Olfactory-Mediated Fear Conditioning in Mice: Simultaneous Measurements of Fear-Potentiated Startle and Freezing (original) (raw)
. Author manuscript; available in PMC: 2010 Jun 7.
Abstract
This study demonstrates that mice display olfactory-cued fear as measured with both freezing and fear-potentiated startle. Following a preconditioning test to measure any unconditioned responses to odor, mice received 5 pairings of a 10-s odor with a 0.25-s, 0.4-mA footshock. The next day, startle and freezing were measured in the presence and absence of the odor. Both fear measures increased after training with amyl acetate (Experiment 1) and acetophenone (Experiment 2). The enhancement of startle did not occur when the same number of odors and shocks were presented in an unpaired fashion (Experiment 3). Furthermore, mice were able to discriminate between an odor paired with shock and a nonreinforced odor (Experiment 4).
Cue-specific conditioned fear in rodents has been used as a powerful model for the study of learning and memory as well as anxiety disorders. In rodents, fear is often studied by means of Pavlovian fear conditioning, in which a previously neutral stimulus such as a light or tone (the conditioned stimulus [CS]) is paired with an aversive stimulus, for example, a footshock (the unconditioned stimulus [US]). The resulting hypothetical state of fear can be assessed with several different behavioral measures (McAllister & McAllister, 1971). Two widely used measures of conditioned fear are fear-potentiated startle (FPS) and freezing (Davis, 2000; Fendt & Fanselow, 1999). FPS is defined as an augmented startle response in the presence of the aversive conditioned cue, whereas freezing is defined as the absence of activity in the presence of the cue (Blanchard & Blanchard, 1969; Fendt & Fanselow, 1999). Usually these measures are taken independently, although techniques have been introduced to measure both simultaneously (Fendt, 2001; Gewirtz, Falls, & Davis, 1997; Leaton & Borscz, 1985). Numerous studies have demonstrated that both these fear responses are dependent on the integrity of the amygdala complex (Davis, 2000; Fanselow & LeDoux, 1999; Fendt, 2001; Fendt & Fanselow, 1999). However, the exact nature of the behavioral relationship between conditioned freezing and FPS remains unclear.
Past studies examining conditioned fear responses in rodents have mainly utilized either auditory or visual stimuli as the CS. Although these studies have elucidated some of the neural mechanisms involved in conditioned fear, many questions remain concerning the precise nature by which a specific stimulus acquires fear-eliciting properties. The use of an olfactory CS may provide an additional tool to further understand the behavioral and neuro-biological aspects of fear in mammals, for several reasons. Olfactory stimuli are particularly salient cues to rodents, and conditioning is most effective when more salient cues are used as the CS during training (Rescorla & Wagner, 1972). For example, previous behavioral studies have shown that rats can acquire conditioned fear to an olfactory stimulus very quickly (Otto, Cousens, & Rajewski, 1997; Paschall & Davis, 2002). The use of an olfactory CS may be especially useful for molecular studies that are optimized by training protocols that induce rapid and robust learning (Ressler, Paschall, Zhou, & Davis, 2002). Furthermore, anatomical and functional studies have revealed that the olfactory sensory system provides relatively immediate sensory projections to subcortical structures involved in processing of aversive stimuli, including the amygdala (Pitkänen, 2000). These sensory efferents appear to maintain an odorant-specific topographical organization that may be uniquely suited for functionally dissecting the circuitry changes that occur with discrete learning events (Zou, Horowitz, Montmayeur, Snapper, & Buck, 2001). In addition, the many commercially available synthetic and natural odors provide a wide array of discrete stimuli with individual topographical representations (Leon & Johnson, 2003).
Limited studies in olfactory fear conditioning have been done in rats. Examination of mice would be useful given the wide variety of transgenic and other genetically engineered mice with targeted disruptions in genes that are involved in olfactory-motivated behaviors, and learning and memory in general. On one hand, olfactory fear conditioning in mice would be expected to have many of the same properties as those already seen in rats. However, species differences are important to investigate because interspecies performance variations have been shown in other behavioral tasks such as the Morris water maze (D’Hooge & De Deyn, 2001) and defensive behaviors (Blanchard, Griebel, & Blanchard, 2003).
In this study, we hypothesized that C57BL/6J mice can be fear conditioned to computer-controlled odorant exposure and can learn to discriminate these odorants. The first two experiments in this study show that C57BL/6J mice can display FPS and freezing when shock is paired with two different odors, amyl acetate (Experiment 1) and acetophenone (Experiment 2). In contrast, FPS is not observed when odors and shocks are presented in an unpaired fashion (Experiment 3). Experiment 4 demonstrates that mice can discriminate between two odors that are either paired (CS+) or not paired (CS−) with shock, as indicated by selective freezing and FPS to the reinforced odor. Together, these findings show that olfactory stimuli can be effectively used in aversive and differential conditioning paradigms to elicit both freezing and FPS in C57BL/6J mice.
