Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules (original) (raw)
Abstract
Using a high-throughput chemical screen, we identified two small molecules that enhance the survival of human embryonic stem cells (hESCs). By characterizing their mechanisms of action, we discovered an essential role of E-cadherin signaling for ESC survival. Specifically, we showed that the primary cause of hESC death following enzymatic dissociation comes from an irreparable disruption of E-cadherin signaling, which then leads to a fatal perturbation of integrin signaling. Furthermore, we found that stability of E-cadherin and the resulting survival of ESCs were controlled by specific growth factor signaling. Finally, we generated mESC-like hESCs by culturing them in mESC conditions. And these converted hESCs rely more on E-cadherin signaling and significantly less on integrin signaling. Our data suggest that differential usage of cell adhesion systems by ESCs to maintain self-renewal may explain their profound differences in terms of morphology, growth factor requirement, and sensitivity to enzymatic cell dissociation.
Keywords: human embryonic stem cell survival, cell-cell adhesion, cell-ECM adhesion
Conventional murine and human embryonic stem cells (hESCs) derived from blastocysts can be propagated indefinitely and have the ability to generate all cell types (1–3). They express pluripotency transcription factors, including the ones that can reprogram somatic cells back to pluripotent states: Oct4, Sox2, Nanog, and Klf4. However, murine and human ESCs respond very differently to several key signaling pathways in self-renewal or differentiation. For example, murine ESCs (mESCs) self-renew under leukemia inhibitory factor (LIF) and bone morphogenic protein (BMP) (4, 5), whereas human ESCs (hESCs) appear dependent on FGF, and TGFβ/Activin/Nodal pathway activity for self-renewal (6–11). These studies clearly suggest that there exist two distinct self-renewal mechanisms. In addition, hESCs grow in vitro as large flattened 2D colonies, whereas mESCs display characteristic small-domed 3D appearances of compact colonies. Moreover, unlike mESCs, hESCs are very vulnerable to single-cell dissociation. Massive cell death occurs after complete single-cell dissociation, which has been a significant hurdle for rapid expansion and genetic manipulation of hESCs. To address this critical challenge and understand the molecular mechanisms that govern hESC survival, we used a high-throughput chemical screening approach and identified two small molecules with distinct mechanisms of action that significantly increase hESC survival after single-cell dissociation. In depth characterizations of compounds’ mechanism of action revealed that hESC survival and self-renewal is regulated by the interplay between two cell adhesion systems: cell-cell adhesion and cell-ECM adhesion. Our studies also uncovered a common mechanism that underlies and integrates two seemingly distinct self-renewal states represented by conventional murine and human ESCs.
Results
Identification of Small Molecules That Promote hESC Survival After Single-Cell Dissociation.
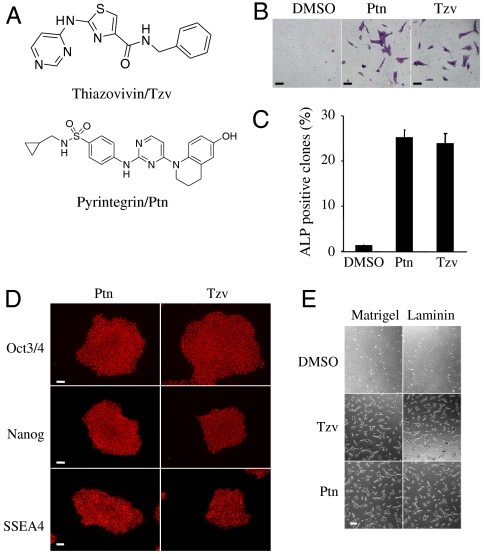
To improve hESC culture and uncover molecular mechanisms underlying hESC death after single-cell dissociation, we performed a high-throughput phenotypic chemical screen of 50,000 synthetic compounds to identify small molecules that promoted hESC survival after trypsin dissociation. From the screen, two compounds, a 2,4-disubstituted thiazole (named Thiazovivin/Tzv) and a 2,4-disubstituted pyrimidine (named Tyrintegin/Ptn) were identified that significantly increased cell survival after dissociation while maintaining pluripotency [i.e., characteristic hESC colony morphology and alkaline phosphatase (ALP) expression] (Fig. 1A).
Fig. 1.
