Ultraviolet A does not induce melanomas in a Xiphophorus hybrid fish model (original) (raw)
Abstract
We examined the wavelength dependence of ultraviolet (UV) ra-diation (UVR)-induced melanoma in a Xiphophorus backcross hybrid model previously reported to be susceptible to melanoma induction by ultraviolet A (UVA) and visible light. Whereas ultraviolet B (UVB) irradiation of neonates yielded high frequencies of melanomas in pigmented fish, UVA irradiation resulted in melanoma frequencies that were not significantly different from unirradiated fish. Spontaneous and UV-induced melanoma frequencies correlated with the degree of pigmentation as expected from previous studies, and the histopathology phenotypes of the melanomas were not found in significantly different proportions in UV-treated and -untreated tumor-bearing fish. Our results support the conclusion that a brief early-life exposure to UVB radiation causes melanoma formation in this animal model. These data are consistent with an essential role for direct DNA damage, including cyclobutane dimers and (6-4) photoproducts, in the etiology of melanoma.
Keywords: ultraviolet B, DNA damage, cyclobutane dimer, reactive oxygen species, melanin
In the late 1980s Setlow and coworkers used genetic hybrids from interspecific crosses involving several species of the fish genus Xiphophorus to investigate the effects of UVR on the induction of cutaneous malignant melanoma (CMM) (1). These pioneering studies demonstrated that ultraviolet B (UVB) irradiation of backcross hybrids generated from a specific genetic crossing scheme induced melanomas at significant frequencies above spontaneous levels. These results were later confirmed, and the genetic basis of UVB-induced melanoma susceptibility in this cross was recognized to be the same as in the well-studied spontaneous Xiphophorus hybrid melanoma model (2). In 1993, Setlow used a different Xiphophorus interspecies cross (designated as Sp-couchianus; Fig. 1) to study the wavelength dependence of melanoma induction and reported that wavelengths in the ultraviolet A (UVA) and visible ranges were effective in inducing melanomas in first-generation backcross (BC1) hybrids generated from this particular cross (3). An action spectrum for melanoma induction was proposed with maxima in the UVB (302/313 nm) and UVA (365 nm) ranges. Because UVA fluence is quantitatively much greater than UVB in sunlight incident to the earth's surface (∼10-fold), Setlow suggested that, on the basis of this action spectrum, UVA was more effective than UVB in causing melanomas in the human population (2, 4). This report had significant public health consequences; it suggested that the use of commercially available sunscreens that effectively blocked UVB but not UVA encouraged more lengthy recreational sunlight exposure and thereby increased the exposure to UVA and its associated risks. Over the past 20 years, these data have become central to the debate on the role of UVA in melanoma and the risks associated with recreational and artificial exposures to UVA wavelengths.
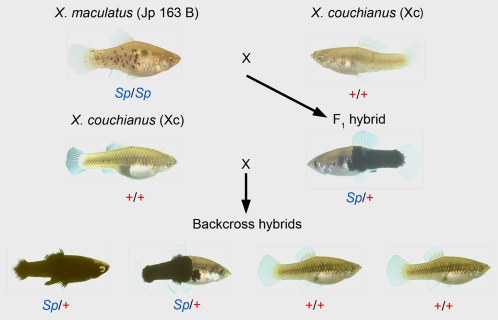
Fig. 1.
The Sp-couchianus backcross hybrid breeding scheme. F1 hybrids are produced by mating a macromelanophore pigmented “spotted side” (Sp/Sp) X. maculatus female from strain Jp 163 B (Upper Left) to a X. couchianus (Xc) male not carrying the Sp allele (+/+) (Upper Right), therefore not exhibiting any macromelanophore pigmentation. F1 hybrids are backcrossed to either male or female X. couchianus individuals and produce BC1 progeny of which ≈50% exhibit the Sp (spotted side) (Sp/+) and 50% the wild-type (+/+) pigment phenotypes.
Debate over the action spectrum for melanoma has only intensified because subsequent research using a variety of animal models has not corroborated these results. Studies in mammalian models, including the South American opossum (Monodelphis domestica) and several genetically modified mouse models, demonstrate that UVA does not induce melanomas (5–7). In contrast, evidence from all of these models, including Xiphophorus, supports a role for UVB in the etiology of melanoma. Unlike placental mammals, fish are very efficient at removing UVB-induced direct damage in DNA using photoenzymatic repair (PER) (8, 9) and this mechanism greatly decreases melanoma formation in the Xiphophorus melanoma model (1, 3). The most abundant damage induced by UVB irradiation results from the direct absorption of photons by DNA and includes the formation of cyclobutane pyrimidine dimers (CPD) and (6-4) pyrimidine dimers [(6-4)PD] (10, 11). Collectively, the results from the vast majority of animal studies strongly suggest that one (or both) of these lesions is required for melanoma formation.
Xiphophorus offers some significant advantages over other animal models for investigating melanoma. The classical, spontaneous Xiphophorus hybrid melanoma model has been studied for over 80 years and offers powerful genetic and biochemical approaches for revealing determinants of melanomagenesis (12–14). The Xi-phophorus melanoma receptor kinase gene (XMRK) behaves as a dominant oncogene in this fish model and is a mutated derivative of the fish ortholog for the human epidermal growth factor receptor (EGFR/ErbB-1). Consistent with the activity of mammalian EGFR in melanocytes (15–17), activation of the XMRK oncoprotein leads to numerous downstream signaling cascades including, but not limited to, the RAS/RAF/MAPK and PI3-K/AKT signaling pathways (12). In addition to these signaling cascades, the transformed phenotype in Xiphophorus also involves participation of transcription factors (e.g., STAT5) and glycoproteins (e.g., osteopontin, OPN) that are intimately involved in cellular proliferation and antiapoptotic responses that characterize numerous human cancers. Hence, Xiphophorus hybrid models offer ideal experimental platforms to further elucidate the biochemistry underlying melanomagenesis within the context of a controlled genetic background.
