Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function (original) (raw)
Abstract
Aims
Cell-based therapy for myocardial infarction (MI) holds great promise; however, the ideal cell type and delivery system have not been established. Obstacles in the field are the massive cell death after direct injection and the small percentage of surviving cells differentiating into cardiomyocytes. To overcome these challenges we designed a novel study to deliver cardiac progenitor cells as a cell sheet.
Methods and results
Cell sheets composed of rat or human cardiac progenitor cells (cardiospheres), and cardiac stromal cells were transplanted onto the infarcted myocardium after coronary artery ligation in rats. Three weeks later, transplanted cells survived, proliferated, and differentiated into cardiomyocytes (14.6 ± 4.7%). Cell sheet transplantation suppressed cardiac wall thinning and increased capillary density (194 ± 20 vs. 97 ± 24 per mm2, P < 0.05) compared with the untreated MI. Cell migration from the sheet was observed along the necrotic trails within the infarcted area. The migrated cells were located in the vicinity of stromal-derived factor (SDF-1) released from the injured myocardium, and about 20% of these cells expressed CXCR4, suggesting that the SDF-1/CXCR4 axis plays, at least, a role in cell migration. Transplantation of cell sheets resulted in a preservation of cardiac contractile function after MI, as was shown by a greater ejection fraction and lower left ventricular end diastolic pressure compared with untreated MI.
Conclusion
The scaffold-free cardiosphere-derived cell sheet approach seeks to efficiently deliver cells and increase cell survival. These transplanted cells effectively rescue myocardium function after infarction by promoting not only neovascularization but also inducing a significant level of cardiomyogenesis.
Keywords: Myocardial infarction, Cardiac progenitor cells, Cardiospheres, Cardiac regeneration, Contractility
1. Introduction
Despite advances in cardiac treatment after myocardial infarction (MI), congestive heart failure remains the number one killer worldwide. MI results in an irreversible loss of functional cardiomyocytes followed by scar tissue formation. To date, heart transplant remains the gold standard for treatment of end-stage heart failure, a procedure which will always be limited by the availability of a donor heart. Hence, developing a new form of therapy is vital.
A number of adult non-cardiac progenitor cells have been tested for myocardial regeneration, including skeletal myoblasts,1 bone-marrow2, and endothelial progenitor cells.3,4 In addition, several cardiac resident stem cell populations have been characterized based on the expression of stem cell marker proteins.5–8 Among these, the c-Kit+ population has been reported to promote myocardial repair.5,9 Recently, an ex vivo method to expand cardiac-derived progenitor cells from human myocardial biopsies and murine hearts was developed.10 Using this approach, undifferentiated cells (or cardiospheres) grow as self-adherent clusters from postnatal atrium or ventricular biopsy specimens.11
To date, the most common technique for cell delivery is direct injection into the infarcted myocardium.12 This approach is inefficient because more than 90% of the delivered cells die by apoptosis and only a small number of the survived cells differentiated into cardiomyocytes.13 An alternative approach to cell delivery is a biodegradable scaffold-based engineered tissue.14,15 This approach has the clear advantage in creating tissue patches of different shapes and sizes and in creating a beating heart by decellularization technology.16 Advances are being made to overcome the issue of small patch thickness and to minimize possible toxicity of the degraded substances from the scaffold.15 Recently, scaffold-free cell sheets were created from fibroblasts, mesenchymal cells, or neonatal myocytes.17,18 Transplantation of these sheets resulted in a limited improvement in cardiac function due to induced neovascularization and angiogenesis through secretion of angiogenic factors.17–19 However, few of those progenitor cells have differentiated into cardiomyocytes.17 The need to improve cardiac contractile function suggests focusing on cells with higher potential to differentiate to cardiomyocytes with an improved delivery method.
In the present study, we report a cell-based therapeutic strategy that surpasses limitation inherent in previously used methodologies. We have created a scaffold-free sheet composed of cardiac progenitor cells (cardiospheres) incorporated into a layer of cardiac stromal cells. The progenitor cells survived when transplanted as a cell sheet onto the infarcted area, improved cardiac contractile functions, and supported recovery of damaged myocardium by promoting not only vascularization but also a significant level of cardiomyogenesis. We also showed that cells from a sheet can be recruited to the site of injury driven, at least partially, by the stromal-derived factor (SDF-1) gradient.
2. Methods
Detailed methods are provided in the Supplementary Methods.
2.1. Animals
Three-month-old Sprague Dawley male rats were used. Study was performed in an American Association for Accreditation of Laboratory Animal Care accredited facility with approval from the animal use committee at Sun Health Research Institute. Animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Rats were randomly placed into four groups: (1) sham-operated rats, n = 12; (2) MI, n = 12; (3) MI treated with rat sheet, n = 10; and (4) MI treated with human sheet, n = 10.
