Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington’s disease transgenic mice (original) (raw)
. Author manuscript; available in PMC: 2010 May 10.
Abstract
While there have been enormous strides in the understanding of Huntington’s disease (HD) pathogenesis, treatment to slow or prevent disease progression remains elusive. We previously reported that dietary creatine supplementation significantly improves the clinical and neuropathological phenotype in transgenic HD mice lines starting at weaning, before clinical symptoms appear. We now report that creatine administration started after onset of clinical symptoms significantly extends survival in the R6/2 transgenic mouse model of HD. Creatine treatment started at 6, 8, and 10 weeks of age, analogous to early, middle, and late stages of human HD, significantly extended survival at both the 6- and 8-week starting points. Significantly improved motor performance was present in both the 6- and 8-week treatment paradigms, while reduced body weight loss was only observed in creatine-supplemented R6/2 mice started at 6 weeks. Neuropathological sequelae of gross brain and neuronal atrophy and huntingtin aggregates were delayed in creatine-treated R6/2 mice started at 6 weeks. We show significantly reduced brain levels of both creatine and ATP in R6/2 mice, consistent with a bioenergetic defect. Oral creatine supplementation significantly increased brain concentrations of creatine and ATP to wild-type control levels, exerting a neuroprotective effect. These findings have important therapeutic implications, suggesting that creatine therapy initiated after diagnosis may provide significant clinical benefits to HD patients.
Keywords: chemotherapy, creatine, Huntington’s disease
Huntington’s disease (HD) is a fatal, autosomal dominant, neurodegenerative disease for which there is no current effective treatment to ameliorate or prevent the insidious progression of the disorder. The disease phenotype in HD is caused by an expansion of a polyglutamine tract in the protein, huntingtin (htt; The Huntington’s Disease Collaborative Research Group 1993), leading to conformational change, abnormal protein–protein interactions, and eventual neuronal death. Mutant htt undergoes proteolytic processing, in part by the proapoptotic enzyme caspase-3, releasing an N-terminal fragment containing the polyglutamine tract. This fragment forms macromolecular aggregates with itself and other proteins which become ubiquitinated and large enough to be visible in the dendritic and axonal arbors, cytoplasm, and nuclei of neurons (Mangiarini et al. 1996; Davies et al. 1997; DiFiglia et al. 1997; Kuemmerle et al. 1999). It has been suggested that htt aggregation may be the trigger for a toxic gain of function, leading to neurodegeneration.
While studies in HD patients and transgenic models of HD have provided hypotheses about the pathogenic mechanisms in HD, it remains unclear how the genetic mutation results in selective cell death. It has been postulated that one effect conferred by the gene defect may be impaired energy metabolism (Beal 2000). There is substantial evidence that bioenergetic dysfunction plays a role in cell death in neurological diseases, particularly in HD (Brouillet et al. 1995; Gu et al. 1996; Koroshetz et al. 1997; Sawa et al. 1999). N-terminal huntingtin fragments, a pathological hallmark of HD, may directly impair mitochondrial function (Panov et al. 2002). In addition, the mitochodrial toxins malonate and 3-nitropropionic acid in experimental animals reproduce the neuropathological phenotype observed in HD patients (Beal et al. 1993; Brouillet et al. 1995). Lesions produced by these compounds involve energy depletion, followed by activation of excitatory amino acid receptors and free radical production (Schulz et al. 1995a, 1995b). The accidental ingestion of 3-nitropropionic acid in man results in selective basal ganglia lesions and delayed dystonia (Ludolph et al. 1991).
If mitochondrial impairment plays a role in neuronal dysfunction in HD, then chemotherapy that buffers intracellular energy levels may ameliorate the neurodegenerative process. Creatine kinase and its substrates creatine and phosphocreatine constitute an energy-buffering and transport system, connecting sites of energy production with sites of energy consumption (Hemmer and Wallimann 1993). Creatine administration increases brain concentrations of phosphocreatine (PCr) and inhibits activation of the mitochondrial permeability transition (MPT), both of which may exert neuroprotective effects (Hemmer and Wallimann 1993; O’Gorman et al. 1996). Previous studies have demonstrated the neuroprotective effects of creatine in in vitro and in vivo models of neurological diseases. Creatine reduces anoxic damage to hippocampal slices in vitro (Carter et al. 1995; Balestrino et al. 2002). It also attenuates MPTP-induced dopamine depletions and substantia nigra neuronal loss (Matthews et al. 1999). Creatine administration increases survival in a transgenic mouse model of amyotrophic lateral sclerosis and ameliorates the ventral horn neurodegeneration (Klivenyi et al. 1999). In addition, creatine exerts neuroprotective effects in mitochodrial-neurotoxin animal models of HD produced by malonate and 3-nitropropionic acid administration (Matthews et al. 1998). We previously showed that pre-symptomatic dietary creatine supplementation started at weaning (21 days) extends survival in the R6/2 and N171–82Q transgenic HD mice while significantly improving the clinical and neuropathological phenotype (Ferrante et al. 2000; Andreassen et al. 2001a). In addition, we have shown that clinical behavioral changes are impaired before 6 weeks in R6/2 mice (Ferrante et al. 2000, 2002; Dedeoglu et al. 2002).
Most of the pre-clinical therapeutic drug trials in transgenic HD mice have been performed prior to the appearance of overt behavioral symptoms. While prophylactic treatment may be considered in patients at risk for HD, usually a diagnosis is followed by treatment. In the present studies, we modeled therapeutic trials, using dietary creatine supplementation in symptomatic HD mice started at time points of disease severity that are analogous to early, middle, and late stages of human HD. We examined whether creatine administration exerts beneficial effects on survival, as well as the behavioral and neuropathological phenotype, in R6/2 mice after symptoms appear.
