Targeted Delivery of siRNA to Macrophages for Anti-inflammatory Treatment (original) (raw)
Abstract
Inflammation mediated by tumor necrosis factor-α (TNF-α) and the associated neuronal apoptosis characterizes a number of neurologic disorders. Macrophages and microglial cells are believed to be the major source of TNF-α in the central nervous system (CNS). Here, we show that suppression of TNF-α by targeted delivery of small interfering RNA (siRNA) to macrophage/microglial cells dramatically reduces lipopolysaccharide (LPS)-induced neuroinflammation and neuronal apoptosis in vivo. Because macrophage/microglia express the nicotinic acetylcholine receptor (AchR) on their surface, we used a short AchR-binding peptide derived from the rabies virus glycoprotein (RVG) as a targeting ligand. This peptide was fused to nona-D-arginine residues (RVG-9dR) to enable siRNA binding. RVG-9dR was able to deliver siRNA to induce gene silencing in macrophages and microglia cells from wild type, but not AchR-deficient mice, confirming targeting specificity. Treatment with anti-TNF-α siRNA complexed to RVG-9dR achieved efficient silencing of LPS-induced TNF-α production by primary macrophages and microglia cells in vitro. Moreover, intravenous injection with RVG-9dR-complexed siRNA in mice reduced the LPS-induced TNF-α levels in blood as well as in the brain, leading to a significant reduction in neuronal apoptosis. These results demonstrate that RVG-9dR provides a tool for siRNA delivery to macrophages and microglia and that suppression of TNF-α can potentially be used to suppress neuroinflammation in vivo.
Introduction
Inflammation is a complex biological reaction of the immune system in response to harmful stimuli including pathogens, damaged cells, or irritants.1 It is a protective attempt by the body to remove harmful stimuli as well as to initiate the healing process.2 However, inflammatory response is often superfluous and, in turn, can cause more damage to the body. Dysregulated inflammatory response can lead to autoimmune disorders such as inflammatory bowel disease, multiple sclerosis, and rheumatoid arthritis.3
Inflammatory processes occurring in the central nervous system (CNS) are closely related to neuronal cell death in many types of neurodegenerative diseases including Alzheimer's disease, Parkinson's disease, and multiple sclerosis.4,5 The inflammatory response in the brain is mediated by activated microglia, the resident macrophages of the nervous system, that normally respond to neuronal damage and phagocytose damaged cells.6 Although microglial activation is critical for host defense, chronic activation of microglia may cause neuronal damage through the release of potentially cytotoxic proinflammatory cytokines and reactive oxygen intermediates.7,8
Macrophages and microglia are the major producers of tumor necrosis factor-α (TNF-α), a proinflammatory cytokine, following exposure to infection and inflammation. Peripheral infection can also initiate the synthesis of TNF-α within the CNS.9,10 In fact, an acute peripheral insult can result in chronic activation of microglial cells. For example, after systemic administration of lipopolysaccharide (LPS) in mice, the peripheral macrophages rapidly produce large amounts of TNF-α, which in turn, activates the microglial cells in the brain to produce TNF-α over long periods of time.6 Chronic microglial activation is seen in several CNS disorders11,12 and the activated “primed” microglial cells respond with exaggerated cytokine secretion upon further peripheral challenge.4 Thus, nonspecific systemic infection or inflammation in people with existing inflammation in the brain may contribute to disease progression through further activation of the already primed microglia in the brain.4 Therefore, suppressing TNF simultaneously in both macrophages and microglial cells should be particularly beneficial in reducing inflammation within the CNS.
There has been a tremendous interest in neutralizing TNF for therapeutic application in a variety of autoimmune diseases including rheumatoid arthritis, inflammatory bowel disease, psoriatic arthritis, and ankylosing spondylitis.13,14,15 Currently, there are three TNF-α antagonists licensed for clinical use in the United States including two monoclonal antibodies (infliximab and adalimumab) and a recombinant soluble TNF-α receptor (etanercept) and treatment with these agents have provided significant benefits for a variety of inflammatory disorders in humans.16,17 However, since the antibodies do not readily cross the blood–brain barrier, they are unlikely to be useful to suppress TNF in microglial cells. Moreover, the possible toxic side effects associated with chronic antibody treatment also necessitate development of newer methods to suppress TNF within the CNS.
