Treatment Options for Sexual Dysfunction in Patients with Chronic Kidney Disease: A Systematic Review of Randomized Controlled Trials (original) (raw)
Abstract
Background and objectives: Sexual dysfunction is very common in patients with chronic kidney disease (CKD), but treatment options are limited. The benefits and harms of existing interventions for treatment of sexual dysfunction were assessed in patients with CKD.
Design, setting, participants, & measurements: MEDLINE (1966 to December 2008), EMBASE (1980 to December 2008), and the Cochrane Trial Registry (Issue 4 2008) were searched for parallel and crossover randomized and quasi-randomized trials. Treatment effects were summarized as mean differences (MD) or standardized mean difference (SMD) with 95% confidence intervals (CI) using a random effects model.
Results: Fourteen trials (328 patients) were included. Phosphodiesterase-5 inhibitors (PDE5i) compared with placebo significantly increased the overall International Index of Erectile Function-5 (IIEF-5) score (three trials, 101 patients, MD 1.81, 95% CI 1.51 to 2.10), all of its individual domains, and the complete 15-item IIEF-5 (two trials, 80 patients, MD 10.64, 95% CI 5.32 to 15.96). End-of-treatment testosterone levels were not significantly increased by addition of zinc to dialysate (two trials, 22 patients, SMD 0.19 ng/dl, 95% CI −2.12 to 2.50), but oral zinc improved end-of-treatment testosterone levels. There was no difference in plasma luteinizing and follicle-stimulating hormone level at the end of the study period with zinc therapy.
Conclusions: PDE5i and zinc are promising interventions for treating sexual dysfunction in CKD. Evidence supporting their routine use in CKD patients is limited. There is an unmet need for studying interventions for male and female sexual dysfunction in CKD considering the significant disease burden.
Sexual dysfunction is a set of disorders characterized by physical and psychologic changes that result in the inability to perform satisfactory sexual activities. The condition has been found to be significantly more common in men and women with chronic kidney disease (CKD) than in the general population (1). Men with CKD frequently suffer from reduced libido, erectile dysfunction, and difficulty reaching orgasm (2). Approximately 50% of male predialysis CKD patients and 80% of male dialysis patients have erectile dysfunction (3–6). Moreover, the prevalence of erectile dysfunction in male dialysis patients has been found to increase with age (63% <50 years versus 90% ≥50 years) (3). Similar results have been reported in women with CKD, with 55% of female dialysis patients reporting difficulty with sexual arousal (2). Dysmenorrhea, delayed sexual development, impaired vaginal lubrication, dyspareunia, and difficulties in reaching orgasm are also frequently observed (7,8).
Multiple factors contribute to the frequent occurrence of sexual dysfunction in CKD patients, including hormonal disturbances (such as hyperprolactinemia, hypogonadism in males, and changes in hypothalamic-pituitary function in women) (9), anemia (10), CKD mineral and bone disorder (4), psychosocial factors (such as depression, anxiety, poor self-esteem, social withdrawal, marital discord, body image issues, fear of disability and death, loss of employment, and financial difficulties) (2,11,12), autonomic neuropathy (13), medications (including antihypertensives, antidepressant, and histamine receptor blockers) (2), and comorbid illness (such as diabetes mellitus, cardiovascular disease, and malnutrition) (2,14). Sexual dysfunction is inversely associated with GFR (7) and is improved after renal transplantation (15,16), suggesting that CKD per se may contribute to sexual dysfunction in these patients (15).
Studies have also identified significant associations between sexual dysfunction in CKD patients and depression (8,17), impaired quality of life (8,17,18), and adverse cardiovascular outcomes (19). Effective treatment of sexual dysfunction in CKD patients may therefore potentially lead to improvement in these patient-level outcomes, although a causal link has not been definitively established (18).
Therapies that have been used to treat sexual dysfunction include phosphodiesterase-5 inhibitors (PDE5i), intracavernosal injections, intraurethral suppositories, hormonal therapy, mechanical devices, and psychotherapy. Although many clinical trials and reviews have explored the role of these interventions for sexual dysfunction in nonuremic patients (20–24), the effectiveness and safety of these interventions in patients with CKD have not yet been studied thoroughly. Therefore, we aimed to evaluate the benefits and harms associated with various interventions for sexual dysfunction in patients with CKD.
Materials and Methods
Search Strategy
We searched MEDLINE (via OvidSP, 1966 to December 2008), EMBASE (via OvidSP, 1980 to December 2008), Cochrane Renal Group's Specialized Register, and the Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library Issue 4, 2008) for relevant trials. CENTRAL and the Renal Group's specialized register contain the handsearched results of conference proceedings from general and specialty meetings. This is an ongoing activity across the Cochrane Collaboration and is retrospective and prospective. The search strategy used to obtain titles and abstracts of studies that may be relevant to the review is reported in Appendix 1.
Types of Studies
All randomized controlled trials (RCTs) and quasi-RCTs of any treatment (hormone therapy, PDE5i, intracavernous injections, intraurethral pellets, mechanical devices, and behavioral therapy) for sexual dysfunction in male and female patients with CKD were included. Trials were considered without language restrictions.
Types of Participants
Patients aged >18 years and with any stage of CKD, including patients who are not receiving renal replacement therapy (predialysis) and those with end-stage kidney disease who are receiving hemodialysis or peritoneal dialysis or have a functioning kidney transplant, were considered for inclusion. Studies that enrolled patients without CKD were excluded.
Types of Interventions
All studies of pharmacologic and nonpharmacologic interventions for treating sexual dysfunction in patients with CKD were considered for inclusion. For treatment of male sexual dysfunction, we explored pharmacologic and nonpharmacologic interventions. Pharmacologic agents included hormonal therapy [oral, injected, or topical (transdermal) testosterone] and drugs [oral (PDE5i, sildenafil, tadalafil, vardenafil, and mirodenafil) or topical (intracavernous injections of alprostadil, α1-antagonist, and intraurethral alprostadil, prazosin, or their combinations)]. Nonpharmacologic strategies included mechanical devices (vacuum constriction device for inducing erection, penile prosthesis) and psychoeducational interventions such as rational emotive therapy, sex group therapy, modified Masters & Johnson–Kaplan's sexual therapy, educational intervention, systematic desensitization, and sexual counseling.