Materials and methods
Transgenic HD mice of the R6/2 strain and littermate controls were obtained from Jackson Laboratories (Bar Harbor, ME, USA). Male R6/2 mice were bred with females from their background strain (B6 CBAFI/J). The offspring were genotyped by PCR assay of DNA obtained from tail tissue. CAG repeat length was examined to ensure that a drift in number of CAG repeats did not play a role in the outcome of the studies. Transgenic mice were housed in microisolator cages in a modified barrier facility. A 12-h light–dark cycle was maintained and animals were given free access to food and water. Groups (n = 20) of transgene negative and positive R6/2 mice from the same ‘f’ generation were placed on either unsupplemented diets or diets supplemented with 2% creatine (4 g/kg, Sigma Chemical, St Louis, MO, USA, formulated into chow by Purina). This resulted in five groups of mice that were followed in the course of this study: a creatine-untreated wild-type littermate control group, a creatine-untreated R6/2 group and a creatine-treated R6/2 group which was started on normal chow at weaning and fed creatine chow starting at 6 weeks (42 days after birth) and followed until death, a creatine-treated R6/2 group which was started on normal chow at weaning and fed creatine chow starting at eight weeks (56 days after birth) and followed until death, a creatine-treated R6/2 group which was started on normal chow at weaning and fed creatine chow starting at 10 weeks (70 days after birth) and followed until death. In order to ensure homogeneity of the cohorts used in these experiments, we have standardized our criteria for placement of mice into testing groups. Mice were randomized, all within 2 days of the same age, from 36 litters of the same ‘f’ generation. Any mice that had altered base-paired banding, identified from PCR analysis, were excluded from the study. All mice were weighed prior to placement and equally distributed according to weight within each cohort. All mice were handled under the same conditions by one investigator. Equal numbers of mice from both genders were included in the experimental paradigm. We did not observe gender differences in survival in the R6/2 transgenic HD mouse model. One hundred mice were used in the survival studies. All animal experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and were approved by both the Veterans Administration and Boston University Animal Care Committees.
Behavioral testing (rotarod)
Motor performance was assessed weekly on the same day. The mice were given two training sessions in order to acclimate them on the rotarod apparatus (Columbus Instruments, Columbus, OH, USA). Testing commenced on days 23–25 for all five groups of mice regardless of when creatine treatment was started. Mice were placed on a rotating rod at 16 rpm. The length of time remaining on the rod was taken as the measure of competency. The maximum score was 60 s, and each mouse performed three separate trials. The three results were averaged and recorded.
Body weights
Mice were weighed twice a week at the same time of day.
Survival
Mice were observed twice daily, in the morning and late afternoon. The criteria for euthanization was the point in time in which the HD mice were unable to right themselves after being placed on their back and initiate movement after being gently prodded for 20 s. HD mice have lost approximately 40–50% of their body weight at this time point. Two independent observers confirmed the criteria for euthanization (RJF and AD). This point was noted as the time of death.
Histological evaluation
Groups of 10 animals were deeply anesthetized and then transcardially perfused with 4% buffered paraformaldehyde at 90 days from wild-type littermate control mice and each treatment paradigm: unsupplemented R6/2 mice, and creatine-treated R6/2 mice in which creatine formulated chow was started at 6 weeks, 8 weeks, and 10 weeks (50 mice were used in the histopathologic analysis). The brains were removed, post-fixed with the perfusant for 2 h, weighed, cryoprotected in a graded series of 10% and 20% glycerol/2% dimethyl sulphoxide (DMSO) solution, subsequently serially frozen sectioned at 50 µm, stored in 6-well tissue collection clusters, and stained for Nissl substance (cresyl violet). Serially cut tissue sections were immunostained using a huntingtin antibody (EM48, dilution, 1 : 1000; courtesy of Dr X-J. Li) as previously published (Dedeoglu et al. 2002).
Stereology/quantitation
Serial-cut coronal tissue-sections from the rostral segment of the neostriatum to the level of the anterior commissure (interaural 5.34 mm/bregma 1.54 mm to interaural 3.7 mm/bregma − 0.10 mm; Franklin and Paxinos 1997) were used for neuronal and htt aggregate analysis. Unbiased stereological counts of htt-positive aggregates (= 1.0 µm) were obtained from the neostriatum in 10 animals from unsupplemented and 2% creatine-supplemented R6/2 mice at 90 days from each treatment paradigm starting at 6, 8, and 10 weeks, using Neurolucida Stereo Investigator software (Microbrightfield, Colchester, VT, USA). The total area of the neostriatum was defined in serial sections in which counting frames were randomly sampled. The dissector counting method was employed in which htt-positive aggregates were counted in an unbiased selection of serial sections in a defined volume of the neostriatum. Striatal neuron areas were analyzed by microscopic videocapture using a Windows-based image analysis system for area measurement (Optimas, Bioscan Incorporated, Edmonds, WA, USA). The software automatically identifies and measures profiles. All computer identified cell profiles were manually verified as neurons and exported to Microsoft Excel. Cross-sectional areas were analyzed using Statview.
High-performance liquid chromatography analysis
Oral creatine dietary supplementation was administered to 6-week-old R6/2 mice for 2 weeks before the mice were killed. Frozen striatal tissue samples from 10 mice each of creatine-supplemented R6/2 mice, unsupplemented R6/2 mice, and unsupplemented littermate control mice were dissected on a freezing cold plate (− 20°C) and placed in 0.4 m perchloric acid (10 mL/mg wet weight), homogenized, and centrifuged. The supernatant was neutralized with 25 mL of 2 m K2CO3 added to 200 mL of the supernatant and recentrifuged. Supernatants were stored at − 80°C until injected. Standards were prepared in 0.4 m perchloric acid at concentrations of 10 mm for creatine and adenosine triphosphate (ATP; based on tissue concentrations). A Perkin-Elmer (Foster City, CA, USA) gradient high-performance liquid chromatography (HPLC) pump and a Waters multiple UV detector were used for analysis and completed by the BMC/HPLC core facility. We have previously described these methods (Matthews et al. 1998).
Statistics
The data are expressed as the mean ± SEM. Statistical comparisons of rotarod, weight data, and histology data were compared by anova or repeated measures anova. Survival data were analyzed using Kaplan–Meier survival curves.
