Wnt/β-Catenin Pathway Activation Is Enriched in Basal-Like Breast Cancers and Predicts Poor Outcome (original) (raw)
Abstract
Although Wnt/β-catenin pathway activation has been implicated in mouse models of breast cancer, there is contradictory evidence regarding its importance in human breast cancer. In this study, invasive and in situ breast cancer tissue microarrays containing luminal A, luminal B, human epidermal growth factor receptor 2 (HER2)+/ER− and basal-like breast cancers were analyzed for β-catenin subcellular localization. We demonstrate that nuclear and cytosolic accumulation of β-catenin, a read-out of Wnt pathway activation, was enriched in basal-like breast cancers. In contrast, membrane-associated β-catenin was observed in all breast cancer subtypes, and its expression decreased with tumor progression. Moreover, nuclear and cytosolic localization of β-catenin was associated with other markers of the basal-like phenotype, including nuclear hormone receptor and HER2 negativity, cytokeratin 5/6 and vimentin expression, and stem cell enrichment. Importantly, this subcellular localization of β-catenin was associated with a poor outcome and is more frequently observed in tumors from black patients. In addition, β-catenin accumulation was more often observed in basal-like in situ carcinomas than other in situ subtypes, suggesting that activation of this pathway might be an early event in basal-like tumor development. Collectively, these data indicate that Wnt/β-catenin activation is an important feature of basal-like breast cancers and is predictive of worse overall survival, suggesting that it may be an attractive pharmacological target for this aggressive breast cancer subtype.
The Wnt/β-catenin pathway was first implicated in mammary tumorigenesis when the Int-1 integration site of the mouse mammary tumor virus was identified as the mammalian homolog of the Drosophila Wingless polarity morphogen.1 To reflect this homology, Int-1 was renamed Wnt-1, and its overexpression in the mammary epithelium was found to be sufficient for mammary tumorigenesis.2 Activation of the pathway by Wnt binding to its Fzd and FRP5/6 coreceptors prevents phosphorylation and degradation of β-catenin, the major pathway effector, by the GSK3β/APC/axin destruction complex. Subsequent cytosolic and nuclear β-catenin accumulation and binding to TCF transcription factors results in the regulation of target genes governing proliferation, survival, and matrix remodeling.3 Dysregulation of several other components of the Wnt/β-catenin pathway, including overexpression of a stabilized mutant of β-catenin and mutation of Apc, results in mammary tumorigenesis in mouse models.4 Moreover, activation of this pathway is associated with both embryonic and postnatal mammary development in vivo,4 suggesting that its regulation is critical for proper mammary epithelial homeostasis.
Despite these strong data in mouse models, Wnt pathway involvement in human breast cancer has not been clearly established. Pathway dysregulation, through expression of Wnts and secreted Wnt antagonists or APC inactivation, has been observed in human breast cancers.5 With respect to β-catenin itself, nuclear β-catenin has been observed in as many as 63% of breast cancers.6 Importantly, Hung and colleagues7 reported that nuclear staining of β-catenin and overexpression of the β-catenin target cyclin D1 was associated with a poorer prognosis of breast cancer patients. Consistently, reduced expression of membranous β-catenin in breast cancer has been correlated with a significantly worse outcome8 and metastasis.9 However, several other reports failed to find an association between β-catenin expression and outcome or metastasis10,11,12,13,14 or even evidence for pathway activation in human breast cancer specimens.8,13,14,15
One factor likely contributing to the discordance between these studies is that the breast cancers analyzed for β-catenin localization were not categorized by molecular subtype. Over the last decade, analysis of gene expression profiles in breast tumors has vastly improved our understanding of the breast cancer subtypes that had been previously based predominantly on histopathological and immunohistochemical criteria.16 Molecular subtyping is not only important for understanding the underlying mechanisms that drive the tumor phenotype but is also critical for predicting prognosis and guiding treatment. For example, basal-like breast cancers are typically very aggressive, are more commonly found in specific ethnic populations, and lack any targeted therapies since they do not express the estrogen receptor (ER) or HER2. Interestingly, the molecular characteristics of each breast cancer subtype are not only restricted to invasive cancers but are also observed in ductal carcinoma in situ and lobular intraepithelial neoplasia, suggesting that these molecular programs distinguish even early breast tumors.17,18
Given these features and the clinical implications of distinct breast cancer subtypes and the conflicting data regarding Wnt pathway activity in human breast cancer, we sought to determine whether it was specifically activated in any molecular subtypes. Our results demonstrate that both cytosolic and nuclear β-catenin were more frequently observed in basal-like invasive breast cancers than any other subtype and were associated with many features of basal-like cancer, including enrichment of the stem/progenitor cell population. Importantly, cytosolic and nuclear β-catenin in breast cancer specimens was predictive of a poor outcome, indicating that Wnt/β-catenin pathway activity is an attractive therapeutic target for basal-like breast cancer.
Materials and Methods
Tumor Samples
Archival formalin-fixed paraffin-embedded tissues from breast cancer patients were obtained from the surgical pathology archive of the University of Chicago for tissue microarray (TMA) construction. The study was approved by the University of Chicago Institutional Review Board. Pathological features, including histological diagnosis, grade, tumor size, and axillary lymph node metastasis, were abstracted from the pathology reports (Table 1). There were survival data on 131 of the 134 invasive breast cancer patients with a median follow-up of 8.3 years. The histological grading of invasive breast cancer and carcinoma in situ was performed using the Elston-Ellis–modified Scarff-Bloom-Richardson method19 and a three-tier grading system World Health Organization–based system modified by Fan and Thomas,20 respectively. Breast cancer subtypes were previously analyzed for the expression of immunohistochemical markers and defined as luminal A (ER)+ and/or progesterone receptor (PR)+, HER2−), luminal B (ER+ and/or PR+, HER2+), basal-like (ER−, PR−, HER2−, cytokeratin [CK] 5/6+ and/or epidermal growth factor receptor [EGFR]+), HER2+ (HER2+, ER−, PR−), or unclassified (negative for all five markers) according to Perou et al.21 Subtyping was also previously confirmed by gene-expression profiling in a subset of specimens.22
Table 1.