General Method
Subjects
Adult (8–12 weeks) male C57BL/6J mice (Jackson Laboratories, Bar Harbor, Maine) housed in standard group cages (≤5 per cage), were given ad-lib access to food and water, on a 12-hr light–dark cycle. All experiments were performed during the light cycle (9 a.m. to 7 p.m.), were approved by the Emory University Institutional Review Board, and followed National Institutes of Health Internal Animal Care and Use Committee standards.
Olfactory Stimuli
Odorants were prepared in advance and in a separate room. Mixtures of 5% (vol/vol) amyl acetate and 10% (vol/vol) acetophenone (Sigma-Aldrich, St. Louis, MO) were dissolved in propylene glycol. A higher concentration of acetophenone was used on the basis of our pilot studies, which indicated that lower concentrations were less effective—possibly because of the difference in volatility between the two odors (Cometto-Muniz, Cain, & Abraham, 2003; Jones, Heldt, Davis, & Ressler, 2003). Odorants were placed in glass sample jars and attached to the odor delivery apparatus (see below).
Olfactory Fear Apparatus
Fear training and testing sessions were conducted in four identical startle response systems (SR-LAB, San Diego Instruments, San Diego, CA). Each consisted of a nonrestrictive Plexiglas cylinder (5.5 cm diameter, 13 cm long) mounted on a Plexiglas platform that was located in a ventilated, sound-attenuating chamber. The floor of each cylinder was a cradle-shaped grid that contained seven 3.0-mm diameter stainless steel bars spaced 1 cm apart, through which shock could be delivered. Cylinder movements were detected by a piezoelectric accelerometer mounted under each platform and were digitized and stored by an interfacing computer assembly as voltage output sampled each millisecond. Startle amplitude was defined as the peak accelerometer voltage that occurred during the first 100 ms after the onset of the startle stimulus. The voltage output was sampled every millisecond during a 5-s “activity window” starting 7 s before the startle stimulus. For each cylinder, a voltage output threshold corresponding to mouse immobility was determined by recording the voltage output of the cylinder while it was empty (without a mouse). Voltage readings above the average threshold response were used as evidence of mobility. Response sensitivities were calibrated (SR-LAB Startle Calibration System) to be nearly identical in each of the startle systems.
Startle and background stimuli were presented through a high-frequency speaker located 15 cm above the chambers. Startle was elicited by a 105-dB, 50-ms white noise burst. A continuous 65-dB white noise background was delivered through chamber speakers during training and testing. Sound intensities were measured by an audiometer (Radio Shack, #33–2055). The footshock US was generated by a programmable animal shocker (San Diego Instruments) located outside the isolation chambers and was delivered through the cage floor bars. Footshock intensity was 0.4 mA. Startle, background, and US stimuli presentation and data acquisition were controlled by an IBM PC-compatible computer using SR-Lab software.
Odor stimuli were delivered to chambers in a manner similar to that described previously (Paschall & Davis, 2002). Briefly, a compressed air tank with a pressure regulator and flow meter delivered a constant flow rate of 40 L/min. The flow meter output was split with a Y-connector to create two separate delivery lines: a clean, odor-free line and an odor-delivery line that was connected to a solenoid valve controlled by a computer running the SR-Lab software. PharMed Tygon tubing (3.2 mm id; Saint-Gobain, Akron, OH) was used to form delivery lines because of its low permeability to vapors. When the valve opened, air flowed through the odor-delivery line into a sealed jar containing the dissolved odorant. Tubing from the jar merged with the odor-free line to form a single 80-cm delivery line that fed into the front of the Plexiglas cylinder. When the valve closed, air flowed thorough the odor-free line only. Opening and closing of the solenoid valve did not produce any difference in rate of air flow to the cylinder. Backflow was prevented by one-way valves. The odor was rapidly removed from the back of the cylinder via an exhaust hose feeding into the room’s ventilation fan.
To test for the discrimination of odors, we modified the odor delivery apparatus to deliver two separate odors. Rather than two lines as above, there were three lines in this configuration: an odor-free line and two odor-delivery lines. Each odor-delivery line was configured to deliver one of two different odors via separately controlled solenoid valves. The two odor-delivery lines and the odor-free line were joined back together by Y-connectors, and an 80-cm common line of tubing led into the Plexiglas cylinder. When both valves were closed, air flowed through the odor-free line and directly to the Plexiglas cylinder.