Results
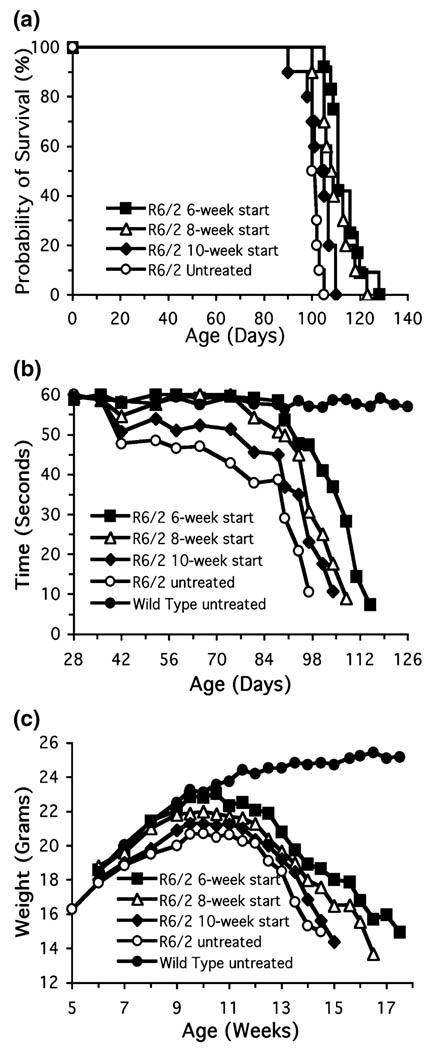
The effects of oral administration of creatine in the diet started at 6, 8, and 10 weeks of age on survival in R6/2 transgenic HD mice are shown in Fig. 1(a). Oral administration of 2%creatine significantly improved survival in the 6-week and 8-week paradigms, in comparison to unsupplemented R6/2 mice (unsupplemented R6/2 mice: 100.3 ± 1.6; 6-week creatine start: 114.7 ± 3.3, _F_3,38 = 14.95, p < 0.01; 8-week creatine start: 110.1 ± 3.9, _F_3,38 = 12.35, p < 0.01). While there was a trend towards extended survival, creatine supplementation started at 10 weeks of age did not reach significance (10 week creatine start: 104.1 ± 3.8, _F_3,38 = 2.15, p < 0.32). The percentage increases in survival for the 6- and 8-week creatine starting points were 14.4% and 9.7%, respectively.
Fig. 1.
The effects of oral administration of creatine in the diet started at 6, 8, and 10 weeks of age on survival (a), motor performance (b), and body weight (c) in R6/2 transgenic HD mice.■, R6/1 6-week start; Δ, R6/2 8-week start; ♦, R6/2 10-week start;○, R6/2 untreated;●, wild-type untreated.
The effects of oral administration of creatine in the diet on rotarod performance between 30 and 114 days are shown in Fig. 1(b). Oral administration of 2% creatine significantly improved rotarod performance throughout the entire measured (4–17 weeks) life span of the R6/2 mice in which dietary creatine supplementation was started at 6 and 8 weeks, in contrast to unsupplemented R6/2 mice (unsupplemented R6/2 mice: 42.8 ± 5.1 s; 6-week creatine start: 52.7 ± 2.8 s, _F_3,30 = 16.37; p < 0.01; 8-week creatine start: 50.9 ± 2.7 s; _F_3,30 = 12.18; p < 0.01, data represent combined means of all time points). Significant improvement in motor performance was not observed at the 10-week creatine starting point (10-week creatine start: 44.5 ± 4.7 s; _F_3,30 = 1.89; p < 0.67). The percentage increases in rotarod performance for the 6- and 8-week creatine starting points were 23.1% and 18.9%, respectively.
The effects of oral administration of creatine on body weight in HD transgenic mice are shown in Fig. 1(c). Creatine supplementation started at 6 weeks resulted in significant improvement of body weight in comparison to unsupplemented R6/2 mice (_F_4,100 = 15.82; p < 0.01). The total percentage increase in body weight from 5 to 15 weeks (data represent combined means of all time points) in comparison to unsupplemented R6/2 mice was 18.7%. While there were significant differences in body weight at individual early and late time points in the 8-week creatine start time, overall body weight loss from 5 to 15 weeks (data represents combined means of all time points) showed an 8.0% cumulative improvement in weight loss which was not significant in comparison to unsupplemented R6/2 mice. No significant body weight differences were observed between the 10-week creatine start time and unsupplemented R6/2 mice.
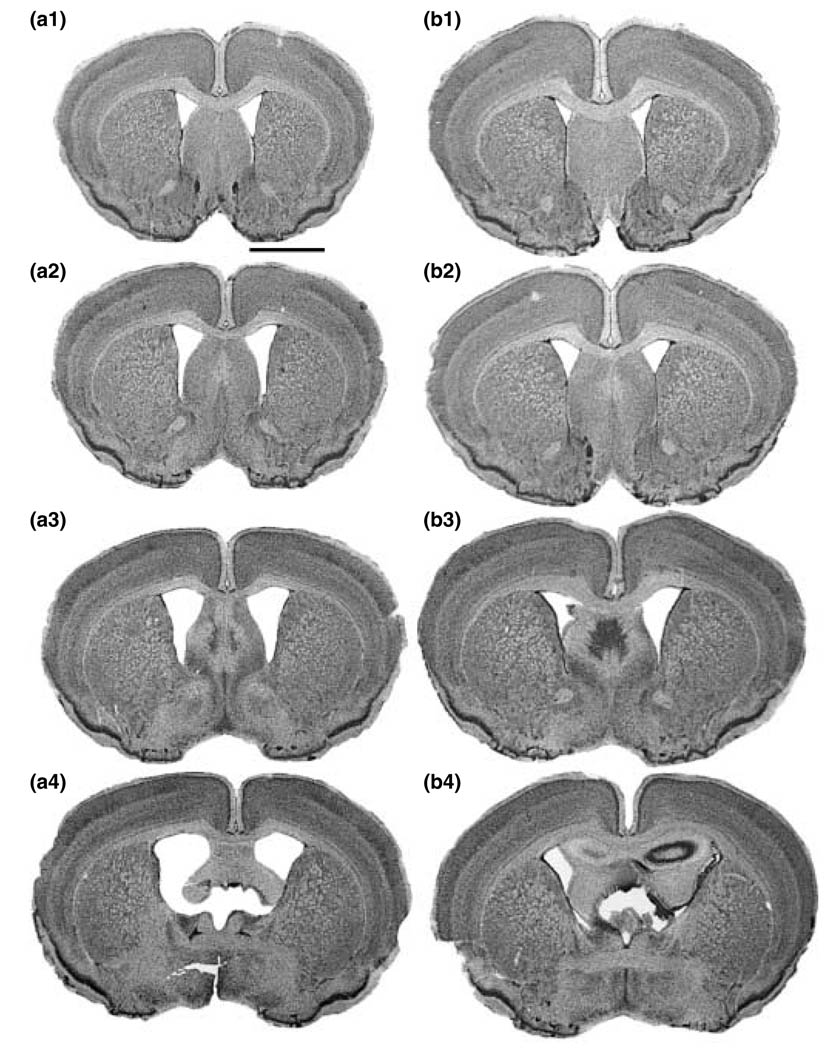
Gross brain weights of unsupplemented R6/2 mice decreased significantly over time until death. At 90 days of age, only brain weights from the 6-week creatine start time showed significant differences, in comparison to the unsupplemented R6/2 mice (unsupplemented R6/2 mice: 343 ± 15 mg; creatine-supplemented R6/2 mice: 398 ± 13 mg, _F_5,60 = 8.74; p < 0.01). There was a 17.4% greater brain weight in the creatine-treated R6/2 mice than the unsupplemented mice at 90 days. Coincident with less brain weight loss, gross brain atrophy was not as prominent in the creatine-treated mice, in comparison to the unsupplemented R6/2 brains. Dietary 2% creatine supplementation reduced gross brain atrophy in R6/2 mice in comparison to the untreated R6/2 mice, however, only within the 6-week creatine paradigm (Fig. 2). Striatal areas were significantly greater in the creatine-treated R6/2 mice, in comparison to untreated R6/2 mice (_F_5,60 = 11.54; p < 0.01).
