Health care utilization history, GOLD guidelines, and respiratory medication prescriptions in patients with COPD (original) (raw)
Abstract
Background:
The relationship between prior health care utilization and respiratory medication prescriptions in an unselected population of patients with COPD is not known.
Methods:
We determined the prescribed respiratory medications and respiratory and nonrespiratory health care encounters in 523 Veterans with COPD at the Cincinnati Veterans Affairs Medical Center between 2000 and 2005. Prescribed treatments were compared with the GOLD guidelines and each patient was classified as receiving less medications than recommended in the guidelines (<G), medications according to the guidelines (=G), or more medications than recommended (>G).
Results:
Respiratory medications were <G for 54%, =G in 33%, and >G for 14% of the patients studied. For GOLD stages 1 and 2, G patients the most prior respiratory encounters during a 12 month period (0.31 ± 0.073 (0.21, 0.47), 0.75 ± 0.5 (0.37, 1.5), 1.1 ± 0.27 (0.74, 1.6) visits/person/year, <G, =G, >G, respectively, mean + standard error of mean (SEM) (95% confidence limits) 2 degrees of freedom (df) ANOVA P < 0.001 for prescription effect). For GOLD stages 3 and 4, <G was associated with significantly fewer prior respiratory visits than was =G (0.78 ± 0.11 (0.6, 1.0) and 2.4 ± 0.47 (1.9, 3.1) visits/person/year, respectively, _P_ < 0.001). There were no differences in nonrespiratory health care visits for GOLD stages 1 and 2 by prescription level (3.1 ± 0.24 (2.6, 3.5), 3.1 ± 0.46 (2.1, 4.6) and 4.1 ± 0.55 (3.3, 5.1) visits/person/year, <G, =G, >G respectively, 2 df ANOVA P = 0.096) or for GOLD stages 3 and 4 (3.6 ± 0.25 (3.2, 4.1) and 4.0 ± 0.44 (3.3, 4.9) visits/person/year, <G and =G, respectively, P = 0.36).
Conclusions:
Respiratory medications prescribed for an unselected population with a broad range of COPD severity complied poorly with the GOLD pharmacologic treatment guidelines but correlated with the number of prior respiratory health care visits.
Keywords: health care, COPD, respiratory visits, GOLD guidelines, prescription
Introduction
Chronic obstructive pulmonary disease (COPD) is a common disease affecting 12–24 million adults in the United States.1,2 COPD is currently the fourth leading cause of mortality and is projected to be the third leading cause of death by 2020.1,2 Outpatient health care encounters, emergency department visits, and hospitalizations prompted by COPD cost more than 32 billion dollars yearly.1,2
Over 50 clinical practice guidelines have been developed for the management of COPD.3,4 The Global Strategy for the Diagnosis and Management of Chronic Obstructive Lung Disease (GOLD), a collaboration of the World Health Organization and the National Heart Lung and Blood Institute of the National Institute of Health, has created evidence-based guidelines with standards for grading evidence, explicit recommendations, and a well organized implementation group with regular, systematic updates and revisions.5 Nevertheless, despite international dissemination and intensive promotion, the GOLD guidelines have not been universally adopted and implemented by primary care physicians or pulmonologists.6–13
The reasons for poor adherence with COPD treatment guidelines are not well understood. Potential factors contributing to noncompliance include low utilization of spirometry, unawareness of guideline recommendations, and perceived ineffectiveness.14 To examine the relationship between prior health care utilization and the prescription of respiratory medications, we determined respiratory health care encounters and the prescription of respiratory medications compared with the GOLD guidelines in an unselected population with COPD.
Patients and methods
Patients
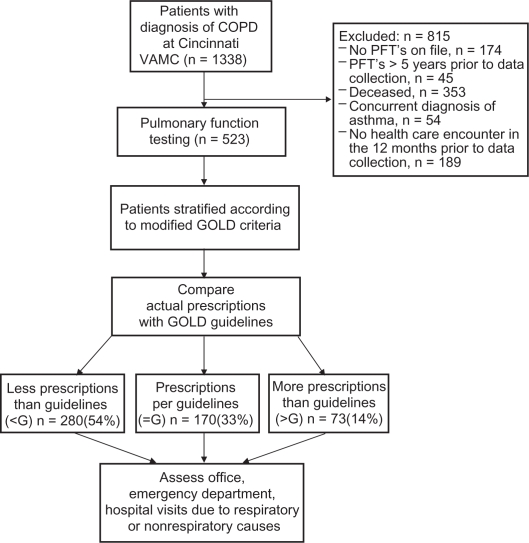
We reviewed, retrospectively, the medical records of all patients at the Cincinnati Veterans Administration Medical Center (VAMC) with a diagnosis of COPD between June 1, 2000 and June 1, 2005 (Figure 1). To ensure that all participants were followed actively, individuals who did not have at least one health care encounter in the 12 months prior to the study were excluded (n = 189, Figure 1). Prescribed therapeutic regimens compared with GOLD treatment guidelines are presented in Figure 1. All spirometry was performed in the Cincinnati VAMC Pulmonary Function Laboratory according to American Thoracic Society (ATS) guidelines. For 131 patients, the forced expiratory volume in 1 second (FEV1) divided by the forced vital capacity (FVC), FEV1/FVC, was ≥0.70 and they were classified as clinical COPD (former GOLD stage 0).5 The remaining 392 patients were classified into 4 stages (modified GOLD 1, modified GOLD 2, modified GOLD 3, modified GOLD 4) defined by the GOLD guidelines based upon prebronchodilator spirometry.5
Figure 1.