Clinical Characteristics of Breast Cancer Patients
| Carcinoma in situ (%) (n = 56) | Invasive cancer (%) (n = 134) | |
|---|---|---|
| Age at diagnosis in years, mean ± SD | 55.1 ± 13.0 | 56.0 ± 15.6 |
| Race, n (%) | ||
| Black | 25 (47) | 80 (60) |
| White | 27 (51) | 49 (37) |
| Other | 1 (2) | 4 (3) |
| Histological type, n (%) | ||
| Ductal | 55 (98) | 110 (82) |
| Lobular | 1 (2) | 14 (10) |
| Other | 10 (7) | |
| Grade, n (%) | ||
| I | 20 (42) | 6 (5) |
| II | 17 (35) | 58 (45) |
| III | 11 (23) | 65 (50) |
| AJCC stage, n (%) | ||
| 0 | 56 (100) | — |
| 1 | — | 32 (25) |
| 2 | — | 71 (55) |
| 3 | — | 22 (17) |
| 4 | — | 4 (3) |
| Tumor size in cm, mean ± SD | 1.5 ± 1.5 | 3.3 ± 2.5 |
| Lymph node involvement, n (%) | 0 | 64 (54) |
TMA Construction
The progression-based TMAs23 were constructed from formalin-fixed paraffin-embedded in situ and invasive carcinomas and lymph node metastases tumor samples and adjacent histological normal epithelium. 1-mm tissue cores were arrayed into a new paraffin block using an automated arrayer (ATA-27, Beecher Instruments, Sun Prairie, WI) as described.24
Immunohistochemistry
4-μm TMA sections were deparaffinized and rehydrated in through graded alcohols. Endogenous peroxidases were blocked with 0.3% hydrogen peroxide; nonspecific staining was prevented by incubation in Protein Block Serum-free Solution (Dako, Carpinteria, CA). Immunohistochemistry assays were performed using a Dako immunostainer, and the antibodies, dilutions, and antigen retrieval methods used are summarized in Table 2. Immunoreactivity was detected using Envision+ reagents (Dako) and 3–3′-diaminobenzidine as the chromogen, followed by counterstaining with hematoxylin. For the dual staining of CD44 and CD24, Bond Polymer Refine Detection (Leica Microsystems, Bannockburn, IL) and 3-3’-diaminobenzidine was used as the detection system for CD24. After treatment with denaturing solution, the slides were incubated with anti-CD44 antibody, and staining was detected with Bond Polymer AP Red Detection (Leica) and Vulcan Fast Red Chromogen Kit 2 (Biocare Medical, Concord, IL). Slides were counterstained with hematoxylin and mounted. Human tonsil, colorectal cancer, breast tissue, and commercially available cell lines were used as positive controls, and negative controls were isotypic IgG or no primary antibody.
Table 2.
Antibodies and Conditions Used for Immunohistochemical Analyses
| Antibody | Clone | Dilution | Source | Pretreatment | Scoring |
|---|---|---|---|---|---|
| ER | SP1 | 1:50 | Lab Vision/Thermo Fisher (Fremont, CA) | Microwave 30 minutes, citrate buffer (pH 6.0) | Nuclear; 0 = ≤10%, 1 = 11–30%, 2 = 30–70%, 3 = ≥70%25 |
| PR | SP2 | 1:50 | Lab Vision/Thermo Fisher | Microwave 30 minutes, citrate buffer (pH 6.0) | Nuclear; 0 = ≤10%, 1 = 11–30%, 2 = 30–70%, 3 = ≥70%25 |
| HER2 | HercepTest | Ready to use | Dako | Microwave 15 minutes, Epitope retrieval solution (HercepTest. cat # K5207) | Membranous; 0, 1+, 2+, 3+26 |
| EGFR | 2-18C9 | Ready to use | Dako | Proteinase K (DAKO, PharmDX, Code K1494) | Membranous; 0, 1+, 2+, 3+ (DAKO, PharmDX); 0 = 0%; positive- if any staining observed |
| CK 5/6 | D5/16 B4 | 1:100 | Dako | Microwave 30 minutes, citrate buffer (pH 6.0) | Cytosolic; 0 = ≤5%, 1 = 6–30%, 2 = 31–60%, 3 = >61%27 |
| Vimentin | V9 | 1:50 | Dako | None | cytosolic; 0 = ≤5%, 1 = 6–30%, 2 = 31–60%, 3 = >61%27 |
| β-Catenin | 14 | 1:200 | Transduction Laboratories/BD Biosciences (San Jose, CA) | Microwave 30 minutes, citrate buffer (pH 6.0) | Nuclear; cytosolic; membranous; 0 = ≤10%, 1 = 11–30%, 2 = 30–70%, 3 = ≥70%25 |
| CD24 | Ab-2, SN3b | 1:200 | Lab Vision/Thermo Fisher | Steamer 20 minutes, target retrieval solution (DAKO, S1699) | Cytosolic; 0 = 0%, 1 = 1–10%, 2 = 11–50%, 3 = 51–75%, 4 = 76–100%28 |
| CD44 | Ab-4, 156-3C11 | 1:200 | Lab Vision/Thermo Fisher | Steamer 20 minutes, target retrieval solution (DAKO, S1699) | Membranous; 0 = 0%, 1 = 1–10%, 2 = 11–50%, 3 = 51–75%, 4 = 76–100%28 |
Immunohistochemistry Evaluation
Two observers (A.I.K., G.F.K.) performed quantitative analysis of the tissue specimen without knowledge of specimen identification. Scoring was based on intensity and percentage of positively stained cells (Table 2); all discrepancies were resolved by a second examination using a multihead microscope. As described above, the tissues were evaluated previously for the expression of ER, PR, HER2, CK 5/6, and EGFR for subtyping, whereas β-catenin, vimentin, and CD44/CD24 were analyzed for the current study. The immunohistochemistry score for ER, PR, and β-catenin was calculated as described25; however, membrane-associated, cytosolic and nuclear β-catenin immunoreactivity were evaluated separately as described in supplemental Figure S1 at http://ajp.amjpathol.org,25 which also shows representative images of each category and score. HER-2 was evaluated by immunohistochemistry according to American Society of Clinical Oncology/College of American Pathologists guidelines.26 EGFR immunostaining was evaluated according to PharmDX recommendations. The vimentin and CK 5/6 staining were evaluated as described.27 CD44 and CD24 scoring was performed as described.28
Data Analysis
Random-effects ordinal regression models29 were used to compare the score of β-catenin among the tissue types, taking into account the within-patient correlation and the order of β-catenin score. Kruska–Wallis tests were used to examine whether β-catenin, CD44+/CD24−, and vimentin scores were different across tumor subtypes. Spearman correlations determined the interrelationships between β-catenin, CD44+/CD24−, vimentin, ER, PR, HER2, EGFR, CK 5/6, age at diagnosis, tumor size, tumor stage, and histological grade. The Wilcoxon rank-sum test was used to examine whether β-catenin score was different between blakcs and whites. Proportional odds models were used to model β-catenin as a function of clinical characteristics and to identify independent associations. Survival curves were generated by the Kaplan–Meier method, and the differences in survival rate were analyzed with the Log-rank test. Cox proportional hazard models were used to control other prognostic factors in survival analysis to examine the independent prognostic value of β-catenin. P values <0.05 were considered statistically significant.