2.2. Myocardial infarction
MI was created by the ligation of the left coronary artery.20 In brief, rats were anesthetized using 1 mL/kg of an MI cocktail composed of ketamine (100 mg/mL), xylazine (20 mg/mL), acepromazine (10 mg/mL), and atropine (0.5 mg/mL). Animals were intubated and ventilated using a small animal ventilator (Harvard Apparatus). A left thoracotomy was performed via the third intercostal rib, and the left coronary artery was ligated. The extent of infarct was verified by measuring the area at risk: heart was perfused with PBS containing 4 mg/mL Evans Blue as previously described by our laboratory.20 The area at risk was estimated by recording the size of the under-perfused (pale-coloured) area of myocardium (see Supplementary material online, Figure S1). Only animals with an area at risk >30% were used in the present study. Post-mortem infarct size was measured using triphenyl tetrazolium chloride staining as previously described by our laboratory.20
2.3. Isolation of cardiosphere-forming cells
Cardiospheres were generated as described10 from atrial tissues obtained from: (1) human atrial resection samples obtained from patients (aged from 53 to 73 years old) undergoing cardiac bypass surgery at Arizona Heart Hospital (Phoenix, AZ) in compliance with Institutional Review Board protocol (n = 10), (2) 3-month-old SD rats (n = 10). Briefly, tissues were cut into 1–2 mm3 pieces and digested with 0.2% trypsin (Invitrogen, Carlsbad, CA) and 0.1% collagenase IV (Invitrogen). The remaining tissue fragments were cultured ‘as explants’ in a complete explants medium for 4 weeks (Supplementary Methods).
2.4. Cell sheet preparation, labelling, handling, and transplantation
Cardiosphere-forming cells (CFCs) combined with cardiac stromal cells were seeded on double-coated plates (poly-l-lysine and collagen type IV from human placenta) in cardiosphere growing medium (Supplementary Methods). The sheets created from the same cell donors were divided into two groups, one for transplantation and the other for characterization by immunostaining and RT–PCR (Supplementary Methods).
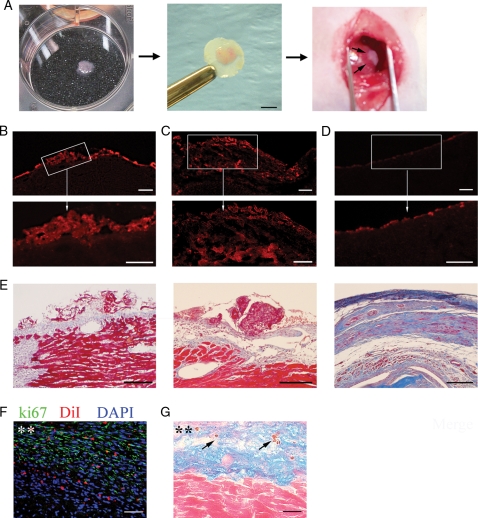
Prior to transplantation, rat cell sheets were labelled with 2 μM 1, 1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine, DiI, for tracking transplanted cells in rat host myocardium (Molecular Probes, Eugene, OR). Sheets created using human cells were transplanted unlabelled. Sheets were gently peeled off the collagen-coated plate and folded twice to form four layers. The entire sheet with 200 μl of media was gently aspirated into the pipette tip, transferred to the supporting polycarbonate filter (Costar) and spread off by adding media drops on the sheet (Figure 2A). Polycarbonate filter was used as a flexible mechanical support for cell sheet to facilitate handling during the transplantation. Immediately after LAD occlusion, the cell sheet was transplanted onto the infarcted area, allowed to adhere to the ventricle for 5–7 min, and the filter was removed before closing the chest (Figure 2A).
Figure 2.
Transplantation and growth of cell sheet after transplantation. (A) Sheet transplantation onto infarcted heart. Detached cell sheet on six-well plate (left); cell sheet folded on filter (middle); and transplanted onto left ventricle (right). Scale bar 2 mm. DiI-labelled cell sheets grafted above MI area at day 3 (B) and day 21 (C) after transplantation. (D) LV section of untreated MI rat at day 21 showing no significant red fluorescence background. Bottom row (B–D) demonstrates the enlargement of box-selected area of corresponding top panels. (E) Similar sections stained with Masson's Trichrome. Section of rat (F) or human (G) sheet treated rat at day 21 after MI. (F) Section was stained with antibody against Ki-67 (green). Cell sheet was pre-labelled with DiI (red). Nuclei stained with blue fluorescence of DAPI. (G) Section was double stained with human nuclei (blue) and caspase 3 (brown, arrows) antibodies and counterstained with eosin. Asterisks (**) indicate cell sheet area. Scale bars 200 μm (B–D, top row), 100 μm (B–D, bottom row, and E) or 50 μm (F, G).
2.5. Cardiac function
Three weeks after MI, closed-chest in vivo cardiac function was measured using a Millar pressure conductance catheter system (Millar Instruments, Houston, TX) (Supplementary Methods).
2.6. Cell sheet survival, engraftment, and cell migration
Rat host myocardium and cell sheet composition after transplantation were characterized by immunostaining (Supplementary Methods). Rat-originated cells were traced by DiI, while human-originated cells were identified by immunostaining with anti-human nuclei or human lamin antibodies.