Flow diagram for inclusion and exclusion of study patients.
Note: The diagnosis of COPD was defined by ICD-9 codes, 491.XX (Chronic Bronchitis), 492.XX (Emphysema) and 496.XX (Chronic Obstructive Lung Disease).
Study design
Each patient’s prescribed medication regimen was compared with the GOLD treatment guidelines. Subjects were classified as less medications than guidelines (<G) if they were prescribed less than the recommended regimen, according to guidelines (=G) if their respiratory medication prescription met the guidelines, and more medications than guidelines (>G) if they were prescribed more respiratory medications than recommended. The Veterans Affairs medical system electronic medical record was reviewed and every outpatient visit, emergency department encounter, and hospitalization during the preceding 60 months was categorized as respiratory or nonrespiratory based upon the chief complaint and diagnostic coding for the encounter. Examples of respiratory chief complaints included breathlessness, cough, or wheezing.
Because the prescription record could be determined most accurately at the time of the chart review, the primary analysis studied health care encounters during the preceding 12 months. In a secondary analysis, we extended the time frame to the preceding 60 months.
This protocol was approved by the Research and Development Committee of the Cincinnati VAMC and the University of Cincinnati Institutional Review Board. The need for consent was waived.
Statistics
The analyses of health care visits were conducted on the counts of encounters over 12 months and 60 months. Based upon the inclusion criteria, all patients were assumed to have been followed actively throughout the 60 month period. Only health care encounters that occurred within the Cincinnati VAMC system were counted and any period with no entry in the electronic medical record was recorded as zero visits for that time span. All visit frequencies were normalized to yearly rates. For many analyses, modified GOLD groups 1 and 2 were pooled, as were groups 3 and 4, because the combined modified GOLD groups shared similar physiologic characteristics and treatment recommendations.4 Patients with clinical COPD could be categorized as =G or >G. Patients in modified GOLD stage 1 and 2 could be classified as <G, =G, or >G, whereas modified GOLD 3 and 4 subjects were grouped as <G or =G.
For all analyses in which the number of visits was the dependent variable, confidence limits and _P_-values were based upon Wald tests within Poisson models with the Poisson variance estimates adjusted by the ratio of the model deviance to the model degrees of freedom. The study alpha was P = 0.05, two-tailed. Results are presented as mean ± SEM (upper and lower 95% confidence limits).
Results
Study population
1338 patient records were evaluated and 523 patients were studied (Figure 1). The clinical characteristics of the study population are presented in Tables 1 and 2.
Table 1.
Characteristics of the 523 study subjects
| Gender | |
|---|---|
| Male | 498 (95%) |
| Female | 25 (5%) |
| Age (years) | 66.5 ± 11.0 |
| Race | |
| White | 444 (85%) |
| African-American | 68 (13%) |
| Asian | 1 (0.2%) |
| Other/not noted | 10 (2%) |
| Spirometry | |
| within 2 years | 321 (61%) |
| within 5 years | 523 (100%) |
| Smoking status | |
| current smoker | 257 (50%) |
| former smoker | 245 (47%) |
| never smoker | 17 (3%) |
| Medications | |
| Short acting beta agonist | 346 (66%) |
| Long acting beta agonist | 114 (22%) |
| Ipratroprium | 181 (35%) |
| Albuteral/Ipratroprium (Combivent®) | 114 (22%) |
| Theophylline | 46 (9%) |
| Tiotroprium | 10 (2%) |
| Inhaled steroid | 158 (30%) |
| Systemic steroid | 31 (6%) |
| Supplemental oxygen | 121 (23%) |
Table 2.
Distribution of patients, FEV1, and FEV1/FVC according to modified GOLD stage
| Modified GOLD stage | n (%) | FEV1 (I) | FEV1/FVC (%) |
|---|---|---|---|
| Clinical | 131 (25%) | 2.47 ± 0.71 (2.35 – 2.59) | 77.2 ± 5.69 (76.27 – 78.22) |
| COPD | |||
| 1 | 28 (5%) | 2.66 ± 0.61 (2.44 – 2.88) | 65.1 ± 3.17 (63.89 – 66.25) |
| 2 | 134 (25.6%) | 2.03 ± 0.50 (1.94 – 2.11) | 58.8 ± 6.57 (57.63 – 59.86) |
| 3 | 113 (21.6%) | 1.29 ± 0.32 (1.23 – 1.35) | 47.9 ± 8.92 (46.2 – 49.49) |
| 4 | 117 (22.4%) | 0.89 ± 0.28 (0.84 – .94) | 42.3 ± 9.31 (40.63 – 44) |
Health care visits
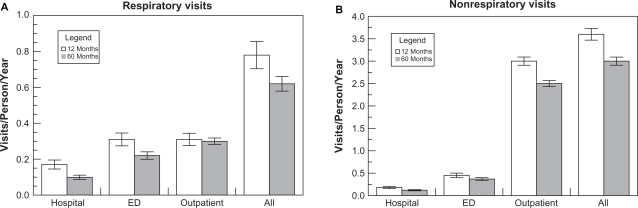
Hospital, emergency department (ED), outpatient respiratory and nonrespiratory visits over the 12 and 60 month periods are shown in Figure 2. ED and outpatient visits comprised similar proportions of total respiratory visits (35% and 48%) whereas there were 6-fold more outpatient visits (83%) than ED visits (12%–13%) for nonrespiratory causes. Hospitalizations represented 16%–17% of respiratory visits but only 4%–5% of nonrespiratory encounters. The number of hospital, ED, and outpatient respiratory visits increased with each modified GOLD stage, whereas the number of nonrespiratory visits/person/year was similar for all groups over the 12 month period. Respiratory and nonrespiratory visits over the 60 month period are presented in Figure 3.
