A Pilot Study of Predictive Markers of Chemotherapy-Related Amenorrhea Among Premenopausal Women with Early Stage Breast Cancer (original) (raw)
. Author manuscript; available in PMC: 2010 Jun 10.
Published in final edited form as: Cancer Invest. 2008 Apr–May;26(3):286–295. doi: 10.1080/07357900701829777
Abstract
Background
Premenopausal women treated for early stage breast cancer (ESBC) are at risk for chemotherapy-related amenorrhea (CRA). Prospectively-validated, predictive markers of CRA are needed.
Patients and Methods
Premenopausal women with ESBC and planned chemotherapy (≥ 25% risk of amenorrhea) were evaluated. Follicle stimulating hormone (FSH), estradiol, Inhibin A and B, anti-Müllerian hormone (AMH), and quality of life (QOL) were prospectively evaluated pre-, post-, 6 months and 1 year post-chemotherapy and correlated with age and menstrual status. CRA was defined as absence of menses 1 year post-chemotherapy.
Results
Forty-four women were evaluated at the time of analysis. Median age at diagnosis and FSH 1 year post-chemotherapy were higher among women with CRA (44 yrs [33–51] vs. 40 yrs [31–43]; p = 0.03; 39.8 vs. 5.0 mLU/mL, p = 0.0058, respectively). Median estradiol 1 year post-chemotherapy was higher among women who resumed menses (108.3 vs. 41.3 pg/mL, p = 0.01). Pre-chemotherapy median Inhibin B and AMH were lower among women with CRA (33.2 vs. 108.8 pg/mL; p = 0.03; 0.16 vs. 1.09 ng/mL, p = 0.02, respectively). The risk of CRA was increased among women with lower pre-chemotherapy Inhibin B (RR = 1.67, p = 0.15) and AMH (RR = 1.83, p = 0.05). Amongst women whose pre-chemotherapy Inhibin B and AMH values were below the median, the incidence of CRA was 87.5%.
Conclusions
Results indicate that pre-chemotherapy Inhibin B and AMH are lower among women experiencing CRA and may be predictive of CRA among premenopausal women facing chemotherapy for ESBC.
Keywords: Amenorrhea, Breast cancer, Chemotherapy, Premenopausal, Quality of life
INTRODUCTION
It is estimated that 178,480 women will be diagnosed with breast cancer in the United States during the year 2007 (1). Approximately one-third of diagnoses were expected among women aged less than 50 years within the premenopausal age-range (2). The Early Breast Cancer Trialists’ Collaborative Group states that women aged less than 50 years derive the greatest benefit from chemotherapy (3). Appropriately, younger, premenopausal women will likely be offered cytotoxic, systemic therapy. Effective chemotherapeutics, known to affect rapidly dividing cells including the ovary, can threaten both future ovarian function and subsequent fertility greatly affecting the experience of survivorship. In clinical practice, resumption of menses and serum hormonal levels, including estradiol and follicle stimulating hormone (FSH), currently serve as indicators of menopausal status at the completion of treatment. To date, prospectively-validated, predictive markers of ovarian function following the administration of chemotherapy do not exist.
Inhibin, a dimer composed of an alpha and beta sub-unit, is produced by the human ovarian granulosa cell. Inhibin A reflects luteal function, whereas Inhibin B reflects granulosa cell and follicular reserve. Expression of both subunits is tightly correlated with granulosa cell activity (4, 5). In women approaching menopause, Inhibin serum levels decrease over time. Therefore, the measurement of serum Inhibin levels should reflect the function and reserve of the ovarian granulosa cell. Interestingly, investigators have observed lower Inhibin levels post-chemotherapy among women who experience chemotherapy-induced, hypergonadotropic amenorrhea compared to stable levels among those who resumed menstrual function following chemotherapy (4, 5).
The anti-Müllerian hormone (AMH) is a member of the transforming growth factor beta (TGF-β) family. In humans, it is expressed in the ovarian granulosa cell of primary, pre-antral and small antral follicles, but not by larger developing follicles. AMH, almost undetectable at birth, increases at puberty, remains stable throughout adulthood, and declines at menopause (6–8). A longitudinal study illustrated that, among healthy, premenopausal females, serum AMH values did not vary significantly during the menstrual cycle in contrast to other steroids/peptides (FSH, luteinizing hormone [LH], estradiol, and Inhibin B) whose rise and fall reflect selection and maturation of the dominant follicle (9). The relative consistency of AMH throughout the ovarian cycle makes it an attractive determinant of ovarian activity, thus reflecting the oocyte/follicular pool.
Although the influence of chemotherapy on ovarian function is an important issue in the care of young breast cancer survivors with implications on both treatment opportunities and quality of life, prospective markers of ovarian reserve have yet to be validated. To date, few studies have prospectively correlated sequential Inhibin (A or B) and AMH levels with established hormonal markers, namely estradiol and FSH, and menstrual status. In the present study, we report that pre-chemotherapy Inhibin B and AMH, in parallel with well-known markers of ovarian reserve, are predictive of future ovarian function among premenopausal women treated with chemotherapy for early stage breast cancer.
PATIENTS AND METHODS
Premenopausal women, aged ≥18 or ≤55 years, with a histologically-confirmed, Stage I-III diagnosis of operable breast adenocarcinoma were eligible for study enrollment. Pre-menopausal status was defined as the presence of two menstrual cycles within 180 days of study enrollment. Additional inclusion criteria included Karnofsky score ≥70, ability to provide informed consent, planned follow-up at Duke University, and a planned course of chemotherapy with a ≥25% risk of permanent amenorrhea. Patients were excluded if they had a history of an ovarian tumor, were currently pregnant, or had received oral contraceptives within 30 days of study enrollment. Human investigations were performed after both protocol approval by the Duke University Institutional Review Board (IRB # 5335) and signed informed consent from all patients allowing for analysis of proteins related to premature ovarian failure.