A recent publication (18) exploited the same Xiphophorus Sp-couchianus hybrid melanoma model used by Setlow for UVA induction studies (3) to investigate photosensitization of melanin as a possible mechanism for melanoma formation. In this report, electron paramagnetic resonance was used to describe the UVR wavelength dependence of reactive melanin radical formation in pigmented fish skin. The action spectrum for melanin-sensitized generation of reactive radicals derived from these experiments positively correlated with the action spectrum for melanoma formation reported by Setlow (3). These results are consistent with a role for UVB and UVA in generating melanin-derived reactive oxygen species (ROS) in melanoma causation. However, the observation that virtually all other animal models developed to study melanoma are refractory to UVA-induced melanoma calls into question the role of melanin-derived ROS in melanoma induction.
With these considerations in mind, we used the Xiphophorus Sp-couchianus model in experiments designed to replicate the results of Setlow's seminal UVA melanoma study (3), with specific attention to the requirement for sufficient sample sizes to allow statistical inferences. Our primary goal was to resolve the ongoing debate regarding the action spectrum for melanoma induction. Specifically, we wanted to address the following questions: (i) Does UVA induce melanomas in this model? (ii) Is there a correlation between the degree of genetically defined pigmentation and melanoma susceptibility? (iii) Are there any differences in the histopathologies of spontaneous and UVR-induced melanomas? By answering these questions we hope to increase our understanding of melanoma causation and the roles played by UVR and DNA damage in melanoma initiation.
Results
UVB but Not UVA Induced Melanomas in the Sp-couchianus Backcross Hybrid Model.
On the basis of the initial UVR melanoma induction results using the Sp-couchianus model (3), we performed an approximate power calculation for a two-sample comparison assuming a background value of 15–20% and a 2- to 3-fold increase in melanoma after treatment. From this calculation we determined that 150–200 fish would be required to achieve statistical significance (P < 0.005). The sample sizes of each treatment group at the end of the experiment were 216 control (−UV) fish, 282 UVA irradiated fish, and 194 UVB irradiated fish. Because tumor latency in this particular hybrid cross can be prolonged, for completeness our study was designed to allow for tumor formation and progression through maturity, and fish were killed at 12–14 months of age. However, exophytic lesions were scored throughout the course of the experiment and typically occurred in fish >6 months of age with the majority of tumors arising between 8–12 months. The mortality rate during the experiment not due to melanoma formation was consistent across the three treatment groups (−UV = 10%, UVA = 6%, and UVB = 11%).
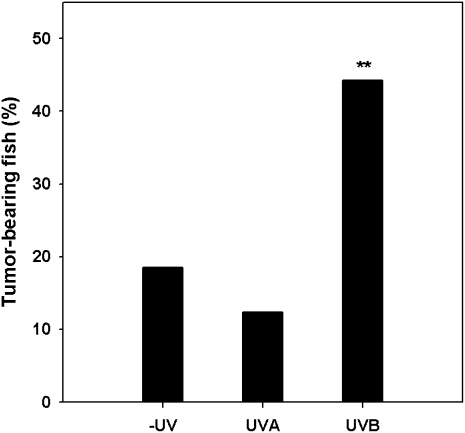
We found the incidence of melanomas to be significantly greater in the UVB-irradiated fish compared with the nonirradiated control fish (UVB: 86 tumor-bearing fish out of 194 individuals, melanoma frequency = 44.3%; control: 40 tumor bearing fish out of 216 individuals, melanoma frequency = 18.5%; χ2 = 31.99, P < 0.0001). However, there was no difference in melanoma incidence between the UVA-irradiated fish and that of the control fish (UVA: 35 tumor-bearing fish out of 282 individuals, melanoma frequency = 12.4%; control: 40 tumor-bearing fish out of 216 individuals, melanoma frequency = 18.5%; χ2 = 3.57, _P_ > 0.05) Therefore, our results contradict the report of Setlow and colleagues (3) and indicate that UVB but not UVA significantly induces melanomas in this Xiphophorus melanoma model (Fig. 2). We used doses of 6.4 and 80 kJ/m2 UVB and UVA, respectively, for each of the five UV treatments; Setlow and colleagues used a dose range of 0.35–3.0 and 0.5–11.75 kJ/m2 313 (UVB) and 365 (UVA), respectively, for their treatments. Because of differences in emission spectra and dosimetry, these doses are not directly comparable. However, on the basis of our toxicity and DNA damage determinations, our incident doses are equal to or exceed those of Setlow et al. (3).
Fig. 2.
Melanoma frequencies in unirradiated and UV-irradiated BC1 pigmented fish. Melanoma frequencies (%) are shown for the −UV control, UVA, and UVB fish. A significant difference (**) was found between the UVB and both the −UV and UVA treatment groups (P < 0.0001). No difference was seen between the −UV and UVA treatment groups (_P_ > 0.05).