Results
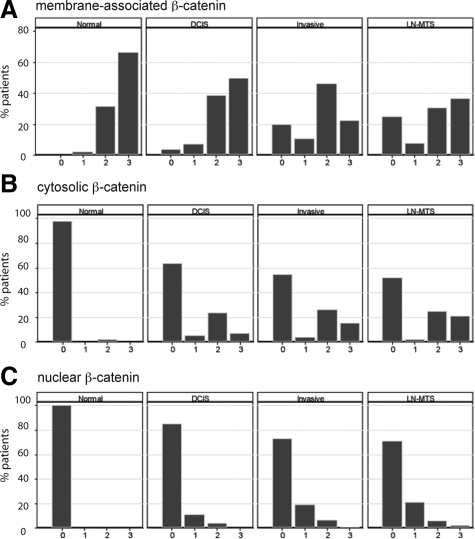
To determine whether Wnt pathway activation is observed in a specific breast cancer subtype, we analyzed β-catenin localization in TMAs constructed from breast cancer specimens collected at the University of Chicago between 1992 and 2004. The TMAs contained 772 core specimens from 190 different patients—56 in situ and 134 invasive breast cancers. The clinical characteristics of the cases are described in Table 1, and details about the scoring of β-catenin immunohistochemistry, as well as representative images, are shown in supplemental Figure S125 (at http://ajp.amjpathol.org). Membrane localization of β-catenin was observed in all normal breast tissues and a majority of in situ specimens, invasive cancers, and metastases (Figure 1A). As predicted from the literature,8,30 there was a statistically significant decrease in membrane-associated β-catenin between normal tissue and the three tumor tissue types and from in situ to invasive cancer (P < 0.0001). In contrast, cytosolic β-catenin was infrequently observed in normal breast but observed in some in situ lesions, invasive cancers, and metastases (Figure 1B). Similar to cytosolic β-catenin, nuclear localization of β-catenin was not observed in normal breast but increased in both frequency and intensity within in situ and invasive cancers and metastases (Figure 1C). These data support disparate roles for distinct subcellular pools of β-catenin in breast cancer and suggest that Wnt signaling may be activated during tumor progression.
Figure 1.
A shift in β-catenin localization from the membrane to the cytosol and nucleus is associated with breast cancer development. The subcellular localization of β-catenin was analyzed in normal breast (n = 95), DCIS lesions (n = 80), invasive breast cancers (n = 119), and lymph node metastases (n = 52) specimens. A: Membranous β-catenin score was higher in normal breast tissue than in carcinoma in situ, invasive cancer, and lymph node metastases (P < 0.0001). Both cytosolic (B) and nuclear (C) β-catenin score were lower in normal breast tissues than in situ and invasive cancers and metastases (P < 0.0001). In all graphs, the x axis describes the immunohistochemical score, ranging from 0 (no staining) to 3 or 4 (highest intensity and/or percentage of positive cells) as described in the Materials and Methods and Table 2.
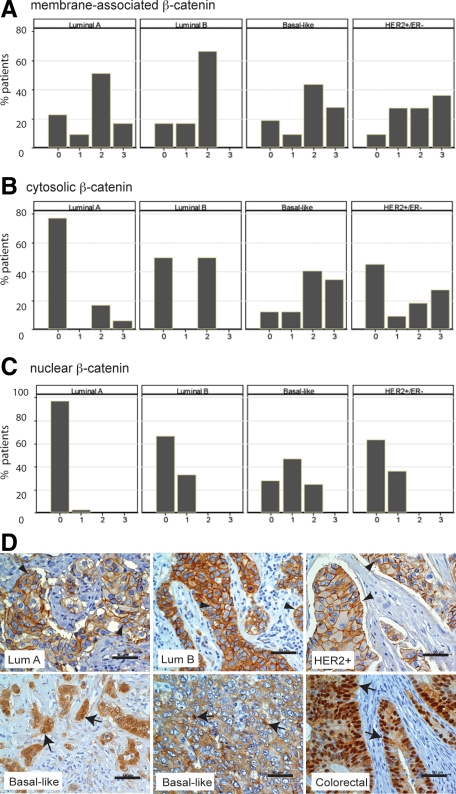
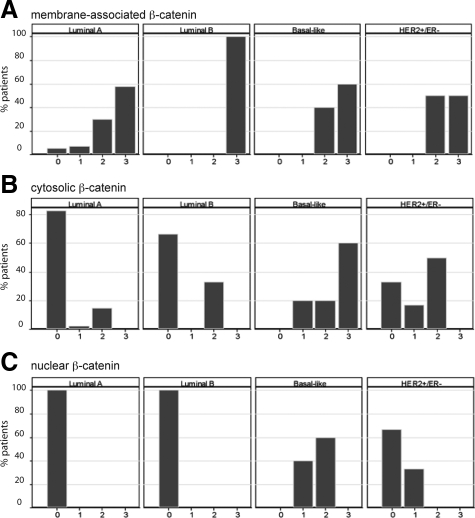
Given that cytosolic and nuclear accumulation of β-catenin was expressed in fewer than half of the breast tumors analyzed, we next determined whether the positive tumors segregated into any particular subtype. Using ER, PR, HER2, EGFR, and cytokeratin 5/6 as biomarkers, most of the invasive breast cancers on the TMA were classified into the luminal A (n = 66), luminal B (n = 6), HER2+/ER− (n = 11), and basal-like (n = 32) subtypes. In invasive breast cancers, membrane staining of β-catenin was not differently represented in any of the tumor subtypes and all subtypes had more than 78% of tumors expressing at least weak membrane-localized β-catenin (Figure 2A). In contrast, cytosolic and nuclear β-catenin was expressed in a majority of basal-like breast cancers (Figures 2, B and C). The proportion of tumors positive for cytosolic or nuclear β-catenin in basal-like tumors was statistically higher than all other tumor subtypes, except HER2+/ER−, for cytosolic β-catenin. There was a low frequency of cytosolic and nuclear β-catenin in luminal A cancers, which was significantly different from any other tumor subtype except cytosolic β-catenin in luminal B tumors. Representative images of β-catenin in each of these subtypes of invasive breast cancers are shown in Figure 2D. It should be noted that the cytosolic and nuclear β-catenin were tightly correlated with each other (r = 0.61, P < 0.0001), and although all tumors with nuclear β-catenin simultaneously had cytosolic localization, not all tumors with cytosolic localization showed nuclear β-catenin.
