OX40 is required for regulatory T cell–mediated control of colitis (original) (raw)
Abstract
The immune response in the gastrointestinal tract is a tightly controlled balance between effector and regulatory cell responses. Here, we have investigated the role of OX40 in influencing the balance between conventional T cells and Foxp3+ regulatory T (T reg) cells. Under steady-state conditions, OX40 was required by T reg cells for their accumulation in the colon, but not peripheral lymphoid organs. Strikingly, under inflammatory conditions OX40 played an essential role in T reg cell–mediated suppression of colitis. OX40−/− T reg cells showed reduced accumulation in the colon and peripheral lymphoid organs, resulting in their inability to keep pace with the effector response. In the absence of OX40 signaling, T reg cells underwent enhanced activation-induced cell death, indicating that OX40 delivers an important survival signal to T reg cells after activation. As OX40 also promoted the colitogenic Th1 response, its expression on T reg cells may be required for effective competition with OX40-dependent effector responses.
These results newly identify a key role for OX40 in the homeostasis of intestinal Foxp3 +T reg cells and in suppression of colitis. These fi ndings should be taken into account when considering OX40 blockade for treatment of IBD.
The immune response in the intestine is a tightly controlled balance between innate and adaptive effector responses and negative regulatory pathways of control (Bouma and Strober, 2003; Izcue et al., 2009). Disruption of this balance by genetic or environmental factors can lead to chronic inflammatory syndromes such as the inflammatory bowel diseases (IBDs).
CD4+Foxp3+ T reg cells (Foxp3+ T reg cells) play a nonredundant role in immune homeostasis, preventing pathological inflammatory responses to environmental and self-antigens (Sakaguchi et al., 2008; Shevach, 2009). Loss of function mutations in the FOXP3 gene lead to impaired T reg cell function and the development of a severe multiorgan inflammatory disease (Bennett et al., 2001). In humans, this is called immune dysregulation polyendocrinopathy enteropathy, X-linked syndrome, and commonly involves severe intestinal inflammation, thus illustrating the importance of T reg cells in control of intestinal immune function.
Mouse models of intestinal inflammation have also pinpointed a key role for T reg cells in intestinal homeostasis, as illustrated in a model of T cell–driven colitis induced by the transfer of naive CD4 T cells into RAG−/− mice (Izcue et al., 2009). Disease development can be prevented and cured by transfer of CD4+CD25+ T reg cells, providing a tractable model to unravel factors controlling the accumulation and function of colitogenic and T reg cells in vivo (Coombes et al., 2005).
Understanding how particular co-stimulatory pathways affect both effector T (T eff) cell and T reg cell responses is crucial for development of antiinflammatory strategies based on co-stimulatory blockade, as interventions that inhibit both types of response may not reset the immunological balance in favor of T reg cells.
OX40 is a member of the large TNF/TNFR family of co-stimulatory molecules that control lymphocyte function. OX40 is expressed primarily on activated T cells and its ligand OX40L on activated APC and LTi cells (Watts, 2005). The OX40–OX40L pathway plays an important role in the sustenance of CD4 Th cell responses and development of memory (Croft, 2003), and targeting this pathway inhibits several autoimmune diseases (Sugamura et al., 2004). Increases in OX40+ and OX40L+ cells are associated with intestinal inflammation in IBD patients and colitis in mice (Souza et al., 1999; Malmström et al., 2001). OX40 signaling plays a functional role in intestinal inflammation and blockade of OX40–OX40L interactions inhibits colitis in various mouse models (Malmström et al., 2001; Takeda et al., 2004).
In contrast with conventional T cells, OX40 is expressed on the majority of CD4+Foxp3+ cells, even in the absence of activation (Fontenot et al., 2005; Vu et al., 2007). Despite these findings, little is known about the role of OX40 signaling in T reg cell function. There are conflicting results from in vitro and in vivo studies, with evidence that OX40 signaling is either neutral or can promote or inhibit T reg cell–mediated suppression (Takeda et al., 2004; Valzasina et al., 2005; Golovina et al., 2008; Hippen et al., 2008; Piconese et al., 2008).
Here, we have examined the role of OX40 in influencing the balance between T eff and T reg cells in the intestine. Our results revealed a requirement for OX40 for the accumulation of T reg cells in the normal colon. Importantly, OX40 expression by T reg cells was indispensable for suppression of OX40-dependent colitogenic T cell responses.
RESULTS AND DISCUSSION
OX40 is preferentially expressed on intestinal T cells and promotes Foxp3+ T reg cell accumulation in the colon
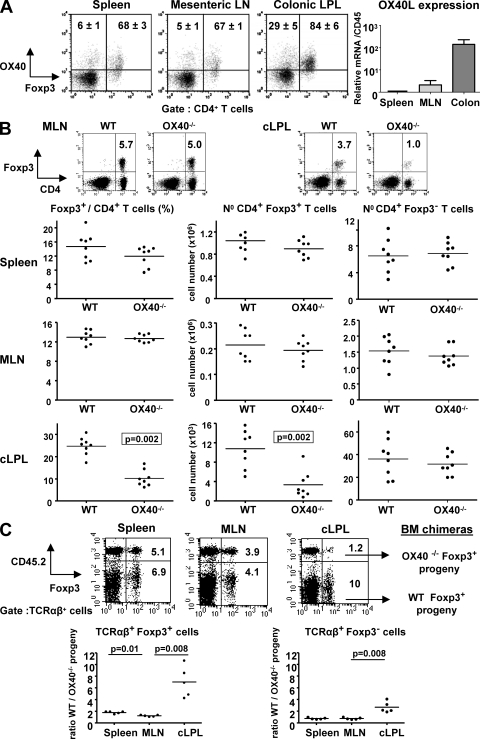
Analysis of OX40 expression among CD4+ T cells in different locations revealed compartmentalization of expression. In the lymphoid organs, OX40 was expressed primarily by CD4+Foxp3+ T cells with only a minority of CD4+Foxp3− T cells showing positive staining (Fig. 1 A). Among colonic lamina propria leukocytes (cLPL), the percentage of OX40+Foxp3+ cells was higher than in lymphoid organs (84%; Fig. 1 A). OX40L mRNA expression was also increased in the colon compared with mesenteric LN (MLN) or spleen, probably reflecting the increased activation of APC in the intestine (Fig. 1 A).