Fear Conditioning
In each experiment, mice were given 3 days of preexposure to the startle cylinders to minimize contextual conditioning and to acclimate them to handling. Two days prior to conditioning, the mice received 15 startle stimulus presentations to habituate startle to a stable baseline. The next day, mice received a preconditioning test (pretest) session to assess whether they displayed unconditioned effects to the odor prior to conditioning. During pretest, mice were placed in the cylinder and, 5 min later, were presented with 12 startle-alone trials. The initial startle-alone trials were intended to habituate startle to a stable baseline and were not used in analyses. Mice were then presented with 10 odor-startle trials randomly intermingled with 10 startle-alone trials and separated by a 90-s intertrial interval (ITI). Odor–startle trials consisted of a 10-s odor presentation that coterminated with a 50-ms, 105-dB noise burst. This pretest is unlikely to result in any effects such as latent inhibition because such effects typically require a large number of preexposures to the CS (Schauz & Koch, 1998). The next day, mice were placed in the startle cylinder and, 5 min later, received the first of 5 pairings of a 10-s odor CS coterminating with a 0.25-s, 0.4-mA footshock, presented with an average 120-s ITI (range = 90 – 150 s). The mice were then returned to their home cage. Twenty-four hours after training, mice were given a postconditioning test (posttest) identical to the pretest.
Data Analysis
Startle and immobility were measured in the presence (odor–startle trials) and absence (startle-alone trials) of the odor CS. For each mouse, a percent FPS and a percent freezing were computed by first subtracting the mean of startle-alone trials from the mean of the odor–startle trials. This difference score was then divided by the mean of the startle-alone trials and multiplied by 100. The presence of associative FPS and freezing were assessed by examining the change in behavior from pretest to posttest. This approach accounts for potential confounding nonassociative effects of the CS on dependent behaviors (Falls, 2002; Heldt, Sundin, Willott, & Falls, 2000). As such, within-subject statistical analyses were performed for each experiment. In Experiments 1 and 2, simple paired-sample t tests were performed. For Experiments 3 and 4, mixed-model analyses of variance (ANOVAs) were performed with training (paired, unpaired) as the between-subjects factor and session (pretest, posttest) as the within-subject factor (Experiment 3), or trial type (CS+, CS−) and session (Pretest, Posttest) as the within-subject factors (Experiment 4). Subsequent analyses were done with simple paired-sample and pairwise t tests.
Freezing scores were determined as follows: Voltage outputs for each cage were first converted to the average voltage output for each second of the 5-s activity window. Averages above or below the mean voltage output of the empty cylinder (without the mouse) were assigned an immobility score of 0 or 1 (0 = mobile, 1 = immobile) for each second of the 5-s activity window. A percent immobility score for each trial was computed by averaging the five immobility scores and multiplying by 100. This score was used as an index of freezing. Pilot studies have found a high correlation between this automated index of freezing and observational ratings of freezing (_r_s > .89).
Experiment 1 and 2
Method
To test whether mice could acquire fear to an olfactory cue, we first administered a pretest (Figures 1a and 1b). The next day, mice were presented with five odor–shock pairings and then given a posttest 24 hr later. For Experiment 1, 5% amyl acetate was used as the CS; and for Experiment 2, 10% acetophenone was used as the CS. For each experiment, n = 8, with 1 mouse excluded from analysis in Experiment 1 because of an equipment malfunction.
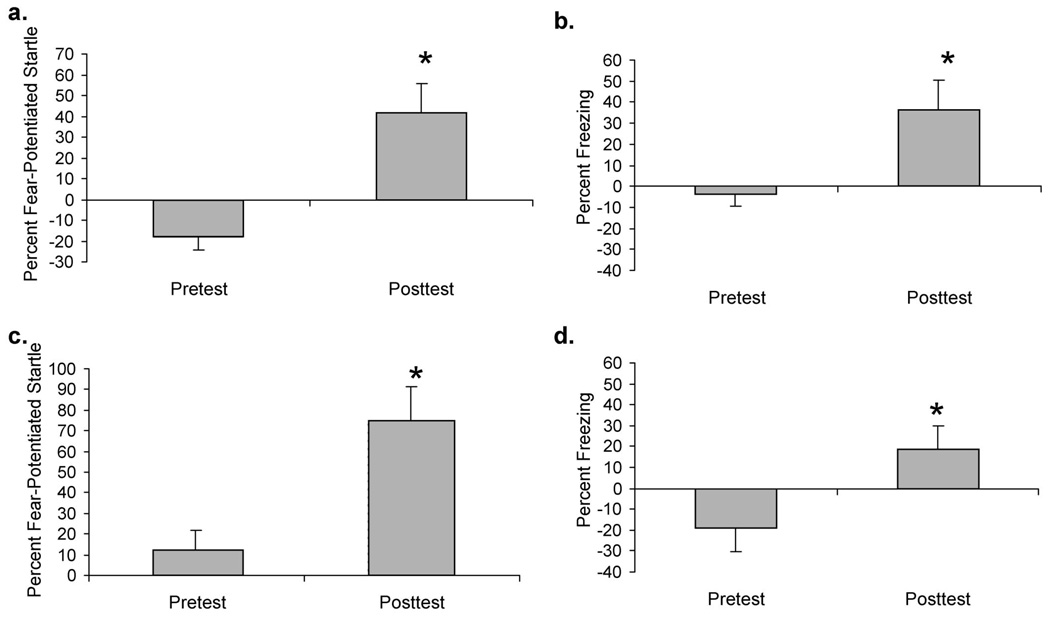
Figure 1.