Similarly, for treatment of female sexual dysfunction, pharmacologic agents included hormonal therapy [oral or topical (transdermal) estrogens, testosterone, progesterone, or tibolone] and drugs (oral PDE5i). Nonpharmacologic strategies included mechanical interventions (estrogen or nonhormonal lubricating vaginal creams and clitoral therapy devices) and psychoeducational interventions (rational emotive therapy, sex group therapy, modified Masters & Johnson–Kaplan's sexual therapy, educational intervention, systematic desensitization, and sexual counseling).
Types of Outcome Measures
We planned to obtain the following outcome measures as reported in the included studies:
Male sexual dysfunction outcomes
- Changes in mean score on any standard validated sexual function scale. The various scales that were considered for inclusion included the 15-item International Index of Erectile Function (IIEF), 5-item International Index of Erectile Function (IIEF-5), Physic Component Score, and Mental Component Score.
- Achievement of prolonged penile rigidity satisfactory to enable complete sexual intercourse (measurement of genital blood flow and nocturnal penile tumescence).
- Number of successful sexual intercourses and number of participants who showed improved sexual function as measured by patient's log and reported by study authors.
- Hormone levels as measured by trialists (including testosterone or other hormone levels).
- Major and minor adverse effects of interventions (coronary ischemia, headache, flushing for PDE5i, and priapism for intracavernous injections).
- Treatment compliance (as reported by study authors).
- Number of participants who dropped out
Female sexual dysfunction outcomes
- Changes in score on any standard validated sexual function scales [Female Sexual Function Index (FSFI), Female Intervention Efficacy Index (FIEI), and Female Sexual Distress Scale (FSDS)]
- Variation of vaginal pressure-volume, genital threshold of perception of vibration, vaginal pH, genital blood flow, and prolactin and zinc concentrations (units as reported by study authors)
- Hormonal levels as measured by trialists [including luteinizing hormone (LH), follicle-stimulating hormone (FSH), and prolactin levels or other]
- Levels of other markers (including zinc or other as reported by study authors)
- Number of participants who showed improved sexual function (as defined by study authors)
- Adverse effects [incidence of coronary ischemia for PDE5i and breast cancer (ductal carcinoma in situ, lobular carcinoma in situ, invasive ductal carcinoma, invasive lobular carcinoma)] for estrogen replacement therapy and headache as reported in the study
- Treatment compliance as defined by study authors
- Number of participants who dropped out
Data Collection
The titles and abstracts were screened independently by two reviewers (M.V., S.D.N.) who discarded studies that were not applicable; however, studies and reviews that might include relevant data or information on trials were initially retained. The reviewers independently assessed retrieved abstracts and the full text of these studies to determine which studies satisfied the inclusion criteria. The same reviewers independently carried out data extraction using standard data extraction forms. Studies reported in non-English language journals were translated before assessment. When more than one publication of one trial existed, only the paper with the most complete data was included. We only included the data from the first part of the crossover trials for this analysis. Further information required from the original author was requested by written correspondence and any relevant information obtained was included in the review. Disagreements were resolved in consultation with G.F.M.S.
Study Quality
The quality of included studies was assessed independently by M.V. and S.D.N. using a checklist that included allocation concealment; blinding of participants, investigators, outcome assessors, and data analysts; use of intention-to treat-analysis; and completeness to follow-up. Any discrepancy was resolved by discussion with G.F.M.S.
Statistical Assessment
For dichotomous outcomes, adverse effects (coronary ischemia due to PDE5i, priapism, or penile pain due to intraurethal injections, vaginal itching due to vaginal cream, study withdrawal rate due to any adverse effect) results were expressed as relative risk (RR) with 95% confidence intervals (CI). Data were pooled using the random effects model, but the fixed effects model was also analyzed to ensure robustness of the model chosen and susceptibility to outliers. Where continuous scales of measurement were used to assess the effects of treatment (15-IIEF, 5-IIEF, FSFI, and FIEI scores; vaginal pH; genital blood flow; variations of vaginal pressure-volume; genital threshold of perception of vibration; and measurement of testosterone, LH, FSH, prolactin, and zinc concentration), the mean difference (MD) was used, or the standardized mean difference (SMD) if different scale units of measurement were used. Heterogeneity was analyzed using the χ2 test on N − 1 degrees of freedom (Cochran Q), with an alpha of 0.05 used for statistical significance and the _I_2 statistic (25). If an adequate number of studies were identified, we planned for subgroup analysis and metaregression to explore the effects of various covariates relating to patient, treatment, and study characteristics such as stage of kidney disease, treatment duration, and various study quality measures on prespecified treatment outcomes. Analyses were performed using Revman 5 (The Cochrane Collaboration, United Kingdom, 2007).
Results
Search Results
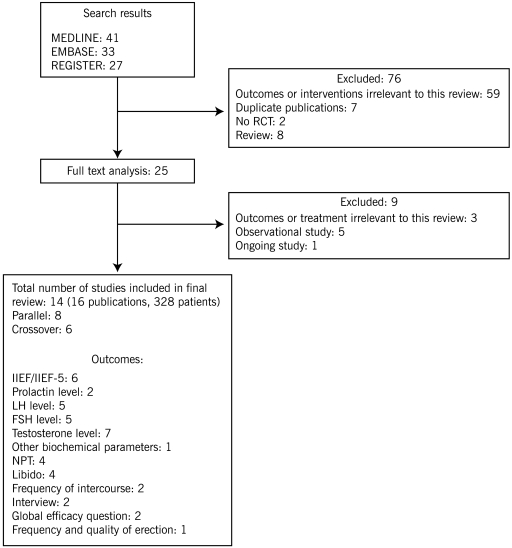
We identified 101 articles in MEDLINE, EMBASE, and in CENTRAL. Seventy-six studies were excluded at the abstract stage because they did not meet the inclusion criteria. Of the remaining 25 citations (full-text analysis), 9 studies were excluded because they assessed treatment or outcomes that were not relevant to this review. Finally, 14 trials reported in 16 publications and enrolling a total of 328 patients were included in the review (Figure 1) (5,26–38). Authors of these trials were contacted for additional details, but none responded.