Patients were evaluated at four study time points: pre-chemotherapy (≤ 3 weeks of chemotherapy), post-chemotherapy (3 to 7 weeks post-chemotherapy), 6 months post-chemotherapy (18–30 weeks post-chemotherapy), and one year post-chemotherapy (48–60 weeks post-chemotherapy). With the exception of informed consent at the pre-chemotherapy study visit, study procedures were identical at each study visit. Each study visit included serum collection (10 mL) and the administration of QOL assessment scales, including the Functional Assessment of Cancer Therapy-Breast (FACT-B; http://www facit.org), menstrual history and family planning questionnaires. Due to the urgency associated with the initiation of chemotherapy, baseline serum specimens were drawn across phases of the menstrual cycle. As the Duke Comprehensive Cancer Center catchment area is quite large, post-, 6 months and 1 year post-chemotherapy visits were coordinated with routine physician follow-up visits. Therefore, serum analyses were not timed with the menstrual cycle among women who continued to menstruate.
Estradiol was measured via radioimmunoassay (RIA, Diagnostic Systems Laboratories, Inc., Webster, Texas, USA). Sensitivity for estradiol was 10 pg/mL. FSH was measured via immunoradiometric assay (IRMA, Diagnostic Systems Laboratories, Inc.) for first interim analysis (10) and chemilumines-cent immunometric assay (Immulite, Siemans Medical Solutions Diagnostics, Malvern, Pennsylvania, USA) for the present analysis. The sensitivities for FSH were 0.11 mIU/mL and 0.05 mIU/mL, respectively. Inhibin A and B were measured via Enzyme-Linked ImmunoSorbent Assay (ELISA, Diagnostic Systems Laboratories, Inc). Sensitivity was 1 pg/mL for Inhibin A and 7 pg/mL for Inhibin B. Finally, AMH was measured via ELISA (Diagnostic Systems Laboratories, Inc). Sensitivity was 0.017 ng/mL for AMH. Serum analyses were performed at Duke University (Durham, North Carolina), Northwestern University (Chicago, Illinois), University of North Carolina (Chapel Hill, North Carolina), and University of Virginia (Grant U54-HD28934, University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, Charlottesville, Virginia).
Statistical analysis
Serum FSH, estradiol, Inhibin A, Inhibin B, AMH, and QOL were assessed at all four study visits. QOL was measured by the FACT-B scale. Raw FACT-B scores were normalized to T-scores in order to place all scores on the same scale as adult patients with cancer (11).
Medians of each serum marker at each visit on all 6 endpoints were calculated according to menstrual status and according to age group. In this analysis, chemotherapy-related amenorrhea (CRA) was defined as the absence of menses one-year post-chemotherapy. Age was categorized into younger (<35 years) and older (≥35 years) cohorts. Throughout the published breast cancer literature, breast cancer arising in “young” women is defined by an age of less than 35 years. Therefore, this age cut-off was selected to define a younger cohort of patients within a population of premenopausal women and remains consistent with previous reports (12–14). Additionally, patients who underwent therapeutic ovarian suppression (i.e., bilateral salpingo-oophorectomy [BSO] or treatment with a gonadotropin-releasing hormone [GnRH] agonist) during the course of this study were excluded from the analysis at the time of intervention. Available hormone levels were evaluated by age (<35 vs ≥35 years), but were not evaluated by CRA status as 1 year follow-up was not attained.
For a number of patients, Inhibin A values were recorded as simply “<0.13”; for the purposes of statistical analysis, these values were imputed to be 0.065. In the analysis of age differences on Inhibin A across the four time points, the percent of patients with imputed values were 3%, 30%, 0%, and 33%, respectively. For many patients, AMH values were recorded as “<0.05”; these values were imputed to be 0.025. In the analysis of age differences on AMH across time, the percent of patients with imputed values were 5%, 81%, 64%, and 65%, respectively.
Differences between CRA and age subgroups on all endpoints were tested with the non-parametric Wilcoxon two-sample test using a two-sided alpha of 0.10. The Wilcoxon test was also used to test for subgroup differences on change in hormone level from pre-chemotherapy to post-chemotherapy.
RESULTS
Patient characteristics
At the time of this analysis, both serum values and corresponding menstrual data were available for 44 patients, of which 41 patients were evaluable pre-chemotherapy, 32 post-chemotherapy, 16 six months post-chemotherapy and 21 one year post-chemotherapy (Table 1). Of the 23 patients who were not evaluable one year post-chemotherapy, 9 had yet to reach one year of follow-up, 6 were no longer receiving care at Duke University, 3 had undergone bilateral salpingo-oophorectomy, 2 received additional chemotherapy for progressive disease, 2 received GnRH agonist therapy, and 1 withdrew from the study. Median age of all evaluable patients (n = 44) was 40 years (range 21–51 years). The median age among patients who developed CRA was 44 years (range 33–51 years, n = 16) versus 40 years (range 31–43 years, n = 5) among those who had resumed menses (p = 0.03).
Table 1.