a: In Experiment 1, mice showed more fear-potentiated startle with an amyl acetate cue after the odor was paired with shock. b: Mice showed increased freezing in the presence of amyl acetate after the odor was paired with a shock. c: In Experiment 2, mice showed an increase in fear-potentiated startle after the odor acetophenone was paired with a shock. d: Mice showed increased freezing in the presence of acetophenone after the odor was paired with a shock. *p < .05
Results
As seen in Figures 1a and 1b, during the pretest, the amyl acetate presentation produced nonsignificant changes in the percent FPS (−17%) and percent freezing (−4%) prior to conditioning (_p_s > .05). After odor-shock pairing, mice showed 41% FPS in the presence of amyl acetate, which represented a significant increase from pretest, t(6) = 2.42, p < .05. Animals also showed a significant increase in freezing to the odor following training, t(6) = 3.09, p < .03.
In the Experiment 2 pretest (Figures 1c and 1d), mice displayed nonsignificant increases in percentage of FPS (12%) and freezing (−19%) in the presence of acetophenone (_p_s > .05; Figures 1c and 1d). After receiving acetophenone paired with shock, the mice showed reliable increases in FPS (74%) and freezing (19%), t(7) = 3.30, p < .02, and t(7) = 2.54, p < .05, respectively.
Experiment 3
Method
To control for nonassociative effects, we compared odor–shock paired mice to a group of mice in which the odor and shock were explicitly unpaired (see Figure 2a). The paired group (n = 14) received five amyl acetate-shock pairings on each of 2 training days. The unpaired group (n = 14) received five shocks and five odor presentations on each day, but these stimuli were not paired. During unpaired training, the time between odor and shock averaged 90 s but was randomized between 60 and 120 s.
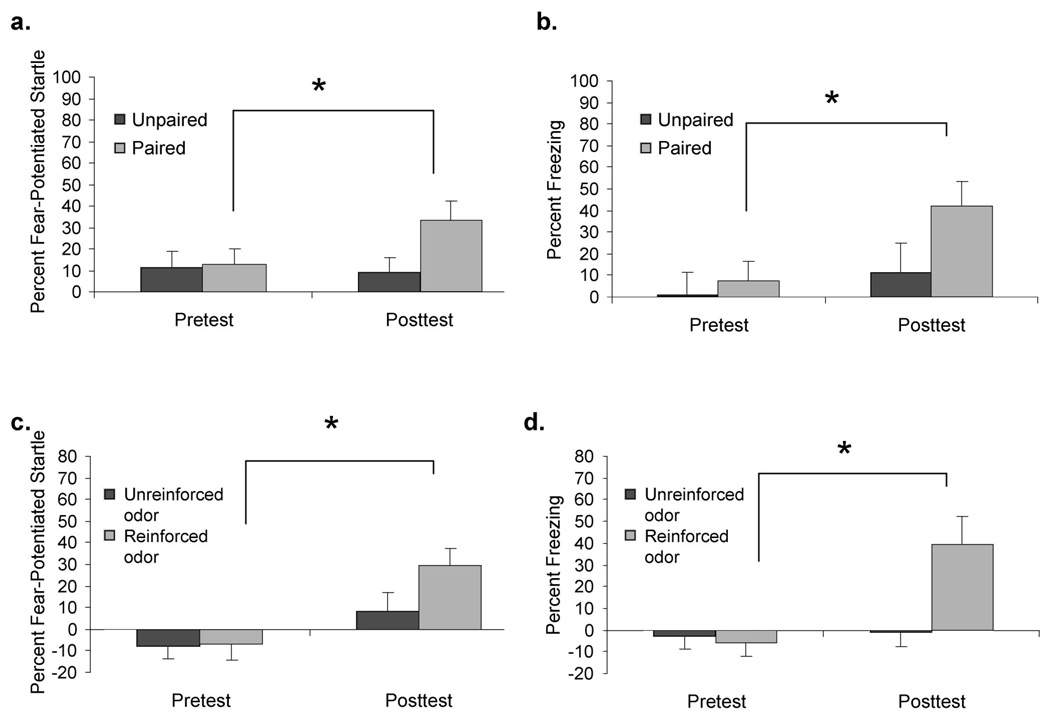
Figure 2.
