Clinical features of hepatitis B virus-related hepatocellular carcinoma (original) (raw)
Abstract
Hepatocellular carcinoma (HCC) is a major cause of cancer death, and chronic hepatitis B is a serious worldwide problem. The epidemiology of HCC is distinctive. Hepatitis B virus (HBV) plays a major role in hepatocarcinogenesis. Prevention of HBV-related HCC is a key issue in current hepatology. This paper describes the prevention and clinical features of HBV-related HCC, along with a short review of the disease.
Keywords: Hepatocellular carcinoma, Hepatitis B virus
INTRODUCTION
Persistent infection by hepatitis C virus (HCV) or hepatitis B virus (HBV) is a background factor in almost all cases of hepatocellular carcinoma (HCC) in Japanese patients, as it causes chronic hepatitis and liver cirrhosis, and ultimately HCC. Several studies have indicated that HCC develops mainly in the cirrhotic liver and alongside chronic liver diseases. It is widely accepted that viral cirrhosis caused by HBV or HCV infection is an important risk factor for the development of HCC. However, HCC can develop in non-cirrhotic livers, particularly in countries where HBV is endemic. The present paper discusses the clinical features of HBV-related HCC and the importance of viral status and viral eradication for improving the prognosis of HBV-related HCC.
CARCINOGENIC FACTORS AFFECTING CHRONIC LIVER DISEASE (TYPE B)
It is believed that the incidence of carcinogenesis is low in cases with fibrotic severity index F0 according to the new Inuyama classification of chronic hepatitis related to HBV, whereas it increases with a decrease in cure rate in association with an increase in the fibrotic severity index F. It is thus believed that the incidence of carcinogenesis also increases with increased severity of cirrhosis in patients with chronic hepatitis.
Accordingly, we examined carcinogenesis-modulating factors by long-term observation of patients with HBV-related liver cirrhosis[1].
Several studies have indicated that HCC develops mainly in the cirrhotic liver. It is widely accepted that viral cirrhosis due to infection by HBV or HCV is an important risk factor for the development of HCC. It has also been indicated that various factors and their interactions are involved in the etiology and pathogenesis of HCC. We thus determined the rate of HCC development to assess the risk factors for hepatocellular carcinogenesis in patients with HBV-related compensated viral cirrhosis.
We previously concluded that a high HBV-DNA titer (more than 3.7 LGE/mL) was the most predictive factor of the development of HCC in HBsAg-positive liver cirrhosis. It was found by univariate analysis that the incidence of carcinogenesis was affected by the following three factors with statistical significance: alanine aminotransferase (ALT) ≥ 100 IU/L (P = 0.008), lactate dehydrogenase (LDH) ≥ 480 IU/L (P = 0.014), and HBV-DNA ≥ 3.7 LGE/mL (P = 0.008). Relative risks for HCC were 2.04 (95% CI: 1.10-4.68), 2.00 (95% CI: 1.08-4.56), and 2.84 (95% CI: 1.27-6.34) for these respective factors[1].
It was found by multivariate analysis using these factors that ALT and HBV-DNA levels were independent significant risk factors for HCC. The risk ratio in patients with ALT ≥ 100 IU/L to that in those with ALT < 100 IU/L was 4.53, while the risk ratio in patients with HBV ≥ 3.7 LGE/mL to that in those with HBV-DNA < 3.7 LGE/mL was 7.71 (Table 1).
Table 1.
Carcinogenic factors involved in hepatocellular carcinoma in patients with type B cirrhosis[1]
| Variable | n | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| P-value1 | RR2 | 95% CI | P-value3 | RR2 | 95% CI | ||
| ALT (IU/L) | |||||||
| ≥ 100 | 14 | 0.0083 | 2.044 | 1.098-4.677 | 0.025 | 4.525 | 1.202-17.030 |
| < 100 | 51 | 1.000 | 1.000 | ||||
| LDH (IU/L) | |||||||
| ≥ 480 | 30 | 0.0138 | 2.000 | 0.878-4.558 | NS (0.081) | 2.880 | 0.881-9.412 |
| < 480 | 35 | 1.000 | 1.000 | ||||
| HBV-DNA (LGE/mL) | |||||||
| ≥ 3.7 | 46 | 0.0082 | 2.836 | 1.269-6.340 | 0.014 | 7.712 | 1.511-39.365 |
| < 3.7 | 19 | 1.000 | 1.000 |
In fact, the interval from suspected diagnosis of cirrhosis to development of HCC in the high-HBV DNA group was shorter than that in the persistently low-HBV DNA group (Figure 1). Moreover, ALT in the high-HBV-DNA titer group was higher than that in the low-HBV-DNA titer group. Despite this finding, serum ALT level was not correlated with HBV-DNA level (Figure 2, P = 0.353)[1].