Clinical characteristics of enrolled patients
| Characteristic | No (%) |
|---|---|
| Age (n = 44) | |
| All patients | 40 years (range 21–51) |
| CRA (n = 21) | |
| Present | 16 (76%) |
| Absent | 5 (24%) |
| Race (n = 44) | |
| White | 32 (73%) |
| Black | 9 (20%) |
| Asian | 3 (7%) |
| Chemotherapy regimens (n = 44) | |
| AC | 13 (30%) |
| AC→Taxol | 9 (21%) |
| TAC | 8 (18%) |
| AC→Taxotere | 5 (11%) |
| ddAC→Taxol | 3 (7%) |
| AC→T (neo), Xeloda (adj) | 4 (9%) |
| FEC | 1 (2%) |
| TCH (neo), AC (adj) | 1 (2%) |
| Trastuzumab | 8 (18%) |
| Adjuvant Endocrine Therapy* (n = 44) | |
| Yes | 27 (61%) |
| No | 17 (39%) |
| Patient Preferences (n = 44) | |
| Interested in Fertility | 8 (18%) |
| Discussed with MD | 12 (27%) |
| Aware of Fertility-Sparing Treatments | 12 (27%) |
The majority of patients enrolled in this study received a combination chemotherapy regimen that incorporated both an anthracycline and a taxane. The incidence of CRA was the same among women who did or did not receive a taxane as part of their standard care (75% [9/12] versus 75% [6/8]). Sixty-one percent (27/44) of patients received adjuvant endocrine therapy at the completion of chemotherapy. Among patients with CRA, 12 of 16 (75%) received adjuvant endocrine therapy (n = 11, Tamoxifen; n = 1, aromatase inhibitor). Among patients who resumed menses at one year post-chemotherapy, 4 of 5 (80%) received Tamoxifen. At the pre-chemotherapy visit, 18% (8/44) of patients indicated that they were interested in future fertility, 27% (12/44) had discussed fertility issues with their treating physician, and 27% (12/44) stated they were aware of fertility-sparing therapies.
Effect of age on serum hormone values
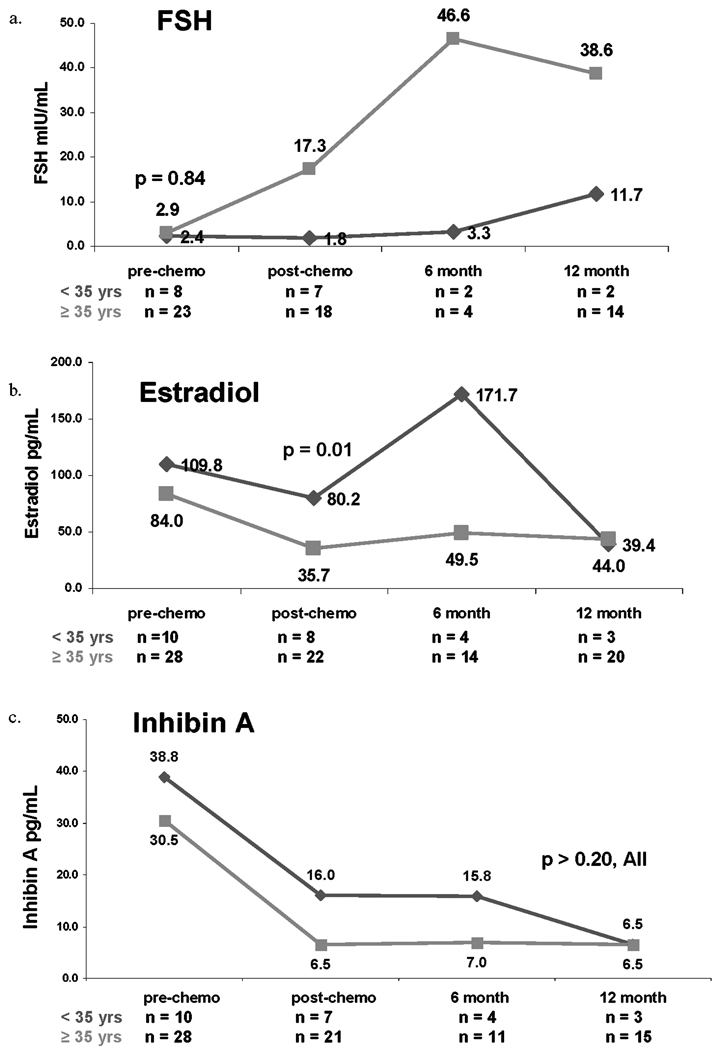
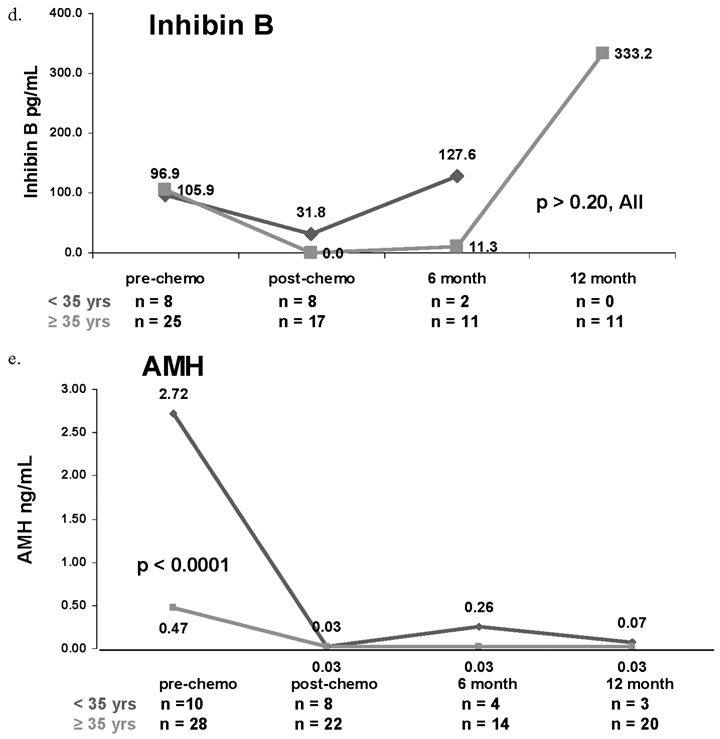
Median pre-chemotherapy FSH was nearly identical for women aged <35 compared to those aged ≥35 years (2.4 versus 2.9 mLU/mL, p = 0.84). In post-, 6 months-, and one year post-chemotherapy, however, median FSH was greater among women aged ≥35 years compared to those <35 years (Figure 1a). While for the first three study time points, median estradiol was numerically greater among women aged < 35 years compared to those aged ≥35 years, only the post-chemotherapy values were significantly different (_p_ = 0.01, Figure 1b). Pre-, post-, and 6 month post-chemotherapy Inhibin A (Figure 1c) and post- and 6 month-post-chemotherapy Inhibin B medians (Figure 1d) were numerically higher among younger women compared with older counterparts (all _p_ values >0.20). Finally, pre-chemotherapy median AMH among women aged <35 years was significantly higher compared with those aged ≥35 years (2.72 versus 0.47 ng/mL, p < 0.0001; Figure 1e).