Novel synthetic small molecules dramatically increase hESC survival after single cell dissociation by enhancing cell-ECM mediated integrin activity. (A) Chemical structures of Thiazovivin/Tzv and Pyrintegrin/Ptn as indicated. (B) ALP staining of hESC colonies that had grown from dissociated single cells seeded in low density for four days and treated as indicated. Bar, 20 μm. (C) Ratio of ALP positive colonies vs. total initially seeded cells (n = 2). (D) Immunostaining of hESCs long-term maintained in chemically defined media containing Ptn or Tzv as indicated. Bar, 50 μm. (E) Representative phase contrast images of hESCs 12 h after seeding on the different matrices and treated with the indicated compounds. Bar, 20 μm. If not specified, all the above hESCs were grown in the chemically defined medium and feeder-free condition on Matrigel coated plates. All error bars indicate ± SEM.
Tzv and Ptn both enhance survival of single hESCs more than 30-fold on matrigel-coated plates after enzymatic dissociation (Fig. 1 B and C). hESCs have been serially passaged in Tzv or Ptn containing chemically defined medium for more than 40 generations. Under such conditions, hESCs homogenously maintained the characteristic colony morphology, expression of typical pluripotency markers, and normal karyotype (Fig. 1D and Fig. S1). When these cells were injected into nude mice, they generated complex teratomas consisting of all three primary germ layer tissues (Fig. S1). These results, confirmed with several independent hESC lines, collectively and convincingly demonstrated that both compounds could substantially promote hESC survival without compromising their self-renewal and full developmental potency.
Tzv and Ptn Enhance Cell-ECM Adhesion-Mediated Integrin Signaling.
Tzv and Ptn have a dramatic effect on cell attachment even within a few hours. And both compounds have little impact on cell proliferation (Fig. S1). Thus the observed survival-promoting effect may be largely attributed to an increase in cell attachment following cell dissociation and seeding processes. To directly examine the compound’s effect on cell attachment, dissociated hESCs were treated with Tzv or Ptn. Within a few hours, treated cells displayed dramatically increased adhesion to matrigel- or laminin-coated plates but not to gelatin-coated plates (Fig. 1E). Integrin signaling, initiated by cell-ECM adhesion, provides a survival mechanism, and β1 integrin is regarded as a major integrin mediator for hESCs (12, 13). The expression of β1 integrin was not affected by trypsin or compound treatment (Fig. S2). The monoclonal antibody HUTS-21, which specifically binds to the activated form of β1 integrin, was used to measure integrin activity (14). Notably, compound treatment increased the level of HUTS-21 binding, suggesting Tzv and Ptn increase cell-ECM adhesion-mediated integrin activity (Fig. S2). Integrin signaling is known to cross-talk with growth factor signaling to promote cell survival by activating phosphatidylinositol-3-kinase and MAPK pathway, which are two master regulators of hESC self-renewal (15–21). Thus, it is not surprising that Ptn treatment significantly enhances phosphorylation of multiple growth factor receptors, including FGFR, IGFR, and EGFR1, and their downstream PI-3 kinase and MAPK activities (Fig. S2). Furthermore, inhibition of FGFR, IGFR, EGFR1, and ErbB2 greatly abolished increased AKT phosphorylation and diminished the survival-promoting effect induced by Ptn treatment (Fig. S2). Collectively, our results demonstrate that compounds can enhance cell-ECM adhesion to activate integrin signaling, which synergizes with growth factors to promote cell survival.
Tzv Protects hESCs in the Absence of ECM by Regulating E-Cadherin-Mediated Cell-Cell Interaction.
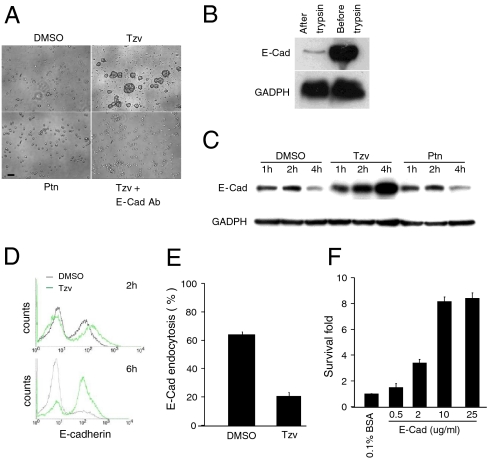
Although Tzv could not promote hESC attachment to gelatin-coated plates, we observed that the treated-cells formed aggregates floating in the plate and were protected from death (Fig. 2A, Fig. S3). This phenomenon was not observed with Ptn treatment. Massive cell death occurred when cells were plated onto gelatin-coated plates even in the presence of Ptn. These observations suggested that Tzv may have another function to promote cell survival in addition to activating integrin, and Tzv and Ptn may have different biological targets. Since cell aggregation is most often mediated through cell-cell adhesion, and E-cadherin is the primary cell-cell adhesion molecule and a highly expressed protein in hESCs (22), we tested the effect of a specific E-cadherin blocking antibody on multicellular aggregate formation. When the cells were cultured in the presence of an antibody, the formation of large, compact aggregates following Tzv treatment was severely inhibited and extensive cell death was observed (Fig. 2A), suggesting that Tzv enhances hESC survival in suspension, presumably acting through E-cadherin-mediated cell-cell adhesion.