To assess sheet-originated cardiomyocytes within the host myocardium, the number of cells positive for both human nuclei and myosin heavy chain (MHC) (human sheet); or both DiI and MHC (rat sheet) were counted. To assess sheet-originated capillaries within the rat host myocardium, the number of cells positive for both human nuclei and von Willebrand factor (vWf) (human sheet); or both DiI and vWf (rat sheet) were counted. Cells were counted in five microscopic fields within cell sheet and area of infarct (n = 5). The number of cells expressing specific markers was normalized to the total number of cells determined by 4′,6-diamidino-2-phenylindole staining of the nuclei DNA.
To assess the survival of transplanted cells, sections were stained with Ki-67 antibody followed by fluorescent detection and caspase 3 primary antibodies followed by DAB detection (Supplementary Methods).
To evaluate human sheet engraftment, sections were stained with human lamin antibody followed by fluorescent detection (Supplementary Methods).
Rat host inflammatory response to the transplanted human cell sheet 21 days after transplantation was evaluated by counting tissue mononuclear phagocytes and neutrophils (Supplementary Methods).
2.7. Imaging
Images were captured using Olympus IX70 confocal microscope (Olympus Corp, Tokyo, Japan) equipped with argon and krypton lasers or Olympus IX-51 epifluorescence microscope using excitation/emission maximum filters: 490/520 nm, 570 /595 nm, and 355 /465 nm. Images were processed using DP2-BSW software (Olympus Corp).
2.8. Statistics
All data are represented as mean ± SE Significance (P < 0.05) was determined using ANOVA (StatView).
3. Results
3.1. Generation of cardiospheres
Cardiospheres were generated from atrial tissue explants. After 7–14 days in culture, a layer of stromal cells arose from the attached explants (Supplementary material online, Figure S2a). CFCs, small phase-bright single cells, emerged from explants and bedded down on the stromal cell layer (Supplementary material online, Figure S2b). After 4 weeks, single CFCs, as well as cardiospheres (spherical colonies generated from CFCs) were observed (Supplementary material online, Figure S2c).
3.2. Cellular characteristics of cardiospheres in vitro
Immunocytochemical analysis of dissociated cardiospheres revealed that 30% of cells were c-Kit+ indicating that the CFCs maintain multipotency. About 22 and 28% of cells expressed α, β-MHC and cardiac troponin I, respectively. These cells represent an immature cardiomyocyte population because they were smaller (10–15 μm in length vs. 60–80 μm for mature cardiomyocytes) and no organized structure of MHC was detected. Furthermore 17% of the cells expressed α-smooth muscle actin (SMA) and 6% were positive for vimentin, both are mesenchymal cell markers (Supplementary material online, Figure S3a and b). Less then 5% of cells were positive for endothelial cell marker; vWf. Cell characteristics of human cardiospheres are similar to those from rat tissues (Supplementary material online, Figure S3c).
Cardiospheres were further characterized based on the expression of c-Kit antigen. RT–PCR analysis was performed on both c-Kit+ and c-Kit− subsets isolated from re-suspended cardiospheres. KDR, kinase domain protein receptor, was recently identified as a marker for cardiovascular lineage progenitors in differentiating embryonic stem cells.21 Here, we found that the c-Kit+ cells were also Nkx2.5 and GATA4-positive, but were low or negative for KDR (Supplementary material online, Figure S3d). In contrast, c-Kit− cells strongly expressed KDR and GATA4, but were negative for Nkx2.5. Both c-Kit+ and c-Kit− subsets did not express Isl1, a marker for multipotent secondary heart field progenitors.22
3.3. Characteristics of cell sheet prior to transplantation
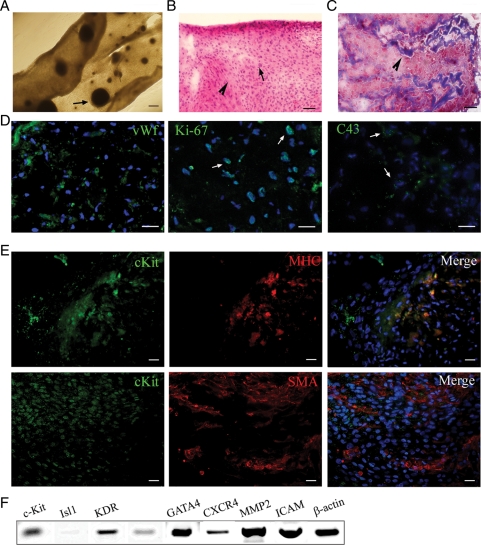
The cell sheet is a layer of cardiac stromal cells in which the cardiospheres were incorporated at a frequency of 21 ± 0.5 spheres per 100,000 viable cells (Figure 1A). The average diameter of cardiospheres within a sheet was 0.13 ± 0.02 mm and their average area was 0.2 ± 0.06 mm2 (Figure 1A). After sheets were peeled off the plate, it exhibited a heterogeneous thickness ranging from 0.05–0.1 mm (n = 10), H&E staining (Figure1B) and Masson's Trichrome staining (Figure 1C) of the sheet sections revealed tissue-like organized structures composed of muscle tissue intertwined with streaks of collagen with no necrotic core. Based on the immunostaining results, sheet compiled of several cell types including SMA+ cardiac stromal cells (50%), MHC+ cardiomyocytes (20%), and vWf+ endothelial cells (10%) (Figure 1D and E). Fifteen percent of the sheet-forming cells were c-Kit+ suggesting the cells multipotency (Figure 1E). Cells within the sheet expressed gap-junction protein C43, an indicator of electromechanical coupling between cells (Figure 1D). In addition, 40% of cells were positive for the proliferation marker Ki-67 suggesting an active cell cycle state (Figure 1D, middle panel). Human sheet expressed genes known to be upregulated in undifferentiated cardiovascular progenitors such as c-Kit and KDR; cardiac transcription factors Nkx2.5 and GATA4; genes related to adhesion, cell homing, and migration such as ICAM (intercellular adhesion molecule), CXCR4 (receptor for SDF-1), and matrix metalloprotease 2 (MMP2). No expression of Isl1 was detected in human sheet (Figure 1F).