Figure 2.
Cytosolic and nuclear β-catenin is most frequently observed in basal-like breast cancers. The subcellular localization of β-catenin was analyzed in 66 luminal A, 6 luminal B, 32 basal-like, and 11 HER+/ER− invasive cancers. A–C: While there was no segregation of membrane-associated β-catenin (A) among different subtypes of invasive breast cancer, a higher percentage of basal-like tumors expressed cytosolic (B) or nuclear (C) β-catenin (28/32 and 23/32, respectively) than luminal A (15/66 and 2/66, respectively), luminal B (3/6 and 2/6, respectively), and HER2+/ER− tumors (6/11 and 4/11, respectively). P < 0.05 for all comparisons between basal-like and other tumor types except with HER2+/ER− for cytosolic β-catenin. D: β-catenin immunolocalization (arrowheads) is observed predominantly at the membrane of luminal A, luminal B, and HER2+/ER− tumors. Nuclear and cytosolic β-catenin (arrows) is most prominent in basal-like breast cancers. A sporadic colon carcinoma is shown as a positive control for β-catenin accumulation in the cytosol and nuclei. Scale bars, 50 μm.
Given that cytosolic and nuclear β-catenin was frequently observed in basal-like breast cancers, we next addressed whether β-catenin localization was associated with other histological markers of the basal-like phenotype. Cytosolic β-catenin localization was inversely correlated with ER (r = −0.46, P < 0.0001) and PR (r = −0.43, P < 0.0001) and positively correlated with EGFR (r = 0.34, P = 0.0002) and cytokeratin 5/6 (r = 0.29, P = 0.002). Similarly, nuclear β-catenin was inversely correlated with ER (r = −0.60, P < 0.0001) and PR (r = −0.55, P < 0.0001) and positively correlated with EGFR (r = 0.36, P = 0.0001) and cytokeratin 5/6 (r = 0.26, P < 0.0001). Additionally, cytosolic and nuclear β-catenin localization were strongly associated with vimentin expression in invasive cancers (r = 0.43, P < 0.0001 for cytosolic and r = 0.63, P < 0.0001 for nuclear), which was also highly enriched in basal-like tumors (see supplemental Figure S2 at http://ajp.amjpathol.org). These correlations were dependent on molecular subtype because these are all hallmarks of the basal-like phenotype. Furthermore, cytosolic, but not nuclear, β-catenin was positively correlated with high grade (Table 3). β-catenin localization was not associated with tumor stage, size, lymph node status, or age at diagnosis (not shown). The association between grade and cytosolic β-catenin was no longer statistically significant after controlling for molecular subtype in a proportional odds model, perhaps because basal-like tumors are more likely to be high grade. Interestingly, high levels of cytosolic β-catenin were more frequently associated with tumors from blacks as compared with whites or other ethnicities (P = 0.048; Table 3), and this racial difference remained statistically significant after controlling for the molecular subtype (P = 0.016). These data underscore the relationship between cytosolic/nuclear accumulation of β-catenin and breast cancers with the histological phenotypes and clinical behaviors associated with basal-like tumors.
Table 3.
β-Catenin and Clinical Characteristics in Patients with Invasive Cancer
| Cytosolic β-catenin score | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | P value | |
| Race, n (raw %) | |||||
| Black | 34 (48) | 4 (6) | 18 (25) | 15 (21) | 0.047 |
| White | 28 (65) | 1 (2) | 11 (25) | 3 (7) | |
| In basal-like tumors | |||||
| Black | 1 (5) | 3 (16) | 7 (37) | 8 (42) | |
| White | 3 (23) | 1 (8) | 6 (46) | 3 (23) | |
| In luminal A tumors | |||||
| Black | 26 (70) | 0 | 7 (19) | 4 (11) | |
| White | 22 (88) | 0 | 3 (12) | 0 | |
| Grade, n (raw %) | |||||
| I | 4 (67) | 0 | 2 (33) | 0 | 0.015 |
| II | 35 (64) | 0 | 16 (29) | 4 (7) | |
| III | 24 (44) | 4 (7) | 13 (24) | 14 (25) |
| Nuclear β-catenin score | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | P value | |
| Race, n (row %) | |||||
| Black | 51 (72) | 17 (24) | 3 (4) | 0 | 0.97 |
| White | 32 (75) | 5 (12) | 5 (12) | 1 (2) | |
| Grade, n (raw %) | |||||
| I | 6 (100) | 0 | 0 | 0 | 0.14 |
| II | 42 (76) | 8 (15) | 5 (9) | 0 | |
| III | 37 (67) | 14 (25) | 3 (5) | 1 (2) |
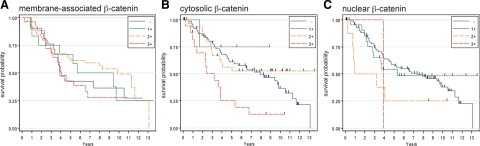
Basal-like breast cancers are typically associated with a worse clinical outcome compared with luminal A and luminal B cancers. Because of the association of cytosolic and nuclear β-catenin with the basal-like phenotype, we asked whether β-catenin localization was predictive of survival in this patient group. Of the 134 patients with invasive breast cancer in our cohort, we had data on overall survival on 131 of them with a median follow-up of 8.3 years in which five-year survival rate was 55.2%, whereas the median survival was 6.6 years. In the 114 samples that had interpretable β-catenin localization, subtype classification, and follow-up, there was no impact of membrane-associated β-catenin expression on overall patient survival (Figure 3A). However, those patients whose tumors had high levels of cytosolic or nuclear β-catenin expression had a significantly reduced overall survival (Figures 3, B and C). The median survival time of patients with high cytosolic β-catenin was only 3.2 years, whereas those with no, weak, or moderate cytosolic β-catenin had a median survival of 8.3 years. Compared with patients with either no or weak/moderate staining of cytosolic β-catenin, the hazard ratio of patients with strong staining was 2.79 (95% confidence interval CI: 1.53–5.10; P = 0.001). After adjustment for ER status, tumor grade, and stage, high cytosolic β-catenin staining remained significantly associated with worse overall survival, with adjusted hazard ratio of 2.91 (95% CI: 1.48–5.73; P = 0.002). Among patients with basal-like invasive cancer (n = 31), the hazard ratio for strong cytosolic β-catenin staining compared with no or weak/moderate staining was 2.32 (95% CI: 0.91–5.89; P = 0.08). Compared with patients with no or weak nuclear β-catenin, those with moderate or strong nuclear β-catenin had a hazard ratio of 2.24 (95% CI: 1.12–4.93; P = 0.045). Among patients with basal-like invasive cancer, the hazard ratio for moderate or strong nuclear β-catenin was 1.83 (95% CI: 0.68–4.89; P = 0.23). Collectively, these data suggest that high cytosolic or nuclear β-catenin may have independent prognostic value for breast cancer, even within the basal-like subtype.