Figure 1.
OX40 is preferentially expressed on intestinal T cells and promotes Foxp3+ T reg cell accumulation in the colon in a cell intrinsic way. (A, left) Tissues from WT mice analyzed by flow cytometry for CD4, Foxp3, and OX40 expression. Percentages of OX40+ cells among CD4+ Foxp3− or Foxp3+ cells are indicated in representative dot plots (n = 5). (right) OX40L mRNA amounts in spleen, MLN, and total colon homogenate (n = 5). Values are normalized to CD45 and are mean values (± SEM) relative to spleen (set to 1). (B) Percentages and total numbers of Foxp3− and Foxp3+ CD4 T cells in tissues of WT and OX40−/− mice. (C) BM cells from WT congenic mice (CD45.1+) mixed in equal proportion with BM cells from OX40−/− mice (CD45.2+) were injected i.v. into irradiated RAG−/− mice. 8 wk after transfer, tissues were harvested and stained for TCR-β, CD45.2, and Foxp3. Representative staining (top) and the ratio of WT/OX40−/− progeny among TCRαβ+ cells are shown (bottom). Each point represents an individual mouse and horizontal bars represent group means (B and C). Data are representative of two to three independents experiments.
Given the large proportion of T reg cells that expressed OX40 in systemic and mucosal compartments, we next examined the requirement for OX40 in T reg cell accumulation in vivo. There were no significant differences in the frequencies or numbers of Foxp3+ T reg cells in the spleen (Fig. 1 B), as previously reported (Vu et al., 2007); there were also no differences in the MLN (Fig. 1 B) or in the BM (Fig. S1). In contrast, frequencies and absolute numbers of CD4+Foxp3+ T cells in the cLPL were 2.5- and 3.3-fold lower, respectively, in OX40−/− compared with WT mice (Fig. 1 B).
To determine whether the influence of OX40 on T reg cell accumulation in the intestine was cell intrinsic, we generated mixed BM chimeras using WT (CD45.1) and OX40−/− (CD45.2) BM cells (Fig. 1 C). 8 wk after BM reconstitution, TCRαβ_+_Foxp3+ T reg cells derived equally from WT or OX40−/− BM in the MLN, and there was only a small increase in the proportion of Foxp3+ cells derived from WT compared with OX40−/− cells in the spleen (mean ratio 1.7:1, WT/OX40−/−; Fig. 1 C). Consistent with results observed in OX40−/− mice, there was a highly significant skew toward WT-derived cells among colonic Foxp3+ T reg cells (average ratio 6:1, WT/OX40−/−; Fig. 1 C). Together, these results show that OX40 acts directly on T reg cells to promote their accumulation in the intestine.
Although the differences were most marked for Foxp3+ cells, TCRαβ+Foxp3− cells in the colon were also preferentially derived from WT progeny (average ratio 2.5:1, WT/OX40−/−; Fig. 1 C). This probably reflects a positive effect of OX40 on the homeostasis of CD4+Foxp3− T cells that express OX40 (29%; Fig. 1 A). In the colon, spleen, and MLN of unmanipulated mice, no differences were observed in the percentages of CD4+Foxp3− T cells presenting an activated/memory phenotype (CD44+CD62L−) between OX40−/− and WT mice (Fig. S1).
The frequency of Foxp3+ T reg cells was also 1.5- and 1.7-fold reduced in the lung and liver, respectively, of OX40−/− compared with WT mice (Fig. S1). These results suggest that OX40 promotes T reg cell accumulation in other tissues, although the effects are modest compared with the colon.
OX40 expression on T reg cells is required to suppress T cell–induced colitis
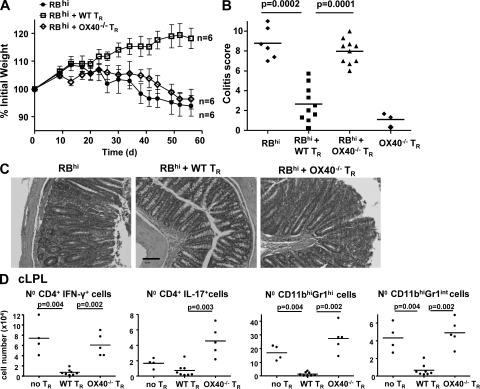
To determine if OX40 expression by T reg cells is necessary for their suppressive function in vivo, we tested their capacity to prevent the development of T cell–induced colitis. RAG−/− mice transferred with naive CD4+CD45RBhi T cells developed wasting disease and colitis (Fig. 2), whereas cotransfer of WT CD4+CD25+ T reg cells with naive CD4+ T cells prevented development of disease. Strikingly, cotransfer of OX40−/− CD4+CD25+ T reg cells failed to prevent systemic or mucosal inflammation. Indeed, the incidence and severity of colitis in this group was indistinguishable from those that received colitogenic T cells alone (Fig. 2, A–D).
Figure 2.
OX40 expression on T reg cells is required to suppress T cell induced colitis. RAG−/− mice received WT CD4+CD45RBhi T cells alone (no TR), or in combination with WT or OX40−/− CD4+CD25+ T reg cells (TR). (A) Mean weight of six animals per group (± SEM). (B) Colitis score at 8 wk. An additional control group showed that OX40−/− T reg cells do not induce colitis by themselves. (C) Photomicrographs of mid-colon sections. Bar, 100 µm. (D) Number of colonic IFN-γ+ and IL-17+CD4+ T cells, neutrophils (CD11c−CD11bhiGr1hiSSChi) and monocytes/macrophages (CD11c−CD11bhiGr1intSSClo) at 8 wk. Each point represents an individual mouse and horizontal bars represent group means (B and D). Results are representative of three independent experiments.