Figure 2.
Distribution of health care encounters over 12 and 60 month intervals.
Notes: Results are mean ± SEM. Panel A shows hospital, ED, outpatient, and total respiratory visits. Panel B shows hospital, ED, outpatient, and total nonrespiratory visits.
Abbreviations: Hospital, hospitalizations; ED, emergency department visits; Outpatient, outpatient health care encounters; All, total health care encounters.
Figure 3.
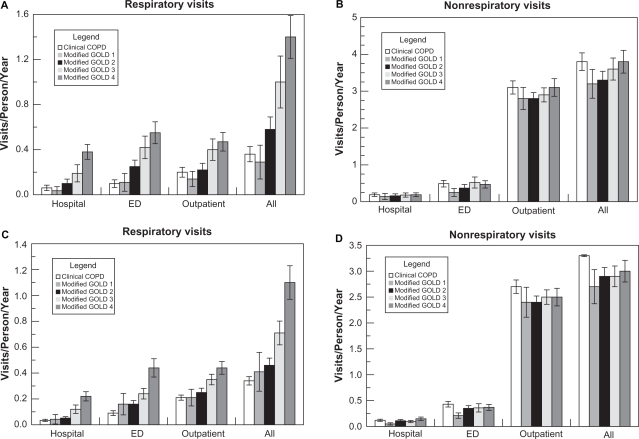
Health care encounters during the 12 month period (A, B) and the 60 month period (C, D) according to modified GOLD stage.
Notes: Results are mean ± SEM (95% confidence interval). Panel A shows hospital, ED, outpatient, and total respiratory visits. Patients with advanced COPD had more respiratory health care visits compared with individuals with a clinical diagnosis of COPD (0.36 ± 0.067 (0.24, 0.54), 0.29 ± 0.15 (0.11, 0.77), 0.58 ± 0.11 (0.42, 0.80), 1.0 ± 0.23 (0.77, 1.3), and 1.4 ± 0.19 (1.1, 1.7) visits/person/year over the 12 month period for clinical COPD (former GOLD stage 0) and modified GOLD stages 1, 2, 3, and 4 respectively, 4 df ANOVA P < 0.001). Panel B shows hospital, ED, outpatient, and total nonrespiratory visits. The frequency of nonrespiratory health care visits was the same in all groups regardless of modified GOLD stage (3.8 ± 0.24 (3.4, 4.3), 3.2 ± 0.39 (2.4, 4.3), 3.3 ± 0.24 (2.9, 3.8), 3.6 ± 0.46 (3.1, 4.1), and 3.8 ± 0.31 (3.3, 4.3) visits/person/year for clinical COPD and modified GOLD stages 1, 2, 3, and 4 respectively, 4 df ANOVA P = 0.51). Panel C shows the number of office, ED, and hospital respiratory visits increasing with each modified GOLD stage from 0.34 ± 0.032 visits/person/year in patients with a clinical diagnosis of COPD to 1.1 ± 0.13 visits/person/year for those individuals in modified GOLD stage 4 (4df ANOVA comparing all groups, P < 0.001). Panel D shows similar numbers of hospital, ED, office, and total nonrespiratory visits in each modified GOLD stage, 4df ANOVA comparing all groups P = 0.45).
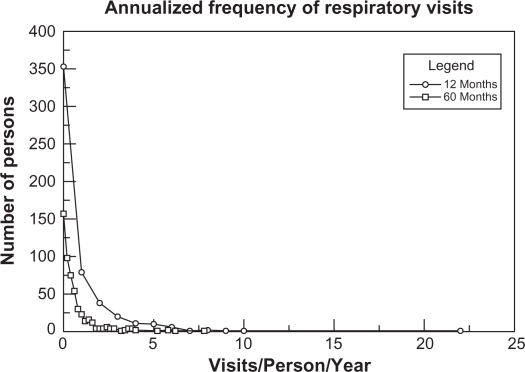
The annualized frequency of prior respiratory encounters is presented in Figure 4. In the 12 month period, the majority of the patients (81%) had 3 or fewer respiratory encounters; 80.7% of all respiratory encounters occurred in 17.5% of the patients. In contrast, approximately two-thirds of patients (70%) had a respiratory encounter during the 60 month period. Approximately half of the patients (53.4%) had 1–5 respiratory encounters accounting for 39.7% of respiratory visits whereas 16.6% of patients had more than 5 encounters and accounted for 60.3% of all respiratory visits.