Fig. 2.
Gross brain atrophy of coronal step serial-sections from the rostral to caudal axis of the neostriatum in untreated (a1–a4) and creatine-treated (b1–b4) R6/2 mice. Dietary 2% creatine supplementation started at 6 weeks reduced gross brain atrophy and ventricular enlargement in R6/2 mice (b), in comparison to the untreated R6/2 mice (a). Bar in (a1) equals 2 mm.
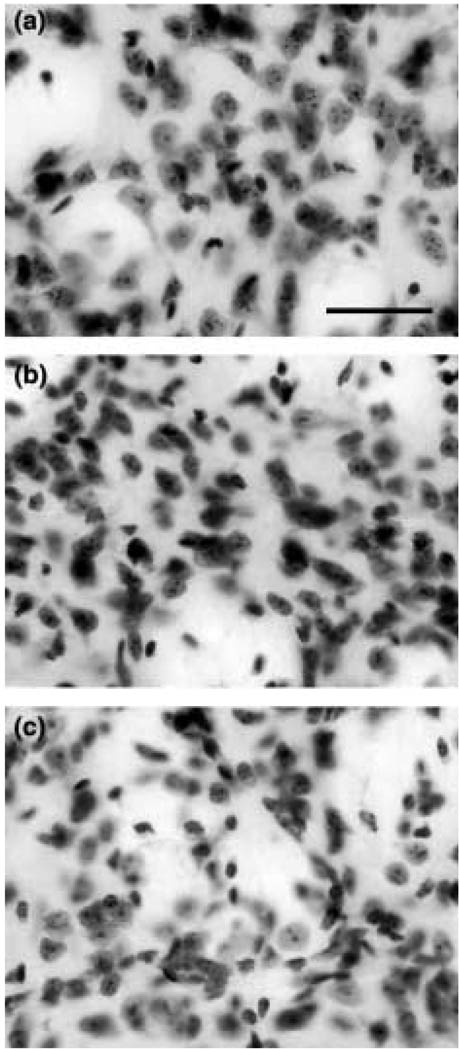
Consistent with the gross brain weight loss and atrophy, we have previously reported that there is significant progressive atrophy of striatal neurons from 21 to 90 days in the unsupplemented R6/2 mice (Ferrante et al. 2000). At 90 days of age, the cytoprotective effect of 2% creatine significantly delayed striatal neuron atrophy only within the 6-week start paradigm, as measured in cross-section (6-week creatine-treated R6/2 mice: 83.6 ± 10.7 µm2; unsupplemented R6/2 mice: 52.1 ± 18.1 µm2; _F_5,60 = 9.67; p < 0.01; Fig. 3).
Fig. 3.
Dorso-medial aspect of the neostriatum in littermate wild-type control mouse (a), creatine-treated R6/2 mouse started at 6 weeks (b), and untreated R6/2 mouse (c). At 90 days of age, marked striatal neuron atrophy was present in the untreated R6/2 mouse (c) that was significantly reduced in the creatine-treated R6/2 mouse (b), in comparison to wild-type littermate control mice (a). Bar in (a) equals 50 µm.