Figure 1.
Cell sheet characteristics. (A) Fully formed cell sheet. Arrow indicates integrated cardiosphere. (B) H&E staining; pink colour (arrowhead) indicates cytosol and blue (arrows) indicates nuclear stain. Note that there is no necrotic core within the cell sheet. (C) Masson's Trichrome staining of sheet section. Arrowhead indicates collagen deposition within the sheet. (D and E) Sheet sections were labelled with antibodies against following markers: (D) vWf (green), Ki-67 (green), C43 (green); (E) c-Kit (green), MHC (red), SMA (red) as indicated on top of each panel. Nuclei were labelled with blue fluorescence of 4′,6-diamidino-2-phenylindole (DAPI). (F) Gene expression analysis of the cell sheet. Scale bars, 200 μm (A) or 50 μm (B–E).
3.4. Cell sheet survival and proliferation
Two approaches were used to track transplanted cells in the host myocardium. First, rat cell sheets were labelled with red fluorescent dye, DiI, prior to the transplantation. Second, the sheet created from human cells (human sheet) were identified in rat host myocardium by immunostaining with human nuclei antibodies.
DiI-labelling together with trichrome staining showed engraftment of the cardiosphere-derived cell sheet to the infarcted myocardium (Figure 2B_–_D). In vivo sheets grew into a stratum with heterogeneous thickness ranging from 0.1–0.5 mm over native tissue. The percentage of Ki-67+ cells within the sheet was 37.5 ± 6.5 (Figure 2F) whereas host tissue was mostly negative (except for the vasculature).
To assess the viability of transplanted cells, the heart sections were stained with the apoptosis marker, caspase 3. A low level of caspase 3 was detected within the sheet, suggesting that the majority of transplanted cells survived after transplantation (Figure 2G).
3.5. Identification of inflammatory response
Twenty-one days after transplantation of human cell sheet, inflammatory response of rat host was examined. Transplantation of human sheet on infarcted rats reduced the number of mononuclear phagocytes (ED1-like positive cells) compared with untreated MI control (Supplementary material online, Figure S4a–e and l). In addition, the number of neutrophils was similar in both control untreated MI and sheet-treated sections (Supplementary material online, Figure S4f–k and m). These data suggest that at 21 days post transplantation, human cell sheet was not associated with significant infiltration of host immune cells.
3.6. Cell sheet engraftment and migration
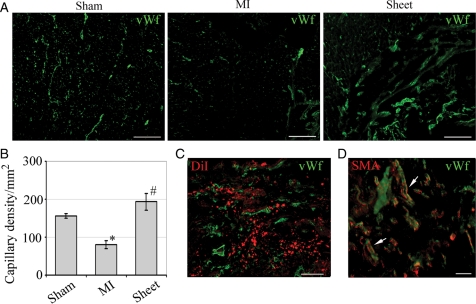
Development of new vasculature was determined in cardiac tissue sections by co-localization of DiI labelling and vWf staining (Figure 3C). Three weeks after transplantation, the capillary density of ischaemic myocardium in the sheet-treated group significantly increased compared with MI animals (194 ± 20 vs. 97 ± 24 per mm2, P < 0.05, Figure 3A and B). The capillaries originated from the sheet ranged in diameter from 10 to 40 μm (n = 30). A gradient in capillary density was observed with higher density in the sheet area which was decreased towards underlying infarcted myocardium. Mature blood vessels were identified within the sheet area and in the underlying myocardium in close proximity to the sheet evident by vWf and SMA double staining (Figure 3D).
Figure 3.
Neovascularization of infarcted wall. (A) Frozen tissue sections stained with vWf antibody (green). LV section of control (sham), infarcted (MI), and MI treated with cell sheet (sheet) rats. Scale bar, 100 μm. (B) Capillary density decreased in the MI compared with sham (*P < 0.05) and improved after cell sheet treatment (#P < 0.05). (C) Neovascularization within cell sheet area was recognized by co-localization of DiI- (red) and vWf (green) staining. Scale bar 100 μm. (D) Mature blood vessels (arrows) were identified by co-localization of SMA (red) and vWf (green) staining. Scale bar 50 μm.