Figure 1.
Median serum concentrations of follicle-stimulating hormone (FSH, 1a), estradiol (1b), Inhibin A (1c), Inhibin B (1d) and AMH (1e) based on age <35 years or ≥35 years. Red lines represent women aged <35 years. Green lines represent women aged ≥35 years.
Figure 1 can also be used to compare the effect of age on change in serum hormone levels from pre-chemotherapy to immediately post-chemotherapy. Serum FSH values increased to a greater degree among women aged ≥35 years compared to women aged <35 years (p = 0.04), whereas changes in AMH were more pronounced among younger women (p = 0.003). Estradiol, Inhibin A and Inhibin B values all declined to a greater degree among women aged ≥35 years compared with younger women; however, differences were not statistically significant.
Effect of CRA on serum hormone values
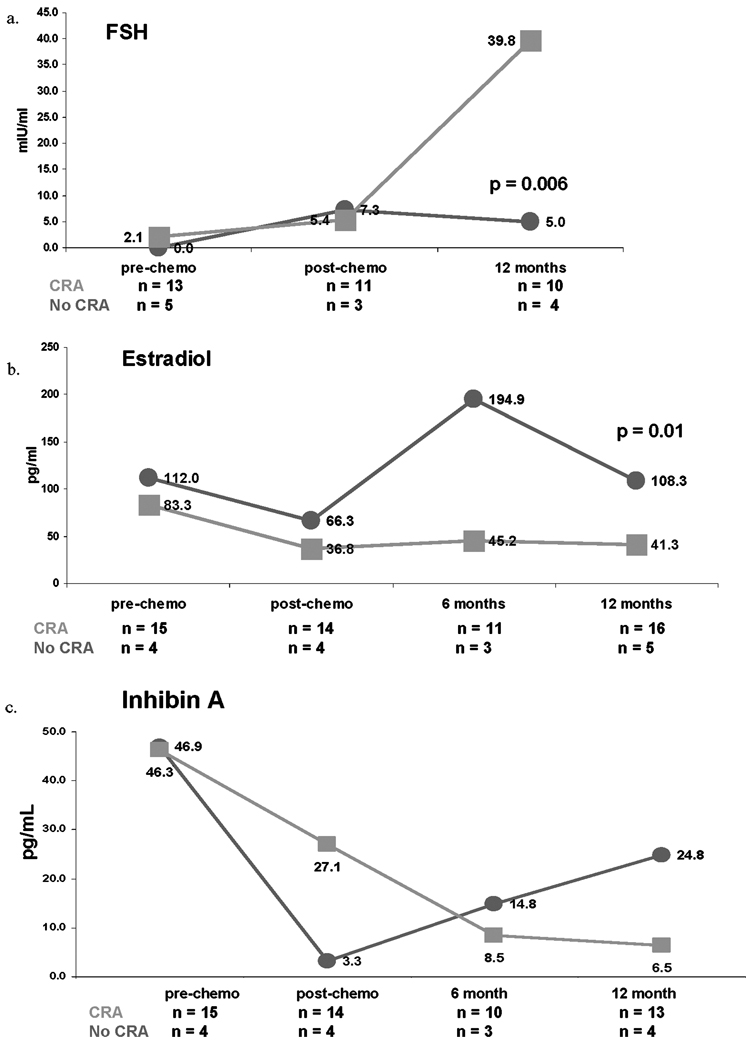
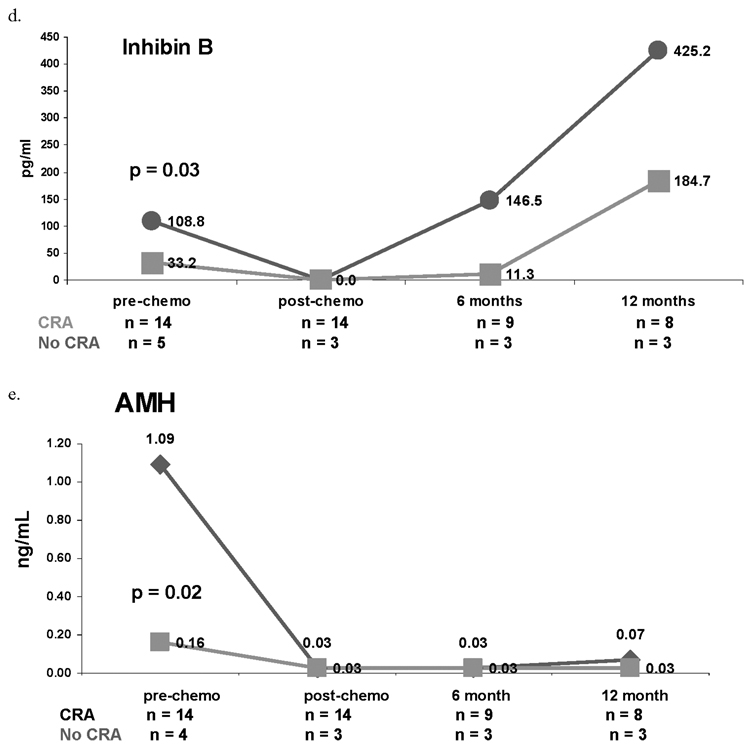
At the time of this analysis, both menstrual status and serum hormone values were available for 21 patients one year post-chemotherapy. Median pre-chemotherapy serum hormone values by CRA status are presented in Table 2. Median FSH was higher one year post-chemotherapy among women who experienced CRA compared to those who resumed menses (39.8 versus 5.0 mIU/mL, p = 0.006) (Figure 2a). Median estradiol was consistently higher among women who resumed menses compared to those who experienced CRA at all study time points; however, only at one year post-chemotherapy differences were significant (108.3 versus 41.3 pg/mL, p = 0.01, Figure 2b). Inhibin A was not significantly associated with CRA status at any time point (Figure 2c). Pre-chemotherapy median Inhibin B was significantly lower among women who experienced CRA compared to those who resumed menses (33.2 versus 108.8 pg/mL; p = 0.03, Figure 2d) but were not significantly different at other study time points. Additionally, pre-chemotherapy median AMH was significantly lower among women who developed CRA compared to those who maintained menses (0.16 versus 1.09 ng/mL, p = 0.02; Figure 2e). While the median AMH values at one-year post chemotherapy do not appear to differ, all 4 of the women who resumed menses had values >0.05, while only 1 of the 14 women with CRA had a value >0.05 (p = 0.002).
Table 2.
Baseline median serum hormone values
| Hormone(Median, Range) | All | CRA* | No CRA* | p value** |
|---|---|---|---|---|
| FSH, mIU/mL | 2.9 (0–25.3) | 2.1 (0–25.3) | 0 (0–1.1) | 0.16 |
| Estradiol, pg/mL | 98.8 (6.5–383.4) | 83.3 (31.3–383.4) | 112.0 (41.8–160.4) | 0.96 |
| Inhibin A, pg/mL | 34.8 (0.7–152.6) | 46.3 (2.6–152.6) | 46.9 (12.3–110.0) | 0.96 |
| Inhibin B pg/mL | 105.9 (0.0–571.8) | 33.2 (0–187.6) | 108.8 (28.6–425.2) | 0.03 |
| AMH, ng/mL | 0.7 (0.006–7.9) | 0.16 (0.006–1.5) | 1.09 (0.64–3.8) | 0.02 |
Figure 2.