a: To control for nonassociative effects in Experiment 3, we compared odor–shock paired mice with mice in which the odor and shock were explicitly unpaired. In the posttest, the paired mice showed an increase in fear-potentiated startle, but the unpaired group did not. b: Mice in the paired, but not the unpaired, group showed increased freezing from the pretest to posttest. c: Experiment 4 tested for discrimination of two odors in fear conditioning. Some mice received amyl acetate paired with shock and acetophenone as the odor-alone stimulus, and others received the opposite. When tested, the mice showed an increase in fear-potentiated startle to the reinforced odor, but not to the nonreinforced odor. d: Mice also showed an increase in freezing to the odor that was paired with shock, but not to the nonreinforced odor. *p < .05
Results
An analysis of the percent FPS with the mixed-model ANOVA revealed a significant Group × Session interaction, F(1, 22) = 5.16, p < .05. Neither the group nor session main effects were significant (_p_s > .05). Within-subject analyses indicated that mice given paired training showed a significant increase in percent FPS after conditioning, t(11) = 2.80, p < .02. In contrast, mice given unpaired training showed no difference from the pretest, _t_(11) = 0.30, _p_ > .05 (Figure 2a).
For percent freezing, the overall analysis revealed only a significant session effect, F(1, 22) = 4.88, p < .04, indicating an overall increase in freezing from the pretest to the posttest. Both the group main effect and Group × Session interaction were nonsignificant (_p_s > .05). Thus, as a combined group, mice given both paired and unpaired training displayed more posttest freezing when compared with the pretest. However, individual evaluations of each group indicated that the reliable session effect was primarily driven by a significant increase in mice given paired training, t(11) = 2.43, p < .04. Mice given unpaired training displayed no increase in percent freezing, _t_(11) = 0.73, _p_ > .05 (Figure 2b).
Experiment 4
Method
The purpose of Experiment 4 was to determine whether mice could learn to discriminate between an odor paired with shock and a nonreinforced odor. Prior to differential conditioning, mice were given a pretest. This test was similar to the pretest described above, except that the mice received 10 odor-startle trials for each of the 2 odors (CS+, CS−) and 10 startle–alone trials. The order of the test trials was randomly assigned. Training was conducted on the following 2 days and involved differential reinforcement of the two odors in a counterbalanced design. On each of 2 days, Group 1 (n = 10) received 5 presentations of acetophenone alone (CS−) interspersed with 5 pairings of amyl acetate and footshock (CS+). Group 2 (n = 6) received acetophenone paired with the shock (CS+) and amyl acetate alone (CS−). The following day, FPS and freezing were measured during the same test session used in the Experiment 4 pretest.
Results
Overall ANOVAs for both percent FPS and freezing revealed no main effect for group, _F_s(1, 13) < 1.88, _p_s > .05. There were also no significant two-way or three-way interactions by group, _F_s(1, 13) < 3.057, _p_s > .05. Therefore, the data obtained from Groups 1 and 2 were collapsed and analyzed with a mixed-model ANOVA including session (pretest, posttest) and trial type (CS+, CS−)as within-subject variables. For percent FPS, this overall analysis revealed a significant trial type effect, F(1, 15) = 7.50, p < .02, and Session × Trial Type interaction, _F_(1, 15) = 8.79, _p_ < .01. Subsequent paired-sample _t_ tests indicated that after conditioning, percent FPS to the CS+ increased from a pretest level of −7% to a posttest level of 29%, a significant change, _t_(15) = 2.63, _p_ < .02 (Figure 2c). On the other hand, percent FPS to the CS− increased nonsignificantly, from −8% in the pretest to 8% in the posttest, _t_(15) = 0.26, _p_ > .05.