Our previous work has shown that T reg cells act on T cells and innate immune cells to inhibit colitis (Maloy et al., 2003). OX40−/− T reg cells on the other hand were inefficient in suppressing both arms of the colitogenic response. Marked increases in CD4+IFN-γ+ T cells (8-fold) and CD4+IL-17+ T cells (6-fold), as well as in inflammatory monocytes/macrophages (7-fold) and neutrophils (20-fold), were observed at wk 8 in the colon of mice restored with colitogenic T cells plus OX40−/− T reg cells compared with mice protected from colitis by transfer of WT T reg cells (Fig. 2 D). Together, these results show that the inability of OX40−/− T reg cells to inhibit colitis involves a complete deficiency in their control of innate and adaptive inflammatory responses in the colon.
OX40 is required for early T reg cell accumulation in the lymphoid organs and colon during control of intestinal inflammation
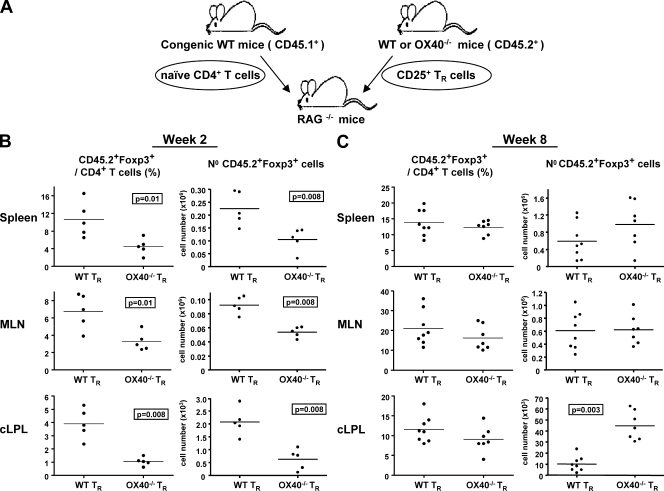
Next, we assessed if OX40 expression by T reg cells was required for their accumulation in the lymphoid organs and colon in T cell transfer colitis. To distinguish Foxp3+ cells derived from T reg progeny from those derived from CD4+CD45RBhi precursors, we isolated colitogenic and regulatory populations from CD45.1+ and CD45.2+ mice, respectively (Fig. 3 A).
Figure 3.
OX40 is required for early Foxp3+ T reg cell accumulation in the lymphoid organs and colon during control of intestinal inflammation. (A) CD45.1+ CD4+CD45RBhi T cells were cotransferred with CD45.2+ WT or OX40−/− T reg cells (TR) into RAG−/− mice. Numbers and percentages of CD45.2+CD4+Foxp3+ cells in tissues 2 wk (B) and 8 wk (C) after cotransfer. Each point represents an individual mouse. Data are representative of three independent experiments.
2 wk after transfer, before disease onset, there was an approximately twofold reduction in the total number and frequency of CD45.2+ CD4+ Foxp3+ cells in the spleen and MLN of mice cotransferred with OX40−/− T reg cells compared with mice cotransferred with WT T reg cells (Fig. 3 B). In the colon, differences were even more marked, as OX40−/− T reg cells were fourfold less frequent and 3.5-fold reduced in total number compared with similarly transferred WT T reg cells (Fig. 3 B). Thus, although OX40 strongly influences T reg accumulation in the colon, it may also promote T reg cell accumulation during inflammation at any site in which T reg cells have to compete with a proliferative T eff cell population. Importantly, 1 mo after transfer, CD45.2+Foxp3+ frequencies among CD4+ T cells were still 2-fold lower in lymphoid organs and 2.5-fold lower in the colon of mice transferred with OX40−/− compared with WT T reg cells (Fig. S2).
8 wk after transfer, the picture was very different, with CD45.2+CD4+Foxp3+ T cells present in spleen, MLN, and colon at a similar frequency in mice cotransferred with OX40−/− or WT T reg cells. Indeed, in the colon the total number of OX40−/− Foxp3+ T reg cells was fourfold increased compared with the number of WT Foxp3+ T reg cells (Fig. 3 C).
These results suggest that T reg cells that do not express OX40 fail to compete effectively with colitogenic T cells up to a month after cotransfer, resulting in the development of colitis. After disease onset, the requirement for OX40 signaling for T reg cell accumulation in the intestine is reduced, but the T reg cells that accumulate under these circumstances fail to suppress the disease.
OX40−/− T reg cells show normal proliferative responses and retain Foxp3 expression after transfer
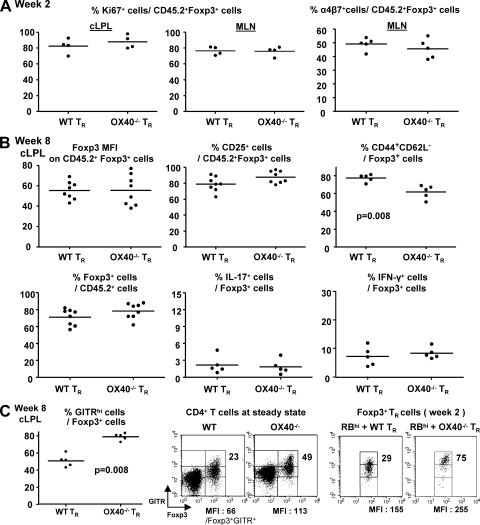
As OX40−/− T reg cells were impaired in their accumulation during the first month after cotransfer, we investigated whether their proliferative capacity was reduced. OX40−/− and WT CD45.2+Foxp3+ T reg cells showed a similar proliferative response as assessed by Ki67 staining 2 wk after cotransfer in MLN and cLPL (Fig. 4 A). This may suggest that the reduction of OX40−/− T reg cells is a consequence of impaired survival signals rather than proliferation defects. Furthermore, the expression of the α4β7 integrin in the MLN was also similar, indicating that OX40−/− T reg cells are not impaired in the up-regulation of colon-homing receptors after activation (Fig. 4 A).
