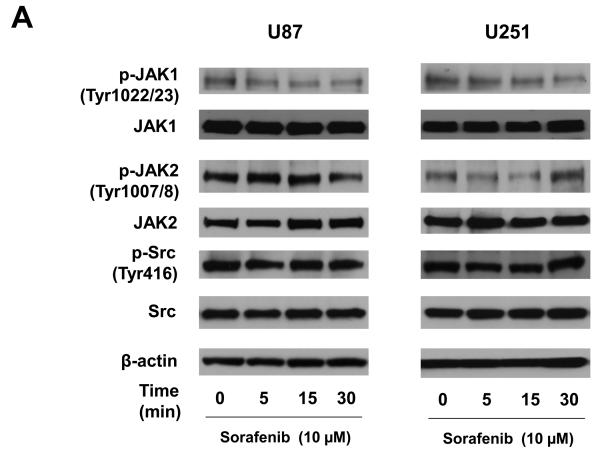
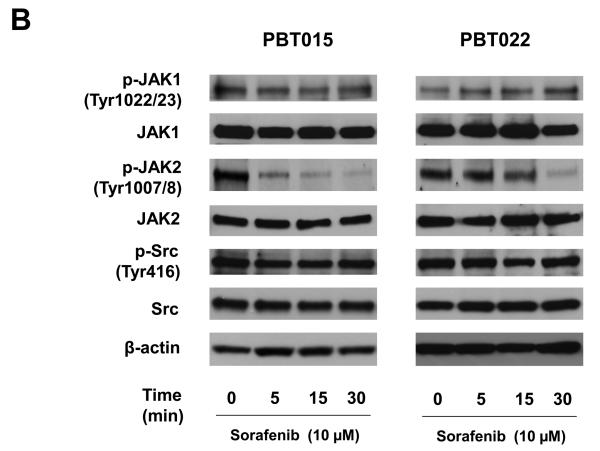
The phosphorylation of STAT3 at Tyr705 is usually mediated by receptor-associated tyrosine kinases, such as the Janus kinases (JAKs), or less frequently by non-receptor tyrosine kinases (Src) (8). Inhibiting phosphorylated STAT3 (Tyr705) by sorafenib was detected at 5 to 15 min treatment (Figure 5A) in glioblastoma cells. To elucidate how sorafenib causes the dephosphorylation of STAT3 at Tyr705, expression of total and phosphorylated proteins of JAK1, JAK2, and Src were examined after 5, 15, and 30 min sorafenib treatment in both cell lines and primary cultures. Figure 6A shows the effects of sorafenib on expression of total and phosphorylated JAK1, JAK2, and Src in two established cell lines, U87 and U251, by immunobloting assays. Expression of phosphorylated Src and total JAK1, JAK2 and Src was not affected by sorafenib. Phosphorylated JAK1 was decreased in a time-dependent manner in both cell lines. Phosphorylated JAK2 was only reduced in U87 cells after 30 min treatment. Interestingly, sorafenib inhibited the phosphorylation of JAK2 in two primary cultures, PBT015 and PBT022, in a time-dependent manner (Figure 6B). However, phosphorylated JAK1, Src and total proteins were not affected by sorafenib in these primary cultures. These results indicate there are intrinsic differences between established cell lines and primary cultures of human glioblastomas.
Figure 6.
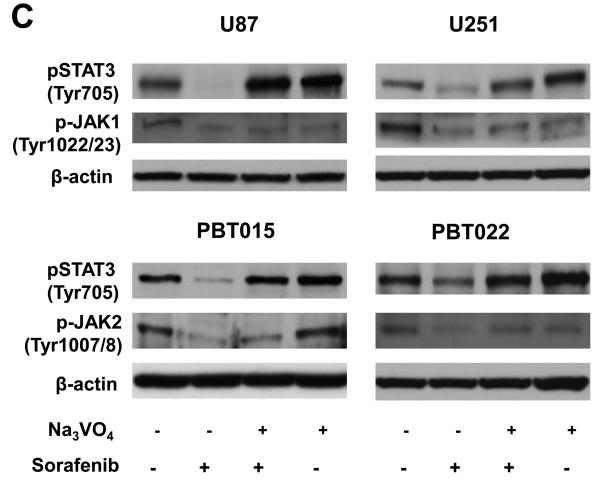
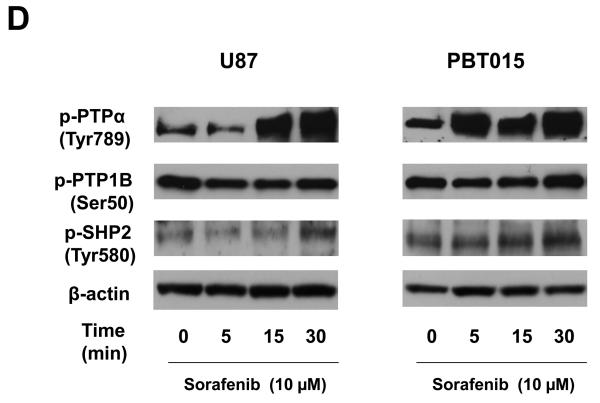
Effects of sorafenib on JAK and Src kinases, and protein tyrosine phosphatases. (A) and (B) Effects of sorafenib on the activities of JAK1, JAK2, and Src after 5, 15, 30 min treatment of sorafenib in U87, U251, PBT015 and PBT022 cells. (C) Effects of sodium vanadate on phosphorylation of STAT3, JAK1 and JAK2. Cells were treated with 0.5 mM sodium orthovanadate (Na3VO4) for 25 min, and then were treated with 10 μM sorafenib for 30 min. (D) Effects of sorafenib on phosphorylation of PTPα, PTP1B and SHP2 by immunobloting assays.