Figure 3.
Invasive breast cancers with high cytosolic and nuclear β-catenin are associated with poor survival. Kaplan–Meier overall survival curves are presented for 117 patients with invasive breast cancers according to expression of membranous (A), cytosolic (B), and nuclear (C) β-catenin. In contrast to a lack of association of membrane-associated β-catenin with overall survival, high levels of cytosolic (score of 3) or nuclear (score of ≥2) β-catenin expression is predictive of poor outcome (P = 0.0005 and P = 0.039, respectively).
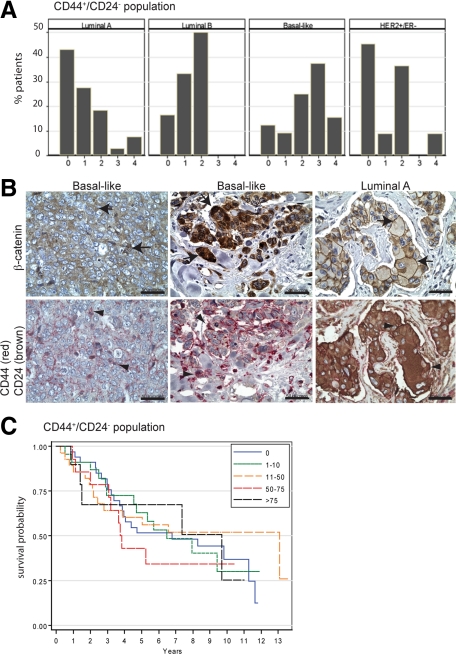
Because basal-like breast cancers are enriched for CD44high/CD24low stem cell-like population of tumor cells,28 the expression of CD44 and CD24 were also examined in these tumors. As expected, high CD44+/CD24− score was specifically associated with the basal-like subtype of invasive breast cancers (Figure 4A). Importantly, in invasive cancers, the CD44+/CD24− profile was correlated with cytosolic (r = 0.31; P = 0.0009) and nuclear (r = 0.38; P < 0.0001) β-catenin localization as well as vimentin expression (r = 0.45; P < 0.0001). Concomitant expression of cytosolic/nuclear β-catenin and CD44, but not CD24, was observed in basal-like tumor cells (Figure 4B). Like β-catenin, CD44+/CD24− population was inversely correlated with ER and PR status (r = −0.28; P = 0.0022 and r = −0.29; P = 0.0015) and positively correlated with EGFR (r = 0.27; P = 0.0046) and cytokeratin 5/6 (r = 0.24; P = 0.012). However, the CD44+/CD24− score was not correlated with age, race, histological grade, and stage (not shown) and not predictive of overall survival (Figure 4C). These data suggest that those tumor cells with cytosolic and nuclear β-catenin are most likely to be of the basal-like phenotype with a CD44+/CD24− profile; yet, β-catenin is a better predictor of outcome than the CD44+/CD24− profile in this set of patients.
Figure 4.
The invasive breast cancers with cytosolic and nuclear β-catenin are enriched in stem cell populations. A: CD44 and CD24 immunohistochemistry was performed to identify a CD44+/CD24− stem cell population in luminal A (n = 65), luminal B (n = 6), HER2+/ER− (n = 11), and basal-like (n = 32) tumors. The percentage of invasive cancers with high CD44+/CD24− scores (>3) is significantly higher in basal-like breast cancers compared with other tumor subtypes (P = 0.0001). B: Immunolocalization of β-catenin and the identification of the CD44+/CD24− profile on serial sections of basal-like breast cancers illustrate that the same tumor cells have cytosolic and nuclear β-catenin (arrows) and are CD44-positive but CD24-negative (arrowheads). In contrast, membrane-associated β-catenin (arrows) colocalizes with CD44-negative/CD24-positive cells (arrowheads) in a luminal A tumor, for example. Scale bars, 50 μm. C: Kaplan–Meier survival curves show that there is no significant difference in overall survival of breast cancer patients when the tumors are stratified by the percentage of CD44+/CD24− tumor cell populations (P = 0.98).
Finally, we addressed how early in tumor progression that accumulation of β-catenin was associated with the basal-like phenotype. Analysis of β-catenin expression in in situ lesions of each molecular subtype (40 luminal A, 3 luminal B, 5 basal-like, and 6 HER2+/ER−) shows that, like invasive cancers, membrane-associated β-catenin was not preferentially expressed in any subtype of in situ carcinoma subtype (Figure 5A). In contrast, cytosolic and nuclear β-catenin was expressed in all basal-like in situ lesions but less frequently in the other subtypes (Figure 5, B and C). Cytosolic and nuclear β-catenin was positively correlated with high-grade (r = 0.43; P = 0.0026 for cytosolic and r = 0.54; P = 0.0001 for nuclear) and EGFR expression (r = 0.31; P = 0.023 for cytosolic and r = 0.53; P < 0.0001 for nuclear) and negatively associated with ER (r = −0.48; P = 0.0002 for cytosolic and r = −0.59; P < 0.0001 for nuclear) and PR (r = −0.45; P = 0.0007 for cytosolic and r = −0.55; P < 0.0001 for nuclear) status. Moreover, nuclear β-catenin correlated with vimentin expression (r = 0.39; P = 0.0044) in in situ carcinomas, and vimentin was expressed in most basal-like in situ lesions but not in other subtypes (see supplemental Figure S3A at http://ajp.amjpathol.org). In contrast to that observed in invasive cancer, there was neither a correlation between the CD44+/CD24− expression profile and cytosolic (r = −0.08; P = 0.50) or nuclear β-catenin (r = −0.07; P = 0.57), nor between the CD44+/CD24− profile and in situ cancer subtype (see supplemental Figure S3B at http://ajp.amjpathol.org). Despite the few number of samples represented by each subtype in this data set, these findings suggest that β-catenin accumulation may be an early marker of the basal-like phenotype.
Figure 5.