Figure 4.
Phenotypic and functional characteristics of WT and OX40−/− T reg cells after transfer. CD45.1+ CD4+CD45RBhi T cells were transferred with CD45.2+ WT or OX40−/− T reg cells (TR) into RAG−/− mice. (A) Ki67 expression in cLPL and MLN and α4β7 integrin expression in MLN on Foxp3+ T reg progeny at wk 2. (B) Foxp3, CD25, CD44, CD62L expression, IL-17, and IFN-γ secretion by Foxp3+ T reg progeny at wk 8 in cLPL. (C) GITR expression by Foxp3+ T reg progeny at 2 wk (dot plots) and 8 wk (graph) and CD4+ T cells from the spleen of normal mice (dot plots). Percentages of GITRhi cells among CD4+Foxp3+ cells and GITR MFI on Foxp3+GITR+cells are indicated in dot plots. Data are representative of two independent experiments.
Despite the late accumulation of T reg cells in the colon of mice cotransferred with OX40−/− T reg cells, there was no suppression of disease. Decreased expression of Foxp3 or CD25 by T reg cells can lead to their loss of suppressor functions, production of inflammatory cytokines, and autoimmune disease (Wan and Flavell, 2007; Tang et al., 2008). This did not seem to be the case as Foxp3 (MFI and percentage of Foxp3+ cells among T reg cell progeny) and CD25 expression were similar between OX40−/− and WT CD45.2+ T reg cells present at 8 wk in the colon, and these cells did not express inflammatory cytokines IL-17 or IFN-γ (Fig. 4 B).
Analysis of other molecules linked to T reg function such as CTLA-4 or IL-10 showed no differences between OX40−/− and WT CD45.2+ T reg cells (unpublished data). However, OX40−/− T reg cells exhibited a less activated/memory phenotype (CD44+CD62L−) than WT T reg cells 8 wk after transfer in the colon (Fig. 4 B) and MLN (unpublished data). OX40 promotes the acquisition of an effector/memory phenotype by activated CD4 T cells (Watts, 2005) and may therefore favor the full activation of T reg cells during control of inflammatory responses. In addition, the frequency of glucocorticoid-induced TNF receptor (GITR)hi T reg cells was significantly higher in OX40−/− compared with WT Foxp3+ T reg cells in both the late and early phase of the disease, as well as in lymphocyte-replete mice at steady-state (Fig. 4 C). This raises the possibility that increased GITR signaling in OX40−/− T reg cells may affect their suppressive function (Shimizu et al., 2002). GITR may also promote the late accumulation of OX40−/− T reg cells in the colon, as a GITR signal can support the in vivo expansion of Foxp3+ T reg cells (Shevach and Stephens, 2006; van Olffen et al., 2009). Further experiments are required to establish any functional role of GITR in this model.
Lack of OX40 signaling renders activated T reg cells more susceptible to apoptosis
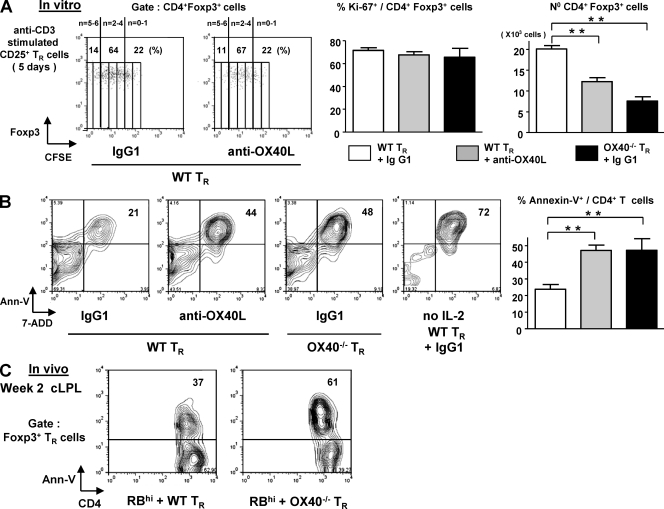
Next, we assessed whether OX40 deficiency in T reg cells was associated with impaired survival after activation. FACS-sorted CD25+ T reg cells were activated with anti-CD3 in the presence of APC and exogenous IL-2. Anti-OX40L–blocking mAb or OX40−/− T reg cells were used to test the influence of OX40 on T reg cell survival after activation. In line with in vivo results, Foxp3+ T reg cell proliferation in vitro as assessed by CFSE dilution and Ki67 expression (Fig. 5 A) was unaffected by the absence of an OX40 signal. Despite this, there was an approximately twofold reduction in the total number of Foxp3+ T reg cells that accumulated after activation in the absence of an OX40 signal, and this was accompanied by a twofold increase in the percentage of apoptotic cells (Annexin V+) at day 5 (Fig. 5 B). Importantly, OX40−/− T reg cells were also more sensitive to apoptosis than WT T reg cells 2 wk after cotransfer with CD4+CD45RBhigh cells as shown by Annexin V staining on colonic Foxp3+ cells after overnight culture (Fig. 5 C). Together, these results show that OX40 promotes the accumulation of TCR-activated T reg cells by rendering them less sensitive to activation-induced cell death.
Figure 5.