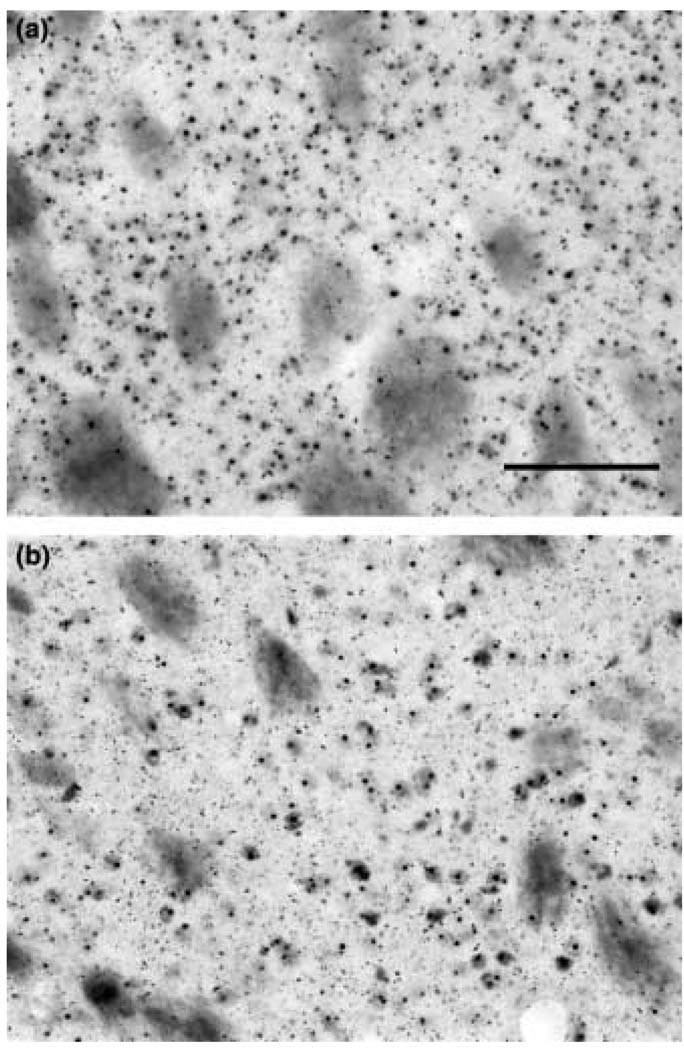
R6/2 mice show an early and progressive accumulation of htt-positive aggregates, as well as an increase in aggregate size, from 21 days of age through 90 days. Histopathological comparison at 90 days showed that dietary supplementation with 2% creatine, initiated after behavioral symptoms were present, resulted in a significant reduction in striatal htt aggregate number only within the 6-week creatine start time (6-week creatine start R6/2 mice: 3.32 · 106 ± 0.87; unsupplemented R6/2 mice: 5.45 · 106 ± 1.21, _F_4,42 = 15.23; p < 0.01; Fig. 4). The percentage decrease in aggregate number, as compared with unsupplemented mice, was 39.1%. A trend towards decreased numbers of htt aggregates in the 8-week creatine start time was present, however, significance was not reached (8-week creatine start R6/2 mice: 4.08 · 106 ± 1.01, _F_4,42 = 2.17; p < 0.38).
Fig. 4.
Huntingtin-positive aggregates in the neostriatum in an R6/2 mouse (a) and creatine supplemented R6/2 mouse (b) at 90 days of age. Histopathological comparison showed that dietary supplementation with 2% creatine initiated at 6 weeks resulted in a significant reduction in striatal aggregate number. Bar in (a) equals 100 µm.
HPLC analysis showed a significant decrease in both creatine and ATP levels in the R6/2 mice, in comparison to littermate control mice, suggesting mitochondrial dysfunction in the R6/2 HD model (_F_4,32 = 10.42, 12.57; p < 0.01; Table 1). There was a 38.9% reduction in creatine levels in the unsupplemented R6/2 mice, in comparison to littermate wild-type control mice. There was a significant increase in both creatine and ATP levels in striatal brain tissue samples from creatine-treated R6/2 mice, in comparison to unsupplemented R6/2 mice, consistent with a neuroprotective effect (_F_4,32 = 8.28, 9.14; p < 0.01). The creatine and ATP levels found in creatine supplemented R6/2 mice were not significantly different than those detected in normal littermate control mice (Table 1). Creatine supplementation improved creatine levels by 48.6%. ATP levels were reduced by 64.6% in the unsupplemented R6/2 mice and were increased by 60.2% after creatine supplementation.
Table 1.
Creatine and ATP levels measured at 8 weeks of age after 2 weeks of creatine supplementation started at 6 weeks of age
| Creatine | ATP levels | |
|---|---|---|
| R6/2 mice | 8.23 ± 0.79 | 0.74 ± 0.17 |
| Creatine-treated R6/2 mice | 12.54 ± 0.51 | 1.86 ± 0.24 |
| Wild-type control mice | 13.49 ± 0.48 | 2.09 ± 0.21 |
Discussion
We previously reported that compounds, which augment bioenergetics, significantly improve the behavioral and neuropathological phenotype in transgenic HD mice (Ferrante et al. 2000, 2002; Andreassen et al. 2001b, 2001c). While dichloroacetate and lipoic acid have modest effects, both creatine and coenzyme Q10 treatment showed greater neuroprotective benefits (Ferrante et al. 2000, 2002). In comparison to the other bioenergetic therapeutic agents, however, dietary creatine supplementation has the greatest efficacy in improving the clinical and neuropathological phenotype in R6/2 mice, extending survival by 17.5%. The present study confirms our previous results that creatine is neuroprotective in R6/2 transgenic HD mice (Ferrante et al. 2000) and extends these findings by demonstrating the efficacy of creatine after symptoms are present in the R6/2 mice. In symptomatic HD mice, oral creatine supplementation started at progressive time points of disease severity, analogous to early, middle, and late stages of human HD, significantly extended survival at both the 6- and 8-week starting points, with a trend towards increased survival at the 10-week starting point. Creatine treatment in symptomatic mice extended survival by 14.4%, started at the early clinical presentation of disease at 42 days. This percentage increase in survival was comparable to coenzyme Q10 treatment (14.5%) started prior to disease onset at 21 days (Ferrante et al. 2002). Creatine delayed the onset of clinical sequelae in early and moderate grades of the disease process in HD mice and slowed the progression of neuropathological changes and htt aggregates in the earliest treatment paradigm starting at 6 weeks. In addition, we show there are significantly reduced levels of creatine and ATP in the R6/2 HD transgenic mouse line and that dietary creatine supplementation increased brain levels of both creatine and ATP, consistent with a neuroprotective effect. These findings suggest that creatine therapy initiated after diagnosis may provide clinical benefits to HD patients and underscore the importance of the power of transgenic mouse models of HD for the screening of therapeutics.
A complementary strategy in understanding the mechanism of pathogenesis in HD has been to study the clinical, neuropathological, and molecular alterations of HD patients, while exploring pathophysiological phenomena in animal models. The development of transgenic mouse models of HD has provided a major advance for studying disease pathogenesis and for developing therapeutics. There is substantial evidence that energy dysfunction occurs in HD, and that this may play a role in cell death (Beal 2000). Consistent with this hypothesis, elevated lactate levels, reduced PCr in resting muscle, defects in mitochodrial enzymes, susceptibility of mitochondria to depolarization in lymphoblasts and fibroblasts, and ultrastructural mitochondrial abnormalities have all been reported in HD patients (Beal 2000).