Cytosolic and nuclear β-catenin is observed in basal-like carcinoma in situ lesions. The subcellular localization of β-catenin was analyzed in 54 in situ carcinomas, composed of four molecular subtypes (40 luminal A, 3 luminal B, 5 basal-like, and 6 HER2+/ER−). Like invasive cancers, there was no segregation of membrane-associated β-catenin (A) among different subtypes of carcinoma in situ. However, a higher percentage of basal-like tumors expressed cytosolic (B) and nuclear (C) β-catenin (5/5 for both) than luminal A (7/40 and 0/40, respectively), luminal B (1/3 and 0/3, respectively), and HER2+/ER− lesions (4/6 and 2/6, respectively). P < 0.001.
Discussion
We report here that cytoplasmic and nuclear accumulation of β-catenin, indicative of Wnt pathway activation, is most frequently observed in the basal-like subtype of in situ and invasive cancers. In in situ and invasive cancers, cytosolic and nuclear β-catenin are associated with other features of basal-like tumors including ER and PR negativity, EGFR, and CK 5/6 expression as well as vimentin expression. In invasive cancers, but not in situ cancer, β-catenin accumulation was correlated with the CD44+/CD24− profile such that Wnt pathway activation in these tumors might be a reflection of their enriched stem cell composition. Importantly, Wnt pathway activation in breast cancer is associated with a poor outcome, suggesting that it might be a valuable therapeutic target for this tumor type.
The role of the Wnt pathway activation in breast cancer has been controversial. There are conflicting reports of nuclear β-catenin in breast cancer specimens,7,8,13,14,15 perhaps because other studies have not classified the tumors by molecular subtype. There are significant limitations to the current study, including modest size of the tumor data set, few cases representing the luminal B and HER2+/ER− subtypes of invasive and in situ cancers, and using immunohistochemical detection of nuclear/cytosolic β-catenin accumulation as the only read-out of Wnt pathway activation. Despite these confounding factors, our data do support the hypothesis that breast cancers with a basal-like phenotype frequently demonstrate Wnt/β-catenin signaling. In mouse models, activation of the Wnt pathway is sufficient for mammary tumors, but most of these tumors exhibit more of a metaplastic than basal-like phenotype.31 Interestingly, human metaplastic breast cancers are, like basal-like tumors, triple-negative and have a distinct gene expression signature indicative of altered differentiation patterns.32 In mouse models of basal-like breast cancer, including conditional Brca1/p53 inactivation33 and adiponectin haploinsufficiency,34 β-catenin accumulation is observed in at least a subset of tumors. These data raise the possibility that activation of signaling via the Wnt pathway is an important component of the basal-like phenotype but not sufficient for initiating this tumor program. Alternative explanations for the lack of basal-like mammary tumors in Wnt pathway genetic models include nonphysiological levels of pathway activation in these systems or that the critical tumor initiating cell compartment has not been properly targeted. Clearly, further studies are required to distinguish among these possibilities using both human cancers and animal models.
What are the implications of these data for the treatment of basal-like breast cancer? Although basal-like breast cancers make up only 15 to 20% of all breast cancers, they present a significant clinical problem because they are aggressive in nature and are not amenable to many targeted therapies available for breast cancer. In addition, basal-like and HER2+/ER− tumors are initially more chemosensitive than other tumor types but have a worse prognosis attributable to higher relapse among those with residual disease.35 Our data are consistent with the possibility that β-catenin might be an attractive therapeutic target for basal-like breast cancer. Although Wnt pathway inhibitors have not been studied extensively in preclinical breast cancer models, there have been significant efforts to test the efficacy of such compounds in colorectal cancer.36,37 Additional studies are necessary to evaluate these compounds, or newer generation inhibitors, in preclinical models to explore their therapeutic potential in breast cancer patients.
We report here that cytosolic and nuclear β-catenin accumulation in breast cancers correlates with several markers of the basal-like phenotype, including vimentin expression. Vimentin, an intermediate filament expressed in mesenchymal cells, is implicated in the epithelial-to-mesenchymal transition from many contexts including breast cancer.38 Up-regulation of vimentin preferentially occurs in breast cancers with the basal-like phenotype and may be related to the aggressive behavior and metastatic spread of these tumors.39 The correlation between vimentin expression and Wnt/β-catenin signaling in basal-like tumors suggests that there may be a direct relationship. In fact, there is evidence that vimentin is a direct transcriptional target of β-catenin/TCF in breast cancer cells.40 Alternatively, it is feasible that vimentin is not a direct β-catenin target but is regulated in a coordinated fashion with Wnt signaling.
The relationship between nuclear and cytosolic β-catenin accumulation and tumor cells with a stem cell profile is consistent with known functions of Wnt/β-catenin signaling in the breast and other tissues in maintaining stem cell self-renewal.41 Moreover, mammary tumor cells from MMTV-Wnt and -ΔNβ-catenin transgenic models are enriched for stem cell populations.42 It is likely that Wnt signaling in tumor cells with stem cell characteristics has implications for therapeutic sensitivity because the pathway mediates radiation resistance of mammary progenitor cells.42,43 It is interesting that many of the in situ lesions in our study, not just those of the basal-like subtype, were enriched for stem cell markers. Perhaps this subpopulation of tumor cells has a broader role in early breast tumor progression than previously appreciated. The lack of an association between Wnt pathway activation and the stem cell profile in in situ cancer suggests that β-catenin may be a more specific feature of early basal-like tumors than stem cell enrichment, although this hypothesis will need to be formally tested by additional analyses of in situ breast cancers.
The data described here raise some key questions that will need to be addressed by further studies. For example, it was unexpected that cytosolic β-catenin was such a strong predictor of poor outcome, even in the absence of nuclear accumulation in some cases. Consistent with our observations, cytosolic β-catenin without nuclear localization has been reported previously in some breast cancers.13,44 Most colorectal cancers, however, express prominent nuclear β-catenin that is associated with cytoplasmic localization.45,46 In fact, Herter et al47 suggested that nuclear β-catenin accumulation is the first event in colorectal tumor development, followed by an increase in the cytosolic pool. The disconnect between these pools of β-catenin in breast tumors leaves open the possibility that there is a unique function of cytosolic β-catenin. Another interesting aspect of these data are the association between the presence of cytosolic and nuclear β-catenin and race. It is not understood why black women are at a higher risk to develop basal-like breast cancers compared with their white counterparts48,49; however, this study raises the possibility that the Wnt/β-catenin pathway may play a role in the underlying biological differences that contribute to varying susceptibility among populations. Finally, the mechanism by which the Wnt/β-catenin pathway is activated in these tumors is unknown. It is possible that the pathway is active through Wnt ligand expression, as in the stem cell niche, or, alternatively, there are acquired genetic or epigenetic alterations selected for during tumor development. Although such events have not been documented specifically in basal-like tumors, loss of APC is observed in some invasive breast cancers,5 and stabilizing mutations in the β-catenin gene, CTNNB1, have been described in triple-negative metaplastic breast cancers.50 It is possible that through further characterization of the role of Wnt/β-catenin signaling in basal-like tumors, β-catenin will emerge as a valuable therapeutic target for this breast cancer subtype.