Figure 4.
Frequency of yearly health care respiratory health care encounters per person during the 12 and 60 month periods.
Prior health care encounters and respiratory medication prescriptions
In the clinical COPD group (former GOLD 0), patients receiving >G had twice as many prior respiratory visits as patients receiving =G, 0.58 ± 0.19 versus 0.27 ± 0.057 visits/person/year, P = 0.02. (Table 3) This increased total health care utilization was caused by more ED visits and hospitalizations. In the composite group modified GOLD 1 and 2, the prescription level increased as the number of prior respiratory event related encounters rose (2 df ANOVA P < 0.0001, Table 3). There were no significant differences in total nonrespiratory visits (2 df ANOVA P = 0.096, Table 3). In the composite group modified GOLD 3 and 4, those individuals receiving = G had three times as many total respiratory visits as those who received <G, P <0.0001 (Table 3). This increase was due to elevated numbers of office visits as well as ED encounters and hospitalizations. There were no differences in hospital, office, or total nonrespiratory visits, P = 0.36. Similar relationships between prescribed treatment and respiratory and nonrespiratory encounters were present during the 60 month period (Table 3).
Table 3.
Respiratory and nonrespiratory health care encounters over the 12 and 60 month periods based upon modified GOLD stage and prescription level
| Health care encounters | Clinical COPD | Modified GOLD 1 + 2 | Modified GOLD 3 + 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prescriptions per guidelines | More prescriptions than guidelines | P value* | Less prescriptions than guidelines | Prescriptions per guidelines | More prescriptions than guidelines | P value** | Less prescriptions than guidelines | Prescriptions per guidelines | P value* | ||
| (=G) | (>G) | (<G) | (=G) | (>G) | (<G) | (=G) | |||||
| Respiratory Visits in 12 months visits/person/year | Hospital | 0.032 ± 0.018 (0.016, 0.061) | 0.14 ± 0.071 (0.084, 0.23) | 0.0002 | 0.018 ± 0.013 (0.0074, 0.045) | 0.19 ± 0.19 (0.089, 0.39) | 0.27 ± 0.011 (0.18, 0.41) | <0.001 | 0.19 ± 0.04 (0.13, 0.26) | 0.56 ± 0.16 (0.4, 0.79) | <0.0001 |
| ED | 0.053 ± 0.023 (0.029, 0.097) | 0.22 ± 0.11 (0.11, 0.36) | 0.0004 | 0.15 ± 0.047 (0.093, 0.23) | 0.31 ± 0.25 (0.14, 0.71) | 0.43 ± 0.12 (0.27, 0.68) | 0.0047 | 0.3 ± 0.058 (0.22, 0.41) | 1 ± 0.2 (0.76, 1.4) | <0.0001 | |
| Outpatient | 0.19 ± 0.05 (0.13, 0.28) | 0.22 ± 0.09 (0.12, 0.41) | 0.67 | 0.15 ± 0.041 (0.094, 0.23) | 0.25 ± 0.11 (0.1, 0.61) | 0.38 ± 0.17 (0.24, 0.61) | 0.02 | 0.3 ± 0.049 (0.22, 0.4) | 0.83 ± 0.19 (0.61, 1.1) | <0.0001 | |
| All | 0.27 ± 0.057 (0.18, 0.41) | 0.58 ± 0.19 (0.37, 0.91) | 0.016 | 0.31 ± 0.073 (0.21, 0.47) | 0.75 ± 0.5 (0.37, 1.5) | 1.1 ± 0.27 (0.74, 1.6) | <0.0001 | 0.78 ± 0.11 (0.6, 1) | 2.4 ± 0.47 (1.9, 3.1) | <0.0001 | |
| Respiratory Visits in 60 months visits/person/year | Hospital | 0.025 ± 0.0091 (0.016, 0.041) | 0.056 ± 0.02 (0.033, 0.094) | 0.001 | 0.022 ± 0.0075 (0.013, 0.037) | 0.063 ± 0.04 (0.028, 0.14) | 0.13 ± 0.037 (0.09, 0.19) | <0.0001 | 0.14 ± 0.025 (0.11, 0.19) | 0.25 ± 0.061 (0.18, 0.36) | 0.016 |