Figure 1.
Flow chart showing the number of citations retrieved by individual searches and number of trials included in the systematic review.
Trial Characteristics
Two groups of trials were identified: eight parallel trials (26–33) and six crossover trials (5,34–38).
Of the eight parallel trials, four (50 patients) compared elementary zinc or zinc chloride to placebo (26,27,30,32). In three of these trials, zinc chloride was added to the dialysis bath (26,27,32); in one trial elementary zinc was administered orally (30). Two trials (99 patients) compared vardenafil with placebo (28,33), and one trial (41 patients) compared sildenafil citrate with placebo (31). One trial (24 patients) compared vitamin E to placebo (29). Of the remaining six crossover trials, three trials (59 patients) compared sildenafil citrate with placebo (5,34,37,). Two trials (40 patients) compared bromocriptine with placebo (36,38), and one trial (15 patients) compared 1,25-dihydroxycholecalciferol with placebo (35). Five trials included diabetic patients. Of these, one enrolled patients with diabetic nephropathy. Eleven trials included patients on hemodialysis, one included patients on peritoneal dialysis, and the remaining two studies included renal transplant recipients. Other characteristics of the included trials are detailed in Table 1.
Table 1.
Characteristics of studies and participants in the included studies
| Study ID | Study Design | Population Characteristics | Type of Renal Replacement Therapy | Interventiona | N | Follow-Up (months) | Key Outcome Measures | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) Mean (SD)/Range | Duration of Dialysis/Transplant (months) Mean (SD)/Range | Percent Diabetics | IIEF/IIEF-5 | Testosterone Level | LH/FSH Level | NTP | Otherb | |||||
| Antoniou 1977 (26) | Parallel | 48.5 (7.9) | 49.1 (14.5) | NA | HD | Zinc chloride in dialysate (400 μg/L) | 8 | 3 to 4 | x | x | ||
| Brook 1980 (27) | Parallel | 37.6 (8.2) | 28.0 (21.2) | NA | HD | Zinc chloride in dialysate (400 μg/L) | 14 | 1.5 | x | x | x | x |
| Demir 2006 (28) | Parallel | 48 (7.4) | 25 | NA | Txp | Vardenafil (10/20 mg) | 60 | 1 | x | x | ||
| Yeksan 1992 (29) | Parallel | 38.4 (10.8) | NA | NA | HD | Vitamin E (300 mg) | 24 | 2 | x | x | x | |
| Mahajan 1982 (30) | Parallel | 38.0 (7.0) | 24.6 (18.5) | 5.0 | HD | Elementary oral zinc or zinc acetate (25/50 mg) | 20 | 6 | x | x | x | |
| Seibel 2002 (31) | Parallel | 49.0 (10.0) | 42.0 (31.0) | NA | HD | Sildenafil (50 mg) | 41 | 1 | x | |||
| Wabrek 1982 (32) | Parallel | 48.7 (8.3) | 6.6 | 25.0c | HD | Zinc in dialysate (400 mg/L) | 8 | NA | x | x | x | |
| Yang 2008 (33) | Parallel | NA | NA | 0.0 | HD | Vardenafil (10/20 mg) | 39 | 1 | x | x | ||
| Bellovich 2000 (34) | Crossover | 52.4 | NA | 28.0 | HD | Sildenafil citrate (25 to 50 mg) | 14 | NA | x | x | x | |
| Blumberg 1980 (35) | Crossover | 37.4 (10.9) | 29.8 (26.1) | 0.0 | HD | 1,25(OH)2D3 (0.25/0.5/1.5 μg/d) | 15 | 2 to 4 | x | x | x | |
| Bommer 1979 (36) | Crossover | 28.0 to 50.0 | 26.0 to 86.0 | NA | HD | Bromocriptine (5 mg/d) | 15 | 8 | x | x | ||
| Mahon 2005 (37) | Crossover | 55.6 (12.0) | 6.0 to 60.0 | 46.0 | PD | Sildenafil citrate (50/100 mg) | 13 | 1 | x | x | ||
| Muir 1983 (38) | Crossover | 44.5 (10.2) | 62.1 (26.2) | NA | HD | Bromocriptine (until 7.5 mg/day) | 25 | 3 | x | x | ||
| Sharma 2006 (5) | Crossover | 40.0 (8.0) | 56.4 (36.0) | 21.8 | Txp | Sildenafil (25/50/100 mg) | 32 | 2 | x | x |
Study Quality
By current methodological standards for reporting, trial quality was variable. Allocation concealment was adequate in only 2 of 14 (14%) trials (30,38) and unclear in 12 of 14 (86%) trials (27–30,32–38,39). Participants were blinded in 3 of 14 (21%) trials (33,35,36), investigators were blinded in 1 of 14 (7%) trials (26), participants and investigators were blinded in 8 of 14 (57%) trials (5,27,29–32,37–38), but outcome assessors were blinded in none of the trials. Two trials (14%) did not blind all different groups (28,34). None of the 14 trials (0%) was analyzed on an intention-to-treat basis. The number of patients lost to follow-up ranged from 0% to 36.0%.
Study Outcomes
PDE5i versus Placebo.