Small interfering RNA (siRNA)-mediated gene silencing offers an alternative therapeutic strategy to overcome inflammatory conditions.18,19,20,21 Several proof of principal studies have demonstrated the potential of RNA interference to suppress proinflammatory cytokines.18,19,20,21 However, the major impediment for therapeutic use of siRNAs is the lack of methods to deliver siRNA to desired cell types in vivo.22 Delivery to microglial cells poses a special challenge because of the presence of the blood–brain barrier.23,24 We have earlier reported that rabies virus glycoprotein (RVG)-9dR peptide may allow siRNA delivery across the blood–brain barrier.22 In this study, we find that macrophages and microglial cells also express α7 subunit of the acetylcholine receptor (AchR) to which RVG peptide binds and that the chimeric RVG-9dR peptide allows siRNA delivery to these cell types in vitro as well as in vivo to suppress neuroinflammation and neuronal apoptosis mediated by TNF-α.
Results
RVG-9dR peptide allows delivery of siRNA to macrophages and microglia cells in vitro
We previously showed that RVG peptide specifically binds AchR α7 subunit-expressing neuronal cells and addition of 9dR residues to its carboxyl-terminus allows siRNA binding so that the chimeric RVG-9dR peptide can transduce siRNA to neuronal cells.22 Because neuronal targeting in this system is dependent on AchR expression, we considered the possibility that RVG-9dR peptide might also target non-neuronal cells if they expressed α7 subunit of AchR. It has recently been reported that AchRs are also expressed on blood-borne macrophages and brain microglia cells.25 We therefore first tested whether cell lines of macrophage (Raw 264.7) and glial (N9) origin express the AchR α7 subunit using specific antibody staining. Both Raw 264.7 and N9 cells were found to express high levels of AchR (Supplementary Figure S1a, upper panel). The receptor was constitutively expressed and the level of expression did not change after cellular activation by LPS treatment. We next tested whether RVG-9dR could transduce fluorescently labeled siRNA into these cells. Fluorescein isothiocyanate (FITC)-conjugated siRNA (siFITC) was mixed with RVG-9dR at a 1:10 molar ratio and the cells were incubated with the complex for 4 hours, washed and cultured overnight in fresh media before flow cytometric analysis. RVG-9dR peptide was able to transduce siFITC into N9 (about 90%) and Raw 264.7 cells (about 65%) (Supplementary Figure S1a, lower panel). To confirm that the transduced siRNA is delivered into the cell cytoplasm, we also tested gene silencing. Green fluorescent protein (GFP)-expressing Raw 264.7 cells were transduced with RVG-9dR-complexed GFP siRNA and gene silencing tested 48 hours later. GFP was silenced with efficiency of nearly 70%, as assessed by the reduction in mean fluorescent intensity of GFP expression (Supplementary Figure S1b,c). However, treatment of cells with siRNA alone without a carrier or transfection with irrelevant anti-luciferase siRNA (siLuc) complexed to RVG-9dR showed negligible silencing effects. RVG-9dR was also superior compared with the conventional transfection reagent Lipofectamine 2000 in inducing gene silencing.