Sodium vanadate blocks dephosphorylation of STAT3 at Tyr705 induced by sorafenib
We also test another possibility that dephosphorylation of STAT3 induced by sorafenib is contributed by direct effects of protein tyrosine phosphatases (PTPs). Tumor cells were pre-treated with 0.5 mM sodium orthovanadate (Na3 VO4), a general inhibitor for tyrosine phosphatases, for 25 min and then 10 μM sorafenib was added to cells for another 30 min. Immunoblotting assays showed that sodium vanadate blocked the effects of sorafenib on dephosphorylation of STAT3 in both cell lines and primary cultures (Fig. 6C). To further confirm the effect of sodium vanadate, we also did the converse experiments, in which the tumor cells were first treated with 10 μM sorafenib for 5 min and then sodium vanadate was added for 30 min. Sodium vanadate showed similar inhibition on de-phosphorylation of STAT3 induced by sorafenib (data not shown), comparable to the results using sodium vanadate first (Fig. 6C). Though phosphorylated JAK1 in U87 and U251 cells or phorsphorylated JAK2 in PBT015 and PBT022 cells was inhibited by sorafenib, sodium vanadate only showed little effects on the activaties of these two kinases (Fig. 6C). These results indicate a role for tyrosine phosphatases in the mechanism of STAT3 dephosphorylation induced by sorafenib.
Sorafenib affects the activities of protein tyrosine phosphatases in glioblastoma cells
Since sodium vanadate can reverse the dephosphorylation of STAT3 (Tyr705) induced by sorafenib (Fig. 6C), we further investigated the effects of sorafenib on the activities of several tyrosine phosphatases which include PTPα, PTP1B and SHP2. Immunoblotting assays with total cell lysates showed that sorafenib increased phosphorylation of PTPα and SHP2 in U87 and PBT015 cells (Fig. 6 D). The phosphorylated PTP1B was not affected by sorafenib treatment in comparison with DMSO control (zero concentration). TC-PTP activity was determined by human TC-PTP activity assay kit (R&D Systems) and its activity was slightly increased by sorafenib treatment in U87 and PBT015 cells (data not shown). These results suggest that protein tyrosine phosphatases may contribute to dephosphorylation of STAT3 induced by sorafenib.
Discussion
In glioblastomas, STAT3 acts a molecular “hub” to link extracellular signals to transcriptional control of proliferation, cell cycle progression, and immune evasion (30). Since STAT3 plays such a central role in glioblastoma signal transduction, inhibiting STAT3 signaling provides a new opportunity for glioblastoma treatment. Here we present evidence that sorafenib inhibits proliferation and induces apoptosis of two established cell lines (U87 and U251) as well as two primary cultures (PBT015 and PBT022) derived from human glioblastomas. Inhibition of cell growth and survival in these glioblastoma tumor cells is associated with inhibition of the STAT3 signaling pathway. Although there are different growth properties among these tumor cells, inhibition of phosphorylated STAT3 at Tyr705 is common to all cells. The formation of STAT3/DNA complexes in nuclei is consequently decreased by sorafenib in these tumor cells. Furthermore, sorafenib inhibits STAT3 signaling within minutes and this inhibition persists for at least 24 h following treatment. Importantly, over-expression of a constitutively activated STAT3 mutant partially blocked the effects of sorafenib.