A secondary effect of the gene defect and formation of N-terminal htt fragments may be impaired energy metabolism. Greenamyre and colleagues have identified mitochondrial calcium handling defects in mitochondria from lymphoblasts of HD patients and transgenic mice that may result from the direct effects of mutant htt, compromising mitochodrial function (Panov et al. 2002). R6/2 mice develop marked decreases in _N_-acetylaspartate concentrations prior to neuronal loss that may be a consequence of mitochondrial dysfunction (Jenkins et al. 2000). Creatine significantly attenuates these decreases in _N_-acetylaspartate concentrations in R6/2 mice (Ferrante et al. 2000). In the present findings, we suggest that both the increased creatine and ATP levels in creatine treatment in R6/2 mice play a significant role in stabilizing the proposed energy dysfunction in these mice. The improved bioenergetic therapeutic-effects in the transgenic mice found in this study may be predictive of beneficial effects of creatine in HD patients.
Creatine is a guanidino compound that is produced endogenously in the body with an exogenous supply arising from meat-containing products. Creatine administration has several potential neuroprotective effects including buffering of intracellular energy reserves, stabilizing intracellular calcium, reducing extracellular glutamate, inhibiting activation of the MPT, and acting as an antioxidant. Within the cell, creatine may be present as free creatine or PCr. PCr provides an energy buffer to rephosphorylate adenosine diphosphate to adenosine triphosphate at sites of energy consumption (Hemmer and Wallimann 1993). Creatine administration increases brain concentrations of PCr (Matthews et al. 1998). PCr concentrations, as assessed by magnetic resonance spectroscopy, are reduced in HD patients and correlate well with clinical severity. If the recovery of PCr is dysfunctional in HD patients or if energy stores are diminished, then creatine supplementation may augment PCr production and provide neuroprotection.
Creatine kinase also appears to be coupled directly or indirectly to energetic processes required for calcium homeostasis (Wallimann et al. 1992; Steeghs et al. 1997). Creatine pre-treatment delays increases in intracellular calcium produced by 3-nitropropionic acid in cortical and striatal astrocytes in vitro (Deshpande et al. 1997).
Another potential neuroprotective mechanism is the ability of PCr to stimulate synaptic glutamate uptake and thereby reduce extracellular glutamate (Xu et al. 1996). Extracellular glutamate can potentiate excitotoxic damage. The uptake of glutamate into synaptic vesicles by ATP has been reported. Glutamate uptake into synaptic vesicles is also stimulated by PCr and has been found to be independent of ATP concentration (Xu et al. 1996).
Creatine may also act as an antioxidant either directly or indirectly by augmenting energy buffering. It has some ability to act as a direct antioxidant against superoxide anions and peroxynitrite (Lawler et al. 2002). Creatine administration in experimental animal models of HD and ALS reduces markers of oxidative stress (Klivenyi et al. 1999; Ferrante et al. 2000). Multiple lines of evidence indicate that oxidative stress may play a role in the etiology of HD either as a cause of cell death or as a secondary component of the cell death cascade. We have reported that molecular epitopes indicative of oxidative damage are greatly increased in the striatum and cortex in HD patients and HD mice (Browne et al. 1999; Bogdanov et al. 2001).
We previously showed that dietary creatine dose-dependently improves survival in R6/2 mice. While all three doses of 1%, 2%, and 3% diet supplement provided neuroprotection, a ‘U’ shaped dose–response curve was observed, with 2% creatine being most effective. It is unclear, however, whether optimal creatine dosing has been accomplished. The creatine transporter plays a role in supplying creatine across the blood–brain barrier (Wallimann et al. 1992; Ohtsuki et al. 2002). Creatine may be continuously transported to the brain against a creatine blood/brain concentration gradient (Ohtsuki et al. 2002). Creatine transport may also be enhanced by insulin and inhibited by high exogenous creatine levels (Xu et al. 1996). The poor efficiency of high levels of creatine may be due to a downregulation of creatine transport. Downregulation of creatine transporters may occur with extended constant dosing at high doses and alter the efficacy of creatine supplementation (Benzi 2000). It is therefore possible that improved efficacy may be accomplished using an intermittent dosing regimen with larger doses of creatine than that used in this study, avoiding downregulation of creatine transport.
The present findings have significant therapeutic implications in treatment strategies for HD patients, suggesting that, while early creatine treatment provides the greatest efficacy in the R6/2 transgenic mice, therapy initiated after diagnosis may provide clinical benefits to HD patients. The finding that creatine started in late stages of disease in HD transgenic mice does not extend survival, may be due to the advanced neuronal dysfunction and loss that is present in the mice. In severe to very severe grades of HD, there is up to 90% loss of neurons in the neostriatum (Vonsattel et al. 1985). No drug treatment may be able to overcome that degree of neuronal deficit and extend life when initiated at late stages of disease. Improving quality of life may be as important as extending it. As suggested in these studies, creatine treatment started in early and mid-stages of the disease in mice improve body weight loss and motor performance. It is possible that creatine treatment in HD patients may be as effective in improving these measures.
Nutritional supplements, such as creatine and coenzyme Q10, have been shown to be effective in slowing the phenotypic progression, ameliorating neuronal damage in experimental models of disease. These compounds are being tested in clinical trials and already are becoming more widely consumed by HD patients because of their reported potential benefits, apparent safety, and ready availability. Creatine is an endogenous, relatively safe compound that has been used as a dietary supplement for extended periods of time by athletes and in the clinical setting by patients, with few reported side-effects (Tarnopolsky and Beal 2001; Wyss and Schulze 2002). Creatine, 3–5 g/day, has been shown to be safe and well tolerated by early-stage HD patients, with blood serum creatine levels increasing over twofold (Kieburtz 2001). An inconclusive underpowered study (Verbessem 2002) of 5 mg/kg of creatine over 1 year did not demonstrate an effect on subjects with HD. While the doses used in these studies are known to increase muscle function in normal healthy subjects, it remains unknown whether they are sufficient to provide neuroprotection in the human central nervous system. The safety of chronic administration of higher doses of creatine in HD patients remains to be established.
Much as treatment for cancer and AIDS has evolved, the most effective neuroprotection for HD will likely come from a cocktail of medications. Combining safe and well-tolerated compounds, such as creatine, with other agents may provide significantly greater efficacy. Creatine is under trial in HD patients to test tolerability and potential efficacy and may be one of a number of drugs that form a base foundation in HD patients for prospective multiple-drug combinations.