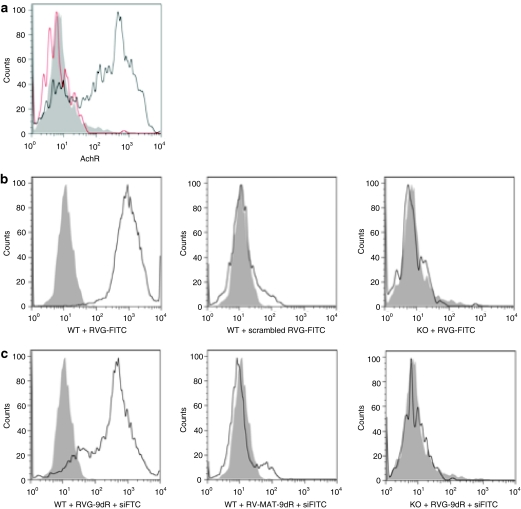
We next tested whether the α7 subunit of AchR is expressed in primary macrophages. Spleen cells from wild type and AchR α7-deficient mice were analyzed by flow cytometry after staining with AchR α7 subunit-specific antibody. The CD11b gated splenic cells from wild type but not knockout mice expressed the AchR α7 subunit (Figure 1a). As expected, CD11b+ microglia cells freshly isolated from the mouse brain also expressed the receptor (data not shown). To confirm that RVG peptide can bind specifically to AchR on primary macrophages, we initially used a FITC-labeled RVG peptide (RVG-FITC) or a FITC-labeled control peptide (scrambled RVG-FITC) to stain the cells. In concordance with receptor expression, RVG-FITC bound macrophages from wild type but not AchR knockout mice (Figure 1b), confirming the specificity of interaction. As expected, scrambled RVG-FITC peptide did not even bind wild-type macrophages. Next, we tested RVG-9dR for siRNA delivery. To test siRNA transduction, we treated spleen cells with siFITC complexed with RVG-9dR or a control (rabies virus matrix (RV-MAT-9dR) peptide. Again, RVG-9dR was able to transduce siFITC into macrophages from wild type, but not AchR knockout mice and the control RV-MAT-9dR peptide was not able to transduce siFITC into wild-type macrophages (Figure 1c). Taken together, these results suggest that RVG-9dR can be used to deliver siRNA to primary macrophages in vitro.
Figure 1.
RVG-9dR peptide allows delivery of siRNA to primary splenic macrophages in vitro. (a) AchR expression in CD11b gated primary macrophages from the spleens of wild type (WT) and AchR knockout (KO) mice was tested using flow cytometry. Filled histogram, isotype control; thick open histogram, wild-type mice; thin open histogram, AchR knockout mice. (b) Spleen cells from wild type and AchR knockout mice were incubated with a FITC-labeled RVG peptide (RVG-FITC) or the control scrambled RVG-FITC peptide along with CD11b antibody for 1 hour, then analyzed by flow cytometry. FITC positivity on CD11b gated cells is shown. Filled histograms represent cells without peptide treatment. (c) Spleen from wild type and AchR knockout mice were treated with FITC-labeled siRNA complexed with RVG-9dR or control RV-MAT-9dR peptides. Twenty-four hours later, cells were stained with CD11b antibody and analyzed by flow cytometry. FITC positivity on CD11b gated cells is shown. Gray histograms represent cells without siRNA treatment. Each figure is representative of at least three experiments with similar results. AchR, acetylcholine receptor; FITC, fluorescein isothiocyanate; RVG, rabies virus glycoprotein; siRNA, small interfering RNA.
RVG-9dR peptide allows delivery of siRNA to macrophages and microglia cells in vivo
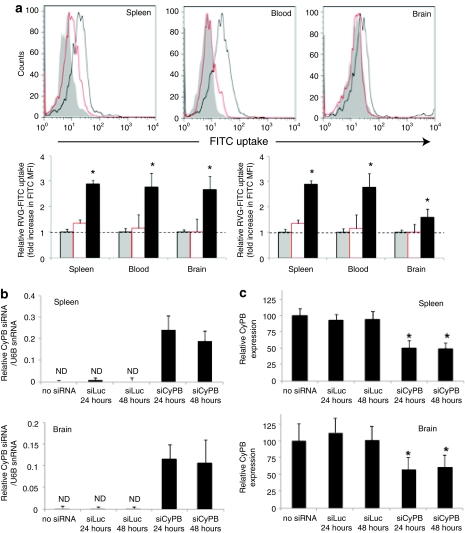
To test in vivo delivery, first we determined whether RVG peptide binds to macrophages and microglia cells in vivo. We injected RVG-FITC peptide intravenously (i.v.) into wild type and AchR knockout mice and after 1 hour, tested for FITC uptake on CD11b+ cells in the blood, spleen, and brain cells. As shown in the overlay histogram (upper panel) and the mean fluorescent intensity shift (lower panel) in Figure 2a, CD11b gated cells from wild type but not knockout mice were FITC positive, suggesting that the i.v. injected peptide is able to bind AchR-bearing cells in vivo. The binding was not as high as seen in vitro studies in Figure 1 probably because of the dilution of the peptide in circulation (only 50 µg of peptide was injected once) as well as the tendency for short peptides