Exposure of Neonates to UVA or UVB Did Not Affect the Degree of Adult Pigmentation.
The degree of pigmentation was visually estimated in each fish by two observers (L.P. and D.T.) as (i) light with 50–70% coverage, (ii) intermediate with 70–90% coverage, and (iii) heavy with >90% coverage. The distribution of pigmentation in the different experimental groups was determined at 4 months and at the time of sacrifice at 12–14 months (Table 1). From Table 1, it is clear that the amount of pigmentation an individual possessed increased with their age in all treatment groups. However, it should be noted that there did not appear to be any differences in the distribution of pigmentation among the treatment groups at the time of sacrifice. Hence, episodic neonatal exposure to either UVA or UVB did not influence the degree of pigmentation in the adult animals.
Table 1.
Distribution of pigmentation in the different treatment groups at 4 and 12 months
| Light, % | Intermediate, % | Heavy, % | |
|---|---|---|---|
| 4 months | |||
| Control | 52.7 | 43.5 | 3.8 |
| UVA | 35.3 | 57.8 | 6.8 |
| UVB | 69.4 | 25.1 | 5.5 |
| 12 months | |||
| Control | 27.1 | 52.5 | 20.4 |
| UVA | 24.8 | 56.7 | 18.4 |
| UVB | 38.4 | 41.8 | 19.8 |
Degree of Adult Pigmentation Was Positively Correlated with the Frequency of Spontaneous and UV-Induced Melanomas.
Melanoma frequencies were positively correlated with pigmentation in all of the treatment groups (Table 2). Approximately 90% of the spontaneous, UVA and UVB tumors occurred in fish with intermediate and heavily pigmented phenotypes. These data indicate that melanoma susceptibility is this animal model is dependent on the level of pigmentation. It should be noted that animals with more melanin pigmentation would also concomitantly increase the number the XMRK oncoproteins because melanocytes are their substrate. It should also be noted that the degree of pigmentation in these BC1 hybrids is genetically regulated (13). We believe the relationship between pigmentation and melanomagenesis is not linear because it appears that a threshold level of pigmentation (i.e., melanocytes, macromelanophores, and melanin) is necessary for tumorigenesis in Xiphophorus. This interpretation is supported by the fact that the great majority of tumors occurred in fish whose bodies were at least 70% pigmented (i.e., intermediate and heavy pigmentation classes). However, the frequencies of melanomas were comparable between the intermediate and heavy pigmentation classes in all three treatment groups (Table 2). For example, in the control treatment, 49% of the tumors occurred in fish with intermediate pigmentation and 46% of the tumors occurred in fish with heavy pigmentation.
Table 2.
Relative induced and background melanoma frequencies in the different pigment groups
| Treatment | |||
|---|---|---|---|
| Pigmentation | Control, % | UVA, % | UVB, % |
| Light | 5.0 | 11.4 | 16.3 |
| Intermediate | 48.8 | 35.7 | 45.3 |
| Heavy | 46.3 | 52.9 | 38.4 |
There Was Variation in Melanoma Histopathology in the Control and UV-Irradiated Treatment Groups.
All tumor-bearing fish that were successfully fixed had their melanomas characterized following the classification system previously described by Gimenez-Conti et al. (19), a brief description of which is given in Materials and Methods. The vast majority of the tumors in not only the control but also the UV-treated groups were spindle cell melanomas (SCM) and melanophorous-macromelanophorous polymorphic melanoma (MMM) (Table 3). In addition, a small number of epithelioid cell melanomas (ECM) were found in all treatment groups at approximately the same frequency. Melanocytic melanomas (MM) were present at very low frequencies (∼3%) in the UVA and UVB treated fish but completely absent in the control group. Hence, SCM and MMM were the predominant malignant lesions in the Sp-couchianus model.
Table 3.
Histopathology of fish melanomas in the different treatment groups
| Tumors | Control | UVA | UVB | ||||
|---|---|---|---|---|---|---|---|
| Class | Stage | N | % | N | % | N | % |
| SCM | I | 2 | 5.9 | 10 | 32.3 | 5 | 6.3 |
| SCM | II | 4 | 11.8 | 4 | 12.9 | 12 | 15 |
| SCM | III | 3 | 8.8 | 1 | 3.2 | 11 | 13.8 |
| SCM | IV | 19 | 55.9 | 3 | 9.7 | 26 | 32.5 |
| Total | 28 | 82.4 | 18 | 58.1 | 54 | 67.5 | |
| ECM | I | 3 | 9.7 | ||||
| ECM | II | 1 | 2.9 | 1 | 3.2 | ||
| ECM | III | 2 | 2.5 | ||||
| ECM | IV | 1 | 2.9 | 2 | 6.5 | 1 | 1.3 |
| Total | 2 | 5.9 | 6 | 19.4 | 3 | 3.8 | |
| MM | I | ||||||
| MM | II | 2 | 2.5 | ||||
| MM | III | 1 | 3.2 | ||||
| MM | IV | 1 | 1.3 | ||||
| Total | 0 | 0 | 1 | 3.2 | 3 | 3.8 | |
| MMM | I | ||||||
| MMM | II | 1 | 3.2 | 5 | 6.3 | ||
| MMM | III | 1 | 2.9 | 2 | 6.5 | 4 | 5 |
| MMM | IV | 3 | 8.8 | 3 | 9.7 | 11 | 13.8 |
| Total | 4 | 11.8 | 6 | 19.4 | 20 | 25 | |
| Totals: | 34 | 100 | 31 | 100 | 80 | 100 |