Lack of an OX40 signal renders activated T reg cells more susceptible to apoptosis. WT or OX40−/− CD25+ FACS sorted T reg cells were cultured in triplicate for 5 d with irradiated APC and soluble anti-CD3, in the presence of an anti-OX40L mAb or isotype control. hIL-2 was added to the cultures (200U/ml). (A) Representative dot plots of CFSE dilution by CD4+Foxp3+ cells. Bar graphs represent means of Ki67 expression by Foxp3+ cells and total number of CD4+Foxp3+ cells at d 5 (mean of triplicate cultures ± SD). (B) Representative Annexin V-7 ADD staining 5 d after T reg cell activation (gated on CD4+ T cells). Bar graph represents mean percentages of apoptotic cells (mean of triplicate cultures ± SD). **, P < 0.001. Results are representatives of two to three independent experiments. (C) CD4+CD45RBhi T cells were transferred into RAG−/− mice with CD25+ TR cells isolated from WT or OX40−/− Foxp3-GFP mice. Numbers in dot plots represent the percentages of Annexin V+ cells at 2 wk among colonic CD4+Foxp3(GFP)+ cells, after overnight culture in medium. Three to four colons were pooled per group, and results are representative of two independent experiments.
OX40 expression on T cells is required for development of colitis
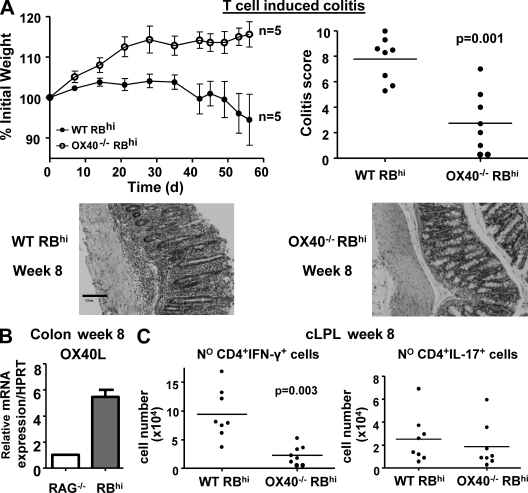
Despite reductions in Foxp3+ T reg cells in the intestine of OX40-deficient mice, there was no sign of colitis in these mice (unpublished data). To test whether OX40 signaling in T cells is also required for colitogenic T cell responses, we transferred OX40−/− and WT CD4+CD45RBhi T cells to RAG−/− mice. Although the latter induced wasting disease and colitis, OX40−/− CD45RBhi T cells failed to induce wasting disease and led to marked amelioration of colitis (Fig. 6 A). Consistent with a requirement for OX40 signaling for a sustained inflammatory response, OX40L mRNA expression was increased in the inflamed colon (Fig. 6 B). Reduced colitis after transfer of OX40−/− CD45RBhi T cells was associated with fourfold fewer CD4+IFNγ+ T cells in the colon (Fig. 6 C). Similar reductions in colonic CD4+TNF+ T cells (unpublished data) were also observed in line with the previously described influence of OX40 on TNF-secreting T cells (Ito et al., 2005). These results show that OX40 expression by T cells promotes colitogenic Th1 responses, which are known to drive colitis in this model (Powrie et al., 1994). The results are also consistent with our earlier findings that anti-OX40L mAb inhibited T cell transfer colitis (Malmström et al., 2001). The requirement for OX40 signaling to sustain colitogenic T cell responses explains the lack of colitis in OX40−/− mice. In addition, an inability of OX40−/− T reg cells to compete for the inflammatory resource OX40L in the gut may well contribute to their inability to control OX40+ effector responses in the gut.
Figure 6.
OX40 is required for the development of colitogenic T cell responses. RAG−/− mice received CD4+CD45RBhi T cells from WT or OX40−/− donors. (A) Mean weight loss (n = 6 ± SEM) and colitis score at wk 8. Representative photomicrographs of mid-colon sections are shown below. Bar, 100 µm. (B) Amount of OX40L mRNA in total colon homogenate from untransferred mice (RAG−/−) or colitic mice (RBhi) at wk 8. Data shown are the mean values (n = 4) relative to untransferred RAG−/− mice (set to 1), after normalization to HPRT expression. (C) Number of colonic IFN-γ+ and IL-17+ CD4+ T cells 8 wk after transfer. Each point in (A, right) and (C) represents an individual mouse. Results are representative of three independent experiments.
Here, we describe an essential role for OX40 in Foxp3+ T reg cell homeostasis and control of intestinal inflammation. OX40 deficiency abolished the ability of T reg cells to suppress T cell transfer colitis. This was associated with an early impairment in the accumulation of Foxp3+ T reg cells in the colon and lymphoid organs. OX40 signaling protected activated T reg cells from apoptosis and promoted their survival in vitro and when analyzed directly ex vivo. An increased sensitivity to activation-induced cell death provides an explanation of why OX40−/− T reg cells are less able to accumulate after transfer and compete with OX40-dependent colitogenic T cell responses. Indeed, an antiapoptotic effect of OX40 was previously described in CD4+ T eff cells, promoting their long-term survival (Song et al., 2005). Interestingly, inflammation overrides the requirement for OX40 signaling, as late accumulation of OX40−/− T reg cells was not impaired in colitic mice. Several inflammatory molecules are able to support the survival of T reg cells and may promote the late accumulation of OX40−/− T reg cells during colitis. These include GITRL, which was increased during colitis (unpublished data), TLR2, and IL-2 (Chen et al., 2009; van Olffen et al., 2009).
The accumulation of Foxp3+ T reg cells in both the lymphoid organs and intestine was highly dependent on OX40 expression upon transfer into lymphopenic mice, whereas under homeostatic conditions, OX40 was not essential for accumulation in lymphoid organs. The majority of T reg cells undergo proliferation upon transfer to RAG−/− mice (∼80%; Fig. 4 A) and this increased pressure, particularly in an environment where IL-2 may be limiting, could explain the more general requirement for OX40 signaling under these conditions. The inability of OX40−/− T reg cells to keep up with OX40-competent colitogenic T cells in the earlier phase of the response could directly account for their inability to control colitis. Indeed, CCR4−/− CD25+ T reg cells were unable to prevent T cell–induced colitis as a consequence of reduced accumulation in the MLN early in the response (Yuan et al., 2007). Furthermore, recent studies have highlighted the importance of the T reg cell/T eff cell ratio for efficient control of colitis (Darrasse-Jèze et al., 2009).