Figure 1.
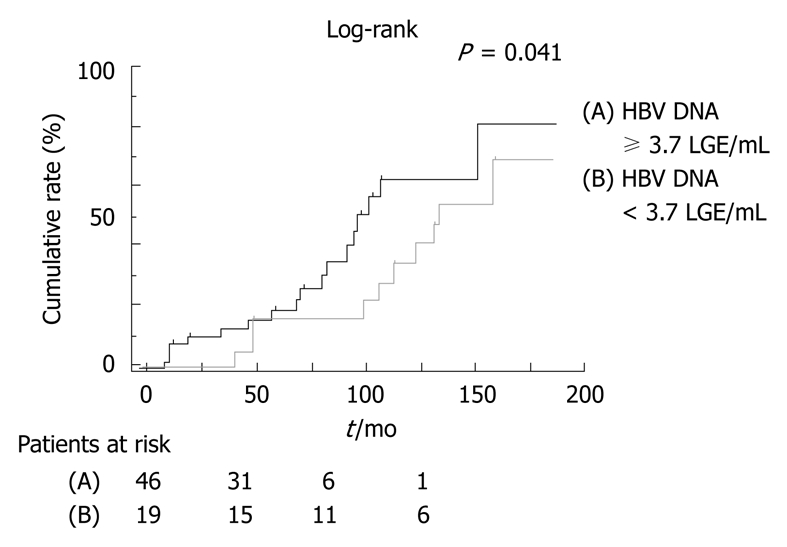
Comparison of the cumulative incidence of hepatocellular carcinoma by viral level in patients with type B cirrhosis[1]. HBV: Hepatitis B virus.
Figure 2.
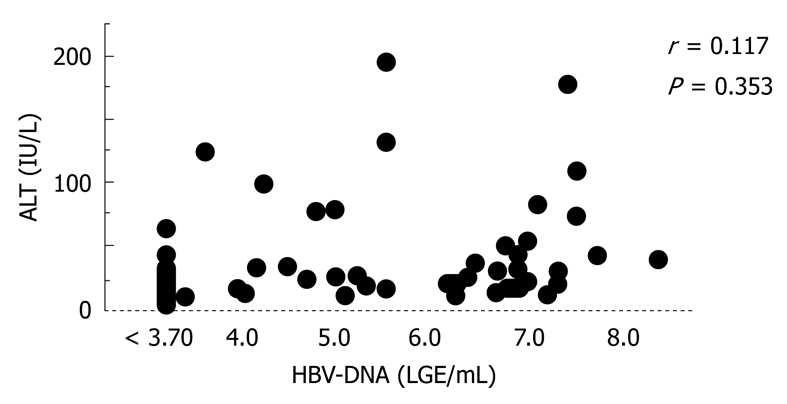
Correlation between HBV-DNA and alanine aminotransferase (ALT) levels in patients with type B cirrhosis[1].
As in our data, Ikeda et al[2] reported that the development of HCC in the high-HBV-DNA group was shorter than that in the low-HBV-DNA group. Moreover, they reported that interferon could be effectively administered in a selected group of patients as a strategy for cancer prevention, even in those with cirrhosis. In addition, many reports have revealed that continuous treatment with lamivudine (LAM) delays clinical progression in patients with chronic hepatitis B and advanced fibrosis or cirrhosis, significantly reducing the incidence of hepatic decompensation and the risk of HCC[3,4].