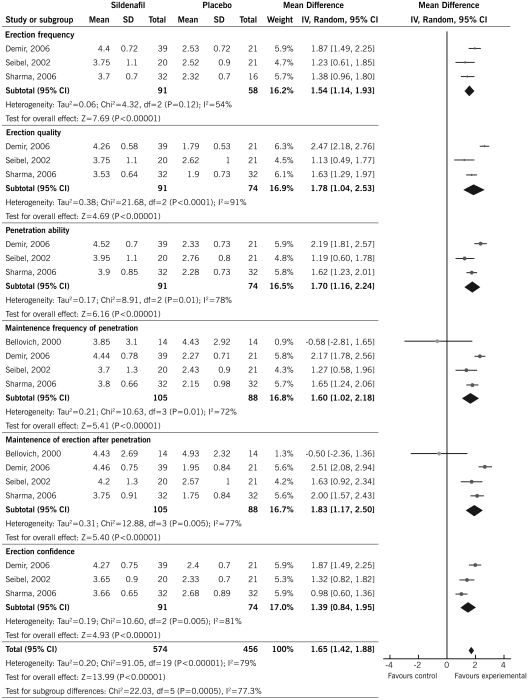
There was a consistent improvement in the overall score of the IIEF-5 with PDE5i compared with placebo (3 RCTs, 101 patients, MD 1.81, 95% CI 1.51 to 2.10) (5,28,31), and a consistent increase of the score of all individual IIEF-5 tool domains: (1) erection frequency (3 RCTs, 149 patients, MD 1.54, 95% CI 1.14 to 1.93) (5,28,31), (2) erection quality (3 RCTs, 165 patients, MD 1.78, 95% CI 1.04 to 2.53) (5,28,31), (3) penetration ability (3 RCTs, 165 patients, MD 1.70, 95% CI 1.16 to 2.24) (5,28,31), (4) maintenance frequency of penetration (4 RCTs, 193 patients, MD 1.60, 95% CI 1.02 to 2.18) (5,28,31,34), (5) maintenance of erection after penetration (4 RCTs, 193 patients, MD 1.83, 95% CI 1.17 to 2.50) (5,28,31,34), and (6) erection confidence (3 RCTs, 165 patients, MD 1.39, 95% CI 0.84 to 1.95) (5,28,31) (Figure 2). When two crossover trials were excluded from the analysis, no significant changes were shown and the resulting effect remained the same for each domain of the overall IIEF-5 score.
Figure 2.
Effect of PDE5i on IIEF-5 scores in CKD patients.
There was significant heterogeneity noted in these analyses (Figure 2). These could be attributed to the differences in the patient characteristics such as stage of kidney disease and baseline comorbid disease conditions, treatment characteristics such as treatment duration and dose of the agent used, and study characteristics such as use of blinding and intention-to-treat analysis. Because of the lack of an adequate number of studies, we could not conduct subgroup analysis or metaregression as planned a priori.
We found a significant increase in the overall satisfaction score of the IIEF-15 sexual assessment tool with sildenafil compared with placebo (1 RCT, 41 patients, MD 2.64, 95% CI 1.32 to 3.96), and a consistent improvement in erectile function (2 RCTs, 80 patients, MD 10.64, 95% CI 5.32 to 15.96), orgasmic function (1 RCT, 41 patients, MD 1.70, 95% CI 0.35 to 3.05), and intercourse satisfaction (1 RCT, 41 patients, MD 1.71, 95% CI 0.11 to 3.31) (31).
Adverse Effects.
There was no significant increase in the risk of headache when PDE5i was compared with placebo (1 RCT, 41 patients, RR 1.05, 95% CI 0.16 to 6.76) (31). Included studies did not report incidence of coronary ischemia for PDE5i, priapism for intracavernous injections, and breast cancer for estrogen replacement therapy.
Zinc versus Placebo
Gonadotropins.
There was no consistent increase in end-of-treatment plasma testosterone concentration by addition of zinc to the dialysate (2 RCTs, 22 patients, SMD 0.19, 95% CI −2.12 to 2.50) (26,27), whereas oral zinc significantly improved end-of-treatment plasma testosterone levels (1 RCT, 20 patients, SMD 1.62, 95% CI 0.58 to 2.66) (29). There was no significant reduction in the end-of-treatment plasma FSH concentration (2 RCTs, 28 patients, MD −9.69 mIU/ml, 95% CI −23.72 to 4.34) (26,30) and plasma LH level (2 RCTs, 20 patients, MD 18.80 mIU/ml, 95% CI −26.16 to 63.76) (26,30) with zinc compared with placebo.
Sexual Function.
One trial showed significant decrease in the frequencies of intercourse (1 RCT, 20 patients, RR 0.22, 95% CI 0.06 to 0.78) and total or partial impotence (1 RCT, 20 patients, RR 0.13, 95% CI 0.02 to 0.82) with zinc compared with placebo (30). There was no consistent variation in libido using zinc compared with placebo (2 RCTs, 34 patients, RR 0.11, 95% CI 0.01 to 1.83) (27,30), nor a significant increase in episodes of nocturnal penile tumescence (1 RCT, 7 patients, RR 0.75, 95% CI 0.07 to 7.73) (32).
Vitamin E versus Placebo
One trial (29) showed a consistent decrease in end-of-treatment prolactin (1 RCT, 24 patients, MD −41.23 ng/ml, 95% CI −50.42 to −32.04), LH (1 RCT, 24 patients, MD −6.77 mU/ml, 95% CI −10.15 to −3.39), and testosterone levels (1 RCT, 24 patients, MD 7.00 pg/ml, 95% CI 4.43 to 9.57) after administration of vitamin E compared with placebo, but no statistically significant decrease in FSH levels (1 RCT, 24 patients, MD −0.65 mU/ml, 95% CI −2.61 to 1.31). No adverse effects were reported in this study.
Other Outcomes
Other outcomes reported in the included studies are summarized in Table 2.
Table 2.