Acknowledgements
We would like to thank Dr Autumn M. Klein for her constructive suggestions and careful review of the manuscript. Karen Smith and Kerry Cormier provided expert assistance in histology preparation and animal husbandry. This work was supported by NIH grants NS37102, AG13846 (RJF), NS35255, AT00613 (SMH and RJF), NS39258, NS38180, AG14930 (MFB), AG12992 (MFB, and RJF), and the Veterans Administration and reap Award (RJF).
Abbreviations used
HD
Huntington’s disease
htt
huntingtin
MPT
mitochondrial permeability transition
PCr
phosphocreatine
References
- Andreassen OA, Dedeoglu A, Ferrante RJ, Jenkins BG, Ferrante KL, Thomas M, Borchelt DR, Hersch SM, Ross CR, Beal MF. Creatine increases survival and delays motor symptoms in a transgenic animal model of Huntington’s disease. J. Neurobiol. 2001a;8:479–491. doi: 10.1006/nbdi.2001.0406. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Ferrante RJ, Huang HM, Dedeoglu A, Park L, Ferrante KL, Kwon J, Borchelt DR, Ross CA, Gibson GE, Beal MF. Dichloroacetate exerts therapeutic effects in transgenic mouse models of Huntington’s disease. Ann. Neurol. 2001b;50:112–117. doi: 10.1002/ana.1085. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Ferrante RJ, Dedeoglu A, Beal MF. Lipoic acid improves survival in transgenic mouse models of Huntington’s disease. Neuroreport. 2001c;29:3371–3373. doi: 10.1097/00001756-200110290-00044. [DOI] [PubMed] [Google Scholar]
- Balestrino M, Lensman M, Parodi M, Perasso L, Rebaudo R, Melani R, Polenov S, Cupello A. Role of creatine and phosphocreatine in neuronal protection from anoxic and ischemic damage. Amino Acids. 2002;23:221–229. doi: 10.1007/s00726-001-0133-3. [DOI] [PubMed] [Google Scholar]
- Beal MF. Energetics in the pathogenesis of neurodegenerative diseases. TINS. 2000;23:298–304. doi: 10.1016/s0166-2236(00)01584-8. [DOI] [PubMed] [Google Scholar]
- Beal MF, Brouillet E, Jenkins B, Henshaw R, Rosen B, Hyman BT. Age-dependent striatal excitotoxic lesions produced by the endogenous mitochondrial inhibitor malonate. J. Neurochem. 1993;61:1147–1150. doi: 10.1111/j.1471-4159.1993.tb03633.x. [DOI] [PubMed] [Google Scholar]
- Benzi G. Is there a rationale for the use of creatine either as nutritional supplementation or drug administration in humans participating in sport? Pharmacol. Res. 2000;41:255–264. doi: 10.1006/phrs.1999.0618. [DOI] [PubMed] [Google Scholar]
- Bogdanov MB, Andreassen OA, Dedeoglu A, Ferrante RJ, Beal MF. Increased oxidative damage to DNA in a transgenic mouse model of Huntington’s disease. J. Neurochem. 2001;79:1246–1249. doi: 10.1046/j.1471-4159.2001.00689.x. [DOI] [PubMed] [Google Scholar]
- Brouillet E, Hantraye P, Ferrante RJ, Dolan R, Leroy-Willig A, Kowall NW, Beal MF. Chronic mitochondrial energy impairment produces selective striatal degeneration and abnormal choreiform movements in primates. Proc. Natl Acad. Sci. USA. 1995;92:7105–7109. doi: 10.1073/pnas.92.15.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne SE, Ferrante RJ, Beal MF. Oxidative stress in Huntington’s disease. Brain Pathol. 1999;9:147–163. doi: 10.1111/j.1750-3639.1999.tb00216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AJ, Muller RE, Pschorn U, Stransky W. Preincubation with creatine enhances levels of creatine phosphate and prevents anoxic damage in rat hippocampal slices. J. Neurochem. 1995;6:2691–2699. doi: 10.1046/j.1471-4159.1995.64062691.x. [DOI] [PubMed] [Google Scholar]
- Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiari L, Bates GP. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- Dedeoglu A, Kubilus JK, Jeitner TM, Matson SA, Bogdanov M, Kowall NW, Matson WR, Cooper AJ, Ratan RR, Beal MF, Hersch SM, Ferrante RJ. Therapeutic effects of cystamine in a murine model of Huntington’s disease. J. Neurosci. 2002;20:8942–8950. doi: 10.1523/JNEUROSCI.22-20-08942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande SB, Fukuda A, Nishino H. 3-Nitropropionic acid increases the intracellular Ca2+ in cultured astrocytes by reverse operation of the Na+–Ca2+ exchanger. Exp. Neurol. 1997;145:38–45. doi: 10.1006/exnr.1997.6457. [DOI] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP, Vonsattel JP, Aronin N. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, Andreassen OA, Jenkins BG, Dedeoglu A, Kuemmerle S, Kubilus JK, Kaddurah-Daouk R, Hersch SM, Beal MF. Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J. Neurosci. 2000;20:4389–4397. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante RJ, Andreassen OA, Dedeoglu A, Ferrante KL, Jenkins BG, Hersch SM, Beal MF. Therapeutic effects of coenzyme Q10 and remacemide in transgenic mouse models of Huntington’s disease. J. Neurosci. 2002;22:1592–1599. doi: 10.1523/JNEUROSCI.22-05-01592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Gu M, Gash MT, Mann VM, Javoy-Agid F, Cooper JM, Schapira AHV. Mitochondrial defect in Huntington’s disease caudate nucleus. Ann. Neurol. 1996;39:385–389. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- Hemmer W, Wallimann T. Functional aspects of creatine kinase in brain. Dev. Neurosci. 1993;15:249–260. doi: 10.1159/000111342. [DOI] [PubMed] [Google Scholar]
- The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Jenkins BG, Klivenyi P, Kustermann E, Andreassen OA, Ferrante RJ, Rosen BR, Beal MF. Non-linear decrease over time in N-acetylaspartate levels in the absence of neuronal loss and increases in glutamine and glucose in transgenic Huntington’s disease mice. J. Neurochem. 2000;74:2108–2119. doi: 10.1046/j.1471-4159.2000.0742108.x. [DOI] [PubMed] [Google Scholar]