Fig. 2.
Tzv stabilizes E-cadherin after cell dissociation to protect hESCs from death under ECM-free conditions. (A) Phase contrast images of hESCs grown on noncoated plates treated with the indicated molecules. Bar, 50 μm. (B) Western blot analysis of full-length E-cadherin in hESCs before and after trypsin. (C) A time-course Western blot analysis of full-length E-cadherin expression in hESCs after trypsin dissociation and treatment with DMSO, Tzv, or Ptn for indicated time. (D) Flow cytometry analysis of E-cadherin surface level in hESCs after trypsin treatment in the presence of Tzv. DMSO was used as a control. (E) E-cadherin endocytosis analysis in the absence or presence of Tzv (n = 2). (F) Cell survival analysis of hESCs grown on BSA or different concentrations of E-cad-Fc chimera-coated plates for five days (n = 2). All error bars indicate ± SEM.
To study the mechanism by which Tzv regulates E-cadherin-mediated cell-cell adhesion, we first examined E-cadherin expression in hESCs after trypsin dissociation. Strikingly, most of the full-length E-cadherin had been cleaved after trypsin dissociation (Fig. 2B). In Tzv-untreated cells, newly synthesized full-length E-cadherins appeared 1 h after enzyme treatment and was significantly reduced after 4 h, suggesting that newly synthesized E-cadherin in dissociated hESCs was not stable. However, in Tzv-treated cells, E-cadherin protein level was significantly increased (Fig. 2C). Furthermore, flow cytometry analysis revealed that cell surface E-cadherin in hESCs was significantly increased by Tzv (Fig. 2D). Therefore, Tzv is likely to affect cell adhesion by modulating the cell surface level of E-cadherin. Semiquantitative RT-PCR revealed comparable amounts of E-cadherin transcripts in mock controls and Tzv-treated cells (Fig. S3), suggesting the difference in E-cadherin protein levels was not due to altered transcription. Finally, an endocytosis assay revealed that internalization of E-cadherin was significantly blocked by Tzv (Fig. 2E). Thus it is likely that Tzv stabilizes E-cadherin on the cell surface through inhibition of endocytosis. To confirm the important role of E-cadherin stabilization for cell survival, we coated plates with a dimeric E-cadherin-Fc chimera protein containing the E-cadherin ectodomain fused to the IgG Fc fragment. Remarkably, dissociated hESCs attached to the coated surface and their survival was significantly increased in a dose-dependent manner (Fig. 2F). Our data implicate that cleavage of E-cadherin itself by trypsin is not the direct cause of cell death but that the disruption of cell-cell interaction by trypsin, which in turn destabilizes E-cadherin, is the main reason. Tzv inhibits endocytosis of E-cadherin on the cell surface, consequently stabilizing E-cadherin and leading to reestablishment of cell-cell interaction, which is essential for hESC survival in ECM-free conditions.
Rho-ROCK Signaling Regulates Cell Adhesion and Cell-Cell Interaction.
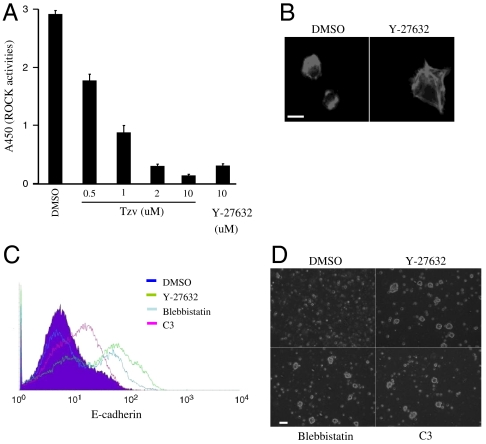
To better understand the molecular mechanism of Tzv, affinity pull-downs using immobilized Tzv were used to identify the putative molecular target of this compound (Fig. S4). It was revealed that Rho-associated kinase (ROCK) is a direct target of Tzv and this was confirmed by an in vitro Rho kinase assay (Fig. 3A). Tzv (2 μM) inhibits ROCK activity and protects hESCs at a similar level as Y-27632 (10 μM), a widely used selective ROCK inhibitor (Fig. 3A and Fig. S4). In contrast, Ptn had no effect on ROCK activity even at 10-μM concentration. Concurrent with our studies, Y-27632 has recently been shown to promote hESC survival; however, its underlying mechanism remains unknown (23, 24).