to be rapidly cleared by glomerular filtration. Next, we tested for siRNA delivery and gene silencing in CD11b+ cells in vivo using RVG-9dR peptide. For this, mice were injected with cyclophilin B (CyPB) siRNA (siCyPB) complexed with RVG-9dR i.v. After 24 and 48 hours of siRNA treatment, CD11b+ macrophages and microglia cells were immunomagnetically isolated from the spleen and brain, respectively. To confirm delivery, small RNA fraction isolated from CD11b selected cells from the spleen and brain were tested for the presence of CyPB siRNA by quantitative reverse transcription (RT)-PCR using CyPB siRNA-specific primers. As shown in Figure 2b, we could detect the presence of siRNA only in siCyPB/RVG-9dR injected mice but not in uninjected and siLuc/RVG-9dR injected mice. Consistent with this, CyPB gene was also effectively silenced after RVG-9dR-mediated siRNA delivery, as measured by quantitative RT-PCR from total RNA extracted from CD11b+ cells from spleen and brain (Figure 2c). In a similar experiment, i.v. injection of naked CyPB siRNA did not result in gene silencing confirming that macrophages do not nonspecifically take up siRNA (Supplementary Figure S2).
Figure 2.
RVG-9dR-mediated in vivo siRNA delivery in macrophages and microglial cells. (a) Mice were i.v. injected with RVG-FITC and FITC uptake by CD11b+ cells in the spleen, peripheral blood, and brain determined by flow cytometry 1 hour after injection. Representative histograms (upper panel) and cumulative data (lower panel) from two independent experiments with three mice each are shown. Mean values were normalized to control. Gray, wild-type mice without RVG-FITC injection; black, wild-type mice injected with RVG-FITC; red, AchR knockout mice injected with RVG-FITC. Error bars indicate SD; *P < 0.05. (b,c) Mice were i.v. injected with cyclophilin B siRNA (siCyPB) complexed with RVG-9dR and CD11b+ macrophages and microglia cells were immunomagnetically isolated from the spleen and brain 24 and 48 hours after siRNA treatment and tested for the presence of (b) specific siRNA and (c) cyclophilin B gene silencing by qRT-PCR. Mean values were normalized to U6B snRNA in b and to β-actin and expressed as percentage of no siRNA control in c. ND, not detected. Error bars indicate SD; *P < 0.05. AchR, acetylcholine receptor; FITC, fluorescein isothiocyanate; qRT-PCR, quantitative reverse transcription-PCR; RVG, rabies virus glycoprotein; siRNA, small interfering RNA.
TNF-α is essential for LPS-induced neuronal cell death in the mouse brain
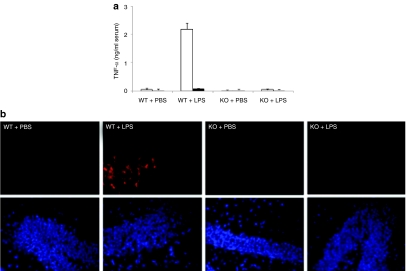
Although it is generally believed that excessive TNF-α might damage neurons in the brain, whether TNF-α is necessary and sufficient to induce neuronal apoptosis is not clear. To address this, we compared wild type and TNF-α-deficient mice for LPS response. LPS injection into wild-type mice induced TNF-α production rapidly and its serum level peaked 1 hour after injection and declined to basal level by 24 hours as assessed by enzyme-linked immunosorbent assay (ELISA). In contrast and as expected, no TNF-α could be detected in TNF-α-deficient mice (Figure 3a). Correspondingly, we found significant brain cell death as assessed by terminal dUTP nick-end labeling staining, in the brains of LPS-injected wild-type mice 24 hours after injection (Figure 3b). However, there were no significant terminal dUTP nick-end labeling positive cells in the brain of LPS-injected TNF-α knockout mice and phosphate-buffered saline (PBS)-injected wild-type mice. Thus, TNF-α appears to be critical to cause neuronal death following LPS injection.
Figure 3.