| ED | 0.061 ± 0.014 (0.04, 0.093) | 0.17 ± 0.055 (0.11, 0.25) | 0.034 | 0.088 ± 0.018 (0.059, 0.13) | 0.16 ± 0.069 (0.076, 0.35) | 0.35 ± 0.093 (0.25, 0.49) | <0.0001 | 0.27 ± 0.04 (0.21, 0.34) | 0.55 ± 0.11 (0.41, 0.74) | 0.0004 | |
| Outpatient | 0.19 ± 0.014 (0.093, 0.25) | 0.27 ± 0.042 (0.19, 0.37) | 0.13 | 0.17 ± 0.027 (0.13, 0.23) | 0.34 ± 0.12 (0.19, 0.59) | 0.42 ± 0.081 (0.3, 0.58) | 0.0003 | 0.36 ± 0.032 (0.3, 0.43) | 0.49 ± 0.081 (0.38, 0.65) | 0.065 | |
| All | 0.28 ± 0.03 (0.22, 0.35) | 0.49 ± 0.084 (0.37, 0.65) | 0.0032 | 0.28 ± 0.042 (0.21, 0.37) | 0.56 ± 0.19 (0.33, 0.95) | 0.9 ± 0.16 (0.69, 1.2) | <0.0001 | 0.77 ± 0.077 (0.64, 0.93) | 1.3 ± 0.21 (1, 1.7) | 0.0016 | |
| Non-respiratory Visits in 12 months visits/person/year | Hospital | 0.14 ± 0.046 (0.085, 0.22) | 0.33 ± 0.12 (0.2, 0.55) | 0.013 | 0.092 ± 0.042 (0.054, 0.16) | 0.063 ± 0.063 (0.011, 0.34) | 0.38 ± 0.16 (0.24, 0.6) | 0.0002 | 0.19 ± 0.046 (0.14, 0.26) | 0.17 ± 0.069 (0.096, 0.3) | 0.76 |
| ED | 0.42 ± 0.1 (0.29, 0.61) | 0.67 ± 0.2 (0.41, 1.1) | 0.15 | 0.29 ± 0.089 (0.16, 0.44) | 0.13 ± 0.13 (0.024, 0.65) | 0.59 ± 0.24 (0.36, 0.98) | 0.036 | 0.42 ± 0.094 (0.31, 0.56) | 0.73 ± 0.22 (0.49, 1.1) | 0.021 | |
| Outpatient | 3.0 ± 0.19 (2.6, 3.4) | 3.6 ± 0.4 (3, 4.4) | 0.078 | 2.7 ± 0.17 (2.3, 3) | 2.9 ± 0.41 (2.1, 4.1) | 3.1 ± 0.34 (2.6, 3.9) | 0.42 | 3 ± 0.18 (2.7, 3.3) | 3.1 ± 0.28 (2.6, 3.7) | 0.73 | |
| All | 3.5 ± 0.26 (3.1, 4) | 4.6 ± 0.56 (3.8, 5.7) | 0.028 | 3.1 ± 0.24 (2.6, 3.5) | 3.1 ± 0.46 (2.1, 4.6) | 4.1 ± 0.55 (3.3, 5.1) | 0.096 | 3.6 ± 0.25 (3.2, 4.1) | 4 ± 0.44 (3.3, 4.9) | 0.36 | |
| Non-respiratory Visits in 60 months visits/person/year | Hospital | 0.099 ± 0.023 (0.069, 0.14) | 0.18 ± 0.047 (0.12, 0.28) | 0.038 | 0.097 ± 0.025 (0.068, 0.14) | 0.038 ± 0.027 (0.0083, 0.17) | 0.15 ± 0.047 (0.092, 0.25) | 0.97 | 0.12 ± 0.024 (0.093, 0.16) | 0.13 ± 0.03 (0.08, 0.2) | 0.91 |
| ED | 0.37 ± 0.059 (0.28, 0.49) | 0.57 ± 0.12 (0.4, 0.83) | 0.077 | 0.3 ± 0.053 (0.23, 0.4) | 0.23 ± 0.068 (0.097, 0.52) | 0.46 ± 0.11 (0.31, 0.68) | 0.14 | 0.33 ± 0.024 (0.26, 0.43) | 0.46 ± 0.12 (0.32, 0.66) | 0.15 | |
| Outpatient | 2.5 ± 0.15 (2.3, 2.8) | 3.2 ± 0.25 (2.7, 3.7) | 0.034 | 2.4 ± 0.14 (2.1, 2.7) | 2.1 ± 0.37 (1.6, 2.9) | 2.5 ± 0.21 (2.1, 3) | 0.67 | 2.5 ± 0.12 (2.2, 2.7) | 2.6 ± 0.24 (2.2, 3.1) | 0.62 | |
| All | 3 ± 0.2 (2.7, 3.4) | 3.9 ± 0.32 (3.3, 4.6) | 0.014 | 2.8 ± 0.018 (2.5, 3.2) | 2.4 ± 0.44 (1.7, 3.4) | 3.1 ± 0.3 (2.6, 3.8) | 0.38 | 2.9 ± 0.16 (2.6, 3.3) | 3.2 ± 0.33 (2.7, 3.8) | 0.42 |
Discussion
Respiratory medications prescribed for patients in this unselected population with a broad range of air flow limitation and COPD severity complied poorly with the GOLD guidelines for the pharmacologic management of COPD. Over half the patients were prescribed less than the recommended respiratory medications. A prior history of reduced numbers of respiratory health care encounters was associated with less than guideline recommended prescription of respiratory medications for all levels of COPD severity. The number of respiratory related health care visits per person per year increased significantly with progressively more severe modified GOLD stage whereas the annual number of nonrespiratory health care visits was similar for individuals in all stages suggesting that these differences were not related to access to care or global health care utilization patterns.