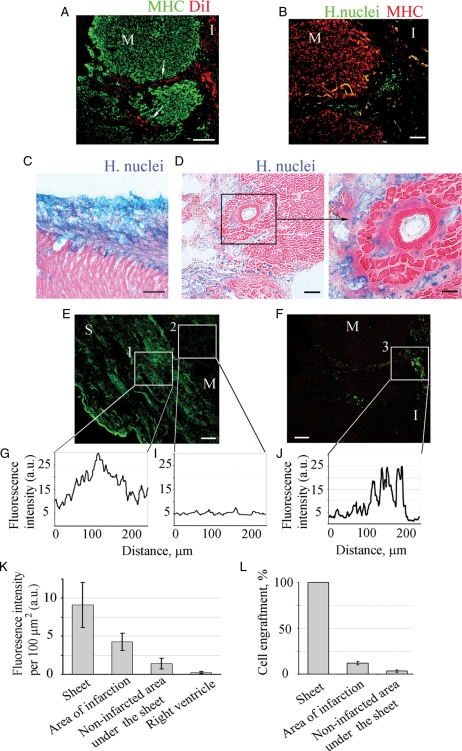
Furthermore, 3 weeks after transplantation, a large number of labelled human nuclei positive or DiI-labelled cells were detected deep within the infarcted area indicating cell migration from the epicardial surface to the infarct (Figure 4A, B, and D). Minor or no migration was detected when the cell sheet was transplanted onto non-infarcted myocardium, sham control (Figure 4C). To evaluate engraftment of sheet-originated cells, sections were labelled with anti-human nuclear lamin antibody. Quantification of engraftment was performed using two approaches: fluorescence intensity and cell counting. Fluorescence intensity of the signal was analysed and compared for different areas of myocardium (Figure 4E_–_J). Since the transplanted sheets are created by human cells and are stained with human nuclear lamin-labelled with green fluorescence, the signal intensity of the sheet is set to 100% (100% of cells are lamin-positive). Myocardial area with no or limited number of labelled cells had the lowest level of fluorescence signal (13%, or 3.2 ± 1.4% of total number of cells), while the area where the cell migrated from the sheet to the infarcted myocardium had higher signal intensity (47%, or 11.9 ± 1.7% of total number of cells), indicating a higher number of sheet-originated cells are engrafted in the infarcted area.) (Figure 4K and L). Migrated cells were positive for KDR (Supplementary material online, Figure S5).
Figure 4.
Engraftment quantification of cells migrated from the sheet into the infarcted area of MI. Animals were treated with rat (A) or human (B_–_F) sheets. Cardiomyocytes were labelled with MHC antibody (A, green or B, red). Rat sheet-originated cells were identified with DiI-labelling, red (A). Arrows indicate the track of migrating cells. Human sheet-originated cells were identified by immunostaining with human nuclei antibody followed by secondary antibodies conjugated with either Alexa 488 (B, E and F, green) or AP (C, D, blue). No migration was detected when the cell sheet was transplanted onto non-infarcted myocardium (C). Heart sections were counterstained with eosin, pink (C–D). Higher magnification of area selected in the box is presented (D, right). Immunofluorescence of sheet (green) grafted to the myocardium surface (E) or cells migrated to the infarction area (F). Fluorescence profiles across the cell sheet itself (G, box 1), area underlying cell sheet (I, box 2) and infarction area with migrated cells (F, box 3). Mean fluorescence intensity of the grafted human (K) cells was determined by outlining the region of interest (ROI) and subtracting the background fluorescence for the same region. Fluorescence intensity was normalized to the area of ROI (n = 6). (L) Percent engraftment was defined as number of lamin-positive cells divided by total number of cells per ROI. ‘M’, myocardium,’S’ sheet, ‘I’ infarction. Scale bars 100 μm (A–C, D, left, E and F), or 50 μm (D, right).
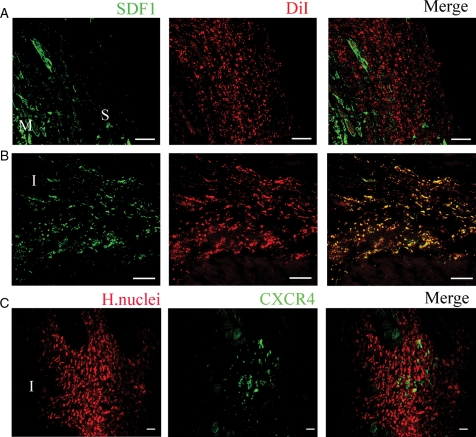
To elucidate a possible mechanism of cell migration, sections were stained to detect SDF1 and its unique receptor CXCR4. The migration patterns of cells from the sheet coincided with SDF-1 expression. Within 3 days after MI, SDF-1 was expressed in the injured myocardium (Figure 5A). At 3 weeks after MI and sheet transplantation, SDF-1 was co-localized with the migrated labelled cells (Figure 5B). PCR analysis revealed CXCR4 expression in cell sheet before transplantation (Figure 1F). However, after transplantation only a fraction of migrated cells expressed CXCR4 (Figure 5C).
Figure 5.