Fig. 3.
Tzv is a novel ROCK inhibitor and Rho-ROCK axis regulates cell-ECM and cell-cell adhesion (A) In vitro Rho-kinase assay treated with indicated compounds (n = 3). (B) Staining of F-actin in hESCs 6 h after seeding on Matrigel-coated plates in the absence or presence of Y-27632, a selective ROCK inhibitor. Bar, 10 μm. (C). Flow cytometry analysis of E-cadherin surface level in hESCs 6 h after trypsin treatment in the presence of indicated compounds. DMSO was used as a control. (D) Phase contrast images of hESCs grown on noncoated plates treated with the indicated molecules. Bar, 100 μm. All error bars indicate ± SEM.
ROCK is a downstream effector of Rho signaling, a master regulator of cytoskeleton remodeling and contractile force generation (25, 26). We hypothesized that Rho-ROCK signaling may regulate cell-ECM interaction and cell-cell interaction in hESC. To investigate the Rho-ROCK mechanism in regulating cell-ECM adhesion, we treated cells with cell-permeable Clostridium botulinum C3 toxin, which inactivates Rho, and Y-27632, a selective ROCK inhibitor, and assessed cell attachment. Inhibition of Rho and ROCK activities resulted in a marked increase of cell attachment to ECM (Fig. S4). To examine the cell adhesion process during replating in detail, we labeled F actin using a fluorescent derivative of phalloidin. As shown in Fig. 3B, cells after dissociation showed prominent stress fiber formation and hypercontraction and were unable to spread even on ECM-coated plates. In contrast, treatment with Y-27632 led to the disappearance of stress fibers and increased focal adhesion. These results suggest that cell hypercontraction induced by Rho-ROCK signaling may be the main cause of cell attachment deficiency.
Next, we investigated whether Rho-ROCK signaling also regulates E-cadherin-mediated cell-cell adhesion in ECM-free conditions. As shown above, three hours after trypsinization, in the untreated cells, E-cadherin levels were very low due to its instability. However, in the cells treated with Clostridium botulinum C3 toxin and Y-27632, E-cadherin total protein and its surface level were significantly increased (Fig. 3C and Fig. S4). Similarly, treatment with blebbistatin, a selective inhibitor of myosin II that is a downstream effector of ROCK, also enhanced the level of E-cadherin (Fig. 3C and Fig. S4). Furthermore, treatment with C3 and blebbistatin led to formation of cell aggregates and protected cell from death in suspension culture (Fig. 3D). Taken together, these results suggest that the Rho-ROCK-Myosin II axis plays an important role in mediating cell-cell adhesion by regulating the stabilization of E-cadherin in ECM-free conditions.
E-Cadherin Signaling and Integrin Signaling Regulate Each Other Through Modulating Rho Activities and Both Are Required for Survival and Self-Renewal of hESCs.
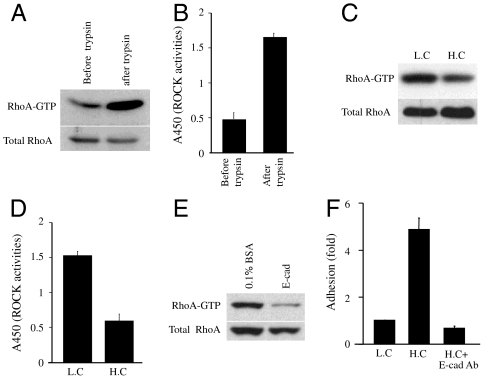
Inhibition of the Rho-ROCK pathway prevents hESCs from death suggests that superactivation of Rho-ROCK signaling induced by single-cell dissociation may be the primary reason why hESCs are vulnerable to trypsin treatment. It was found that Rho and ROCK activities were truly significantly induced by trypsin treatment (Fig. 4 A and B). Next, we investigated the mechanism by which cell dissociation induced activation of the Rho-ROCK pathway. Cell-cell adhesion has been shown to regulate Rho signaling (27), and trypsin treatment induces irreparable cell-cell adhesion in hESCs. Thus we investigate the regulation of Rho-ROCK by cell contact in hESCs. hESCs were plated at low density or at 10-fold higher density. RhoA·GTP and ROCK activity were significantly lower in the postconfluent culture than in the subconfluent culture, suggesting that cell-cell interaction could suppress Rho and ROCK activity (Fig. 4 C and D). Moreover, when cells were plated onto the E-cadherin-coated plate, Rho activity was significantly reduced, providing direct evidence that E-cadherin-mediated cell-cell interaction could regulate Rho activity in hESCs (Fig. 4E). Based on our results/model, down-regulation of Rho-ROCK signaling would reduce cell hypercontraction induced by trypsin treatment and lead to enhancement of cell attachment. Consistent with this, the cell attachment rate was about 5-fold higher in the postconfluent culture compared with the subconfluent culture, indicating that cell-cell interactions may regulate cell-ECM interaction (Fig. 4F). Furthermore, increased hESC attachment in the postconfluent culture was abolished by incubation with E-cadherin blocking antibodies (Fig. 4F). Collectively, our results suggest that cell-cell adhesion regulates cell-ECM adhesion through repressing Rho-ROCK activities.