These findings suggest that clinical efforts for viral eradication or reduction of viral load will reduce the risk of HCC in both HBV and HCV-related liver disease. However, in contrast to chronic hepatitis C, the carcinogenesis in chronic hepatitis B in many cases is not correlated with the severity of the fibrotic changes. These epidemiological results suggest that there may be differences in disease activity and mode of disease progression, as well as cancer development, between hepatitis B and hepatitis C viral infections.
HBV-related hepatocarcinogenesis is associated with viral DNA integration into the host genome. Suppression of inflammation and regeneration may reduce the opportunity for DNA integration and, hence, the risk of HCC, although this effect remains to be confirmed in clinical trials[5,6]. Most cases of late-phase recurrence are thought to be due to metachronous multicentric or de novo carcinogenesis.
HBV-related hepatocellular carcinogenesis appears to be caused by the hepatitis virus itself rather than the inflammatory state characteristic of hepatitis. Persistent high HBV-DNA concentration may itself increase the risk of carcinogenesis. Furthermore, clinical and pathological differences exist between HBV genotypes B and C, and genotype C has a higher risk of HCC development than genotype B[7]. Genotype B-related HCC is less associated with liver cirrhosis and has a higher frequency of solitary tumor as well as more satellite nodules than genotype C-related HCC[7].
Sequential analysis of HBV-DNA is the key to assessing the risk of carcinogenesis. HCC develops in younger patients at a higher incidence and more rapidly due to HBV-DNA integration in the early stages of chronic infection.
Previous studies have reported that the incidence of HCC is higher in males than in females, and that the rate of progression of HBV infection is faster in males. As to HBV genotype, on the other hand, no Japanese HCC patients under 35 years in age had the B genotype (genotype Bj), which is different from the B genotype identified in Taiwan (genotype Ba): the genotype Bj is believed to give a relatively good prognosis[8].
Furthermore, HBV-related HCC often occurs in children, and HCC induced by chronic HBV infection also tends to occur much earlier in patients infected in childhood than that in those infected in adulthood.
CLINICAL CHARACTERISTICS OF HBV-RELATED HCC
HBV-related HCC occurs more often in younger patients with mild inflammation and fibrosis than in patients with HCV-related HCC. It has been reported in a domestic multicenter clinical study that liver function is relatively good in patients with HBV-related HCC and they are in disease stage I in many cases, and that the diagnostic age of HBV-related HCC is 52.1 years, while that of HCV-related HCC is 62.9 years, about 10 years later than in HBV-related HCC patients[9].
However, in many cases of HBV-related HCC, the tumor disease stage is advanced (stage III/IV), presenting an undifferentiated cancer type with portal vein infiltration, suggesting that HBV-related HCC has a worse prognosis and a more refractory character than HCV-related HCC. Moreover, HBV-related HCC is infiltrative, showing multinodular growth, unlike HCV-related HCC[10-12].
HBV-related HCC is often accompanied by portal vein tumor thrombus (PVTT). In our study, HBsAg-positive cases were found in 70% of PVTT patients, while anti-HCV serology was positive in 30% of them. Although the prognosis was poor in most patients with HCC accompanied by PVTT, our combination chemotherapy is likely to be effective in achieving long-term survival and appears to exert anti-tumor activities with tolerable adverse effects in patients with advanced HCC accompanied by PVTT[13].
Although recurrence of HBV-related HCC occurs often within 3 years of hepatic resection, the rate of recurrence decreases thereafter. In addition, large tumors are found in many cases of HBV-related HCC, but there are few cases of multicentric recurrence, suggesting that hepatic resection should be the first-choice therapy in such cases[14].
On the other hand, multicentric recurrence occurs at a high incidence after resection in HCV-related HCC patients with impaired liver function, infrequently showing multicentric carcinogenesis at the time of resection in elderly patients, which is a characteristic of HCV-related HCC. Nagasue et al[15] reported that the 5-year disease-free survival rate was 58% in patients with HBV infection, whereas it was 6% in those with HCV infection (P < 0.001). Thus, major hepatectomy is recommended for treating HBV-related HCC without cirrhosis to prevent early recurrence.
Furthermore, Roayaie et al[16] have reported the 5-year disease-free survival rate to be significantly higher in HBV patients treated with resection than in patients with HCV (49% vs 7%, P = 0.0480). Depending on preoperative liver function and tumor location, a much higher proportion of patients with HBV-related HCC may become candidates for hepatic resection. There are significant differences in preoperative status, tumor characteristics and disease-free survival rate between HCC patients with chronic HBV infection and those with HCV infection whose liver disease has not yet reached the end stage.
In light of these points, curative treatments such as hepatectomy may become the first choice therapy of HBV-related HCC, since many large tumors are found and there are few cases of multicentric carcinogenesis, suggesting that systematic hepatectomy focusing on the morphological type of the portal vein branch may be desirable.
Hepatitis B status, tumor factors, and surgical type affected cancer recurrence after resection in patients with HCC related by concurrent chronic HBV infection, whereas only tumor factors affected recurrence in patients with HCC related by previous chronic HBV infection. Furthermore, Kubo et al[14] have reported viral status, defined by a high viral load, the presence of HBeAg, the absence of anti-HBe antibody, and the absence of precore mutant type HBV, as well as low platelet count and positive surgical margin, to be risk factors for recurrence after resection of HCC in HBsAg-positive patients.
In response to these findings, viral elimination has been suggested as a strategy for improving recovery from HBV-related HCC. This prompted Koike et al[17] to conduct a univariate analysis, in which they reported that degree of fibrosis, pathological grade of HCC, and serum AFP levels were significantly linked to intrahepatic recurrence. In their multivariate analysis, degree of fibrosis was the strongest factor in the recurrence of HCV-related HCC (P = 0.004). In contrast, degree of fibrosis did not influence the recurrence of HBV-related HCC (P = 0.51) or NBNC-related (P = 0.77) HCC. Risk factors for HCC recurrence varied according to the state of viral infection.
De novo carcinogenesis may be present not only in well-differentiated HCC, but also in less-differentiated HCC, especially in HBV-related HCC; and the carcinogenesis in HBV-related HCC may have different impacts from that in HCV-related HCC. The clinical conditions of hepatitis virus have an impact on the outcomes of resection of HBV-related HCC, making it necessary to position antiviral therapy for prevention of recurrence as one therapeutic option for treating HBV-related HCC.
ANTIVIRAL THERAPY OF HBV-RELATED HCC
Kubo et al[18] concluded that the prognosis after resection of HCC was worse in HBeAg-positive patients than in HBeAg-negative patients. Among patients who had undergone transarterial chemolipiodolization, high HBV viral load was the most important risk factor for post-treatment recurrence in cases of complete necrosis, irrespective of the locational pattern of recurrence. The current findings underscore the need for future studies to examine the applicability of antiviral therapy for reducing the risk of HCC recurrence[19].
Recently, LAM, a nucleoside analog that inhibits reverse transcriptase, has been developed. Long-term treatment with LAM has been found to inhibit HBV replication, attenuate hepatitis and improve histological findings of the liver. However, LAM therapy is not free from problems that require resolving, such as relapse of hepatitis as a consequence of the emergence of YMDD viral mutants. The effects of LAM on the therapeutic outcomes of HBV-related HCC patients have not been confirmed in randomized clinical trials (RCTs).
Kubo et al[20] have reported that LAM therapy improves the tumor-free survival rate after curative resection in HBV-related HCC patients showing a high serum concentration of HBV DNA, although careful follow-up was necessary for detection of YMDD viral mutants. Kuzuya et al[21] reported that there was no significant difference in cumulative recurrence rate of HCC between the LAM group and the control group (P = 0.622). However, the median Child-Pugh score at the time of HCC recurrence was significantly different, being 5 (range: 5-6) in the LAM group and 7 (range: 5-12) in the control group (P = 0.005).
LAM therapy may be beneficial in patients with HBV-related HCC after initial treatment, since the treatment may contribute to improvement in remnant liver function; therefore, LAM therapy may reduce the risk of liver failure and extend the therapeutic options available for treatment of recurrent HCC.
It is likely that other antiviral therapies such as interferon (IFN) treatment will be effective in HCV-related HCC[22].