Migration of sheet-originated cells into the infarcted area. Confocal images of MI animals treated with sheets from rats (A and B) or human (C). SDF1 (green) was detected at border zone of the infarct at day 3 (A) and day 21 (B). Rat sheet-originated cells were identified with DiI-labelling (red). Note co-localization of DiI-positive sheet-originated cells with SDF1 at 21 days after MI (B). Human cells were identified by immunostaining with human nuclei antibody, red, (C). Note human cells that migrated to the area of infarct express CXCR4 (green) (C). Scale bar, 200 μm (A, B) or 50 μm (C). ‘M’, myocardium, ‘S’ sheet, ‘I’ infarct.
3.7. Cardiac regeneration
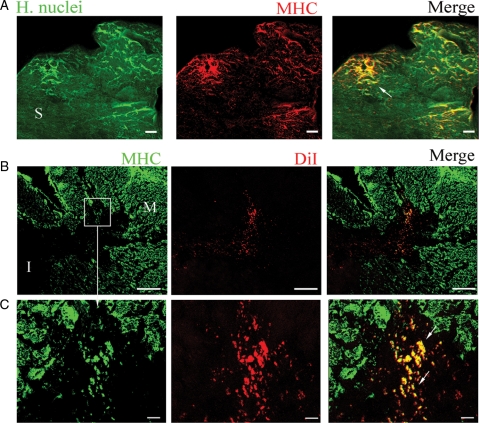
The differentiation of migrating cells into cardiomyocytes was evident by the co-localization of MHC staining with either human nuclei (Figure 6A) or DiI (Figure 6B and C). In contrast to the immature cardiomyocyte-like cells within the pre-transplanted cell sheet, the migrated and newly differentiated cells within the myocardium were about 30–50 μm in size and co-expressed C43 (see Supplementary material online, Figure S6). Cardiomyogenesis within the infarcted myocardium was observed in the sheets created from either rat or human cells.
Figure 6.
Cardiac regeneration. Sections of MI animals treated with human (A) or rat (B, C) sheets. Human sheet was identified by immunostaining with human nuclei antibody (green). Section was double-stained with MHC (red) antibody. Newly formed cardiomyocytes was identified by co-localization of human nuclei and MHC (yellow, arrow). (B) Rat sheet-originated cells were identified by DiI labelling (red). Section was double-stained with MHC (green) antibody. Newly formed cardiomyocytes were detected by co-localization of DiI with MHC (yellow, arrows). (C) Higher magnification of area selected in the boxes (B). Scale bars 200 μm (B), or 20 μm (A, C). ‘M’, myocardium, ‘S’ sheet, ‘I’ infarct.
3.8. Cell sheet improved cardiac contractile function and retarded LV remodelling after MI
Closed-chest in vivo cardiac function was derived from left ventricle (LV) pressure–volume loops (PV loops), which were measured using a solid-state Millar conductance catheter system. MI resulted in a characteristic decline in LV systolic parameters and an increase in diastolic parameters (Table 1). Cell sheet treatment improved both systolic and diastolic parameters (Table 1). Specifically, load-dependent parameters of systolic function: ejection fraction (EF), d_P_/d_T_max, and cardiac index (CI) were decreased in MI rats and increased towards sham control with the cell sheet treatment (Table 1). Diastolic function parameters, d_P_/d_T_min, relaxation constant (Tau), EDV, and EDP were increased in the MI rats and returned towards sham control parameters after sheet treatment (Table 1). However, load-independent systolic function, Emax, was decreased after MI. Treatment with human sheet improved Emax, while treatment with rat sheet had no effect (Table 1). Treatment with either rat or human sheets retarded LV remodelling; as such that it increased the ratio of anteriolateral wall thickness/LV inner diameter (t/_D_i) and wall thickness/LV outer diameter (t/_D_o) (see Supplementary material online, Table S3). However, human sheets appear to further improve LV remodelling compared with rat sheets as indicated by increased ratio of wall thickness to ventricular diameter and decreased both EDV and EDP (Table 1 and see Supplementary material online, Table S3).
Table 1.