Despite extensive publicity and promotion, clinical practice guidelines for the management of COPD have been poorly adopted by primary care practitioners and pulmonologists.6–13 The COPD Resource Network Needs Assessment Survey found that although 54% of generalists and 94% of pulmonologists were aware of published COPD guidelines, they often under-prescribed recommended pharmacologic treatments.8 We found that 54% of patients were prescribed less medications than recommended. Although unawareness of COPD guidelines may be one factor contributing to the lack of adherence, independent prescribing habits and absence of apparent effect on patient outcomes may also influence physician utilization of practice guidelines.15 A history of fewer respiratory encounters correlated with less than recommended prescription of respiratory medications suggesting that providers and possibly patients perceived less need for these treatments. Conversely, our findings indicate that physicians prescribe more than recommended medications for patients who have more frequent respiratory health care encounters. Thus, prior health care visits for respiratory symptoms may influence caregivers to prescribe more respiratory treatments in an effort to alleviate symptoms and improve respiratory health regardless of a patient’s lung function measured by spirometry. Similarly, the Resource Use Study in COPD demonstrated that patients experiencing COPD exacerbations were prescribed significantly more medications than those who did not have respiratory symptoms.16
In our unselected population, most health care encounters for COPD were confined to a small fraction of patients with COPD and this population may be disproportionately represented in some studies of respiratory medications.17 The results of these studies provide, in part, the evidence for spirometry-based COPD management guidelines and may, potentially, bias recommendations toward more treatment. Increased health care utilization may be a distinguishing clinical characteristic that differentiates a phenotypically distinct group of individuals with COPD.18 Our results suggest that studies including only individuals with prior respiratory health care encounters may not be applicable to a general, unselected COPD population and may provide a partial explanation for the apparent less than recommended prescription of respiratory medications.19
There is a paucity of available data to demonstrate that COPD treatment guidelines reduce health care utilization by individuals with COPD or affect mortality.20 An observational study comparing patients managed in general practices according to the British Thoracic Society guidelines or usual care found no differences in general practitioner or nurse visits, outpatient referrals, or hospitalizations.21 Similarly, we did not see fewer prior health care encounters in patients prescribed respiratory medications per guideline recommendations. In fact, for the entire cohort over 12 months, more total respiratory visits occurred in those receiving = G than for those who received G (0.6 ± 0.071(0.48, 0.75), 1.06 ± 0.19(0.85, 1.3), and 0.84 ± 0.17(0.58, 1.2) visits/person/year, for <G, =G, and >G, respectively (2 df ANOVA P = 0.002). However, this study’s methodology did not allow us to determine whether this effect was caused by prescription of fewer respiratory medications for less symptomatic patients and prescription of increased treatments for more symptomatic patients regardless of their pulmonary function or whether step-wise pharmacologic management strategy based upon spirometry does not reduce respiratory health care encounters.
A systematic review to develop clinical practice guidelines for the diagnosis and management of stable COPD revealed insufficient evidence to support using spirometry to guide therapy.22,23 Furthermore, spirometry is not an effective guide for the management of patients with COPD and frequent exacerbations.24 The BODE index, a score based upon FEV1, 6 minute walk test, level of dyspnea, and body mass index, predicts mortality, hospitalization, and COPD exacerbation frequency and severity better than FEV1 alone.25,26 Analysis of patients enrolled in the National Emphysema Treatment Trial suggests that multifactorial models incorporating physiologic parameters, breathlessness, prior exacerbations, and comorbidities may prognosticate emergency department visits or hospitalizations.27 It is not yet known whether these factors or a multivariable index that includes prior respiratory health care visits can be used to guide the pharmacologic management of individuals with COPD.
The frequency of respiratory health care visits by individuals with COPD increased as the FEV1 declined and the modified GOLD stage increased whereas the number of nonrespiratory visits was the same regardless of GOLD stage. Although other investigators have demonstrated that the number of COPD exacerbations increases as the FEV1 decreases, the relationship between spirometry and exacerbation frequency is not consistent.24,28–34 Others have shown that multivariable indices such as the BODE index alone or combined with other parameters predict COPD exacerbations.27,35 However, these studies are difficult to compare because the clinical characteristics of the studied populations varied greatly as did the definitions of COPD exacerbation. In our population of unselected patients with a broad range of disease severity, the frequency of respiratory health care encounters increased as FEV1 declined.
In this study, we utilized only the Veterans Affairs medical record system and may have underestimated total health care utilization by omitting any encounters that occurred outside of the Veterans Health care Administration (VHA). Approximately 30%–40% of health care encounters occurred outside the VHA in a multicenter trial evaluating COPD medications based in Veteran Affairs medical centers.17 We required that all subjects have at least one encounter in the prior year to insure that they were actively receiving care at the Cincinnati VAMC and our results were similar for both the 12 and 60 month time periods suggesting stable respiratory health care utilization within our cohort. Furthermore the data within the electronic medical records available for our review was the same information that was available to the prescribing caregiver so it is representative of the clinical data upon which respiratory medications were prescribed.