We also investigated whether or not UVR affected the degree to which the tumors progressed. To this end, we stratified the different melanoma phenotypes into four stages that take into consideration the tumor size and depth of penetration into the musculature as well as invasion of adjacent structures. This staging system is specific for each type of melanoma and reflects the severity and aggressiveness of a particular tumor. Representative histological sections of the different stages for SCM are shown in Fig. 3: stage I is characterized by vertical and lateral growth of the tumor into the connective tissue with the tumor confined to the dermis without any infiltration into the musculature; stage II melanoma extends beyond the dermis and shows a few individual cells extending into muscle cell bundles; stage III melanoma shows the invasiveness of the tumor into the muscle cell bundles with a certain degree of necrosis; and stage IV melanoma is characterized by the presence of unilateral, bilateral, and/or multiple tumors. This final stage represents the most aggressive form of CMM in this system. The identification of tumors in this study according to this hierarchical system proved informative. Melanomas in the control and UVB treatments had a higher incidence of the more advanced stage IV SCMs when compared with the UVA treatment (Table 3; control vs. UVA: χ2 = 9.55, P < 0.002; UVB vs. UVA: χ2 = 4.33, P < 0.03). Furthermore, melanomas in the UVA treatment group had significantly more early stage (i.e., stage I) SCMs than either the control or UVB treatment groups (Table 3; UVA vs. control: χ2 = 10.93, P < 0.001; UVA vs. UVB: χ2 = 14.85, P < 0.001). The differences in the stages of the UVA and control/UVB SCM are intriguing and suggest that UVA may delay either the onset or progression of CMM.
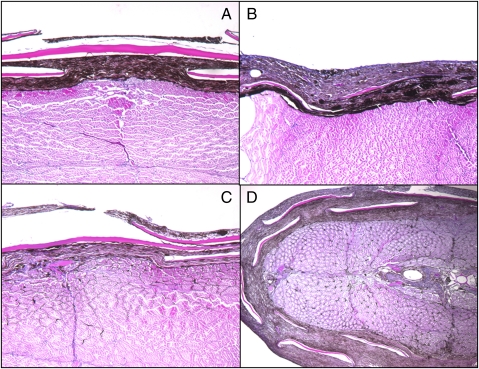
Fig. 3.
Spindle cell melanomas (SCM) stages I–IV. Stage I tumor shows lateral growth located entirely in the dermis without infiltration into the muscle bundles (A). Cross-section of a stage II tumor showing lateral growth with a small proportion of tumor cells infiltrating the underlying tissue (B). Stage III tumor showing more extensive invasion of the musculature (compared with stage II) (C) and stage IV exophytic melanoma shows extensive invasion into the muscles bundles (D). (Magnification: ×100.)
Discussion
UVB but not UVA-induced melanomas in the Sp-couchianus backcross hybrid fish model. Melanomas arise in this system after a short series of daily exposures of neonates to UVB doses approaching the lethal threshold. This exposure protocol is similar to those used in other animal melanoma models and mimics the exposures associated with human risk (i.e., episodes of sunburn in childhood). Several mouse melanoma models have been created in which the RAS/RAF/MAPK or PI3K/AKT pathways have been up-regulated by activating mutations. UVB induces CMM in HGF/SF (5), HRAS/p19(ARF)+/− (20), and CDK4/HRAS mice (21). UVA does not induce melanomas in the albino HGF/SF mouse (5) or in the opossum (7). Consistent with these mammalian models we show that UVA does not induce melanomas in a backcross hybrid fish model. Hence, UVA does not induce melanomas in animal models in which UVB melanomas are induced.
More specifically, UVA does not induce melanomas in the same animal model (Sp-couchianus hybrids) and within the same dose and wavelength ranges used by Setlow and coworkers in 1993 (3). Our study differed from the previous study in several ways. First, we used a broad spectrum UVA source with a 353-nm peak that exposed several free-swimming fry at the same time from both sides in a UV-transparent chamber. Using this facile technique we could treat a large number of fish and accumulate animal numbers that would give us statistical confidence in our results. Second, we allowed our fish to develop tumors for 12–14 months as opposed to 4 months postirradiation. We believe that most of the potential spontaneous (background) and UV-induced melanomas would be fully expressed in older animals, thus avoiding any effects of latency on the melanoma frequency. Third, nearly all of the tumors scored were examined by histopathology and clearly shown to be melanomas of various histological types (Table 1 and Fig. 3).
In view of the disparity between the present results and those of Setlow and colleagues (3), it is informative to reconsider their results (as set out in their table 1 and figure 2 in the 1993 paper). We reviewed their action spectrum data using careful estimates graphically derived from their published dose–response curves (Table S1 and Fig. S1). In their study, two to six groups (depen-ding on the number of available fish) of ∼5 fish each were irradiated in a 1-cm quartz cuvettete with vortex mixing. A background level of 24% was derived from 124 untreated fish and used for the UVB (302 and 313 nm) and UVA (365 nm) portion of the action spectrum; a different background level was used for 405- and 436-nm data (see below). On the basis of these results, and before our study, we performed an approximate power computation for a two-sample comparison using a melanoma incidence of 45% for the treated fish and 24% for the untreated fish.We determined that at least 150 fish were required for statistical reliability. For each of the two UVB (302 and 313 nm) and single UVA (365 nm) experiments the total number of fish was 123, 124, and 85, respectively. However, unlike the control experiment, the treated fish were subgrouped into multiple doses for the UVB, UVA, and blue light dose–response curves. Assuming that comparable numbers of animals were used at each dose level, the 302- and 313-nm UVB treatments would have consisted of ∼31 fish per dose, the 365-nm UVA treatment of ∼14 fish per dose, and the 405- and 436-nm treatments of ∼15 and ∼10 fish per dose, respectively. These sample sizes are considerably lower than what is required for statistical inference to compare each dose to the control group.