Phosphotyrosine (pTyr705) in STAT3 mediates dimer formation, which is required for the binding of STAT3 to DNA (8). Our results show that sorafenib inhibited the tyrosine phosphorylation of STAT3 in both established cell lines and primary cultures of glioblastomas. Tyrosine residue in STAT3 is phosphorylated by JAK or Src family kinases in response to cytokines or growth factors. Sorafenib inhibited phosphorylated JAK1 in U87 and U251 cells, whereas it inhibited phosphorylated JAK2 in primary cultures. Sodium vanadate, a general inhibitor of protein tyrosine phosphatases, reverses the dephosphorylation of STAT3 at Tyr705 induced by sorafenib in both cell lines and primary cultures of glioblastomas. These results indicate that both JAKs and protein tyrosine phosphatases are involved in the mechanisms of action of sorafenib on dephosphorylation of STAT3.
STAT3 regulates basic biologic processes important in tumorigenesis including cell-cycle progression, apoptosis, tumor angiogenesis, and tumor-cell evasion of the immune system (8, 9). Key genes in cell-cycle control, such as cyclin D1, are regulated by STAT3 (8). Thus, inhibition of STAT3 signaling by sorafenib is likely to contribute to inhibition of cell proliferation. Our data indicate that D-type and E-type cyclins, which are involved in cell-cycle control, are common downstream targets for sorafenib in glioblastomas. The expression of cyclin E and three types (D1, D2, D3) of cyclin D are decreased by sorafenib in a dose-dependent manner. These results are consistent with the inhibition of proliferation by sorafenib in tumor cells from glioblastomas.
Expression of Mcl-1, an anti-apoptotic protein, is also regulated by STAT3 signaling (8). Sorafenib has been reported to down-regulate the expression of Mcl-1 in several kinds of tumor cells (17, 31). Here, we show that sorafenib inhibited Mcl-1 expression in both established cell lines and primary cultures from human glioblastomas. The STAT3 and Mcl-1 proteins are the only ones inhibited in common among the established cell lines and primary cultures of glioblastomas. Therefore, down-regulation of Mcl-1 through inhibition of phosphorylated STAT3 may be an important mechanism of action of sorafenib in glioblastomas.
Even though sorafenib was originally developed for inhibition of Raf-MEK-MAPK signaling (14), sorafenib only inhibits phosphorylation of MAPK(44/42) in two primary cultures, but not in established cell lines (U87 and U251). Thus, the antitumor activity of sorafenib is dissociated from inhibition of MAPK in established cell lines of glioblastoma. Since sorafenib inhibits vascular endothelial growth factor receptors (VEGFRs) (14), which are highly expressed in glioblastomas (32), it is possible that sorafenib could inhibit angiogenesis in glioblastomas.
Treatment of glioblastoma is complicated by the blood-brain barrier, which serves as physiologic obstacle for delivery of drugs to the central nervous system. Various approaches have been developed for local delivery of drugs to brain tumors, including convection-enhanced delivery (33). Therefore, local delivery of sorafenib to the malignant cells in the brain may result in more effective antitumor activity with reduced systemic toxicity. Sorafenib shows good tolerability and promising antitumor activity from clinical trials in several types of solid tumors (19-21). Thus, sorafenib is potentially a promising drug for the treatment of malignant gliomas.
Acknowledgments
Supported by the Sunshine Project of the Pediatric Cancer Foundation and NCI grant CA1155674 (to R.J.).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Sathornsumetee S, Reardon DA, Desjardins A, Quinn JA, Vredenburgh JJ, Rich JN. Molecularly targeted therapy for malignant glioma. Cancer. 2007;110:13–24. doi: 10.1002/cncr.22741. [DOI] [PubMed] [Google Scholar]
- 2.Nakada M, Nakada S, Demuth T, Tran NL, Hoelzinger DB, Berens ME. Molecular targets of glioma invasion. Cell Mol Life Sci. 2007;64:458–78. doi: 10.1007/s00018-007-6342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohgaki H. Genetic pathways to glioblastomas. Neuropathology. 2005;25:1–7. doi: 10.1111/j.1440-1789.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- 4.Newton HB. Molecular neuro-oncology and development of targeted therapeutic strategies for brain tumors. Part 1: Growth factor and Ras signaling pathways. Expert Rev Anticancer Ther. 2003;3:595–614. doi: 10.1586/14737140.3.5.595. [DOI] [PubMed] [Google Scholar]
- 5.Newton HB. Molecular neuro-oncology and development of targeted therapeutic strategies for brain tumors. Part 2: PI3K/Akt/PTEN, mTOR, SHH/PTCH and angiogenesis. Expert Rev Anticancer Ther. 2004;4:105–28. doi: 10.1586/14737140.4.1.105. [DOI] [PubMed] [Google Scholar]
- 6.Rich JN, Bigner DD. Development of novel targeted therapies in the treatment of malignant glioma. Nat Rev Drug Discov. 2004;3:430–46. doi: 10.1038/nrd1380. [DOI] [PubMed] [Google Scholar]