Although our results are comparable to findings from other VAMC14,17 the findings of this study are restricted to a predominantly male population from a single medical center and will require validation in a larger, more diverse population of individuals with COPD. We used the pre-bronchodilator FEV1 rather than the post-bronchodilator FEV1 to classify patients according to the modified GOLD guidelines because the majority of patients had not undergone bronchodilator testing. We did not assess compliance with the prescribed medication regimen so it is possible that differences in patients’ adherence with their medications within the various GOLD defined groups might have contributed to the different rates of respiratory health care encounters and, possibly, to the prescription of more respiratory medications. Future prospective trials will be necessary to assess these factors.
This study demonstrates that respiratory medications prescribed for an unselected population of individuals with COPD of diverse severity complied poorly with the GOLD treatment guidelines. The majority of patients were prescribed fewer medications than recommended and these patients had fewer prior respiratory health care visits. Prescription of more than the recommended respiratory medications correlated with a history of increased frequency of respiratory health care encounters suggesting that more respiratory medications are prescribed for patients with increased health care utilization regardless of their lung function measured by spirometry. In addition, the majority of respiratory health care encounters occurred in a minority of patients and these phenotypically distinct patients may be over-represented in some studies of respiratory medications that provide, in part, the evidence for spirometry-based COPD treatment guidelines, potentially biasing recommendations toward increased prescription of respiratory medications. Prior respiratory-related health care encounters may provide a measure of a patient’s respiratory symptoms and may be an important factor influencing caregiver prescription of respiratory medications. In an unselected population with COPD, both a history of fewer respiratory health care encounters in an individual patient and guidelines based upon studies of patients with increased respiratory health care utilization may provide potential explanations for the prescription of less than recommended respiratory medications compared with the spirometry-based GOLD guidelines.
Acknowledgments
The authors thank Frank McCormack, MD for his comments and review of the manuscript.
References
- 1.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Global Initiative for Chronic Obstructive Lung Disease. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 3.Fabbri L, Caramori G, Beghe B, Papi A, Ciaccia A. Chronic obstructive pulmonary disease international guidelines. Curr Opin Pulm Med. 1998;4:76–84. doi: 10.1097/00063198-199803000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Pierson DJ. Clinical practice guidelines for chronic obstructive pulmonary disease: a review and comparison of current resources. Respiratory Care. 2006;51:277–288. [PubMed] [Google Scholar]
- 5.The Global Initiative for Chronic Obstructive Lung Disease (GOLD) www.goldcopd.com, accessed July 24, 2009.
- 6.Ramsey SD. Suboptimal medical therapy in COPD Exploring the causes and consequences. Chest. 2000;117:33S–37S. doi: 10.1378/chest.117.2_suppl.33s. [DOI] [PubMed] [Google Scholar]
- 7.Roche N, Lepage T, Bourcereau J, Terrioux P. Guidelines versus clinical practice in the treatment of chronic obstructive pulmonary. Eur Respir J. 2001;18:903–908. doi: 10.1183/09031936.01.00213701. [DOI] [PubMed] [Google Scholar]
- 8.Barr RG, Celli BR, Martinez FJ, et al. Physician and patients perceptions in COPD: the COPD resource network needs assessment survey. Am J Medicine. 2005;118:1415.e9–1415.e17. doi: 10.1016/j.amjmed.2005.07.059. [DOI] [PubMed] [Google Scholar]
- 9.Fritsch K, Jacot M-L, Klarer A, et al. Adherence to the Swiss guidelines for management of COPD: experience of a Swiss teaching hospital. Swiss Med Wkly. 2005;135:116–121. doi: 10.4414/smw.2005.10844. [DOI] [PubMed] [Google Scholar]
- 10.Glaab T, Banik N, Rutschmann OT, Wencker M. National survey of guideline-compliant COPD management among pneumologists and primary care physicians. COPD. 2006;3:141–148. doi: 10.1080/15412550600829299. [DOI] [PubMed] [Google Scholar]
- 11.Bourbeau J, Sebaldt RJ, Bouchard J, et al. Practice patterns in the management of chronic obstructive pulmonary disease in primary practice: the CAGE study. Can Respir J. 2008;15:13–19. doi: 10.1155/2008/173904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsagaraki V, Markantonis SL, Amfilochiou A. Panrmacotherapeutic management of COPD patients in Greece – adherence to international guidelines. J Clin Pharm Ther. 2006;31:369–374. doi: 10.1111/j.1365-2710.2006.00752.x. [DOI] [PubMed] [Google Scholar]
- 13.Mularski RA, Asch SM, Shrank WH, et al. The quality of obstructive lung disease care for adults in the United States as measured by adherence to recommended processes. Chest. 2006;130:1844–1850. doi: 10.1378/chest.130.6.1844. [DOI] [PubMed] [Google Scholar]