We replotted close estimates of the values from the published 313-nm and 365-nm plots (figure 2 B and C in ref. 3) on a semilog plot (i.e., log fraction of fish with tumors vs. dose) (Fig. S1). Setlow suggested that the sensitivity of the pigmented Xiphophorus BC1 fish to melanoma was consistent with single-hit kinetics and based his action spectrum on the _k_-values (slopes) derived from dose–response curves at each wavelength. Linearity on a semilog plot is characteristic of a single-hit response. Unfortunately, the dose–response curves shown in figure 2 of the 1993 paper (3) were not presented on semilog plots nor were any correlation coefficients associated with these lines given. From our reanalysis of the 313 and 365 dose–response curves (Figs. S1 and S2 and Table S1) we did not observe linearity on a semilog plot. Whereas the UVB dose–response appeared positive, the UVA response appeared to maintain a slope of 0 for doses >0.5 kJ/m2. The error bars for the melanoma frequency at 365 nm for the lowest dose extended below background and showed considerable overlap with the higher doses. It is possible that the melanoma response saturated at <1 kJ/m2, suggesting that UVA-induced melanoma is extremely sensitive to low doses. Our 353-nm dose was on the shoulder of the survival curve, hence was as high as we could use without killing substantial numbers of animals (i.e., doubling this dose resulted in considerable mortality). Hence, we believe that our UVA dose is at or greater than the 365-nm doses used by Setlow and colleagues (3). However, we saw no UVA-induced melanoma formation above background at this dose. Indeed, melanoma formation at low UVA doses is particularly disconcerting because melanoma formation is associated with high episodic sunlight exposures (i.e., sunburn) of young individuals.
Similar problems are evident for the blue light portion of the Setlow action spectrum (i.e., 405 and 436 nm). First, a small number of experimental animals were used; that is, 15 fish/dose for 405 (total = 61 fish) and 10 fish/dose for 436 nm (total = 21 fish). All of the melanoma frequencies at these wavelengths appear very similar to the original 24% background used for the UVB and UVA experiments. The authors suggested, however, that housing the fish in a greenhouse exposed to diffuse shaded sunlight could induce melanomas and could, therefore, be responsible for the high background frequency. An additional control experiment using only yellow lights for housing was performed on fish several months after the UVB/UVA portion of the experiment. Of the 20 fish used, one melanoma was observed at the termination of the experiment (after 2 months) (3). From this experiment a 5% background was calculated and used for the 405- and 436-nm dose–responses. Two problems come to mind regarding this experiment: (i) the animal numbers were very low and (ii) melanomas were scored at half the age of the other control experiment, thereby discounting any melanomas arising between 2 and 4 months. Our fish were housed indoors under fluorescent lighting and showed a background of 18.5% in 12- to 14-month-old fish (Fig. 2). The background in the initial study performed by Setlow and colleagues (1989) (1) was 12.7% (n = 79). Background frequencies in other Xiphophorus backcross systems vary, with low background in some systems (e.g., 7% in the X. maculatus 163B × X. helleri cross) and higher backgrounds in others (e.g., 25% in the X. maculatus 163A × X. helleri cross) (22). Given the variation, a 5% background in the X. maculatus 163B × X. couchianus system is not unreasonable but would require 100–150 animals for statistical justification.
Given our data, the action spectrum for melanoma does not correlate with the action spectrum for melanin radicals in Xiphophorus adult and neonatal fish (18). Hence, UVA-induced melanin radicals do not appear to be involved in melanomagenesis in this animal model. The free radical action spectrum shows ∼2-fold difference in the frequency of melanin radicals generated by 313 and 365 nm, proximal to the two peak wavelengths used in our study. Hence, there is no significant difference in the amount of melanin radicals produced by UVA and UVB at comparable doses. Given the quantitative similarity in the melanin radicals induced by UVA and UVB (18) and the relative inefficiency of ROS induction by UVB (23, 24), we suggest that free radicals are not significantly involved in UVB-induced melanoma in the fish model. If ROS do not induce melanomas, it follows that the direct DNA damage associated with UVB plays a major role in the initiation of melanomas. This idea is strongly supported by the two PER experiments performed in the earlier melanoma studies using the Sp-couchianus model (1, 3). In both experiments the exclusive removal of direct damage, including CPDs and (6-4)PDs, significantly reduced melanoma frequencies. It should be kept in mind that UVA does induce T<>T and T<>C CPDs in DNA as shown by Douki and colleagues (25). Using the same analytical system, we quantified the DNA photoproduct spectrum in pigmented and nonpigmented epidermis of F1 hybrid fish exposed to the same dose used for our carcinogenesis experiments (9). Although we were able to detect T<>T lesions in purified DNA dosimeters after the accumulated 5-day UVA treatment, the frequency of these lesions in fish skin was below the limits of detection, indicating that, at these doses, a threshold for melanoma initiation had not been reached.