The overall analysis of percent freezing resulted in a significant main effect of session, F(1, 15) = 8.83, p < .01, and a Session × Trial Type interaction, _F_(1, 15) = 5.00, _p_ < .05. Follow-up statistics indicated that after training mice froze significantly more to the trained odor (CS+) than they did in the pretest, _t_(15) = 3.87, _p_ < .02 (Figure 2d). Freezing to the nonreinforced odor (CS−) increased slightly, but nonsignificantly, _t_(15) = 1.54, _p_ > .05.
General Discussion
These experiments demonstrate that olfactory cues can reliably elicit conditioned fear in mice after aversive classical conditioning, as measured with both FPS and freezing in the same test session. Using rats, past research has demonstrated that an olfactory CS elicits a number of Pavlovian conditioned responses, including conditioned freezing (Richardson & McNally, 2003; Cousens & Otto, 1998; Otto et al., 1997), FPS (Paschall & Davis, 2002), analgesia, and cardiac responses (Hunt, Hess, & Cambell, 1997; Richardson & McNally, 2003). Our results show that an olfactory CS can reliably be used in mice and extend the finding of past studies showing learned-fear responses in mice with either an auditory and/or visual stimuli CS (Falls, Carlson, Turner, & Willott, 1997; McCaughran, Bell, & Hitzemann, 2000; Risbrough, Brodkin, & Geyer, 2003; Willott et al., 1998).
In Experiments 1 and 2, significant freezing and FPS were observed after pairing of the odor with shock, but not before. However, these results could be due to a number of nonassociative or pseudoconditioning effects, including a generalized increase in anxiety or vigilance, sensitization, or context conditioning (Rescorla & Wagner, 1972).
Therefore, we tested two groups in Experiment 3: one in which the odor was paired with shock, and a second in which the same number of odors and shocks were presented separately. As shown in Figures 2a and 2b, when assessed by means of FPS, mice in the paired group learned the association between odor and shock, whereas mice in the unpaired group did not. In contrast, the evaluation of freezing behavior in our paradigm revealed that as a combined group, both paired and unpaired mice displayed conditioned freezing. Post hoc analyses, however, showed that this overall effect was primarily driven by a significant increase in freezing in paired subjects. Unpaired subjects displayed only a mild, nonsignificant increase in freezing. The evidence of a low level of freezing acquisition in the unpaired group may be due to the lack of complete odor clearance prior to shock. This explanation is consistent with a recent finding that unpaired odor + shock conditioning in rats produces mild CS conditioning (Sorg, Swindell, & Tschirgi, 2004). Efforts to increase the time between CS and shock presentations in the present study may have eliminated signs of acquisition in the unpaired group. Nevertheless, the possibility that mild conditioning produced evidence of conditioned freezing but not FPS may be an indication of differential behavioral thresholds.
To exclude the possibility that the mice were using some cue produced by the mechanism of the odor delivery apparatus as the CS, we gave mice differential conditioning in which one odor was paired with shock (CS+), and the presentation of another odor was nonreinforced (CS−, Experiment 4). During the test session, mice showed a significant increase in both FPS and freezing to the trained odor (CS+) but not to the nonreinforced odor (CS−). This finding suggests that mice acquired odor-specific fear and that differences between reinforced and nonreinforced odors were unlikely to have been due to other nonolfactory factors.
FPS and freezing are arguably the most commonly used measures of conditioned fear and have been used independently by different labs with slightly varying procedural protocols. In these experiments, immobility measurements were assessed from automated recordings of cage cylinder movements, which correlate well with freezing behavior measured by a trained observer. Video recordings from pilot studies revealed that presentation of the odor CS elicited an orientation response toward the odor source, accompanied by sniffing. A past report using similar protocol methodology indicated that auditory and visual CSs do not induce prominent orienting responses (Sundin, Heldt, Willott, Buck, & Falls, 1998). Thus, as seen in appetitive conditioning (Holland, 1977), it appears that stimulus modality influences the topography of the conditioned fear response in mice. The initial orientation reaction subsided after about 2 s. To avoid confounding our freezing measurement with this orienting activity, we recorded activity 3–7 s after odor CS onset.
Olfactory cue conditioning may provide a powerful approach to dissecting the functional neurocircuitry of fear. From a sensory standpoint, odors provide discrete cues that are detected by individual receptors on sensory neurons dedicated to that receptor (Ressler, Sullivan, & Buck, 1993). This molecular discrimination is maintained at the level of the olfactory bulb, and there is some evidence that functional topography representing different odors is present in the olfactory piriform cortex (Illig & Haberly, 2003). Thus, there exists a topographical representation of olfactory sensory inputs in brain regions only one synapse away from the amygdala. This organization may be uniquely suited for functionally dissecting the circuitry changes that occur with discrete learning events.