Fig. 4.
Cell-cell interaction regulates cell-ECM interaction and contributes to survival and self-renewal of hESCs. (A) Western blot showing the active Rho level in hESCs before or after trypsin. (B) Rho-kinase assay showing the ROCK activity in hESCs before or after trypsin treatment (n = 3). (C) Western blot showing the active Rho level in hESCs 30 min after replating at the two different densities on the Matrigel-coated plates. (D) Rho-kinase assay showing the ROCK activity in hESCs 30 min after replating at the two different densities on Matrigel-coated plates (n = 3). (E) Western blot showing the active Rho level in hESCs 30 min after replating on BSA or different concentrations of E-cad-Fc chimera-coated plates. (F) Cell attachment on Matrigel-coated plates for different densities of hESCs (n = 2). LC, low-density culture (1.5 × 104 cells per well of 6-well plate). HC, higher-density culture (15 × 104 cells per well of 6-well plate). All graphs show mean ± SEM.
Under ECM-free conditions, Ptn treatment could not stabilize E-cadherin in hESCs after trypsinization. However, in the presence of ECM, treatment of hESCs with Ptn led not only to cell attachment to ECM-coated plates but also to rapid stabilization of E-cadherin (Fig. S5). And E-cadherin was more stable in cells seeded on the matrigel-coated plate than in cells on gelatin-coated plate (Fig. S5). Furthermore, Rho activity was significantly reduced when cells were plated on a matrigel-coated plate instead of a gelatin-coated plate, suggesting that cell-ECM interaction could also regulates cell-cell interaction by modulating Rho-ROCK pathway (Fig. S5).
Differential Usage of Cell Adhesion Systems May Contribute to Functional Differences Between Distinct Self-Renewal States of Pluripotent Stem Cells.
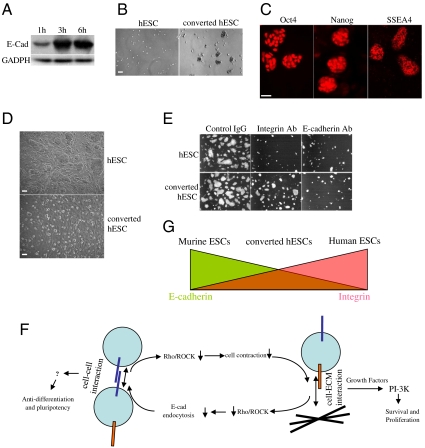
In contrast to hESCs, mESCs are much more tolerant of single-cell dissociation and can be clonally expanded. When treated with trypsin, most E-cadherin in mESCs is degraded as in hESCs (Fig. S6). However, newly synthesized E-cadherin is much more stable than that in hESCs (Fig. 5A). Because E-cadherin stability is regulated by Rho-ROCK signaling as we have shown above, we examined the activity of Rho and ROCK in mESCs after trypsin dissociation. In contrast to hESCs, Rho and ROCK activity were not increased significantly in mESCs (Fig. S6), suggesting that mESCs have a distinct mechanism to regulate Rho and ROCK activities, by which E-cadherin is stabilized. Because human and murine ESCs are maintained in distinct culture conditions, we hypothesized that the stability of E-cadherin in hESCs and mESCs may be regulated by signaling environments of their unique culture conditions, and it may be possible to improve cell survival when culturing hESCs under mouse signaling conditions. While LIF alone is not sufficient for long-term maintenance of hESCs, we found that addition of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase (MEK) inhibitor PD0325901 and p38 inhibitor SB203580 (28, 29), known to enhance/stabilize self-renewal of mESCs, could maintain hESCs under mouse conditions for many passages. E-cadherin in the newly converted cells is more stable than that in conventional hESCs when cells were dissociated by trypsin (Fig. S6). Cell survival after trypsin dissociation was also significantly improved for the converted hESCs under the mouse conditions, and this improvement was more dramatic when cells were grown under ECM-free conditions (Fig. 5B and Fig. S6). Taken together, these results demonstrate that different properties of E-cadherin (e.g., stability) in mESCs, hESCs, and the converted hESCs may be the primary determinant of their distinct sensitivity toward single-cell dissociation. The converted hESCs have been homogenously expanded for more than 20 passages. They maintain typical features of pluripotent stem cells, including the expression of Oct4, Nanog, SSEA4, and the ability to generate derivatives of all three germ layers through in vitro differentiation (Fig. 5C and Fig. S6). However, the converted hESCs are more similar to mESCs. They grow much faster and display as more compact, domed, and smaller colonies than conventional hESCs (Fig. 5D). Comparison of recently derived rodent epiblast stem cells with conventional hESCs has suggested that hESCs may represent a later stage of pluripotency developmentally (i.e., postimplantation epiblast stage) than mESCs (which are at the preimplantation inner cell mass stage) (30, 31). However, this view is also challenged by the fact that hESCs could differentiate into trophoblast cells that first diverge at the blastocyst stage before epiblast formation. While only a chimerism test would definitively approve such developmental stage differences for pluripotency, it can be performed only for animal studies. A requirement of LIF but not of FGF suggests that the converted hESCs may share a similar self-renewal mechanism with mESCs. Self-renewal of hESCs is known to be dependent on integrin signaling, whereas self-renewal of mESCs is not (32). To compare the contribution of two cell adhesion signaling to the self-renewal of the converted hESCs and conventional hESCs, we incubated them in blocking antibodies against E-cadherin and integrin. As predicted, attachment and proliferation of conventional hESCs were strongly affected by blocking integrin signaling. A similar effect of blocking E-cadherin was observed in conventional hESCs, further confirming the essential regulatory role of cell-cell interaction on cell-ECM interaction. More interestingly, attachment and proliferation of the converted hESCs were significantly less affected by the integrin blocking antibody but strongly inhibited by the E-cadherin blocking antibody (Fig. 5E). Furthermore, the converted hESCs were more resistant to differentiation when growing on gelatin-coated plates than conventional hESCs (Fig. S6). These results suggested that integrin signaling is less important and E-cadherin is more important for survival and self-renewal of the converted hESCs. Moreover, we compared E-cadherin expression and the focal adhesion kinase (FAK) phosphorylation level, indicators of E-cadherin and integrin signaling, respectively, in these two cell lines: The converted hESCs have a higher level of E-cadherin expression and a lower level of phosphorylated FAK than conventional hESCs (Fig. S6). Taken together, our data suggest that mouse conditions favor E-cadherin signaling, whereas human conditions favor integrin signaling, and self-renewal of distinct states of pluripotent stem cells could be achieved by different combinations of the two cell adhesion systems.
Fig. 5.
hESCs maintained in mESCs culture condition have better survival and are dependent on different cell adhesion signaling for survival and self-renewal. (A) A time-course Western blot analysis of full-length E-cadherin expression in mESCs after replating for the indicated time. (B) Phase contrast images of the conventional hESCs and converted hESCs 24 h after replating on noncoated plates. Bar, 100 μm. (C) Immunostaining of converted hESCs with typical pluripotency markers. Bar, 50 μm. (D) Phase contrast images of the conventional hESCs and converted hESCs. Bar, 50 μm. (E) Fluorescent images of Oct4-GFP hESCs and converted Oct4-GFP hESCs two days after replating on the Matrigel-coated plates in the presence of control IgG, integrin β1-blocking antibodies, and E-cadherin blocking antibodies (5–10 μg/mL). Representative pictures are shown from three independent experiments. (F) Cell-cell adhesion and cell-ECM adhesion regulate each other through Rho-ROCK signaling. Two cell adhesion systems cooperate with growth factor signaling to control survival, self-renewal of hESCs. (G) Pluripotent stem cell states may exhibit a broad range, and distinct cell states within this dynamic range could be created by different inputs from the two cell adhesion signaling pathway. All graphs show mean ± SEM.