In conclusion, our data refute the only direct evidence that UVA causes melanoma. This is not to say that UVA is harmless. UVA is a complete carcinogen, able to initiate and promote squamous cell carcinomas in the hairless mouse (26, 27). Furthermore, it is thought that UVA plays a significant role in photodermatoses (i.e., polymorphous light eruption) and photoaging (27, 28), as well as a possible role in immunosuppression (29, 30). It is also very possible that long-term chronic exposure to UVA can enhance the progression of incipient melanomas to malignancy through free radical mechanisms or direct formation of CPDs. The World Health Organization's International Agency for Research on Cancer (IARC) recently reported that the use of tanning beds before age 30 is associated with a 75% increase in melanoma (31) and, that similar to tobacco smoke, the UVA used in these devices is a class I carcinogen and should be avoided (32). Our data do not lessen the potential risks of UVA exposure for skin cancer, but do lessen the likelihood that UVA and reactive oxygen species contribute to the etiology of melanomas resulting from early-life exposure to episodic high-dose solar UVR.
Materials and Methods
Animals.
Parental strains were originally obtained from the Xiphophorus Genetic Stock Center located at Texas State University in San Marcos, Texas and have been maintained in our facility since 2000. The X. couchianus stock was collected in 1961 from the Huasteca canyon (Nuevo Leon, Mexico). Progenitors of X. maculatus strain Jp 163 B were obtained in 1939 from the Rio Jamapa (Veracruz, Mexico). This stock is highly inbred, currently in its 100+ generation of full sibling inbreeding. More formal descriptions of hybrid crossing schemes and nomenclature are available at www.xiphophorus.org.
UV Sources.
Xiphophorus are live bearers and were treated on day 5 postparturition and daily for 4 additional days for a total of five treatments. Fry were ∼0.5 cm in length at the time of exposure. An irradiation chamber was designed and constructed in which up to 10 unanesthetized, free-swimming fish could be exposed in each UV transparent chamber to lamps from both sides simultaneously. A fan was mounted at one end of the apparatus to provide airflow over a bed of crushed ice to prevent heating during the longer exposure times (i.e., UVA). For UVB treatment, groups of fry were exposed to a total dose of 6.4 kJ/m2 at a fluence rate of 12.2 J/m2/sec from two unfiltered Philips TL01 bulbs mounted on either side of the irradiation chamber (four bulbs total). The total UVB exposure time was 8 min 45 s. For UVA treatment, groups of fry were exposed to 80 kJ/m2 at a fluence rate of 25.8 J/m2/sec from four Alisun-S (Cosmolux) bulbs (eight bulbs total). The total UVA exposure time was 52 min. Temperature within the irradiation box was stabilized by drawing external air over a bed of ice. The peak emission of the UVB and UVA bulbs were 311 and 353 nm, respectively (Fig. 4). Four sheets of Mylar 500D plastic were used to filter the UVA to reduce UVB wavelengths (<320 nm) to negligible levels. Dose rates were measured using a Model IL 1400A radiometer/photometer coupled to either a UVB or UVA detector (International Light). Dose rates along the entire length of the irradiation boxes were verified using DNA damage dosimeters suspended in the UV-transparent chambers. Negligible attenuation by the plastic chamber or water was observed. To prevent unwanted white light effects like light-inducible PER (33–35), fry were kept in the dark for 24 h before the first exposure until 24 h after the last exposure.
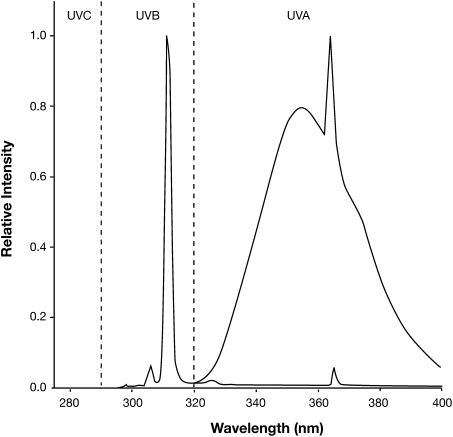
Fig. 4.
Emission spectra for UVA and UVB lamps. Spectra shown are for narrow-band Philips TL01 UVB lamps (peak = 311 nm) and broad-band Alisun-S (Cosmolux) UVA lamps (peak = 353 nm).
UV Exposure Protocol.
Subsequent to treatment, fish from different broods born within a 2-week period were combined into large (55 gal) community tanks. Pigmented fish were easily identified at sexual maturity (∼4 months of age) and segregated from nonpigmented fish. At this time, all pigmented fish were scored on the basis of their degree of melanin pigmentation (i.e., initial pigmentation, Table 1). The degree of pigmentation was estimated in each fish in the tumor study as heavy with >90% coverage, intermediate with 70–90% coverage, and light with 50–70% coverage. Interestingly, we observed relatively few tumors during the segregation of pigmented fish, which coincides with early adulthood in these fish and is within the time frame that Setlow and colleagues (2) scored their treatment groups for melanomas. For the remainder of the experiment, these pigmented fish, which have the Xmrk oncogene, were housed as small groups (four to six fish) in 5.5-gal tanks to facilitate observing tumors. All exophytic tumors were noted at the time of detection and a final count of all tumor-bearing fish was conducted at the end of the experiment (i.e., 12–14 months of age). Upon conclusion, fish were killed using a lethal dose of anesthesia (MS-222) and were preserved in 10% neutral buffered formalin. All tumor-bearing fish and heavily pigmented fish were sent to pathology for internal analysis and tumor identification once preserved.