Haemodynamic parameters
| Group | Sham | MI | MI + rat sheet | MI + human sheet |
|---|---|---|---|---|
| Parameter | ||||
| HR (min−1) | 245 ± 18 | 238 ± 11 | 254 ± 16 | 180 ± 25 |
| ESP (mmHg) | 122 ± 7 | 112 ± 11 | 114 ± 11 | 99 ± 16 |
| EDP (mmHg) | 6 ± 0.3 | 14 ± 3* | 11 ± 3*** | 9 ± 4** |
| ESV (μL) | 69 ± 30 | 241±27* | 114 ± 27*** | 53 ± 15** |
| EDV (μL) | 157 ± 29 | 308 ± 35* | 171 ± 20*** | 109 ± 22** |
| SV (μL) | 109 ± 28. | 98±15 | 79 ± 12 | 68 ± 34 |
| CO (mL/min) | 26 ± 6 | 23 ± 3 | 20 ± 3 | 17 ± 6 |
| CI | 60 ± 0.3 | 52 ± 0.1* | 54 ± 0.3 | 49 ± 3 |
| Ea (mmHg/min) | 1.62 ± 0.5 | 1.34 ± 0.3 | 1.65 ± 0.3 | 2.4 ± 0.6 |
| EF (%) | 66 ± 10 | 30 ± 3* | 49 ± 12*** | 58 ± 15 |
| d_P_/d_T_max (mmHg/s) | 6524 ± 388 | 4926 ± 606* | 5932 ± 584 | 4860 ± 1630 |
| SW (mmHg × μL) | 10494 ± 3167 | 7505 ± 1573 | 6554 ± 1169 | 4974 ± 3936 |
| Emax (mmHg/μL) | 4.1 ± 1.4 | 1.1 ± 0.06 | 1.4 ± 0.2 | 2.9 ± 0.4** |
| −d_P_/d_T_min (mmHg/s) | 4849 ± 454 | 2800 ± 591* | 4445 ± 567 | 2685 ± 1293 |
| Tau (W) (ms) | 15 ± 1 | 22 ± 3* | 16 ± 1*** | 17 ± 6 |
| Tau (G) (ms) | 19 ± 1 | 49 ± 12* | 20 ± 2*** | 35 ± 12 |
4. Discussion
The majority of the cardiac progenitor cells delivered using our scaffold-free cell sheet survived after transplantation onto the infarcted heart. A significant percentage of transplanted cells migrated from the cell sheet to the site of infarction and differentiated into cardiomyocytes and vasculature leading to improving cardiac contractile function and retarding LV remodelling. Thus, delivery of cardiac progenitor cells together with cardiac mesenchymal cells in a form of scaffold-free cell sheet is an effective approach for cardiac regeneration after MI.
Consistent with previous studies,5,11 here we showed that cardiospheres are composed of multipotent precursors, which have the capacity to differentiate to cardiomyocytes and other cardiac cell types. When we fractioned cardiospheres based on c-Kit expression, we identified two subsets: Kit+ /KDR−/low/Nkx2.5+ and Kit−/KDR+/Nkx2.5−(Supplementary material online, Figure S3d), which are likely reflecting cardiac and vascular progenitors.20
In the present study, delivery of cardiac progenitor cells as a cell sheet facilitates cell survival after transplantation. Necrotic cores, commonly observed in tissue engineered patches,23,24 are absent in cardiosphere sheets prior to transplantation (Figure 1B and C). Poor cell survival is caused by multiple processes such as: ischemia from the lack of vasculature and anoikis due to cell detachment from substrate.25 A possible mechanism of cell survival within the sheet is the induction of neo-vessels soon after transplantation due to the presence of endothelial cells within the sheet before transplantation (Figure 1D). The cell sheet continued to grow in vivo (Figure 2B and C), suppressed cardiac wall thinning, and prevented LV remodelling at 21 days after transplantation (see Supplementary material online, Table S3). This maybe due to the induction of neovascularization (Figure 3), which may prevents ischemia-induced cell death (Figure 2G). Another likely mechanism of cell survival is that the cells within the scaffold-free sheet maintained cell-to-cell adhesion16 as shown by ICAM expression (Figure 1F). The cells also exhibit C43-positive junctions (Figure 1D, see Supplementary material online, Figure S6), which may facilitate electromechanical coupling between the transplanted cells and the native myocardium.
We observed cell migration from the sheet to the infarcted myocardium (Figure 4A and B, E and F), which may be facilitated by the strong expression of MMP2 in the cell sheet (Figure 1F). Although, the mechanism of cardiac progenitor cell migration remains unclear, previous observations showed that SDF-1 is upregulated after MI and plays a role in bone-marrow and cardiac stem cell migration.26,27 Our data suggest that SDF-1-CXCR4 axis plays, at least in part, a role in cardiac progenitor cell migration from cell sheet to the infarcted myocardium. This conclusion is based on the following observations: (1) cell sheet expresses CXCR4 prior to transplantation (Figure 1F), (2) migrated cells are located in the vicinity of SDF-1 release (Figure 5A and B), and (3) about 20% of migrated cells expressed CXCR4. Note, not all the migrated cells expressed CXCR4 suggesting other mechanisms are involved in cell migration (Figure 5C).
Here we report that implanting cardiosphere-generated cell sheet onto infarcted myocardium not only improved vascularization but also promoted cardiogenesis within the infarcted area (Figure 6). A larger number of newly formed cardiomyocytes were found deep within the infarct compared with the cell sheet periphery. Notably the transplantation of the cell sheet resulted in a significant improvement of the cardiac contractile function after MI, as was shown by an increase of EF and decrease of LV end diastolic pressure (Table 1).
The beneficial effect of cell sheet is, in part, due to the presence of a large number of activated cardiac mesenchymal stromal cells (myofibroblasts) within the sheet. Myofibroblasts are known to provide a mechanical support for grafted cells, facilitating contraction28 and to induce neo-vascularization through the release of cytokines.17 In addition, mesenchymal cells are uniquely immunotolerant. In xenograft models unmatched mesenchymal cells transplanted to the heart of immunocompetent rats were shown to suppress host immune response29 presumably due to inhibition of T-cell activation.30 Consistently with previous study from our laboratory,31 here, we demonstrated host tolerance to the cell sheet 21 days after MI. Finally, phase II and III clinical trials are currently undergoing in which allogeneic MSCs are used to treat MI in patients (Osiris Therapeutic, Inc.).