- 14.Joo MR, Lee TA, Bartle B, van de Graaff WB, Weiss KB. Patterns of health care utilization by COPD severity: a pilot study. Lung. 2008;186:307–312. doi: 10.1007/s00408-008-9095-5. [DOI] [PubMed] [Google Scholar]
- 15.Smith BJ, Dalziel K, McElroy HJ, et al. Barriers to success for an evidence-based guideline for chronic obstructive pulmonary disease. Chron Respir Dis. 2005;2:121–131. doi: 10.1191/1479972305cd075oa. [DOI] [PubMed] [Google Scholar]
- 16.FitzGerald JM, Haddon JM, Bradly-Kennedy C, et al. Resource use study in COPD (RUSIC): A prospective study to quantify the effects of COPD exacerbations on health care resource use among COPD patients. Can Respir J. 2007;14:145–152. doi: 10.1155/2007/921914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niewoehner DE, Rice K, Cote C, et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotroprium, a once-daily inhaled anticholinergic bronchodilator. Ann Intern Med. 2005;143:317–326. doi: 10.7326/0003-4819-143-5-200509060-00007. [DOI] [PubMed] [Google Scholar]
- 18.Rabe KF. Guidelines for chronic obstructive pulmonary disease treatment and issues of implementation. Proc Am Thorac Soc. 2006;3:641–644. doi: 10.1513/pats.200604-099SS. [DOI] [PubMed] [Google Scholar]
- 19.Herland K, Akselsen J-P, Skjonsberg OH, Bjermer L. How representative are clinical study patients with asthma or COPD for a larger “real life” population of patients with obstructive lung disease? Respiratory Medicine. 2005;99:11–19. doi: 10.1016/j.rmed.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 20.Heffner JE, Ellis R. The guideline approach to chronic obstructive pulmonary disease: How effective? Respiratory Care. 2003;48:1257–1266. [PubMed] [Google Scholar]
- 21.Guest JF, Varney SJ, Diggle J. Impact of the British Thoracic Society chronic pulmonary disease guidelines on patients’ status, health care resource use and health-related quality of life. Prim Care Respir J. 2005;14:242–251. doi: 10.1016/j.pcrj.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilt TJ, Niewoehner D, MacDonald R, Kane RL. Management of stable chronic obstructive pulmonary disease: a systematic review for a clinical practice guideline. Ann Intern Med. 2007;147:639–653. doi: 10.7326/0003-4819-147-9-200711060-00009. [DOI] [PubMed] [Google Scholar]
- 23.Qaseem A, Snow V, Shekelle P, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007;147:633–638. [PubMed] [Google Scholar]
- 24.O’Reilly JF, Williams AE, Holt K, Rice L. Defining COPD exacerbations: impact on estimation of incidence and burden in primary care. Primary Care Respiratory J. 2006;15:346–353. doi: 10.1016/j.pcrj.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 26.Marin JM, Carrizo Sj, Casanova C, et al. Prediction of risk of COPD exacerbations by the BODE index. Respir Med. 2009;103:373–378. doi: 10.1016/j.rmed.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Fan VS, Ramsey SD, Make BJ, Martinez FJ. Physiologic variables and functional status independently predict COPD hospitalizations and emergency department visits in patients with severe COPD. COPD. 2007;4:29–39. doi: 10.1080/15412550601169430. [DOI] [PubMed] [Google Scholar]
- 28.Miravitlles M, Calle M, Alvarez-Gutierrez F, Gobartt E, López F, Martín A. Exacerbations, hospital admissions and impaired health status in chronic obstructive pulmonary disease. Quality of Life Research. 2006;15:471–480. doi: 10.1007/s11136-005-3215-y. [DOI] [PubMed] [Google Scholar]
- 29.Vestbo J, Rasmussen FV. Respiratory symptoms and FEV1 as predictors of hospitalization and medication in the following 12 years due to respiratory disease. Eur Respir J. 1989;2:710–715. [PubMed] [Google Scholar]
- 30.Schermer TR, Sarix CG, van den Bosch WJ, et al. Exacerbations and associated health care cost in patients with COPD in general practice. Monaldi Arch Chest Dis. 2006;65:133–140. doi: 10.4081/monaldi.2006.558. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Aymerich J, Monso E, Marrades RM, et al. Risk factors for hospitalization for a chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2001;164:1002–1007. doi: 10.1164/ajrccm.164.6.2006012. [DOI] [PubMed] [Google Scholar]
- 32.Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:158–164. doi: 10.1164/ajrccm.159.1.9803117. [DOI] [PubMed] [Google Scholar]
- 33.Decramer M, Gosselink R, Troosters T, Verschueren M, Evers G. Muscle weakness is related to utilization of health care resources in COPD patients. Eur Respir J. 1997;10:417–423. doi: 10.1183/09031936.97.10020417. [DOI] [PubMed] [Google Scholar]
- 34.Lusuardi M, Lucioni C, De Benedetto F, et al. GOLD severity stratification and risk of hospitalization for COPD exacerbations. Monaldi Arch Chest Dis. 2008;69:164–169. doi: 10.4081/monaldi.2008.378. [DOI] [PubMed] [Google Scholar]
- 35.Ong KC, Earnest A, Lu SJ. A multidimensional grading system (BODE index) as predictor of hospitalization for COPD. Chest. 2005;128:3810–3806. doi: 10.1378/chest.128.6.3810. [DOI] [PubMed] [Google Scholar]