Histology.
Tumor-bearing fish were killed and the lesions and surrounding tissues removed by dissection. Depending on the size, the excised tumors were divided in several pieces. NBF-fixed pieces of the tumors were embedded in paraffin wax and sectioned by conventional histology techniques. The slides were stained with hematoxylin and eosin. For each sample, slides were prepared from several different areas, so that we had a representative picture of the morphology of the entire tumor. Other pieces of these tumors were reserved for genetic characterization, which we hope to correlate with our histological observations in future studies. The histopathologies of Xiphophorus melanomas have been well categorized in a prior study (19) and, therefore, we have provided only a brief description of type and stage classifications. Tumor types in the Xiphophorus model include: SCM, characterized by the presence of fusiform spindle cells with elongated nuclei, confined to the demis or invading the muscles bundles; MMM are characterized by the presence of many different cell types, including fusiform spindle, round, epithelioid cells as well as melanophores and macromelanophores cells; ECM containing cells that resemble epithelial cells; and MM, which are characterized by the presence of cells with dendritic morphology. The stages of the tumors were determined in all of the different melanoma phenotypes, taking into consideration the size and the invasiveness into the muscle bundles (Results).
Supplementary Material
Supporting Information
Acknowledgments
We acknowledge National Cancer Institute (NCI) Research Grant CA113671, NCI Training Grant CA009480, and National Institute of Environmental Health Sciences Center Grant ES07784 as funding sources.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Setlow RB, Woodhead AD, Grist E. Animal model for ultraviolet radiation-induced melanoma: Platyfish-swordtail hybrid. Proc Natl Acad Sci USA. 1989;86:8922–8926. doi: 10.1073/pnas.86.22.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nairn RS, et al. A CDKN2-like polymorphism in Xiphophorus LG V is associated with UV-B-induced melanoma formation in platyfish-swordtail hybrids. Proc Natl Acad Sci USA. 1996;93:13042–13047. doi: 10.1073/pnas.93.23.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Setlow RB, Grist E, Thompson K, Woodhead AD. Wavelengths effective in induction of malignant melanoma. Proc Natl Acad Sci USA. 1993;90:6666–6670. doi: 10.1073/pnas.90.14.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Setlow RB, Woodhead AD. Temporal changes in the incidence of malignant melanoma: Explanation from action spectra. Mutat Res. 1994;307:365–374. doi: 10.1016/0027-5107(94)90310-7. [DOI] [PubMed] [Google Scholar]
- 5.De Fabo EC, Noonan FP, Fears T, Merlino G. Ultraviolet B but not ultraviolet A radiation initiates melanoma. Cancer Res. 2004;64:6372–6376. doi: 10.1158/0008-5472.CAN-04-1454. [DOI] [PubMed] [Google Scholar]
- 6.Ley RD. Dose response for ultraviolet radiation A-induced focal melanocytic hyperplasia and nonmelanoma skin tumors in Monodelphis domestica. Photochem Photobiol. 2001;73:20–23. doi: 10.1562/0031-8655(2001)073<0020:drfura>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Robinson ES, Hill RH, Jr, Kripke ML, Setlow RB. The Monodelphis melanoma model: Initial report on large ultraviolet A exposures of suckling young. Photochem Photobiol. 2000;71:743–746. doi: 10.1562/0031-8655(2000)071<0743:tmmmir>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Meador JA, Walter RB, Mitchell DL. Induction, distribution and repair of UV photodamage in the platyfish, Xiphophorus signum. Photochem Photobiol. 2000;72:260–266. doi: 10.1562/0031-8655(2000)072<0260:idarou>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell DL, Paniker L, Douki T. DNA damage, repair and photoadaptation in a Xiphophorus fish hybrid. Photochem Photobiol. 2009;85:1384–1390. doi: 10.1111/j.1751-1097.2009.00591.x. [DOI] [PubMed] [Google Scholar]
- 10.Cadet J, Vigny P. In: Bioorganic Photochemistry: Photochemistry and the Nucleic Acids. Morrison H, editor. New York: Wiley; 1990. pp. 1–272. [Google Scholar]
- 11.Wang SY. In: Photochemistry and Photobiology of Nucleic Acids. Wang SY, editor. New York: Academic Press; 1976. pp. 326–356. [Google Scholar]
- 12.Meierjohann S, Schartl M. From Mendelian to molecular genetics: The Xiphophorus melanoma model. Trends Genet. 2006;22:654–661. doi: 10.1016/j.tig.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Nairn RS, et al. Genetic analysis of susceptibility to spontaneous and UV-induced carcinogenesis in Xiphophorus hybrid fish. Mar Biotechnol (NY) 2001;3(Suppl1):S24–S36. doi: 10.1007/s1012601-0004-7. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell D, Paniker L, Sanchez G, Trono D, Nairn R. The etiology of sunlight-induced melanoma in Xiphophorus hybrid fish. Mol Carcinog. 2007;46:679–684. doi: 10.1002/mc.20341. [DOI] [PubMed] [Google Scholar]