Median serum concentrations of FSH (2a), estradiol (2b), Inhibin A (2c), Inhibin B (2d), and AMH (2e) by chemotherapy-related amenorrhea (CRA) status. Red lines represent women who resumed menses at one year post-chemotherapy. Green lines represent women who experienced CRA.
Finally, the association of CRA with Inhibin B and AMH dichotomized at their medians was calculated. Among women with pre-chemotherapy Inhibin B values below the median, 83% (10/12) experienced CRA compared to 50% (3/6) of women with values above the median (Risk Ratio [RR] = 1.67, p = 0.15). Similarly, among women with pre-chemotherapy AMH values below the median, 92% (11/12) experienced CRA compared to 50% (3/6) of women with values above the median (RR = 1.83, p = 0.05). Amongst women whose pre-chemotherapy serum Inhibin B and AMH values both fell below the median, the incidence of CRA was 87.5% (7/8). This compares to 62.5% (5/8) who were above the median for at least one of the two hormones.
Effects on quality of life
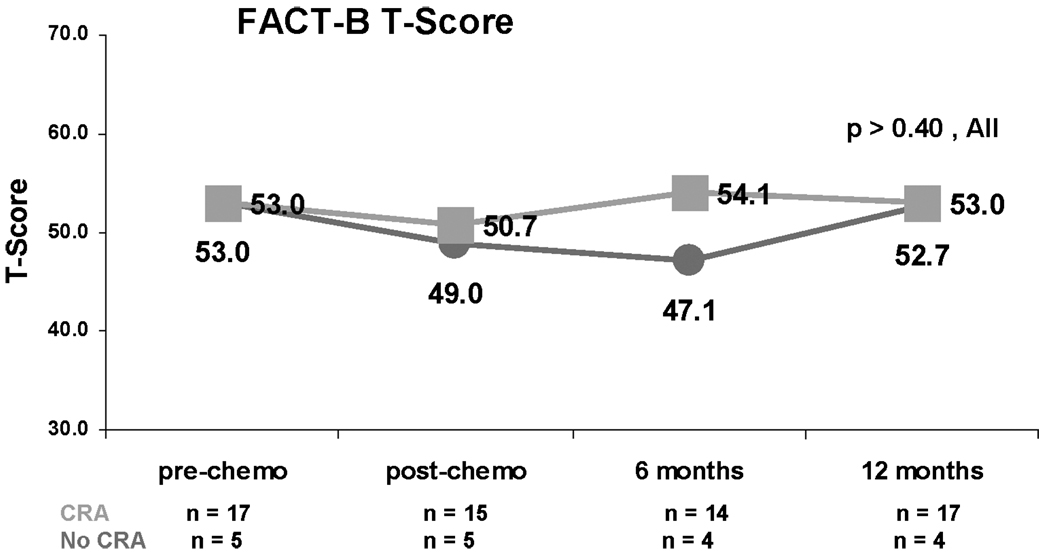
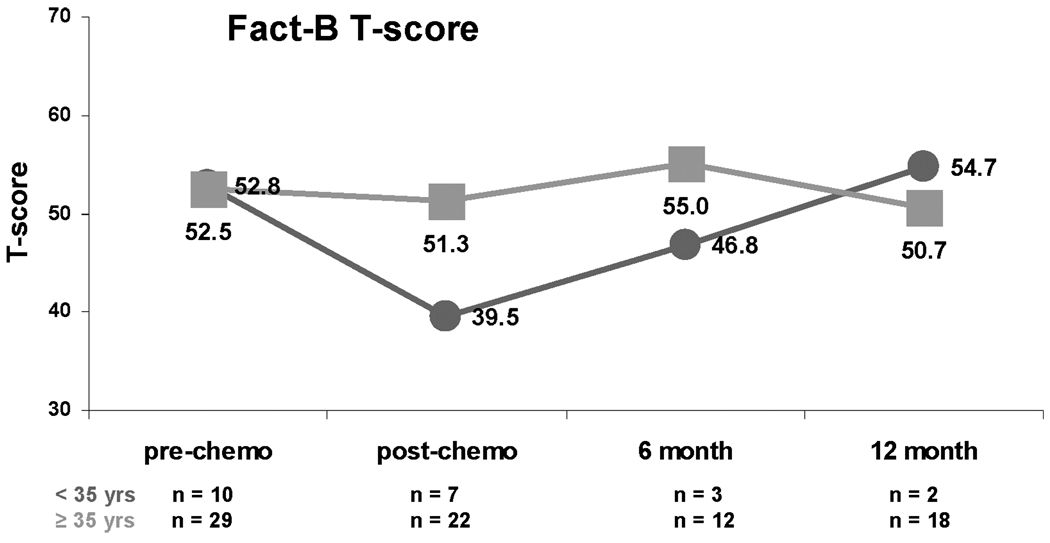
Quality of life (QOL) was not related to CRA status (Figure 3; all p values >0.40). Although median Fact B T scores of the younger and older women were nearly identical at baseline, QOL declined to a greater degree post-chemotherapy among younger women (Figure 4).
Figure 3.
Median FACT-B normalized T scores by CRA status. Red lines represent women who resumed menses at one year post-chemotherapy. Green lines represent women who experienced CRA.
Figure 4.
Median FACT-B normalized T scores by age. Red lines represent women who are aged <35 years. Green lines represent women who are aged ≥35 years of age.
DISCUSSION
Young, premenopausal women facing chemotherapy for early stage breast cancer (ESBC) are at substantial risk for chemotherapy-related amenorrhea (CRA) and subsequent ovarian failure (15). CRA has the potential to affect quality of life, future fertility options and important treatment decisions at the completion of cytotoxic therapies, including the selection of tamoxifen, aromatase inhibitors and/or gonadotropin-releasing hormone (GnRH) agonists. Although this aspect of patient care is of paramount importance, prospectively-defined, predictive markers of CRA are not currently available to the patient or practicing clinician as they face important treatment decisions. The purpose of our analysis was to prospectively evaluate the relationship of both established hormonal markers (FSH and estradiol) and more modern indicators of ovarian reserve (AMH, Inhibin A and B) with that of CRA among premenopausal women with ESBC treated with cytotoxic agents. Our results indicate that both pre-chemotherapy Inhibin B and AMH levels are statistically lower among women who develop CRA compared to those who resume menses. Importantly, the risk of CRA was increased among women with lower pre-chemotherapy serum Inhibin B and AMH values.