Acetophenone was used as one CS odorant because of findings that it may activate only a small number of olfactory bulb glomeruli and that a putative odorant receptor has been identified that is activated by this odorant (Bozza, Feinstein, Zheng, & Mombaerts, 2002). Future studies comparing olfactory fear learning with acetophenone to learning with other odorants that do not activate the same receptor may allow for the combination of molecular anatomical approaches with sophisticated learning paradigms. For example, transgenic mice containing manipulations of the M71 odorant receptor can be examined in a stimulus-specific way comparing odorant ligands that activate this receptor with other odors that do not. The choice of amyl acetate as another CS was based on the effectiveness of this stimulus in other rodent olfactory learning paradigms using this concentration (5%; Paschall & Davis, 2002; Yuan, Harley, McLean, & Knopfel, 2002).
In summary, the current study demonstrates olfactory-mediated FPS and freezing measured simultaneously in C57 mice. Although both measures are widely accepted indices of a central state of fear, the exact relationship between these behavioral responses is presently unclear. It is generally assumed that freezing and potentiated startle co-occur (Leaton & Borscz, 1985). However, few studies have closely investigated this relationship, and in some cases manipulations of a fear state affect only one of these two fear responses. The establishment and use of a protocol that measures both freezing and FPS in the same animals may shed further light on the correlation of these two fear-related behaviors. Finally, this study lays the groundwork for experimental manipulations using transgenic mice to functionally dissect the olfactory system’s role in learning, as well as to further examine the role of brain areas that mediate fear conditioning.
Acknowledgments
This research was supported by the Science and Technology Center Program (Center for Behavioral Neuroscience) of the National Science Foundation under Agreement IBN-987675, and by National Institutes of Health Grant MH69884, the Woodruff Foundation, and a Goldman Philanthropic Partnerships Culpeper Medical Scholarship to Kerry J. Ressler.
References
- Blanchard DC, Blanchard RJ. Crouching as an index of fear. Journal of Comparative Physiology & Psychology. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. The Mouse Defense Test Battery: Pharmacological and behavioral assays for anxiety and panic. European Journal of Pharmacology. 2003;463:97–116. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- Bozza T, Feinstein P, Zheng C, Mombaerts P. Odorant receptor expression defines functional units in the mouse olfactory system. Journal of Neuroscience. 2002;22:3033–3043. doi: 10.1523/JNEUROSCI.22-08-03033.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cometto-Muniz JE, Cain WS, Abraham MH. Quantification of chemical vapors in chemosensory research. Chemical Senses. 2003;28:467–477. doi: 10.1093/chemse/28.6.467. [DOI] [PubMed] [Google Scholar]
- Cousens G, Otto T. Both pre- and posttraining excitotoxic lesions of the basolateral amygdala abolish the expression of olfactory and contextual fear conditioning. Behavioral Neuroscience. 1998;112:1092–1103. doi: 10.1037//0735-7044.112.5.1092. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The amygdala: A functional analysis. 2nd ed. New York: Oxford University Press; 2000. pp. 213–287. [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Research Reviews. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Falls WA. Fear-potentiated startle in mice. In: Crawley J, editor. Current protocols in neuroscience. New York: Wiley; 2002. pp. Unit 8.11-B1–Unit 8.11-B16. [DOI] [PubMed] [Google Scholar]
- Falls WA, Carlson S, Turner J, Willott JE. Fear-potentiated startle in two strains of inbred mice. Behavioral Neuroscience. 1997;111:855–861. [PubMed] [Google Scholar]
- Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron. 1999;23:229–232. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- Fendt M. Injections of the NMDA receptor antagonist aminophos-phonopentanoic acid into the lateral nucleus of the amygdala block the expression of fear-potentiated startle and freezing. Journal of Neuroscience. 2001;21:4111–4115. doi: 10.1523/JNEUROSCI.21-11-04111.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neuroscience & Biobehavioral Reviews. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Falls WA, Davis M. Normal conditioned inhibition and extinction of freezing and fear-potentiated startle following lesions of the medial prefrontal cortex. Behavioral Neuroscience. 1997;111:712–726. doi: 10.1037//0735-7044.111.4.712. [DOI] [PubMed] [Google Scholar]