- 15.de Wit PE, et al. Increasing epidermal growth factor receptor expression in human melanocytic tumor progression. J Invest Dermatol. 1992;99:168–173. doi: 10.1111/1523-1747.ep12616793. [DOI] [PubMed] [Google Scholar]
- 16.Huang TS, Rauth S, Das Gupta TK. Overexpression of EGF receptor is associated with spontaneous metastases of a human melanoma cell line in nude mice. Anticancer Res. 1996;16(6B):3557–3563. [PubMed] [Google Scholar]
- 17.Volff JN, Schartl M. Evolution of signal transduction by gene and genome duplication in fish. J Struct Funct Genomics. 2003;3:139–150. [PubMed] [Google Scholar]
- 18.Wood SR, et al. UV causation of melanoma in Xiphophorus is dominated by melanin photosensitized oxidant production. Proc Natl Acad Sci USA. 2006;103:4111–4115. doi: 10.1073/pnas.0511248103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gimenez-Conti I, et al. A proposed classification scheme for Xiphophorus mela-nomas based on histopathologic analyses. Mar Biotechnol (NY) 2001;3(Suppl 1):S100–S106. doi: 10.1007/s10126001-0031-4. [DOI] [PubMed] [Google Scholar]
- 20.Sharpless NE, Kannan K, Xu J, Bosenberg MW, Chin L. Both products of the mouse Ink4a/Arf locus suppress melanoma formation in vivo. Oncogene. 2003;22:5055–5059. doi: 10.1038/sj.onc.1206809. [DOI] [PubMed] [Google Scholar]
- 21.Hacker E, Muller HK, Hayward N, Fahey P, Walker G. Enhancement of DNA repair using topical T4 endonuclease V does not inhibit melanoma formation in Cdk4/Tyr-Nras mice following neonatal UVR. Pigment Cell Melanoma Res. 2010;23:121–128. doi: 10.1111/j.1755-148X.2009.00643.x. [DOI] [PubMed] [Google Scholar]
- 22.Walter RB, Kazianis S. Xiphophorus interspecies hybrids as genetic models of induced neoplasia. ILAR J. 2001;42:299–321. doi: 10.1093/ilar.42.4.299. [DOI] [PubMed] [Google Scholar]
- 23.Cadet J, Douki T, Ravanat JL, Di Mascio P. Sensitized formation of oxidatively generated damage to cellular DNA by UVA radiation. Photochem Photobiol Sci. 2009;8:903–911. doi: 10.1039/b905343n. [DOI] [PubMed] [Google Scholar]
- 24.Cadet J, Sage E, Douki T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat Res. 2005;571:3–17. doi: 10.1016/j.mrfmmm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Douki T, Reynaud-Angelin A, Cadet J, Sage E. Bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation. Biochemistry. 2003;42:9221–9226. doi: 10.1021/bi034593c. [DOI] [PubMed] [Google Scholar]
- 26.de Gruijl FR. Photocarcinogenesis: UVA vs. UVB radiation. Skin Pharmacol Appl Skin Physiol. 2002;15:316–320. doi: 10.1159/000064535. [DOI] [PubMed] [Google Scholar]
- 27.de Laat A, van der Leun JC, de Gruijl FR. Carcinogenesis induced by UVA (365-nm) radiation: The dose-time dependence of tumor formation in hairless mice. Carcinogenesis. 1997;18:1013–1020. doi: 10.1093/carcin/18.5.1013. [DOI] [PubMed] [Google Scholar]
- 28.Krutmann J. Ultraviolet A radiation-induced biological effects in human skin: Relevance for photoaging and photodermatosis. J Dermatol Sci. 2000;23(Suppl 1):S22–S26. doi: 10.1016/s0923-1811(99)00077-8. [DOI] [PubMed] [Google Scholar]
- 29.Halliday GM. Inflammation, gene mutation and photoimmunosuppression in response to UVR-induced oxidative damage contributes to photocarcinogenesis. Mutat Res. 2005;571:107–120. doi: 10.1016/j.mrfmmm.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Phan TA, Halliday GM, Barnetson RS, Damian DL. Spectral and dose dependence of ultraviolet radiation-induced immunosuppression. Front Biosci. 2006;11:394–411. doi: 10.2741/1807. [DOI] [PubMed] [Google Scholar]
- 31.Cancer IUA. The association of use of sunbeds with cutaneous malignant melanoma and other skin cancers: A systematic review. Int J Cancer. 2006;120:1116–1122. doi: 10.1002/ijc.22453. [DOI] [PubMed] [Google Scholar]
- 32.El Ghissassi F, et al. WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens—part D: Radiation. Lancet Oncol. 2009;10:751–752. doi: 10.1016/s1470-2045(09)70213-x. [DOI] [PubMed] [Google Scholar]
- 33.Mitani H, Yasuhira S, Komura J, Shima A. Enhancement of repair of UV-irradiated plasmids in cultured fish cells by fluorescent light preillumination and growth arrest. Mutat Res. 1991;255:273–280. doi: 10.1016/0921-8777(91)90031-j. [DOI] [PubMed] [Google Scholar]
- 34.Yasuhira S, Mitani H, Shima A. Enhancement of photorepair of ultraviolet-induced pyrimidine dimers by preillumination with fluorescent light in the goldfish cell line. The relationship between survival and yield of pyrimidine dimers. Photochem Photobiol. 1992;55:97–101. doi: 10.1111/j.1751-1097.1992.tb04214.x. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell DL, Scoggins JT, Morizot DC. DNA repair in the variable platyfish (Xiphophorus variatus) irradiated in vivo with ultraviolet B light. Photochem Photobiol. 1993;58:455–459. doi: 10.1111/j.1751-1097.1993.tb09590.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information