- 7.Omuro AM, Faivre S, Raymond E. Lessons learned in the development of targeted therapy for malignant gliomas. Mol Cancer Ther. 2007;6:1909–19. doi: 10.1158/1535-7163.MCT-07-0047. [DOI] [PubMed] [Google Scholar]
- 8.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 9.Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315–24. doi: 10.1038/ncponc0195. [DOI] [PubMed] [Google Scholar]
- 10.Pathak AK, Bhutani M, Nair AS, Ahn KS, Chakraborty A, Kadara H. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol Cancer Res. 2007;5:943–55. doi: 10.1158/1541-7786.MCR-06-0348. [DOI] [PubMed] [Google Scholar]
- 11.Cattaneo E, Magrassi L, De-Fraja C, Conti L, Di Gennaro I, Butti G, et al. Variations in the levels of the JAK/STAT and ShcA proteins in human brain tumors. Anticancer Res. 1998;18:2381–87. [PubMed] [Google Scholar]
- 12.Rahaman SO, Harbor PC, Chernova O, Barnett GH, Vogelbaum MA, Haque SJ. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21:8404–13. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- 13.Iwamaru A, Szymanski S, Iwado E, Aoki H, Yokoyama T, Fokt I, et al. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene. 2007;26:2435–44. doi: 10.1038/sj.onc.1210031. [DOI] [PubMed] [Google Scholar]
- 14.Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–44. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 15.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–8. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 16.Chang YS, Adnane J, Trail PA, Levy J, Henderson A, Xue D, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007;59:561–74. doi: 10.1007/s00280-006-0393-4. [DOI] [PubMed] [Google Scholar]
- 17.Rahmani M, Nguyen TK, Dent P, Grant S. The multikinase inhibitor sorafenib induces apoptosis in highly imatinib mesylate-resistant bcr/abl+ human leukemia cells in association with signal transducer and activator of transcription 5 inhibition and myeloid cell leukemia-1 down-regulation. Mol Pharmacol. 2007;72:788–95. doi: 10.1124/mol.106.033308. [DOI] [PubMed] [Google Scholar]
- 18.Yang F, Van Meter TE, Buettner R, Hedvat M, Liang W, Kowolik CM, et al. Sorafenib inhibits signal transducer and activator of transcription 3 signaling associated with growth arrest and apoptosis of medulloblastomas. Mol Cancer Ther. 2008;7:3519–26. doi: 10.1158/1535-7163.MCT-08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 20.Strumberg D, Clark JW, Awada A, Moore MJ, Richly H, Hendlisz A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–37. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 21.Gridelli C, Maione P, Del Gaizo F, Colantuoni G, Guerriero C, Ferrara C, et al. Sorafenib and sunitinib in the treatment of advanced non-small cell lung cancer. Oncologist. 2007;12:191–200. doi: 10.1634/theoncologist.12-2-191. [DOI] [PubMed] [Google Scholar]
- 22.Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20:2499–513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- 23.Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J, et al. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81–3. doi: 10.1126/science.7541555. [DOI] [PubMed] [Google Scholar]
- 24.Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, et al. Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 25.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 26.Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–72. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 27.Maddika S, Ande SR, Panigrahi S, Paranjothy T, Weglarczyk K, Zuse A, et al. Cell survival, cell death and cell cycle pathways are interconnected: implications for cancer therapy. Drug Resist Updat. 2007;10:13–29. doi: 10.1016/j.drup.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–12. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Chang CY, Li MC, Liao SL, Huang YL, Shen CC, Pan HC. Prognostic and clinical implication of IL-6 expression in glioblastoma multiforme. J Clin Neurosci. 2005;12:930–3. doi: 10.1016/j.jocn.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Brantley EC, Benveniste EN. Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas. Mol Cancer Res. 2008;6:675–84. doi: 10.1158/1541-7786.MCR-07-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu C, Bruzek LM, Meng XW, Gores GJ, Carter CA, Kaufmann SH, et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene. 2005;24:6861–9. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- 32.Joensuu H, Puputti M, Sihto H, Tynninen O, Nupponen NN. Amplification of genes encoding KIT, PDGFRalpha and VEGFR2 receptor tyrosine kinases is frequent in glioblastoma multiforme. J Pathol. 2005;207:224–31. doi: 10.1002/path.1823. [DOI] [PubMed] [Google Scholar]
- 33.Sawyer AJ, Piepmeier JM, Saltzman WM. New methods for direct delivery of chemotherapy for treating brain tumors. Yale J Biol Med. 2006;79:141–52. [PMC free article] [PubMed] [Google Scholar]