T reg cells can suppress T eff cell responses by modulating APC (Cederbom et al., 2000) and by out-competing conventional T cells for interactions with APC (Onishi et al., 2008). Because the majority of T reg cells express OX40, they may be particularly poised to interact with OX40L+ DCs, which are abundant in the MLN of colitic mice (Malmström et al., 2001). In contrast, OX40−/− T reg cells may be impaired in their ability to compete with activated OX40+ T eff cells for interactions with OX40L+ DC, resulting in sustained OX40 signaling into T eff cells, which promotes colitis. Recent studies have shown that to suppress particular Th responses, T reg cells need to express signature transcription factors that drive distinct T eff cell responses. Thus, to inhibit Th1, Th2, or Th17 responses, T reg cells need to express T-bet (Koch et al., 2009), IRF-4 (Zheng et al., 2009), and Stat3 (Chaudhry et al., 2009), respectively. These results emphasize the functional specialization of T reg responses and indicate that to compete effectively with distinct types of effector responses, T reg cells need to be able to compete in a particular niche. Our data suggest that along these lines, OX40 expression on T reg cells may be required to effectively compete with OX40-dependent colitogenic T cell responses.
Further understanding of the factors that influence the balance between effector and T reg cells in the gut is required for the rational design of therapies that inhibit intestinal inflammation while promoting T reg cell activity.
MATERIALS AND METHODS
Mice.
Wild-type C57BL/6, congenic B6.SJL-Cd45, C57BL/6 RAG−/−, C57BL/6 OX40−/− mice, and Foxp3-GFP knock-in mice were bred and maintained under specific pathogen–free conditions in accredited animal facilities at the Sir William Dunn School of Pathology (University of Oxford, Oxford, England, UK). OX40−/− mice (Pippig et al., 1999) were crossed nine times on the C57BL/6 background. OX40−/− Foxp3-GFP mice were generated by crossing OX40−/− mice with C57BL/6 Foxp3-GFP knock-in mice (Fontenot et al., 2005). All procedures involving animals were conducted according to the requirements and with the approval of the UK Home Office Animals (Scientific Procedures) Acts, 1986. Mice were negative for Helicobacter spp. and other known intestinal pathogens and were 6–10 wk old when first used.
Purification of CD4+ T cell subsets.
CD4+ T cells were purified from spleens by negative depletion using rat anti–mouse CD8 (clone YTS 169), B220 (RA3-6B2), MHC class II (TIB 120), and Mac-1 (M1-70) together with anti–rat coated Dynabeads (Invitrogen). Enriched CD4+ single-cell suspensions were stained with anti-CD4, anti-CD25, and anti-CD45RB (all obtained from BD). Naive CD4+CD25−CD45RBhi T cells and regulatory CD4+CD25+CD45RBlo T cells were purified (>99%) on a cell sorter (MoFlo; Dako). Subsequent FACS analysis showed that at least 95% of CD4+CD25+CD45RBlo cells were Foxp3+ and 99% of CD4+CD25−CD45RBhi cells were Foxp3− (Izcue et al., 2008).
T cell transfer model of colitis.
Naive CD4+CD25−CD45RBhi T cells were isolated from congenic B6.SJL-Cd45 (CD45.1+) mice, WT or OX40−/− C57BL/6 mice and injected i.p. into 6–10-wk-old C57BL/6 RAG−/− immunodeficient recipients (4 × 105 cells/mouse). WT and OX40−/− CD4+CD45RBloCD25+ T reg cells (2 × 105/mouse) were co-injected i.p. where indicated. Mice were monitored weekly for wasting disease, and any mice losing >20% of its starting body weight or showing severe signs of disease were killed.
Histological assessment of intestinal inflammation.
Mice were killed 7–8 wk after T cell reconstitution, and samples of proximal colon, mid-colon, and distal colon were fixed in buffered 10% formalin solution. 5-µm paraffin-embedded sections were cut and stained with hematoxylin and eosin and inflammation was scored in a blinded fashion using a previously described scoring system (Izcue et al., 2008).
Generation of BM chimeras.
BM was isolated from congenic B6.SJL-Cd45 mice and C57BL/6 OX40−/− age-matched mice and mixed at a 1:1 ratio. γ-Irradiated (5.5 Gy, 550 rad) C57BL/6 RAG−/− recipients were injected with 5.106 mixed BM cells/mouse i.v.
Isolation of leukocyte subpopulations and FACS.
Cell suspensions were prepared from spleen, MLN, BM, and cLPL as previously described (Izcue et al., 2009). Lymphocytes from lung and liver were isolated as previously described (Sather et al., 2007). The following antibodies were used for flow cytometry: anti-Gr1 and -CD45.2 conjugated to FITC, anti-CD4 conjugated to PerCP or FITC, anti–TCR-β, anti-α4β7, anti-CD11c, and anti-CD62L conjugated to PE, anti-CD11b conjugated to APC, biotinylated anti-CD45.2, anti-OX40, anti-GITR, anti-CD44 (all from BD), or appropriate isotype controls. Biotinylated antibodies were detected with PerCP or FITC-conjugated streptavidin (BD). Intracellular cytokines staining after 4 h PMA and ionomycin restimulation and Foxp3 staining were performed as previously described (Izcue et al., 2008). Apoptosis sensitivity was measured by Annexin V staining (BD) of CD4 T cell enriched samples after 15 h of culture in medium without restimulation or cytokines, as previously described (Ruby et al., 2008).
T reg cell cultures.