In summary, our results show that cardiac progenitor cells can be delivered as a cell sheet, composed of a layer of cardiac stromal cells impregnated with cardiospheres. After transplantation, cells from the cell sheet migrated to the infarct, partially driven by SDF-1 gradient, and differentiated into cardiomyocytes and vasculature. Transplantation of cell sheet was associated with prevention of LV remodelling, reconstitution of cardiac mass, reversal of wall thinning, and significant improvement in cardiac contractile function after MI. Our data also suggest that strategies, which utilize undigested cells, intact cell–cell interactions, and combined cell types such as our scaffold-free cell sheet should be considered in designing effective cell therapy.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the National Institute of Aging [NIH RO1 AG027263] and fund from Banner Sun Health Research Institute, Sun City, AZ.
Supplementary Material
Supplementary Data
Acknowledgements
We thank Joe Bahl for critically reading the manuscript, M.M. and Josh Gher for their technical assistance.
Conflict of interest: none declared.
References
- 1.Fuchs JR, Nasseri BA, Vacanti JP, Fauza DO. Postnatal myocardial augmentation with skeletal myoblast-based fetal tissue engineering. Surgery. 2006;140:100–107. doi: 10.1016/j.surg.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Bone marrow stem cells regenerate infarcted myocardium. Pediatr Transplant. 2003;7(Suppl. 3):86–88. doi: 10.1034/j.1399-3046.7.s3.13.x. [DOI] [PubMed] [Google Scholar]
- 3.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, et al. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–468. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki H, Kawamoto A, Ishikawa M, Oyamada A, Nakamori S, Nishimura H, et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–1325. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 5.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 6.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci USA. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, et al. CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 9.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, et al. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci USA. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, et al. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 11.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 12.Heldman AW, Hare JM. Cell therapy for myocardial infarction: Special delivery. J Mol Cell Cardiol. 2008;44:473–476. doi: 10.1016/j.yjmcc.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giraud MN, Armbruster C, Carrel T, Tevaearai HT. Current state of the art in myocardial tissue engineering. Tissue Eng. 2007;13:1825–1836. doi: 10.1089/ten.2006.0110. [DOI] [PubMed] [Google Scholar]
- 15.Jawad H, Lyon AR, Harding SE, Ali NN, Boccaccini AR. Myocardial tissue engineering. Br Med Bull. 2008;87:31–47. doi: 10.1093/bmb/ldn026. [DOI] [PubMed] [Google Scholar]
- 16.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 17.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 18.Sekine H, Shimizu T, Hobo K, Sekiya S, Yang J, Yamato M, et al. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118:S145–S152. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- 19.Iwaguro H, Yamaguchi J, Kalka C, Murasawa S, Masuda H, Hayashi S, et al. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105:732–738. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- 20.Gaballa MA, Sunkomat JN, Thai H, Morkin E, Ewy G, Goldman S. Grafting an acellular 3-dimensional collagen scaffold onto a non-transmural infarcted myocardium induces neo-angiogenesis and reduces cardiac remodeling. J Heart Lung Transplant. 2006;25:946–954. doi: 10.1016/j.healun.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–528. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 22.Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, Shao Y, et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–117. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 23.Li RK, Yau TM, Weisel RD, Mickle DA, Sakai T, Choi A, et al. Construction of a bioengineered cardiac graft. J Thorac Cardiovasc Surg. 2000;119:368–375. doi: 10.1016/S0022-5223(00)70193-0. [DOI] [PubMed] [Google Scholar]
- 24.Stevens KR, Pabon L, Muskheli V, Murry CE. Scaffold-free human cardiac tissue patch created from embryonic stem cells. Tissue Eng Part A. 2009;15:1211–1222. doi: 10.1089/ten.tea.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 26.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 27.Liang SX, Tan TY, Gaudry L, Chong B. Differentiation and migration of Sca1 + /CD31− cardiac side population cells in a murine myocardial ischemic model. Int J Cardiol. 2010;138:40–49. doi: 10.1016/j.ijcard.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 28.Cai D, Marty-Roix R, Hsu HP, Spector M. Lapine and canine bone marrow stromal cells contain smooth muscle actin and contract a collagen-glycosaminoglycan matrix. Tissue Eng. 2001;7:829–841. doi: 10.1089/107632701753337762. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald DJ, Luo J, Saito T, Duong M, Bernier PL, Chiu RC, et al. Persistence of marrow stromal cells implanted into acutely infarcted myocardium: observations in a xenotransplant model. J Thorac Cardiovasc Surg. 2005;130:1114–1121. doi: 10.1016/j.jtcvs.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 30.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 31.Thai HM, Juneman E, Lancaster J, Hagerty T, Do R, Castellano L, et al. Implantation of a three-dimensional fibroblast matrix improves left ventricular function and blood flow after acute myocardial infarction. Cell Transplant. 2009;18:283–295. doi: 10.3727/096368909788535004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data