Other outcomes related to sexual dysfunction as reported in the included studies
| Study ID | N | Intervention | Outcome | Mean ± SD (treatment group or baseline value) or RR (95% CI) | Mean ± SD (control group or after treatment) |
|---|---|---|---|---|---|
| Antoniou 1977 (26) | 8 | Oral zinc versus placebo | Testosterone concentration (ng/ml)b | 8.00 ± 3.50 | 3.20 ± 2.00 |
| LH (mIU/ml)b | 85.30 ± 81.00 | 47.30 ± 26.90 | |||
| FSH (mIU/ml)b | 24.00 ± 24.30 | 33.00 ± 8.70 | |||
| Bellovich 2000 (34) | 14 | Sildenafil citrate | IIEF | ||
| Frequency of penetration | 3.85 ± 3.10 | 4.43 ± 2.92 | |||
| Maintenance of erection penetration | 4.43 ± 2.69 | 4.93 ± 2.32 | |||
| Brook 1980 (27) | 14 | Zinc chloride versus placebo to dialysate | Improvement of libido | 1.00 (0.08, 13.02) | NA |
| Plasma testosterone (nmol/L)a | 9.00 ± 4.23 | 12.00 ± 1.32 | |||
| Mahajan 1982 (30) | 20 | Oral zinc acetate versus placebo | Total/partial impotence | 0.13 (0.02, 0.82) | NA |
| Decreased libido | 0.11 (0.01, 1.83) | NA | |||
| Decreased frequency of intercourse | 0.22 (0.06, 0.78) | NA | |||
| Increased plasma testosteroned | 5.20 ± 1.58 | 3.00 ± 0.95 | |||
| Decreased plasma FSHd | 25.00 ± 22.14 | 35.00 ± 15.81 | |||
| Decreased plasma LHd | 49.00 ± 82.22 | 38.00 ± 25.3 | |||
| Mahon 2005 (37) | 13 | Sildenafil citrate versus placebo | Global efficacy question | 2.50 (1.05, 5.96) | NA |
| Muir 1983 (38) | 14 | Bromocriptine versus placebo | Testosterone (nmol/L)b | 16.80 ± 4.49 | 17.00 ± 4.11 |
| Sharma 2006 (5) | 32 | Sildenafil Citrate versus placebo | Global efficacy question | 4.33 (2.07, 9.08) | |
| Blood urea nitrogen (mg/dl)a | 18.3 ± 7.6 | 17.9 ± 51.0 | |||
| Creatinine (mg/dl)a | 1.48 ± 0.4 | 1.4 ± 0.4 | |||
| Hemoglobin (g/dl)a | 12.3 ± 1.5 | 13.2 ± 1.4 | |||
| Wabrek 1982 (32) | 8 | Oral zinc versus placebo | Tumescence episodes | 0.75 (0.07, 7.73) | NA |
| Yeksan 1992 (29) | 24 | Vitamin E versus placeboc | Prolactin (ng/ml) | 15.00 ± 4.28 | 56.23 ± 15.66 |
| LH (mU/ml) | 4.66 ± 1.80 | 11.43 ± 5.70 | |||
| FSH (mU/ml) | 4.23 ± 1.83 | 4.88 ± 2.94 | |||
| Testosterone (pg/ml) | 11.79 ± 4.16 | 4.79 ± 1.82 |
Discussion
Key Findings
Our systematic review demonstrated that in small clinical trials, PDE5i improved various aspects of erectile function in CKD patients. No data about safety of these agents have been reported in these studies. Oral zinc supplementation resulted in a significant increase in plasma testosterone concentration along with an increase in the potency and frequency of intercourse. However, administration of zinc in the dialysate did not improve testosterone or the other biochemical parameters of gonadal failure. Only sparse data were available for vitamin E, bromocriptine, and dihydroxycholecalciferol in CKD patients and no trials assessed intracavernous injections, transurethral injections, mechanical devices, or behavioral therapy in CKD. The safety and efficacy of interventions for sexual dysfunction in women with CKD were poorly studied.
Comparison with Other Studies
PDE5i have been extensively studied in the general population and have been generally shown to improve erectile response and to be well tolerated in men with mild to severe erectile dysfunction due to varying etiologies (24,39–46). In a recent systematic review of 130 mostly short-term (≤12 weeks) RCTs of treatments for erectile dysfunction in men, Tsertsvadze et al. (41) reported that PDE5i were significantly more effective than placebo in improving sexual intercourse success (69% versus 35%) and resulted in a higher proportion of men with improved erections (range 67% to 89% versus 27% to 35%) in mixed study populations and in study populations of men with specific comorbid conditions, such as diabetes mellitus, stable cardiovascular disease, hypertension, depression, multiple sclerosis, rectal excision for bowel cancer, and radical prostatectomy for prostate cancer. The magnitude of improvement in erectile function was comparable between sildenafil, vardenafil, and tadalafil. Balanced against these benefits, PDE5i were associated with an increased risk of any adverse event (RR 1.72, 95% CI 1.53 to 1.93), the most common of which were headaches, flushing, dyspepsia, myalgia, and back pain. Although the reporting of all serious adverse or cardiovascular adverse events was inconsistent and incomplete, the overall rate of serious adverse events in men randomly assigned to PDE5i was ≤2% and comparable to those assigned to placebo. There was insufficient evidence to determine whether treatment with PDE5i increased the risks of serious cardiovascular events or nonarteritic anterior ischemic optic neuropathy. On the basis of these findings, the American College of Physicians issued clinical practice guidelines strongly recommending PDE5i for men who seek treatment for erectile dysfunction and who do not have contraindications to PDE5i use (47).
In keeping with the findings of Tsertsvadze et al. in non-CKD populations, we found that administration of PDE5i to men with CKD caused clinically meaningful and statistically significant improvements in general sexual satisfaction and erectile dysfunction. However, despite the high rate of sexual dysfunction in CKD patients and concerns about the safety of pharmacologic treatments in the setting of renal impairment, we found only six small clinical trials (including comparative and cross-over trials) that assessed PDE5i in CKD. The longest study duration was 8 months. Comparison of the efficacy of different PDE5i was not possible because only limited data were available for sildenafil and vardenafil and no data were available for tadalafil or mirodenafil. Unfortunately, there was also a complete lack of safety data for PDE5i in CKD patients, a population that is at high risk for silent cardiovascular disease.