CD4+CD25+ T reg cells (5 × 104/well) were FACS sorted from the spleen of WT or OX40−/− mice (95% Foxp3+) and labeled with CFSE where indicated. T reg cells were stimulated with irradiated APC (105/well; T cell free splenocytes), soluble anti-CD3 (145-2c11; 1 µg/ml), recombinant hIL-2 (PeproTech, 200 U/ml) and either blocking anti-OX40L antibody (OX89; 10 µg/ml) or IgG1 isotype control. Cells were cultured in 96-well round-bottom plates for 5 d in complete RPMI (Invitrogen) containing 5% FCS, 2 mM l-glutamine, 0.05 mM 2-mercaptoethanol, and 100 U/ml each of penicillin and streptomycin. Live cells at d 5 were counted using trypan blue. Proliferation of CD4+Foxp3+ was assessed by CFSE dilution and Ki67 staining; apoptosis was assessed using Annexin V and 7-AAD staining.
Quantitation of gene expression using real-time PCR.
Total RNA was purified from frozen tissue samples using RNAeasy kits (QIAGEN). Homogenization was performed using a Fastprep 24 Homogenizer (MP Biomedicals). cDNA synthesis was performed using Superscript III reverse transcription and Oligo dT primers (both from Invitrogen). Quantitative PCR reactions were performed using SYBR green PCR SensiMix (Quantace) and the following reagents: OX40L primers, 5′-GTTCTGCACCTCCATAGTTTGA-3′ and 5′-GGATGCTTCTGTGCTTCATCT-3′. cDNA samples were assayed in triplicate using a Chromo4 detection system (MJ Research), and gene expression levels for each individual sample were normalized to HPRT (QuantiTect; QIAGEN). Mean relative gene expression was determined, and the differences were calculated using the 2ΔC(t) method (Pfaffl, 2001).
Statistical analysis.
Statistical analysis was performed with Prism 5.0 (GraphPad Software). The nonparametric Mann-Whitney test was used for all statistical comparisons, except for the T reg cell cultures where an unpaired Student’s t test was used. Differences were considered statistically significant when P < 0.05.
Online supplemental material.
Fig. S1 shows that the frequency of Foxp3+ T reg cells was decreased in the lung and liver of OX40−/− mice compared with WT mice and that the frequency of activated/memory CD4+Foxp3− T cells in spleen, MLN and colon were similar between WT and OX40−/− mice. Fig. S2 shows that the frequency of CD45.2+Foxp3+ T cells 4 wk after cotransfer is reduced in the spleen, MLN and cLPL of mice transferred with OX40−/− compared with WT T reg cells. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20091618/DC1.
Acknowledgments
We thank N.Rust for assistance with cell sorting, R.Stillion for histology, and the staff of our animal facility. We thank our colleagues N.Killeen and A.Rudensky for the provision of the OX40−/− mice and Foxp3-GFP knockin mice, respectively. We thank N. Robinson, A. Izcue, P. Ahern, and A. Plüddemann for technical advice.
This work was supported by grants from the Wellcome Trust to T. Griseri and F. Powrie (Senior Fellowship) and by an AstraZeneca PhD studentship to M. Asquith.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
cLPL
colonic lamina propria leukocyte
GITR
glucocorticoid-induced TNF receptor
IBD
inflammatory bowel disease
MLN
mesenteric LN
T eff cell
effector T cell
T reg cell
regulatory T cell
References
- Bennett C.L., Christie J., Ramsdell F., Brunkow M.E., Ferguson P.J., Whitesell L., Kelly T.E., Saulsbury F.T., Chance P.F., Ochs H.D. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27:20–21 10.1038/83713 [DOI] [PubMed] [Google Scholar]
- Bouma G., Strober W. 2003. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 3:521–533 10.1038/nri1132 [DOI] [PubMed] [Google Scholar]
- Cederbom L., Hall H., Ivars F. 2000. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur. J. Immunol. 30:1538–1543 [DOI] [PubMed] [Google Scholar]
- Chaudhry A., Rudra D., Treuting P., Samstein R.M., Liang Y., Kas A., Rudensky A.Y. 2009. CD4+ Regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 326:986–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Davidson T.S., Huter E.N., Shevach E.M. 2009. Engagement of TLR2 does not reverse the suppressor function of mouse regulatory T cells, but promotes their survival. J. Immunol. 183:4458–4466 10.4049/jimmunol.0901465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes J.L., Robinson N.J., Maloy K.J., Uhlig H.H., Powrie F. 2005. Regulatory T cells and intestinal homeostasis. Immunol. Rev. 204:184–194 10.1111/j.0105-2896.2005.00250.x [DOI] [PubMed] [Google Scholar]
- Croft M. 2003. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat. Rev. Immunol. 3:609–620 10.1038/nri1148 [DOI] [PubMed] [Google Scholar]
- Darrasse-Jèze G., Deroubaix S., Mouquet H., Victora G.D., Eisenreich T., Yao K.H., Masilamani R.F., Dustin M.L., Rudensky A., Liu K., Nussenzweig M.C. 2009. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J. Exp. Med. 206:1853–1862 10.1084/jem.20090746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J.D., Rasmussen J.P., Williams L.M., Dooley J.L., Farr A.G., Rudensky A.Y. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341 10.1016/j.immuni.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Golovina T.N., Mikheeva T., Suhoski M.M., Aqui N.A., Tai V.C., Shan X., Liu R., Balcarcel R.R., Fisher N., Levine B.L., et al. 2008. CD28 costimulation is essential for human T regulatory expansion and function. J. Immunol. 181:2855–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippen K.L., Harker-Murray P., Porter S.B., Merkel S.C., Londer A., Taylor D.K., Bina M., Panoskaltsis-Mortari A., Rubinstein P., Van Rooijen N., et al. 2008. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 112:2847–2857 10.1182/blood-2008-01-132951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Wang Y.H., Duramad O., Hori T., Delespesse G.J., Watanabe N., Qin F.X., Yao Z., Cao W., Liu Y.J. 2005. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 202:1213–1223 10.1084/jem.20051135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcue A., Hue S., Buonocore S., Arancibia-Cárcamo C.V., Ahern P.P., Iwakura Y., Maloy K.J., Powrie F. 2008. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 28:559–570 10.1016/j.immuni.2008.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcue A., Coombes J.L., Powrie F. 2009. Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 27:313–338 10.1146/annurev.immunol.021908.132657 [DOI] [PubMed] [Google Scholar]