- Heldt S, Sundin V, Willott JF, Falls WA. Posttraining lesions of the amygdala interfere with fear-potentiated startle to both visual and auditory conditioned stimuli in C57BL/6J mice. Behavioral Neuroscience. 2000;114:749–759. [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. Journal of Experimental Psychology: Animal Behavior Processes. 1977;3:77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Hess MF, Cambell BA. Conditioned cardiac and behavioral response topography to an olfactory CS dissociates with age. Animal Learning & Behavior. 1997;25:53–61. [Google Scholar]
- Illig KR, Haberly LB. Odor-evoked activity is spatially distributed in piriform cortex. Journal of Comparative Neurology. 2003;457:361–373. doi: 10.1002/cne.10557. [DOI] [PubMed] [Google Scholar]
- Jones SV, Heldt S, Davis M, Ressler K. Fear potentiated startle and freezing in mice using olfactory cues. Paper presented at the annual meeting of the Society for Neuroscience; 2003, November; New Orleans, LA. [Google Scholar]
- Leaton RN, Borscz GS. Potentiated startle: Its relation to freezing and shock intensity in rats. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:421–428. [Google Scholar]
- Leon M, Johnson BA. Olfactory coding in the mammalian olfactory bulb. Brain Research Reviews. 2003;42:23–32. doi: 10.1016/s0165-0173(03)00142-5. [DOI] [PubMed] [Google Scholar]
- McAllister WR, McAllister DE. Behavioral measurements of conditioned fear. In: Brush FR, editor. Aversive conditioning and learning. New York: Academic Press; 1971. pp. 105–179. [Google Scholar]
- McCaughran JA, Jr, Bell J, III, Hitzemann RJ. Fear-potentiated startle response in mice: Genetic analysis of the C57BL/6J and DBA/2J intercross. Pharmacology Biochemistry and Behavior. 2000;65:301–312. doi: 10.1016/s0091-3057(99)00216-6. [DOI] [PubMed] [Google Scholar]
- Otto T, Cousens G, Rajewski K. Odor-guided fear conditioning in rats: I. Acquisition, retention, and latent inhibition. Behavioral Neuroscience. 1997;111:1257–1264. [PubMed] [Google Scholar]
- Paschall GY, Davis M. Olfactory-mediated fear-potentiated startle. Behavioral Neuroscience. 2002;116:4–12. doi: 10.1037//0735-7044.116.1.4. [DOI] [PubMed] [Google Scholar]
- Pitkänen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The amygdala: A functional analysis. 2nd ed. New York: Oxford University Press; 2000. pp. 31–115. [Google Scholar]
- Rescorla RA, Wagner AR, editors. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and non-reinforcement. New York: Appleton-Century-Crofts; 1972. [Google Scholar]
- Ressler KJ, Paschall G, Zhou XL, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. Journal of Neuroscience. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler K, Sullivan S, Buck L. A zonal organization of odorant receptor gene expression in the olfactory epithelium. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- Richardson R, McNally GP. Effects of an odor paired with illness on startle, freezing, and analgesia in rats. Physiology & Behavior. 2003;78:213–219. doi: 10.1016/s0031-9384(02)00974-5. [DOI] [PubMed] [Google Scholar]
- Risbrough VB, Brodkin JD, Geyer MA. GABA-A and 5-HT1A receptor agonists block expression of fear-potentiated startle in mice. Neuropsychopharmacology. 2003;28:654–663. doi: 10.1038/sj.npp.1300079. [DOI] [PubMed] [Google Scholar]
- Schauz C, Koch M. Latent inhibition of fear potentiated startle in rats. Behavioural Pharmacology. 1998;9:175–178. [PubMed] [Google Scholar]
- Sorg BA, Swindell S, Tschirgi ML. Repeated low level formaldehyde exposure produces enhanced fear conditioning to odor in male, but not female, rats. Brain Research. 2004;1008:11–19. doi: 10.1016/j.brainres.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Sundin VS, Heldt SA, Willott JE, Buck WW, Falls WA. Fear-potentiation and conditioned freezing to a visual and auditory stimuli are blocked by lesions of the amygdala in C57 mice. Society for Neuroscience Abstracts. 1998;24:926. [Google Scholar]
- Willott JF, Turner JG, Carlson S, Ding D, Seegers Bross L, Falls WA. The BALB/c mouse as an animal model for progressive sensorineural hearing loss. Hearing Research. 1998;115:162–174. doi: 10.1016/s0378-5955(97)00189-5. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, McLean JH, Knopfel T. Optical imaging of odor preference memory in the rat olfactory bulb. Journal of Neurophysiology. 2002;87:3156–3159. doi: 10.1152/jn.00917.2001. [DOI] [PubMed] [Google Scholar]
- Zou Z, Horowitz LF, Montmayeur JP, Snapper S, Buck LB. Genetic tracing reveals a stereotyped sensory map in the olfactory cortex. Nature. 2001 November 8;414:173–179. doi: 10.1038/35102506. [DOI] [PubMed] [Google Scholar]