Since the HBV-DNA level is a recurrence factor after transcatheter arterial chemoembolization (TACE) in patients with HBV-related HCC, IFN therapy may suppress recurrence and improve convalescence. IFN is believed to suppress tumor recurrence after treatment of HCC in patients with HBV-related cirrhosis, especially in the high-aspartate transaminase (AST) subgroup. In any case, the usability of the prescription of LAM in the following therapy should be reviewed from the viewpoint of versatility. It is expected that the usefulness of LAM will be verified by RCTs, although there is the potential issue of drug tolerance stock.
Therefore, it is likely that LAM will be replaced by entecavir (ETV), the appearance rate of tolerance stock of which is low, as a standard remedy in the future. In this context, it may be necessary to review anew the cancer-suppressive effects as well as the recurrence-suppressive effects of ETV. On the other hand, with respect to HBV-related HCC, IFN was assumed to suppress tumor recurrence after treatment of HCC in patients with HBV-related cirrhosis, especially in the high-AST subgroup[23].
It appears that IFN may suppress recurrence by reducing the viral level or by direct antitumor effects in both HBV-related HCC and HCV-related HCC. Recently, Sun et al[24] conducted an RCT, reporting that IFN therapy improved overall survival of patients with HBV-related HCC after curative resection, probably by delay in recurrence.
It will be necessary to review the significance of antiviral therapy in the future, focusing on whether antiviral drugs such as LAM, ETV and IFN can contribute to improved convalescence from HBV-related HCC[25]. Futhermore, LAM will most likely be replaced by tenofovir. Although ETV and pegylated interferon are the other 2 drugs recommended as first-line therapy for HBV, tenofovir remains the first choice. In the future, tenofovir may prove to be effective in preventing recurrence and further investigations involving more cases are needed.
CONCLUSION
Chronic HBV infection is the primary risk factor for the future development of HCC worldwide. Prevention of HBV-related HCC is accomplished by preventing HBV infection via HBV vaccination. Broad public health strategies should include routine testing to identify chronic HBV infection, improved health infrastructures including human resources to provide diagnosis and treatment assessment. For persons who are already chronically infected, development of more effective antiviral therapies that can result in sustained suppression of viral replication and hepatic necroinflammation may reduce the incidence of HCC. In the future, it will be necessary to review the significance of antiviral therapy to improve convalescence from HBV-related HCC.
Footnotes
Peer reviewers: Sandeep Mukherjee, MBBCh, MPH, FRCPC, Associate Professor of Internal Medicine, Section of Gastroenterology and Hepatology, Nebraska Medical Center, Omaha, Nebraska, NE 68198-3285, United States; Satoru Kakizaki, MD, PhD, Assistant Professor, Department of Medicine and Molecular Science, Gunma University, Graduate School of Medicine, 3-39-15 Showa-machi, Maebashi, Gunma 371-8511, Japan
S- Editor Tian L L- Editor O'Neill M E- Editor Lin YP
References