- Koch M.A., Tucker-Heard G., Perdue N.R., Killebrew J.R., Urdahl K.B., Campbell D.J. 2009. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat. Immunol. 10:595–602 10.1038/ni.1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmström V., Shipton D., Singh B., Al-Shamkhani A., Puklavec M.J., Barclay A.N., Powrie F. 2001. CD134L expression on dendritic cells in the mesenteric lymph nodes drives colitis in T cell-restored SCID mice. J. Immunol. 166:6972–6981 [DOI] [PubMed] [Google Scholar]
- Maloy K.J., Salaun L., Cahill R., Dougan G., Saunders N.J., Powrie F. 2003. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 197:111–119 10.1084/jem.20021345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi Y., Fehervari Z., Yamaguchi T., Sakaguchi S. 2008. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl. Acad. Sci. USA. 105:10113–10118 10.1073/pnas.0711106105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M.W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piconese S., Valzasina B., Colombo M.P. 2008. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J. Exp. Med. 205:825–839 10.1084/jem.20071341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pippig S.D., Peña-Rossi C., Long J., Godfrey W.R., Fowell D.J., Reiner S.L., Birkeland M.L., Locksley R.M., Barclay A.N., Killeen N. 1999. Robust B cell immunity but impaired T cell proliferation in the absence of CD134 (OX40). J. Immunol. 163:6520–6529 [PubMed] [Google Scholar]
- Powrie F., Leach M.W., Mauze S., Menon S., Caddle L.B., Coffman R.L. 1994. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1:553–562 10.1016/1074-7613(94)90045-0 [DOI] [PubMed] [Google Scholar]
- Ruby C.E., Montler R., Zheng R., Shu S., Weinberg A.D. 2008. IL-12 is required for anti-OX40-mediated CD4 T cell survival. J. Immunol. 180:2140–2148 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Yamaguchi T., Nomura T., Ono M. 2008. Regulatory T cells and immune tolerance. Cell. 133:775–787 10.1016/j.cell.2008.05.009 [DOI] [PubMed] [Google Scholar]
- Sather B.D., Treuting P., Perdue N., Miazgowicz M., Fontenot J.D., Rudensky A.Y., Campbell D.J. 2007. Altering the distribution of Foxp3+ regulatory T cells results in tissue-specific inflammatory disease. J. Exp. Med. 204:1335–1347 10.1084/jem.20070081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E.M. 2009. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 30:636–645 10.1016/j.immuni.2009.04.010 [DOI] [PubMed] [Google Scholar]
- Shevach E.M., Stephens G.L. 2006. The GITR-GITRL interaction: co-stimulation or contrasuppression of regulatory activity? Nat. Rev. Immunol. 6:613–618 10.1038/nri1867 [DOI] [PubMed] [Google Scholar]
- Shimizu J., Yamazaki S., Takahashi T., Ishida Y., Sakaguchi S. 2002. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 3:135–142 10.1038/ni759 [DOI] [PubMed] [Google Scholar]
- Song J., So T., Cheng M., Tang X., Croft M. 2005. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 22:621–631 10.1016/j.immuni.2005.03.012 [DOI] [PubMed] [Google Scholar]
- Souza H.S., Elia C.C., Spencer J., MacDonald T.T. 1999. Expression of lymphocyte-endothelial receptor-ligand pairs, alpha4beta7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 45:856–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugamura K., Ishii N., Weinberg A.D. 2004. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat. Rev. Immunol. 4:420–431 10.1038/nri1371 [DOI] [PubMed] [Google Scholar]
- Takeda I., Ine S., Killeen N., Ndhlovu L.C., Murata K., Satomi S., Sugamura K., Ishii N. 2004. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J. Immunol. 172:3580–3589 [DOI] [PubMed] [Google Scholar]
- Tang Q., Adams J.Y., Penaranda C., Melli K., Piaggio E., Sgouroudis E., Piccirillo C.A., Salomon B.L., Bluestone J.A. 2008. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 28:687–697 10.1016/j.immuni.2008.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valzasina B., Guiducci C., Dislich H., Killeen N., Weinberg A.D., Colombo M.P. 2005. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 105:2845–2851 10.1182/blood-2004-07-2959 [DOI] [PubMed] [Google Scholar]
- van Olffen R.W., Koning N., van Gisbergen K.P., Wensveen F.M., Hoek R.M., Boon L., Hamann J., van Lier R.A., Nolte M.A. 2009. GITR triggering induces expansion of both effector and regulatory CD4+ T cells in vivo. J. Immunol. 182:7490–7500 10.4049/jimmunol.0802751 [DOI] [PubMed] [Google Scholar]
- Vu M.D., Xiao X., Gao W., Degauque N., Chen M., Kroemer A., Killeen N., Ishii N., Chang Li X. 2007. OX40 costimulation turns off Foxp3+ Tregs. Blood. 110:2501–2510 10.1182/blood-2007-01-070748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y.Y., Flavell R.A. 2007. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 445:766–770 10.1038/nature05479 [DOI] [PubMed] [Google Scholar]
- Watts T.H. 2005. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 23:23–68 10.1146/annurev.immunol.23.021704.115839 [DOI] [PubMed] [Google Scholar]
- Yuan Q., Bromley S.K., Means T.K., Jones K.J., Hayashi F., Bhan A.K., Luster A.D. 2007. CCR4-dependent regulatory T cell function in inflammatory bowel disease. J. Exp. Med. 204:1327–1334 10.1084/jem.20062076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y., Chaudhry A., Kas A., deRoos P., Kim J.M., Chu T.T., Corcoran L., Treuting P., Klein U., Rudensky A.Y. 2009. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 458:351–356 10.1038/nature07674 [DOI] [PMC free article] [PubMed] [Google Scholar]