Acknowledgments
We thank Shihong Li and Can Gong for their excellent technical assistance, Chuanhong Liao for assistance with data management, and Drs. Jenifer Prosperi and Donald Vander Griend for critical review of the manuscript.
Footnotes
Address reprint requests to Kathleen H. Goss, Ph.D., University of Chicago, Department of Surgery, 5841 S. Maryland Ave., Chicago, IL 60637. E-mail: kgoss@surgery.bsd.uchicago.edu.
Supported by NCI R03 CA132143-01A1 (to D.H.), NCI P50 CA125183, Lee Jeans Translational Research and Entertainment Industry Foundation Fund (to O.I.O.), and AACR-Komen Breast Cancer Career Development Award (to K.H.G.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Rijsewijk F, Schuermann M, Wagenaar E, Parren P, Weigel D, Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987;50:649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- Tsukamoto AS, Grosschedl R, Guzman RC, Parslow T, Varmus HE. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988;55:619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Hatsell S, Rowlands T, Hiremath M, Cowin P. Beta-catenin and Tcfs in mammary development and cancer. J Mammary Gland Biol Neoplasia. 2003;8:145–158. doi: 10.1023/a:1025944723047. [DOI] [PubMed] [Google Scholar]
- Zardawi SJ, O'Toole SA, Sutherland RL, Musgrove EA. Dysregulation of Hedgehog. Wnt and Notch signalling pathways in breast cancer. Histol Histopathol. 2009;24:385–398. doi: 10.14670/HH-24.385. [DOI] [PubMed] [Google Scholar]
- Ryo A, Nakamura M, Wulf G, Liou YC, Lu KP. Pin1 regulates turnover and subcellular localization of beta-catenin by inhibiting its interaction with APC. Nat Cell Biol. 2001;3:793–801. doi: 10.1038/ncb0901-793. [DOI] [PubMed] [Google Scholar]
- Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung MC. Beta-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci U S A. 2000;97:4262–4266. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolled-Filhart M, McCabe A, Giltnane J, Cregger M, Camp RL, Rimm DL. Quantitative in situ analysis of beta-catenin expression in breast cancer shows decreased expression is associated with poor outcome. Cancer Res. 2006;66:5487–5494. doi: 10.1158/0008-5472.CAN-06-0100. [DOI] [PubMed] [Google Scholar]
- Nakopoulou L, Gakiopoulou H, Keramopoulos A, Giannopoulou I, Athanassiadou P, Mavrommatis J, Davaris PS. c-met tyrosine kinase receptor expression is associated with abnormal beta-catenin expression and favourable prognostic factors in invasive breast carcinoma. Histopathology. 2000;36:313–325. doi: 10.1046/j.1365-2559.2000.00847.x. [DOI] [PubMed] [Google Scholar]
- Dillon DA, D'Aquila T, Reynolds AB, Fearon ER, Rimm DL. The expression of p120ctn protein in breast cancer is independent of alpha- and beta-catenin and E-cadherin. Am J Pathol. 1998;152:75–82. [PMC free article] [PubMed] [Google Scholar]
- Karayiannakis AJ, Nakopoulou L, Gakiopoulou H, Keramopoulos A, Davaris PS, Pignatelli M. Expression patterns of beta-catenin in in situ and invasive breast cancer. Eur J Surg Oncol. 2001;27:31–36. doi: 10.1053/ejso.1999.1017. [DOI] [PubMed] [Google Scholar]
- Bukholm IK, Nesland JM, Karesen R, Jacobsen U, Borresen-Dale AL. E-cadherin and alpha-, beta-, and gamma-catenin protein expression in relation to metastasis in human breast carcinoma. J Pathol. 1998;185:262–266. doi: 10.1002/(SICI)1096-9896(199807)185:3<262::AID-PATH97>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Wong SC, Lo SF, Lee KC, Yam JW, Chan JK, Wendy Hsiao WL. Expression of frizzled-related protein and Wnt-signalling molecules in invasive human breast tumours. J Pathol. 2002;196:145–153. doi: 10.1002/path.1035. [DOI] [PubMed] [Google Scholar]
- Gillett CE, Miles DW, Ryder K, Skilton D, Liebman RD, Springall RJ, Barnes DM, Hanby AM. Retention of the expression of E-cadherin and catenins is associated with shorter survival in grade III ductal carcinoma of the breast. J Pathol. 2001;193:433–441. doi: 10.1002/path.831. [DOI] [PubMed] [Google Scholar]
- Pedersen KB, Nesland JM, Fodstad O, Maelandsmo GM. Expression of S100A4. E-cadherin, alpha- and beta-catenin in breast cancer biopsies. Br J Cancer. 2002;87:1281–1286. doi: 10.1038/sj.bjc.6600624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadare O, Tavassoli FA. Clinical and pathologic aspects of basal-like breast cancers. Nat Clin Pract Oncol. 2008;5:149–159. doi: 10.1038/ncponc1038. [DOI] [PubMed] [Google Scholar]
- Bryan BB, Schnitt SJ, Collins LC. Ductal carcinoma in situ with basal-like phenotype: a possible precursor to invasive basal-like breast cancer. Mod Pathol. 2006;19:617–621. doi: 10.1038/modpathol.3800570. [DOI] [PubMed] [Google Scholar]
- Mohsin SK, O'Connell P, Allred DC, Libby AL. Biomarker profile and genetic abnormalities in lobular carcinoma in situ. Breast Cancer Res Treat. 2005;90:249–256. doi: 10.1007/s10549-004-4493-8. [DOI] [PubMed] [Google Scholar]
- Elston EW, Ellis IO. Method for grading breast cancer. J Clin Pathol. 1993;46:189–190. doi: 10.1136/jcp.46.2.189-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Thomas P. Tumors of the breast. Cancer Grading Manual. 2007:75–81. [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Hu Z, Fan C, Oh DS, Marron JS, He X, Qaqish BF, Livasy C, Carey LA, Reynolds E, Dressler L, Nobel A, Parker J, Ewend MG, Sawyer LR, Wu J, Liu Y, Nanda R, Tretiakova M, Ruiz Orrico A, Dreher D, Palazzo JP, Perreard L, Nelson E, Mone M, Hansen H, Mullins M, Quackenbush JF, Ellis MJ, Olopade OI, Bernard PS, Perou CM. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96–108. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajdacsy-Balla A, Geynisman JM, Macias V, Setty S, Nanaji NM, Berman JJ, Dobbin K, Melamed J, Kong X, Bosland M, Orenstein J, Bayerl J, Becich MJ, Dhir R, Datta MW. Practical aspects of planning, building, and interpreting tissue microarrays: the Cooperative Prostate Cancer Tissue Resource experience. J Mol Histol. 2007;38:113–121. doi: 10.1007/s10735-006-9054-5. [DOI] [PubMed] [Google Scholar]
- Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- Reiner A, Neumeister B, Spona J, Reiner G, Schemper M, Jakesz R. Immunocytochemical localization of estrogen and progesterone receptor and prognosis in human primary breast cancer. Cancer Res. 1990;50:7057–7061. [PubMed] [Google Scholar]
- Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- Dabbs DJ, Chivukula M, Carter G, Bhargava R. Basal phenotype of ductal carcinoma in situ: recognition and immunohistologic profile. Mod Pathol. 2006;19:1506–1511. doi: 10.1038/modpathol.3800678. [DOI] [PubMed] [Google Scholar]
- Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lovgren K, Grabau D, Ferno M, Borg A, Hegardt C. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeker D, Gibbons RD. A random-effects ordinal regression model for multilevel analysis. Biometrics. 1994;50:933–944. [PubMed] [Google Scholar]
- Hao X, Tomlinson I, Ilyas M, Palazzo JP, Talbot IC. Reciprocity between membranous and nuclear expression of beta-catenin in colorectal tumours. Virchows Arch. 1997;431:167–172. doi: 10.1007/s004280050084. [DOI] [PubMed] [Google Scholar]
- Rosner A, Miyoshi K, Landesman-Bollag E, Xu X, Seldin DC, Moser AR, MacLeod CL, Shyamala G, Gillgrass AE, Cardiff RD. Pathway pathology: histological differences between ErbB/Ras and Wnt pathway transgenic mammary tumors. Am J Pathol. 2002;161:1087–1097. doi: 10.1016/S0002-9440(10)64269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, Fridlyand J, Sahin A, Agarwal R, Joy C, Liu W, Stivers D, Baggerly K, Carey M, Lluch A, Monteagudo C, He X, Weigman V, Fan C, Palazzo J, Hortobagyi GN, Nolden LK, Wang NJ, Valero V, Gray JW, Perou CM, Mills GB. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69:4116–4124. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy A, Savage K, Gabriel A, Naceur C, Reis-Filho JS, Ashworth A. A mouse model of basal-like breast carcinoma with metaplastic elements. J Pathol. 2007;211:389–398. doi: 10.1002/path.2124. [DOI] [PubMed] [Google Scholar]
- Lam JB, Chow KH, Xu A, Lam KS, Liu J, Wong NS, Moon RT, Shepherd PR, Cooper GJ, Wang Y. Adiponectin haploinsufficiency promotes mammary tumor development in MMTV-PyVT mice by modulation of phosphatase and tensin homolog activities. PLoS ONE. 2009;4:e4968. doi: 10.1371/journal.pone.0004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, Ollila DW, Sartor CI, Graham ML, Perou CM. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Small-molecule antagonists of the oncogenic TCF/beta-catenin protein complex. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, Ha JR, Kahn M. A small molecule inhibitor of beta-catenin/CREB-binding protein transcription. Proc Natl Acad Sci U S A. 2004;101:12682–12687. doi: 10.1073/pnas.0404875101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinos MI, Wafai R, Wong MK, Newgreen DF, Thompson EW, Waltham M. Vimentin and epithelial-mesenchymal transition in human breast cancer–observations in vitro and in vivo. Cells Tissues Organs. 2007;185:191–203. doi: 10.1159/000101320. [DOI] [PubMed] [Google Scholar]
- Sarrio D, Rodriguez-Pinilla SM, Hardisson D, Cano A, Moreno-Bueno G, Palacios J. Epithelial-mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 2008;68:989–997. doi: 10.1158/0008-5472.CAN-07-2017. [DOI] [PubMed] [Google Scholar]
- Gilles C, Polette M, Mestdagt M, Nawrocki-Raby B, Ruggeri P, Birembaut P, Foidart JM. Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res. 2003;63:2658–2664. [PubMed] [Google Scholar]
- Woodward WA, Chen MS, Behbod F, Rosen JM. On mammary stem cells. J Cell Sci. 2005;118:3585–3594. doi: 10.1242/jcs.02532. [DOI] [PubMed] [Google Scholar]
- Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci USA. 2007;104:618–623. doi: 10.1073/pnas.0606599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MS, Woodward WA, Behbod F, Peddibhotla S, Alfaro MP, Buchholz TA, Rosen JM. Wnt/beta-catenin mediates radiation resistance of Sca1+ progenitors in an immortalized mammary gland cell line. J Cell Sci. 2007;120:468–477. doi: 10.1242/jcs.03348. [DOI] [PubMed] [Google Scholar]
- Chung GG, Zerkowski MP, Ocal IT, Dolled-Filhart M, Kang JY, Psyrri A, Camp RL, Rimm DL. Beta-catenin and p53 analyses of a breast carcinoma tissue microarray. Cancer. 2004;100:2084–2092. doi: 10.1002/cncr.20232. [DOI] [PubMed] [Google Scholar]
- Hugh TJ, Dillon SA, Taylor BA, Pignatelli M, Poston GJ, Kinsella AR. Cadherin-catenin expression in primary colorectal cancer: a survival analysis. Br J Cancer. 1999;80:1046–1051. doi: 10.1038/sj.bjc.6690461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto M, Ahnen DJ, Franklin WA, Maltzman TH. Expression of beta-catenin and full-length APC protein in normal and neoplastic colonic tissues. Carcinogenesis. 2000;21:1935–1940. doi: 10.1093/carcin/21.11.1935. [DOI] [PubMed] [Google Scholar]
- Herter P, Kuhnen C, Muller KM, Wittinghofer A, Muller O. Intracellular distribution of beta-catenin in colorectal adenomas, carcinomas and Peutz-Jeghers polyps. J Cancer Res Clin Oncol. 1999;125:297–304. doi: 10.1007/s004320050277. [DOI] [PubMed] [Google Scholar]
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. J Am Med Assoc. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- Hayes MJ, Thomas D, Emmons A, Giordano TJ, Kleer CG. Genetic changes of Wnt pathway genes are common events in metaplastic carcinomas of the breast. Clin Cancer Res. 2008;14:4038–4044. doi: 10.1158/1078-0432.CCR-07-4379. [DOI] [PMC free article] [PubMed] [Google Scholar]