Discussion
Using high-throughput chemical screening, we have identified two small molecules with distinct mechanisms of action that greatly enhance hESC survival after single-cell dissociation. Such chemical tools would enable more robust hESC culture and significantly facilitate applications of hESCs such as gene targeting or drug discovery. Stem cell fate/function in vivo is influenced by their microenvironment (i.e., niche), which consists of signaling factors, surrounding cells, and ECM (33–35). Cell-ECM interaction has been implicated to play an important role for hESC survival and self-renewal (36). Here, we discovered and mechanistically characterized that E-cadherin-mediated cell-cell interaction between hESCs constitutes an essential regulatory mechanism to control hESCs survival and self-renewal (Fig. 5F). Why hESCs are sensitive to cell dissociation but mESCs are not is a long-standing mystery. Our results suggest that nonrecoverable cell-cell interaction due to unique growth factor signaling in hESC culture media, which leads to irreparable destruction of the cell survival niche (integrin signaling), is the main reason that hESCs are sensitive to single-cell dissociation. In contrast to hESCs, E-cadherin can fully recover after trypsin treatment in mESCs due to their specific cytokine signaling. Thus, mESCs are more resistant to cell-dissociation treatment.
Survival of hESCs could be achieved by utilizing either the cell-ECM interaction mediated integrin pathway (in the presence of ECM) or the cell-cell interaction mediated E-cadherin pathway (in the absence of ECM). Our studies also indicate that self-renewal of pluripotent stem cells can be maintained by utilizing a different combination of cell adhesion systems. The reason why different cytokines are required for self-renewal of murine and human ESCs remains poorly understood. FGF and TGF beta signaling are known as the major pathways promoting ECM expression and the epithelial-mesenchymal transition process (37), which enhance cell-ECM adhesion and reduce cell-cell adhesion. Here, we demonstrate that growing hESC in conditions favoring mouse ESCs significantly increases E-cadherin levels and inhibits integrin signaling. Accordingly, self-renewal/survival of hESCs under mouse conditions becomes significantly less dependent on integrin signaling and relies more on E-cadherin signaling. Coupled with the fact that E-cadherin signaling and not integrin signaling is required for self-renewal of mESCs, our studies suggest that the different requirements for cytokines by these pluripotent stem cells (i.e., conventional mESCs, hESCs, and converted hESCs) may correspond to their utilization of different cell adhesion systems to maintain their cell identity. In addition to varying cytokine requirements, some key pathways have been shown to play opposite roles in murine and human ESCs: Inactivation of ERK1/2 is required for self-renewal of mESCs, whereas it induces differentiation of hESCs (18, 28). Inhibition of ERK and/or TGF has been shown to increase cell-cell adhesion and inhibit cell-ECM adhesion (38, 39), providing further support for the notion that different usage of cell adhesion systems may define two types of self-renewal states for pluripotent stem cells. Moreover, the converted hESCs still need integrin although they are significantly less dependent on integrin signaling than conventional hESCs, suggesting there may be a broad range of pluripotent stem cell states and that distinct cell states within this dynamic range can be created by different inputs from the two cell adhesion signaling pathways (Fig. 5G).
Finally, our studies also indicate that the physical structure of ESCs (e.g., 2D or 3D and compactness) may not only be the result of their self-renewal states but also may actively play an important role in regulating self-renewal. Cell-ECM interaction modulates growth factor signaling to promote hESC survival and self-renewal. The role of E-cadherin signaling for self-renewal is still unknown. However, the fact that both E-cadherin and integrin signaling could regulate Rho activity suggests that cytoskeletal regulation may be the key. Further study along these lines will provide additional insights into these complex regulatory networks.
Materials and Methods
Cell Culture.
Human ESC lines H1, HES2, HUES7, and HUES9 were cultured on irradiated mouse embryonic fibroblast feeder cells in DMEM-F12 supplemented with 2 mM L glutamine, 1× nonessential amino acids, 20% serum replacement (Invitrogen), and 10 ng/mL basic Fibroblast growth factor (Invitrogen). Chemically defined and feeder-free hESC culture was described previously (40).
More cell culture methods in this study are described in SI Text.
Reagents.
The reagents used in this study are described in SI Text.
High-Throughput Chemical Screen.
The trypsinable hESC lines HUES9 were used for the screen. hESCs were cultured in chemically defined media on the Matrigel-coated plates as described above. Then cells were harvested by trypsin. hESCs were plated at 4,000 cells per well onto Matrigel-coated 384-well plates. After 1 h when cells settled down, compounds from an in-house library of 50,000 discrete heterocycles were added to each well (2-μM final concentration). After an additional six days of incubation, in which media and compounds were changed on day 3, cells were stained for ALP expression and examined for compact colony morphology.
In Vitro Differentiation.
For spontaneous differentiation, cells were treated with collagenase or trypsin and cultured in suspension in ultralow adhesion plates in hESC or mESC growth media in the absence of bFGF or LIF. Media were refreshed every day, and embryoid bodies (EB) were allowed to grow for five days. EBs were then replated onto matrigel-coated plates. Spontaneous differentiations were examined at various time points. Definitive endoderm differentiation was carried out as previously described (41).