Similar to the general population, our review did not identify any clinical trial analyzing the safety and efficacy of PDE5i in female CKD patients, despite the ubiquitous occurrence of sexual dysfunction in this group. Biologic plausibility exists to support the use of PDE5i in female patients with sexual dysfunction (48), but efficacy and safety are unclear and there is no consensus on the best treatment options for sexual dysfunction in female patients (49). Further studies in this important clinical area are warranted.
Our review found some earlier studies supporting the use of oral zinc therapy in CKD to improve sexual dysfunction. These were short-term investigations that assessed the effect of zinc on surrogate end points, such as gonadal hormone levels. With the declining use of zinc in clinical practice, further studies were not conducted. Most trials enrolled dialysis patients, and we did not identify studies enrolling predialysis patients. Because the prevalence of sexual dysfunction remains high in predialysis CKD patients whereas the prevalence of cardiovascular disease is lower than in dialysis patients, this group may represent an opportunity to safely conduct clinical trials assessing the safety and efficacy of PDE5i.
Our systematic review also identified important opportunities for examining the effects of treatments for sexual dysfunction on patient-level outcomes, such as quality of life and cardiovascular events. Previous studies have observed strong associations between erectile dysfunction, depression, and adverse cardiovascular outcomes (8,17–19,50), although a causal link has not been definitively established (18). Of the trials included in our meta-analysis, none considered these patient-level outcome parameters.
Strengths and Weaknesses
Our review had several strengths and weaknesses. The strengths included systematic searches of medical databases, data extraction, analysis, and trial quality assessment by two independent reviewers. The key findings were limited by the lack of long-term studies analyzing interventions targeting erectile dysfunction/sexual dysfunction in CKD patients. The included studies had relatively small sample sizes and were powered to observe differences in surrogate end points rather than patient-focused outcomes. Five studies had a crossover design and most did not adequately report study methods to determine trial quality. Although it is plausible that treatment effects of various agents might differ depending on the stage of the kidney disease, on the basis of the current available data, it is unclear whether such effects truly exist. Further, significant heterogeneity was observed for many outcomes but further analysis to explore the reasons for this heterogeneity could not be conducted because of the small number of studies. Publication bias might exist; however, given the lack of adequate number of studies, formal tests could not be conducted. In the general population, a higher PDE5i dose provides a better response, but whether such effects existed in CKD was unclear. In short, these issues highlight the fact that treatment of sexual dysfunction in CKD has received inadequate attention by researchers to date.
Conclusions
Implications for Practice
Modest evidence exists for the efficacy of PDE5i in CKD patients. The safety profile of these agents has not been extensively analyzed in CKD. Clinicians may use PDE5i in CKD patients who do not have any contraindications for PDE5i use. Oral zinc seems to increase testosterone levels and improve sexual dysfunction, but this evidence needs to be confirmed in future larger trials.
Implications for Research
Given the high prevalence of sexual dysfunction in CKD and lack of clinical trials, further and larger trials exploring various treatment options in male and female CKD patients are needed. These studies should focus on biochemical and patient-centered end points along with establishing their safety profile in dialysis and predialysis patients. Comparative studies of the efficacy and safety of different PDE5i in CKD patients are also warranted.
Disclosures
None.
Acknowledgments
The authors thank Narelle Willis (Review Group Coordinator of the Cochrane Renal Group), Gail Higgins and Ruth Mitchell (Trials Search Coordinators of the Cochrane Renal Group), and the Cochrane Renal Group for assistance with preparation of this study.
Appendix 1: Search Strategy
We searched MEDLINE using the following search strategy:
- exp Sexual Dysfunction, Physiologic/
- exp Sexual Dysfunctions, Psychologic/
- Orgasm/
- sexual dysfunction$.tw.
- sex$ disorder$.tw.
- frigid$.tw.
- (erectile$ adj (disorder$ or dysfunction$)).tw.
- (sexual adj (arousal or aversion$)).tw.
- fsfi.tw.
- Female Sexual Function Index.tw.
- International Index of Erectile Function.tw.
- iief.tw.
- Female Sexual Distress Score.tw.
- FSDS.tw.
- (impotent or impotence).tw.
- dyspareunia.tw.
- orgasm$.tw.
- or/1 to 17
- exp Renal Replacement Therapy/
- (hemodialysis or hemodialysis).tw.
- dialysis.tw.
- (PD or CAPD or CCPD or APD).tw.
- Renal Insufficiency/
- Kidney Failure/
- exp Renal Insufficiency, Chronic/
- Kidney Diseases/
- Uremia/
- (end stage renal or end stage kidney or endstage renal or endstage kidney).tw.
- (ESRF or ESKF or ESRD or ESKD).tw.
- (chronic kidney or chronic renal).tw.
- (CKF or CKD or CRF or CRD).tw.
- ur?emi$.tw.
- or/19 to 32
- and/18,33
We searched EMBASE using the following search strategy:
- exp Sexual Dysfunction/
- sexual dysfunction$.tw.
- sex$ disorder$.tw.
- frigid$.tw.
- (erectile$ adj (disorder$ or dysfunction$)).tw.
- (sexual adj (arousal or aversion$)).tw.
- fsfi.tw.
- Female Sexual Distress Score.tw.
- International Index of Erectile Function.tw.
- iief.tw.
- Female Sexual Function Index.tw.
- fsds.tw.
- (impotent or impotence).tw.
- dyspareunia.tw.
- orgasm$.tw.
- or/1 to 15
- exp Renal Replacement Therapy/
- (hemodialysis or hemodialysis).tw.
- (hemofiltration or hemofiltration).tw.
- (hemodiafiltration or hemodiafiltration).tw.
- dialysis.tw.
- (PD or CAPD or CCPD or APD).tw.
- Kidney Disease/
- Chronic Kidney Disease/
- Kidney Failure/
- Chronic Kidney Failure/
- Uremia/
- (chronic kidney or chronic renal).tw.