Chemotherapy is known to deplete the ovary’s finite primordial follicular pool through direct toxic effects on maturing follicles. Thus, ovarian reserve falls below a threshold and premature ovarian failure ensues (16). The risk of ovarian failure following chemotherapy has been positively correlated with both advancing age (i.e., ≥40 years) and higher cumulative doses of chemotherapy. Combination chemotherapy regimens in comparison to single agents pose a greater risk on subsequent ovarian function, as does particular type of chemotherapy (17). Historically, non-cell-cycle-specific, alkylating agents (i.e., cyclophos-phamide) have proven to be particularly toxic to the ovary. For instance, rates of amenorrhea one year post-chemotherapy were 69% among women having received 6 months of oral CMF (cyclophosphamide, methotrexate, and 5-fluorouracil) chemotherapy. This compares to a 34% risk among those receiving 12 weeks of AC (adriamycin, cyclophosphamide) chemotherapy (18). Finally, it has been postulated that chemotherapy administered during the follicular phase of the menstrual cycle is more ovarian-toxic (15, 16).
Breast cancer patients are acutely concerned about the implications of chemotherapy on ovarian function as it relates to early menopause, future fertility, sexuality and physiologic side-effects (i.e., hot flashes, osteoporosis). This concern has been well-illustrated in over 1,000 young women surveyed regarding the treatment-related effects on menstrual status following chemotherapy for ESBC (19). Over half of surveyed patients reported a desire for future fertility and concerns about early menopause at diagnosis and three-fourths reported concern about becoming infertile. Moreover, approximately 30% stated that fertility concerns affected their treatment choices. As follows, the effects of chemotherapy on future ovarian function has the potential to affect thousands of breast cancer survivors in terms of treatment choices, quality of life, and survivorship.
In clinical practice, hormonal profiles, including FSH and estradiol, and the presence or absence of menses serve as markers of ovarian reserve at the completion of cytotoxic therapies. The availability of predictive markers at the time of diagnosis could have tremendous clinical applicability in guiding the choice of chemotherapeutic agents, use of GnRH agonists during chemotherapy in hopes of protecting future fertility, and as appropriate, timely referrals to specialists in reproductive endocrinology and infertility.Additionally, information regarding potential for future ovarian function and fertility could be emotionally beneficial for young women facing chemotherapy for ESBC.