See SI Materials and Methods for additional information.
Supplementary Material
Supporting Information
Acknowledgments.
We thank Dr. Kent Osborn for histological analysis of teratomas. We also thank Drs. Jem Efe, Wen Xiong, Caroline Desponts, Yuan Xu, Mr. Ron Coleman, and other members of the Ding lab for their technical helps. This work was supported by grants from The Scripps Research Institute (to S.D.) and Larry Hillblom Foundation (to A.H.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292(5819):154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78(12):7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 4.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12(13):2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115(3):281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 6.Beattie GM, et al. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23(4):489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- 7.James D, Levine AJ, Besser D, Hemmati-Brivanlou A. TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development. 2005;132(6):1273–1282. doi: 10.1242/dev.01706. [DOI] [PubMed] [Google Scholar]
- 8.Xu RH, et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Methods. 2005;2(3):185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- 9.Levenstein ME, et al. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24(3):568–574. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells. 2006;24(6):1476–1486. doi: 10.1634/stemcells.2005-0299. [DOI] [PubMed] [Google Scholar]
- 11.Greber B, Lehrach H, Adjaye J. Fibroblast growth factor 2 modulates transforming growth factor beta signaling in mouse embryonic fibroblasts and human ESCs (hESCs) to support hESC self-renewal. Stem Cells. 2007;25(2):455–464. doi: 10.1634/stemcells.2006-0476. [DOI] [PubMed] [Google Scholar]
- 12.Giancotti FG. Integrin signaling: Specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9(5):691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- 13.Xu C, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19(10):971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 14.Luque A, et al. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355-425) of the common beta 1 chain. J Biol Chem. 1996;271(19):11067–11075. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong L, et al. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum Mol Genet. 2006;15(11):1894–1913. doi: 10.1093/hmg/ddl112. [DOI] [PubMed] [Google Scholar]
- 16.Paling NR, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem. 2004;279(46):48063–48070. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- 17.Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nat Biotechnol. 2006;24(3):344–350. doi: 10.1038/nbt1189. [DOI] [PubMed] [Google Scholar]
- 18.Li J, et al. MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation. 2007;75(4):299–307. doi: 10.1111/j.1432-0436.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 19.Comoglio PM, Boccaccio C, Trusolino L. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr Opin Cell Biol. 2003;15(5):565–571. doi: 10.1016/s0955-0674(03)00096-6. [DOI] [PubMed] [Google Scholar]
- 20.Bendall SC, et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448(7157):1015–1021. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, et al. Self-renewal of human embryonic stem cells requires insulin-like growth factor-1 receptor and ERBB2 receptor signaling. Blood. 2007;110(12):4111–4119. doi: 10.1182/blood-2007-03-082586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eastham AM, et al. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67(23):11254–11262. doi: 10.1158/0008-5472.CAN-07-2253. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe K, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25(6):681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 24.Damoiseaux R, Sherman SP, Alva JA, Peterson C, Pyle AD. Integrated chemical genomics reveals modifiers of survival in human embryonic stem cells. Stem Cells. 2009;27(3):533–542. doi: 10.1634/stemcells.2008-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 26.Riento K, Ridley AJ. Rocks: Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4(6):446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 27.Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem. 2001;276(36):33305–33308. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- 28.Burdon T, Stracey C, Chambers I, Nichols J, Smith A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev Biol. 1999;210(1):30–43. doi: 10.1006/dbio.1999.9265. [DOI] [PubMed] [Google Scholar]
- 29.Qi X, et al. BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proc Natl Acad Sci USA. 2004;101(16):6027–6032. doi: 10.1073/pnas.0401367101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tesar PJ, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448(7150):196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 31.Brons IG, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448(7150):191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi Y, et al. Integrins regulate mouse embryonic stem cell self-renewal. Stem Cells. 2007;25(12):3005–3015. doi: 10.1634/stemcells.2007-0103. [DOI] [PubMed] [Google Scholar]
- 33.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25. [PubMed] [Google Scholar]
- 34.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 35.Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290(5490):328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 36.Braam SR, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells. 2008;26(9):2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- 37.Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24(37):5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]
- 38.Lu Q, Paredes M, Zhang J, Kosik KS. Basal extracellular signal-regulated kinase activity modulates cell-cell and cell-matrix interactions. Mol Cell Biol. 1998;18(6):3257–3265. doi: 10.1128/mcb.18.6.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- 40.Yao S, et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci USA. 2006;103(18):6907–6912. doi: 10.1073/pnas.0602280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Amour KA, et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23(12):1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information