- 1.Ishikawa T, Ichida T, Yamagiwa S, Sugahara S, Uehara K, Okoshi S, Asakura H. High viral loads, serum alanine aminotransferase and gender are predictive factors for the development of hepatocellular carcinoma from viral compensated liver cirrhosis. J Gastroenterol Hepatol. 2001;16:1274–1281. doi: 10.1046/j.1440-1746.2001.02616.x. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Fukuda M, Koida I, Arase Y, Chayama K, Murashima N, et al. Interferon decreases hepatocellular carcinogenesis in patients with cirrhosis caused by the hepatitis B virus: a pilot study. Cancer. 1998;82:827–835. doi: 10.1002/(sici)1097-0142(19980301)82:5<827::aid-cncr5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto A, Tanaka E, Rokuhara A, Kiyosawa K, Kumada H, Omata M, Okita K, Hayashi N, Okanoue T, Iino S, et al. Efficacy of lamivudine for preventing hepatocellular carcinoma in chronic hepatitis B: A multicenter retrospective study of 2795 patients. Hepatol Res. 2005;32:173–184. doi: 10.1016/j.hepres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Shafritz DA, Shouval D, Sherman HI, Hadziyannis SJ, Kew MC. Integration of hepatitis B virus DNA into the genome of liver cells in chronic liver disease and hepatocellular carcinoma. Studies in percutaneous liver biopsies and post-mortem tissue specimens. N Engl J Med. 1981;305:1067–1073. doi: 10.1056/NEJM198110293051807. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Chenivesse X, Henglein B, Bréchot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990;343:555–557. doi: 10.1038/343555a0. [DOI] [PubMed] [Google Scholar]
- 7.Lin CL, Chen JD, Liu CJ, Lee PH, Chen PJ, Lai MY, Kao JH, Chen DS. Clinicopathological differences between hepatitis B viral genotype B- and C-related resectable hepatocellular carcinoma. J Viral Hepat. 2007;14:64–69. doi: 10.1111/j.1365-2893.2006.00776.x. [DOI] [PubMed] [Google Scholar]
- 8.Orito E, Sugauchi F, Tanaka Y, Ichida T, Sata M, Tanaka E, Okanoue T, Sakugawa H, Watanabe H, Miyakawa H, et al. Differences of hepatocellular carcinoma patients with hepatitis B virus genotypes of Ba, Bj or C in Japan. Intervirology. 2005;48:239–245. doi: 10.1159/000084601. [DOI] [PubMed] [Google Scholar]
- 9.Tanizaki H, Ryu M, Kinoshita T, Kawano N, Konishi M, Cho A, Nakatsura T, Natsume T, Takahashi S, Sugita M, et al. Comparison of clinical features and survival in patients with hepatitis B and C virus-related hepatocellular carcinoma. Jpn J Clin Oncol. 1997;27:67–70. doi: 10.1093/jjco/27.2.67. [DOI] [PubMed] [Google Scholar]
- 10.Tanabe G, Nuruki K, Baba Y, Imamura Y, Miyazono N, Ueno K, Arima T, Nakajyou M, Aikou T. A comparison of hepatocellular carcinoma associated with HBV or HCV infection. Hepatogastroenterology. 1999;46:2442–2446. [PubMed] [Google Scholar]
- 11.Okuda H, Obata H, Motoike Y, Hisamitsu T. Clinicopathological features of hepatocellular carcinoma--comparison of hepatitis B seropositive and seronegative patients. Hepatogastroenterology. 1984;31:64–68. [PubMed] [Google Scholar]
- 12.Shijo H, Okazaki M, Koganemaru F, Higashi M, Sakaguchi S, Okumura M. Influence of hepatitis B virus infection and age on mode of growth of hepatocellular carcinoma. Cancer. 1991;67:2626–2632. doi: 10.1002/1097-0142(19910515)67:10<2626::aid-cncr2820671038>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa T, Imai M, Kamimura H, Tsuchiya A, Togashi T, Watanabe K, Seki K, Ohta H, Yoshida T, Kamimura T. Improved survival for hepatocellular carcinoma with portal vein tumor thrombosis treated by intra-arterial chemotherapy combining etoposide, carboplatin, epirubicin and pharmacokinetic modulating chemotherapy by 5-FU and enteric-coated tegafur/uracil: a pilot study. World J Gastroenterol. 2007;13:5465–5470. doi: 10.3748/wjg.v13.i41.5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubo S, Hirohashi K, Tanaka H, Tsukamoto T, Shuto T, Yamamoto T, Ikebe T, Wakasa K, Nishiguchi S, Kinoshita H. Effect of viral status on recurrence after liver resection for patients with hepatitis B virus-related hepatocellular carcinoma. Cancer. 2000;88:1016–1024. doi: 10.1002/(sici)1097-0142(20000301)88:5<1016::aid-cncr10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 15.Nagasue N, Ono T, Yamanoi A, Kohno H, El-Assal ON, Taniura H, Uchida M. Prognostic factors and survival after hepatic resection for hepatocellular carcinoma without cirrhosis. Br J Surg. 2001;88:515–522. doi: 10.1046/j.1365-2168.2001.01732.x. [DOI] [PubMed] [Google Scholar]