To date, few studies have prospectively evaluated markers of ovarian reserve in the setting of ESBC. Our results are in accordance with data recently published by Anderson et al. (20). In this analysis, among approximately 40 premenopausal women treated with various chemotherapy regimens for ESBC, pre-chemotherapy AMH levels were lower among women who became amenorrheic at 6 months compared to those who resumed menses (0.58 versus 1.9 ng/mL, p = 0.0007). Among this cohort of patients, in contrast to our results, there was no significant difference in pre-chemotherapy Inhibin B levels between groups (51 ng/mL versus 68 ng/mL, p value not reported) (20). We anticipate that the accurate prediction of CRA among patients treated for ESBC will ultimately rely on a compilation of multiple factors, including hormonal parameters, age at diagnosis and chemotherapeutic agent(s), including type, cumulative dose and duration of treatment.
This analysis is one of the largest studies prospectively evaluating markers of ovarian reserve among premenopausal women receiving chemotherapy for ESBC; however, we recognize several limitations inherent to this study. The results of this preliminary analysis are limited by both a small sample size and the heterogeneity of treatment regimens. To address these issues, we specifically plan to expand this cohort to more accurately evaluate markers of ovarian function in a larger patient cohort. Additionally, the effect of taxanes, commonly-used chemotherapeutics in the adjuvant treatment of node-positive and high-risk node negative ESBC, on subsequent ovarian function has been controversial throughout the literature. Several trials have reported a lower incidence, while others a higher incidence of amenorrhea, among women who received a taxane as part of their adjuvant chemotherapy regimen (17, 21–23). Among a larger cohort of patients, we plan to evaluate the specific interactions of treatment regimens, namely taxanes, on CRA in the setting of ESBC.
Additionally, it is well-known that many steroids and peptides reflective of ovarian reserve, namely FSH, estradiol, and Inhibin A & B, vary across and within the menstrual cycle (9). In this analysis, baseline serum hormones were drawn across the menstrual cycle due to the urgency associated with the initiation of chemotherapy. Subsequent serum collections were performed in conjunction with routine physician visits as the Duke Comprehensive Cancer Center encompasses a large catchment area and many patients travel long distances to receive care. Although we recognize this limitation in the interpretation of our findings, this pattern likely reflects that of “real-time” practice and again speaks to the strength of developing AMH as a predictive marker of ovarian reserve due to its stability across the menstrual cycle (9). AMH, initially a marker of ovarian reserve primarily studied in the reproductive endocrinology literature, has recently gained favor in the chemotherapy literature due to superior cycle-to-cycle reproducibility and improved sensitivity in comparison to traditional testing with FSH. Additionally, decrements in AMH are seen prior to elevations in FSH as illustrated in many recent observational chemotherapy studies, including breast cancer protocols with intravenous cyclophosphamide (20, 24).
Finally, Tamoxifen, a partial estrogen receptor agonist and antagonist, is known to influence both menstrual patterns, including induction of amenorrhea, and hormonal profiles among premenopausal women following therapy for ESBC (17). We recognize that approximately 2/3 of patients in this trial were receiving standard adjuvant endocrine therapy at the one year post-chemotherapy visit. In our analysis, however, a similar proportion of women with CRA and those who resumed menses at one year post-chemotherapy were prescribed adjuvant endocrine therapy (75% vs. 80%, respectively). Furthermore, prior reports have illustrated marked increases in estradiol, with unaltered FSH values, among premenopausal women treated with Tamoxifen for ESBC (25). Therapy with Tamoxifen may be accounting for median estradiol levels above the expected post-menopausal range (i.e., <10 pg/mL) among amenorrheic women one year post-chemotherapy. It is important to recognize that the endpoint of this study was amenorrhea at one year, not permanent ovarian failure. Prior to deeming these patients post-menopausal, a longer duration of follow-up following the cessation of endocrine therapy would be required.
In summary, this pilot study demonstrates that pre-chemotherapy Inhibin B and AMH values are significantly lower among women who experience CRA and that lower baseline values place a woman at higher risk for subsequent CRA. Importantly, this study illustrates an ability to correlate markers of ovarian reserve with an individual woman’s risk of amenorrhea following chemotherapy. Although this data requires validation prior to incorporation into routine clinical care, these results offer hope that clinically useful biomarkers to predict CRA will be available to both breast cancer patients and their clinicians in the near future.
Footnotes
This article funded by Duke Comprehensive Cancer Center Development Funds.
Research previously presented at the 30th Annual San Antonio Breast Cancer Symposium; December 2007, Abstract 4025.