- (CKF or CKD or CRF or CRD).tw.
- (end-stage renal or end-stage kidney or endstage renal or endstage kidney).tw.
- (ESRF or ESKF or ESRD or ESKD).tw.
- ur?emi$.tw.
- exp Kidney Transplantation/
- or/17 to 33
- and/16,34
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States. Prevalence and predictors. JAMA 281: 6, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein F, Shirani S, Wuerth D, Finkelstein SH: Therapy insight: Sexual dysfunction in patients with chronic kidney disease. Nat Clin Pract Nephrol 3: 200–207, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Rosas S, Joffe M, Franklin E, Strom BL, Kotzker W, Brensinger C, Grossman E, Glasser D, Feldman H: Prevalence and determinants of erectile dysfunction in hemodialysis patients. Kidney Int 59: 2259–2266, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Anantharaman P, Schmidt RJ: Sexual function in chronic kidney disease. Adv Chronic Kid Dis 14: 119–125, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Sharma RK, Prasad N, Gupta A, Kapoor R: Treatment of erectile dysfunction with sildenafil citrate in renal allograft recipients: A randomized double-blind, placebo-controlled, cross-over trial. Am J Kidney Dis 48: 128–133, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Procci WR, Goldstein DA, Adelstein J, Massry SG: Sexual dysfunction in the male patients with uremia: A reappraisal. Kidney Int 19: 317–323, 1981 [DOI] [PubMed] [Google Scholar]
- 7.Bellinghieri G, Santoro D, Mallamace A, Savica V: Sexual dysfunction in chronic renal failure. J Nephrol 21: s113–s117, 2008 [PubMed] [Google Scholar]
- 8.Peng YS, Chiang CK, Kao TW, Hung KY, Lu CS, Chiang SS, Yang CS, Huang YC, Wu KD, Wu MS, Lien YR, Yang CC, Tsai DM, Chen PY, Liao CS, Tsai TJ, Chen WY: Sexual dysfunction in female haemodialysis patients: A multicenter study. Kidney Int 68: 760–765, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Palmer BF: Sexual dysfunction in uremia. J Am Soc Nephrol 10: 1381–1388, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Lawrence IG, Price DE, Howlett TA, Harris KP, Feehally J, Walls J: Erythropoietin and sexual dysfunction. Nephrol Dial Transplant 12: 741–747, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Kutner NG: Quality of life and daily haemodialysis. Sem Dial 17: 92–98, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Kimmel P, Peterson RA, Weihs KL, Immense SJ, Boyle WO, Kovac JA, Alleyne S, Cruz I, Veis JH. Psychologic functioning, quality of life, and behavioural compliance in patients beginning haemodialysis. J Am Soc Nephrol 7: 2152–2159, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Campese VM. Autonomic nervous system dysfunction in uraemia. Nephrol Dial Transplant 5[Suppl 1]: s98–s101, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Naya Y, Soh J, Ochiai A, Ushijima S, Kamoi K, Ukimura O, Kawauchi A, Fujito A, Ono T, Iwamoto N, Aoki T, Imada N, Marumo M, Miki T: Significant decrease of the international index of erectile function in male renal failure patients treated with haemodialysis. Int J Impot Res 14: 172–177, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Mehrsai A, Mousavi S, Nikoobakht M, Khanlarpoor T, Shekarpour L, Pourmand G: Improvement of erectile dysfunction after kidney transplantation: The role of the associated factors. Urol J 3: 240–244, 2006 [PubMed] [Google Scholar]
- 16.Al Khallaf HH. Analysis of sexual functions in male nondiabetic hemodialysis patients and renal transplant recipients. Transpl Int 23: 176–181, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Seidman SN, Roose SP: The sexual effects of testosterone replacement in depressed men: Randomized, placebo-controlled clinical trial. J Sex Marital Ther 32: 267–273, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Turk S, Guney I, Altintepe L, Tonbul Z, Yildiz A, Yeksan M: Quality of life in male haemodialysis patients. Nephron Clin Pract 96: c21–c27, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Goldestein I. The mutually reinforcing triad of depressive symptoms, cardiovascular disease, and erectile dysfunction. Am J Cardiol 86[Suppl]: 41F–45F, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Padma-Nathan H, Stecher VJ, Sweeney M, Orazem J, Tseng LJ, Deriesthal H: Minimal time to successful intercourse after sildenafil citrate: Results of a randomized, double-blind, placebo-controlled trial. Urology 62: 400–403, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Linet O, Ogrinc F. Efficacy and safety of intracavernosa alprostadil in men with erectile dysfunction. N Engl J Med 334: 873–877, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D'Andrea F, D'Armiento M, Giugliano D: Effect of lifestyle changes on erectile dysfunctions in obese men: A randomized controlled trial. JAMA 291: 2978–2984, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Melnik T, Garcia Oliveira Soares B, Nasello AG. Psychosocial interventions for erectile dysfunction. In: Cochrane Database of Systematic Review 2007, Issue 3, CD004825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vardi M, Nono A. Phosphodiesterase inhibitors for erectile dysfunction in patients with diabetes mellitus. In: Cochrane Database of Systematic Review 2007, Issue 1, CD002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG: Quantifying heterogeneity in a meta-analysis. Stats Med 21: 1539–1558, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Antoniou LD, Shalhoub RJ, Sudhakar T, Smith JC, Jr: Reversal of uraemic impotence by zinc. Lancet 2: 895–898, 1977 [DOI] [PubMed] [Google Scholar]