- 16.Roayaie S, Haim MB, Emre S, Fishbein TM, Sheiner PA, Miller CM, Schwartz ME. Comparison of surgical outcomes for hepatocellular carcinoma in patients with hepatitis B versus hepatitis C: a western experience. Ann Surg Oncol. 2000;7:764–770. doi: 10.1007/s10434-000-0764-8. [DOI] [PubMed] [Google Scholar]
- 17.Koike Y, Shiratori Y, Sato S, Obi S, Teratani T, Imamura M, Hamamura K, Imai Y, Yoshida H, Shiina S, et al. Risk factors for recurring hepatocellular carcinoma differ according to infected hepatitis virus-an analysis of 236 consecutive patients with a single lesion. Hepatology. 2000;32:1216–1223. doi: 10.1053/jhep.2000.20237. [DOI] [PubMed] [Google Scholar]
- 18.Kubo S, Hirohashi K, Yamazaki O, Matsuyama M, Tanaka H, Horii K, Shuto T, Yamamoto T, Kawai S, Wakasa K, et al. Effect of the presence of hepatitis B e antigen on prognosis after liver resection for hepatocellular carcinoma in patients with chronic hepatitis B. World J Surg. 2002;26:555–560. doi: 10.1007/s00268-001-0267-1. [DOI] [PubMed] [Google Scholar]
- 19.Jang JW, Choi JY, Bae SH, Yoon SK, Woo HY, Chang UI, Kim CW, Nam SW, Cho SH, Yang JM, et al. The impact of hepatitis B viral load on recurrence after complete necrosis in patients with hepatocellular carcinoma who receive transarterial chemolipiodolization: implications for viral suppression to reduce the risk of cancer recurrence. Cancer. 2007;110:1760–1767. doi: 10.1002/cncr.22984. [DOI] [PubMed] [Google Scholar]
- 20.Kubo S, Tanaka H, Takemura S, Yamamoto S, Hai S, Ichikawa T, Kodai S, Shinkawa H, Sakaguchi H, Tamori A, et al. Effects of lamivudine on outcome after liver resection for hepatocellular carcinoma in patients with active replication of hepatitis B virus. Hepatol Res. 2007;37:94–100. doi: 10.1111/j.1872-034X.2007.00013.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuzuya T, Katano Y, Kumada T, Toyoda H, Nakano I, Hirooka Y, Itoh A, Ishigami M, Hayashi K, Honda T, et al. Efficacy of antiviral therapy with lamivudine after initial treatment for hepatitis B virus-related hepatocellular carcinoma. J Gastroenterol Hepatol. 2007;22:1929–1935. doi: 10.1111/j.1440-1746.2006.04707.x. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa T. Secondary prevention of recurrence by interferon therapy after ablation therapy for hepatocellular carcinoma in chronic hepatitis C patients. World J Gastroenterol. 2008;14:6140–6144. doi: 10.3748/wjg.14.6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Someya T, Ikeda K, Saitoh S, Kobayashi M, Hosaka T, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Arase Y, et al. Interferon lowers tumor recurrence rate after surgical resection or ablation of hepatocellular carcinoma: a pilot study of patients with hepatitis B virus-related cirrhosis. J Gastroenterol. 2006;41:1206–1213. doi: 10.1007/s00535-006-1912-0. [DOI] [PubMed] [Google Scholar]
- 24.Sun HC, Tang ZY, Wang L, Qin LX, Ma ZC, Ye QH, Zhang BH, Qian YB, Wu ZQ, Fan J, et al. Postoperative interferon alpha treatment postponed recurrence and improved overall survival in patients after curative resection of HBV-related hepatocellular carcinoma: a randomized clinical trial. J Cancer Res Clin Oncol. 2006;132:458–465. doi: 10.1007/s00432-006-0091-y. [DOI] [PubMed] [Google Scholar]
- 25.Mutimer D, Naoumov N, Honkoop P, Marinos G, Ahmed M, de Man R, McPhillips P, Johnson M, Williams R, Elias E, et al. Combination alpha-interferon and lamivudine therapy for alpha-interferon-resistant chronic hepatitis B infection: results of a pilot study. J Hepatol. 1998;28:923–929. doi: 10.1016/s0168-8278(98)80338-3. [DOI] [PubMed] [Google Scholar]