REFERENCES
- 1.ACS. American Cancer Society: Cancer Facts and Figures. 2007 [Google Scholar]
- 2.Hensley ML, Reichman BS. Fertility and pregnancy after adjuvant chemotherapy for breast cancer. Crit. Rev. Oncol. Hematol. 1998;28:121–128. doi: 10.1016/s1040-8428(98)00013-4. [DOI] [PubMed] [Google Scholar]
- 3.EBCTCG. Polychemotherapy for Early Stage Breast Cancer: An Overview of the Randomised Trials. Early Breast Cancer Trialist’ Collaborative Group. Lancet. 352:930–942. [PubMed] [Google Scholar]
- 4.Blumenfeld Z. Ovarian rescue/protection from chemotherapeutic agents. J. Soc. Gynecol. Investig. 2001;8:S60–S64. doi: 10.1016/s1071-5576(00)00112-x. [DOI] [PubMed] [Google Scholar]
- 5.Blumenfeld Z, Ritter M, Shen-Orr Z, et al. Inhibin A concentrations in the sera of young women during and after chemotherapy for lymphoma: correlation with ovarian toxicity. Am. J. Reprod. Immunol. 1998;39:33–40. doi: 10.1111/j.1600-0897.1998.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 6.Rajpert-De Meyts E, Jorgensen N, Graem N, Muller J, Cate R, Skakkebaek M. Expression of anti-Mullerian hormone during normal and pathological gonadal development: association with differentiation of Sertoli and granulosa cells. Journal of Clinical Endocrinology and Metabolism. 1999;84:3836–3844. doi: 10.1210/jcem.84.10.6047. [DOI] [PubMed] [Google Scholar]
- 7.Hudson P, Douglas I, Donahoe P, Cate R, Epstein J, Pepinsky R, MacLaughlin D. An immunoassay to detect human Mullerian inhibiting substance in males and females during normal development. Journal of Clinical Endocrinology and Metabolism. 1990;70:16–22. doi: 10.1210/jcem-70-1-16. [DOI] [PubMed] [Google Scholar]
- 8.Lee M, Donahoe P, Hasegawa T, Silverman B, Crist B, Best S, Hasegawa Y, Noto R, Scheonfeld D, MacLaughlin D. Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. Journal of Clinical Endocrinology and Metabolism. 1996;81:571–576. doi: 10.1210/jcem.81.2.8636269. [DOI] [PubMed] [Google Scholar]
- 9.La Marca A, Stabile G, Carducci Artenisio A, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Human Reproduction. 2006;12:3103–3107. doi: 10.1093/humrep/del291. [DOI] [PubMed] [Google Scholar]
- 10.Anders C, Snyder S, Barrier R, Demark-Wahnefried W, Welch R, Marcom P, Shaw H, Chui S, Blackwell K. Inhibin A and B as predictive markers for chemotherapy-induced premature ovarian failure (POF) among pre-menopausal women with operable breast cancer. Breast Cancer Research and Treatment; 28th Annual San Antonio Breast Cancer Symposium. 2005;94(Suppl 1) [Google Scholar]
- 11.Brucker P, Yost K, Cashy J, et al. General Population and Cancer Patient Norms for the Functional Assessment of Cancer Therapy-General (FACT-G) per Erratum. Evalulations and the Health Professions. 2005;28(3):370. doi: 10.1177/0163278705275341. [DOI] [PubMed] [Google Scholar]
- 12.Host H, Lund E. Age as a Prognostic Factor in Breast Cancer. Cancer. 1986;57:2217–2221. doi: 10.1002/1097-0142(19860601)57:11<2217::aid-cncr2820571124>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 13.Holli K, Isola J. Effect of age on the survival of breast cancer patients. Eur. J. Cancer. 1997;33:425–428. doi: 10.1016/s0959-8049(97)89017-x. [DOI] [PubMed] [Google Scholar]
- 14.Kollias J, Elston CW, Ellis IO, et al. Early-onset breast cancer–histopathological and prognostic considerations. Br. J. Cancer. 1997;75:1318–1323. doi: 10.1038/bjc.1997.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walshe J, Denduluri N, Swain S. Amenorrhea in Pre-menopausal Women After Adjuvant Chemotherapy for Breast Cancer. Journal of Clinical Oncology. 2006;24:5769–5779. doi: 10.1200/JCO.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- 16.Bines J, Oleske DM, Cobleigh MA. Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. Journal of Clinical Oncology. 1996;14:1718–1729. doi: 10.1200/JCO.1996.14.5.1718. [DOI] [PubMed] [Google Scholar]
- 17.Petrek JA, Naughton MJ, Case D, Paskett ED, Naftalis EJ, Singletary SE, Sukumvanich P. Incidence, Time Course and Determinants of Menstrual Bleeding after Breast Cancer Treatment: A Prospective Study. Journal of Clinical Oncology. 2006;24:1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 18.Cobleigh MA. Amenorrhea Following Adjuvant Chemotherapy for Breast Cancer. Proc. Am. Soc. Clin. Oncol. 1995;14(115):158. [Google Scholar]
- 19.Partridge A, Gelber S, Peppercorn J, et al. Web-Based Survey of Fertility Issues in Young Women With Breast Cancer. Journal of Clinical Oncology. 2004;22:4174–4183. doi: 10.1200/JCO.2004.01.159. [DOI] [PubMed] [Google Scholar]
- 20.Anderson R, Themmen A, Al-Qahtani A, Groome N, Cameron D. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve of premenopausal women with breast cancer. Human Reproduction. 2006;21:2583–2592. doi: 10.1093/humrep/del201. [DOI] [PubMed] [Google Scholar]
- 21.Stone E, Slack R, Novielli A, et al. Rate of chemotherapy-related amenorrhea associated with adjuvant adriamycin and cytoxan and adriamycin followed by taxane in the early stage breast cancer. Breast Cancer Research and Treatment. 2000;64 [Google Scholar]
- 22.Alton J, Jacobs L, Fox K. Chemotherapy-related amenorrhea (CRA) in breast cancer survivors: Impact of taxanes on ovarian function. Breast Cancer Research and Treatment. 2004;88 [Google Scholar]
- 23.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. New England Journal of Medicine. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 24.Lutchman Singh K, Muttukrishna S, et al. Predictors of ovarian reserve in young women with breast cancer. Br. J. Cancer. 2007;96(12):1808–1816. doi: 10.1038/sj.bjc.6603814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bianco A, De Placido S, Pagliarulo C, Fasano S, D’Istria M, De Sio L, Ricciardi I, Delrio G. Effect of adjuvant tamoxifen and CMF on endocrine function of patients with operable breast cancer. Chemioterapia. 1985;4:252–255. [PubMed] [Google Scholar]