- Kieburtz K. Placebo-controlled trial of creatine in Huntington’s disease. Neurol. Abstract. 2001;56:A386. [Google Scholar]
- Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, Andreassen OA, Mueller G, Wermer M, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat. Med. 1999;5:347–350. doi: 10.1038/6568. [DOI] [PubMed] [Google Scholar]
- Koroshetz WJ, Jenkins BG, Rosen BR, Beal MF. Energy metabolism defects in Huntington’s disease and possible therapy with coenzyme Q10. Ann. Neurol. 1997;41:160–165. doi: 10.1002/ana.410410206. [DOI] [PubMed] [Google Scholar]
- Kuemmerle S, Gutekunst CA, Klein AM, Li XJ, Li SH, Beal MF, Hersch SM, Ferrante RJ. Huntington aggregates may not predict neuronal death in Huntington’s disease. Ann. Neurol. 1999;46:842–849. [PubMed] [Google Scholar]
- Lawler JM, Barnes WS, Wu G, Song W, Demaree S. Direct antioxidant properties of creatine. Biochem. Biophys. Res. Commun. 2002;1:47–52. doi: 10.1006/bbrc.2001.6164. [DOI] [PubMed] [Google Scholar]
- Ludolph AC, He F, Spencer PS, Hammerstad J, Sabri M. 3-Nitropropionic acid-exogenous animal neurotoxin and possible human striatal toxin. Can. J. Neurol. Sci. 1991;4:492–498. doi: 10.1017/s0317167100032212. [DOI] [PubMed] [Google Scholar]
- Mangiarini L, Sathasivam K, Seller M, Cozens B, Harper A, Hetherington C, Lawton M, Trottier Y, Lehrach H, Davies SW, Bates GP. Exon 1 of the HD gene with an expanded CAG repeat is sufficient to cause a progressive neurological phenotype in transgenic mice. Cell. 1996;87:493–506. doi: 10.1016/s0092-8674(00)81369-0. [DOI] [PubMed] [Google Scholar]
- Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington’s disease. J. Neurosci. 1998;18:156–163. doi: 10.1523/JNEUROSCI.18-01-00156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, Ferrante RJ, Klivenyi P, Yang L, Klein AM, Mueller G, Kaddurah-Daouk R, Beal MF. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp. Neurol. 1999;157:142–149. doi: 10.1006/exnr.1999.7049. [DOI] [PubMed] [Google Scholar]
- O’Gorman E, Beutner G, Wallimann T, Brdiczka D. Differential effects of creatine depletion on the regulation of enzyme activities and on creatine-stimulated mitochondrial respiration in skeletal muscle, heart, and brain. Biochim. Biophys. Acta. 1996;1276:161–170. doi: 10.1016/0005-2728(96)00074-6. [DOI] [PubMed] [Google Scholar]
- Ohtsuki S, Tachikawa M, Takanaga H, Shimizu H, Watanabe M, Hosoya K, Terasaki T. The blood–brain barrier creatine transporter is a major pathway for supplying creatine to the brain. J. Cereb. Blood Flow Metab. 2002;22:1327–1335. doi: 10.1097/01.WCB.0000033966.83623.7D. [DOI] [PubMed] [Google Scholar]
- Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat. Neurosci. 2002;8:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- Sawa A, Wiegand GW, Cooper J, Margolis RL, Sharp AH, Lawler JF, Jr, Greenamyre JT, Snyder SH, Ross CA. Increased apoptosis of Huntington’s disease lymphoblasts associated with repeat length-dependent mitochondrial depolarization. Nat. Med. 1999;5:1194–1198. doi: 10.1038/13518. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Matthews RT, Jenkins BG, Ferrante RJ, Siwek D, Henshaw DR, Cipolloni PB, Mecocci P, Kowall NW, Rosen BR, Beal MF. Blockade of neuronal nitric oxide synthase protects against excitotoxicity in vivo. J. Neurosci. 1995a;12:8419–8429. doi: 10.1523/JNEUROSCI.15-12-08419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JB, Henshaw DR, Siwek D, Jenkins BG, Ferrante RJ, Cipolloni PB, Kowall NW, Rosen BR, Beal MF. Involvement of free radicals in excitotoxicity in vivo. J. Neurochem. 1995b;5:2239–2247. doi: 10.1046/j.1471-4159.1995.64052239.x. [DOI] [PubMed] [Google Scholar]
- Steeghs K, Benders A, Oerlemans F, de Haan A, Heerschap A, Ruitenbeek W, Jost C, van Deursen J, Perryman B, Pette D, Bruckwilder M, Koudijs J, Jap P, Veerkamp J, Wieringa B. Altered Ca2+ responses in muscles with combined mitochondrial and cytosolic creatine kinase deficiencies. Cell. 1997;89:93–103. doi: 10.1016/s0092-8674(00)80186-5. [DOI] [PubMed] [Google Scholar]
- Tarnopolsky MA, Beal MF. Potential for creatine and other therapies targeting cellular energy dysfunction in neurological disorders. Ann. Neurol. 2001;5:561–574. [PubMed] [Google Scholar]
- Verbessem P. Oral creatine supplementation in patients with Huntington’s disease. Neurol. Abstract. 2002;58:A334. [Google Scholar]
- Vonsattel JP, Myers RH, Stevens TJ, Ferrante RJ, Bird ED, Richardson EP., Jr Neuropathological classification of Huntington’s disease. J. Neuropathol. Exp. Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the ‘phosphocreatine circuit’ for cellular energy homeostasis. Biochem. J. 1992;281:21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss M, Schulze A. Health implications of creatine: can oral creatine supplementation protect against neurological and atherosclerotic disease? Neuroscience. 2002;2:243–260. doi: 10.1016/s0306-4522(02)00088-x. [DOI] [PubMed] [Google Scholar]
- Xu CJ, Klunk WE, Kanfer JN, Xiong Q, Miller G, Pettegrew JW. Phosphocreatine-dependent glutamate uptake by synaptic vesicles. J. Biol. Chem. 1996;271:13435–13440. doi: 10.1074/jbc.271.23.13435. [DOI] [PubMed] [Google Scholar]