- 27.Brook AC, Johnston DG, Ward MK, Watson MJ, Cook DB, Kerr DN: Absence of a therapeutic effect of zinc in the sexual dysfunction of haemodialysed patients. Lancet 2: 618–620, 1980 [DOI] [PubMed] [Google Scholar]
- 28.Demir E, Balal M, Paydas S, Sertdemir Y, Erken U: Efficacy and safety of vardenafil in renal transplant recipients with erectile dysfunction. Transplant Proc 38: 1379–1381, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Yeksan M, Polat M, Türk S, Kazanci H, Akhan G, Erdogan Y, Erkul I: Effect of vitamin E therapy on sexual functions of uremic patients in hemodialysis. Int J Artif Organs 15: 648–652, 1992 [PubMed] [Google Scholar]
- 30.Mahajan SK, Abbasi AA, Prasad AS, Rabbani P, Briggs WA, McDonald FD: Effect of oral zinc therapy on gonadal function in hemodialysis patients. A double-blind study. Ann Intern Med 97: 357–361, 1982 [DOI] [PubMed] [Google Scholar]
- 31.Seibel I, Poli de Figueiredo CE, Telöken C, Moraes JF: Efficacy of oral sildenafil in hemodialysis patients with erectile dysfunction. J Am Soc Nephrol 13: 2770–2775, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Wabrek AJ: Zinc: A possible role in the reversal of uremic impotence. Sexuality e Disability 5: 213–221, 1982 [Google Scholar]
- 33.Yang J, Ju W, Zeng FQ, Xiao YJ, Zhang XP, Xiao CG: Efficacy and safety of vardenafil for kidney transplant recipients with erectile dysfunction. Zhonghua Nan Ke Xue 14: 911–913, 2008 [PubMed] [Google Scholar]
- 34.Bellovich K, Provenzano R, Wankhede S. Effectiveness of sildenafil citrate in male hemodialysis patients. J Am Soc Nephrol 11: 139A, 2000 [Google Scholar]
- 35.Blumberg A, Wildbolz A, Descoeudres C, Hennes U, Dambacher MA, Fischer JA, Weidmann P: Influence of 1,25 dihydroxycholecalciferol on sexual dysfunction and related endocrine parameters in patients on maintenance hemodialysis. Clin Nephrol 13: 208–214, 1980 [PubMed] [Google Scholar]
- 36.Bommer J, Ritz E, del Pozo E, Bommer G: Improved sexual function in male haemodialysis patients on bromocriptine. Lancet 2: 496–497, 1979 [DOI] [PubMed] [Google Scholar]
- 37.Mahon A, Sidhu PS, Muir G, Macdougall IC: The efficacy of sildenafil for the treatment of erectile dysfunction in male peritoneal dialysis patients. Am J Kidney Dis 45: 381–387, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Muir JW, Besser GM, Edwards CR, Rees LH, Cattell WR, Ackrill P, Baker LR: Bromocriptine improves reduced libido and potency in men receiving maintenance hemodialysis. Clin Nephrol 20: 308–314, 1983 [PubMed] [Google Scholar]
- 39.Keating GM, Scott LJ: Vardenafil: A review of its use in erectile dysfunction. Drugs 63: 2673–2703, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Markou S, Perimenis P, Gyftopoulos K, Athanasopoulos A, Barbalias G: Vardenafil (Levitra) for erectile dysfunction: A systematic review and meta-analysis of clinical trial reports. Int J Impot Res 16: 470–478, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Tsertsvadze A, Fink HA, Yazdi F, Macdonald R, Bella AJ, Ansari MT, Garritty C, Soares-Weiser K, Daniel R, Sampson M, Fox S, Moher D, Wilt TJ. Oral phosphodiesterase-5 inhibitors and hormonal treatments for erectile dysfunction: A systematic review and meta-analysis. Ann Intern Med 151: 650–661, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Miles CL, Candy B, Jones L, Williams R, Tookman A, King M. Interventions for sexual dysfunction following treatments for cancer. In: Cochrane Database of Systematic Review 2007, Issue 4, CD005540. [DOI] [PubMed] [Google Scholar]
- 43.Porst H, Rosen R, Padma-Nathan H, Goldstein I, Giuliano F, Ulbrich E, Bandel T: The efficacy and tolerability of vardenafil, a new phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction: The first at home clinical trial. Int J Impot Res 13: 192–199, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Carson CC, Rajfer J, Eardley I, Carrier S, Denne JS, Walker DJ, Shen W, Cordell WH: The efficacy and safety of tadalafil: An update. BJU Int 93: 1276–1281, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Hellstrom WJ, Gittelman M, Karlin G, Segerson T, Thibonnier M, Taylor T, Padma-Nathan H: Vardenafil for treatment of men with erectile dysfunction: Efficacy and safety in a randomized, double-blind, placebo-controlled trial. J Androl 23: 763–771, 2002 [PubMed] [Google Scholar]
- 46.Aranda P, Ruilope LM, Calvo C, Luque M, Coca A, Gil de Miguel A: Erectile dysfunction in essential arterial hypertension and effects of sildenafil: Results of a Spanish national study. Am J Hypertens 17: 139–145, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Qaseem A, Snow V, Denberg TD, Casey DE, Jr, Forciea MA, Owens DK, Shekelle P; for the Clinical Efficacy Assessment Subcommittee of the American College of Physicians Hormonal testing and pharmacologic treatment of erectile dysfunction: A clinical practice guideline from the American College of Physicians. Ann Intern Med 151: 639–649, 2009 [DOI] [PubMed] [Google Scholar]
- 48.D'Amati G, di Gioia CR, Bologna M, Giordano D, Giorgi M, Dolci S, Jannini EA. Type 5 phosphodiesterase expression in the human vagina. Urology 60: 191–195, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Brown DA, Kyle JA, Ferrill MJ. Assessing the clinical efficacy of sildenafil for the treatment of female sexual dysfunction. Ann Pharmacother 43: 1275–1285, 2009 [DOI] [PubMed] [Google Scholar]
- 50.Peng YS, Chiang CK, Hung KY, Chiang SS, Lu CS, Yang CS, Wu KD, Yang CC, Lin RP, Chang CJ, Tsai TJ, Chen WY: The association of higher depressive symptoms and sexual dysfunction in male haemodialysis patients. Nephrol Dial Transplant 22: 857–861, 2007 [DOI] [PubMed] [Google Scholar]