LPS-induced neuronal cell death in mice brain is TNF-α dependent. (a) LPS-induced production of TNF-α was measured by ELISA in the serum of TNF-α knockout (KO) and wild-type (WT) mice 1 hour and 24 hours after administration of LPS (5 mg/kg, i.p.). Open bars, 1 hour; solid bars, 24 hours. Error bars indicate SD. N = 3. (b) Representative images of TUNEL staining of brain tissue sections from TNF-α knockout and wild-type mice 24 hours after LPS injection. PBS was injected as a control. DAPI (blue), TUNEL (red). n = 3. DAPI, 4′,6-diamidino-2-phenylindole; ELISA, enzyme-linked immunosorbent assay; i.p., intraperitoneal; LPS, lipopolysaccharide; PBS, phosphate-buffered saline; TNF-α, tumor necrosis factor-α TUNEL, terminal dUTP nick-end labeling.
RVG-9dR mediates siRNA delivery to microglia and macrophages and silences TNF-α production in vitro and in vivo
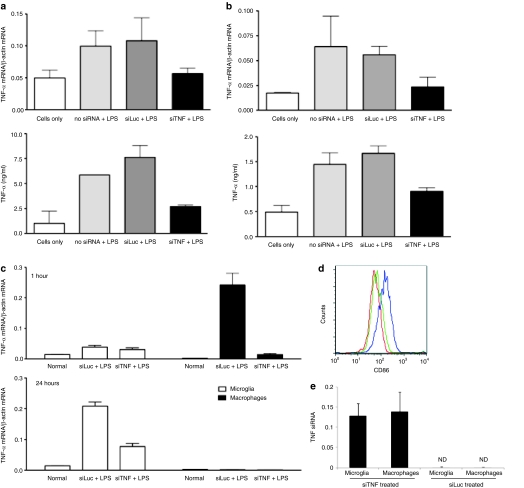
Since TNF-α plays an important role in mediating neuronal apoptosis, we next tested whether we could block TNF-α secretion using RVG-9dR peptide to deliver anti-TNF-α siRNA (siTNF) to macrophages and microglial cells. Initially, we tested for siRNA effects on the LPS response in cell lines. Raw 264.7 and N9 cells were treated with RVG-9dR/siTNF for 4 hours, cultured overnight and then stimulated with LPS. Ten hours later, TNF-α mRNA levels in the cell pellet were measured by RT-PCR and secreted TNF-α levels in the culture supernatants were quantitated by ELISA. Both TNF-α mRNA and secreted TNF-α were significantly reduced in Raw 264.7 and N9 cells when treated with RVG-9dR/siTNF, but not with an irrelevant siLuc bound to RVG-9dR (Figure 4a,b).
Figure 4.
In vitro and in vivo TNF-α silencing using RVG-9dR/siTNF. (a) N9 and (b) Raw 264.7 cells were transduced with siTNF or siLuc complexed with RVG-9dR peptide followed by LPS treatment (5 µg/ml) 24 hours after transduction. TNF-α mRNA levels in cells (upper panels) and secreted TNF-α protein in culture medium (lower panels) were monitored 10 hours after LPS treatment. Error bars indicate SD. N = 3. (c) Mice were i.v. injected with siTNF or siLuc complexed to RVG-9dR peptide followed by LPS (5 mg/kg, i.p.) 24 hours later. TNF-α mRNA levels in microglia and macrophages isolated 1 hour (upper panel) and 24 hours (lower panel) after LPS injection were tested by qRT-PCR. Error bars indicate SD. N = 3. (d) CD86 expression in microglia in the siRNA treated mice 24 hours after LPS injection. Far left histogram: isotype control, far right histogram: siLuc-treated, Middle histogram: siTNF-treated (e) Splenic macrophages and microglia cells isolated from siTNF/RVG-9dR treated mice were tested for the physical presence of siRNA by qRT-PCR. i.p., intraperitoneal; i.v., intravenous; LPS, lipopolysaccharide; ND, not detected; qRT-PCR, quantitative reverse transcription-PCR; RVG, rabies virus glycoprotein; TNF-α, tumor necrosis factor-α.
Next, we tested whether siTNF delivered by RVG-9dR peptide was able to silence TNF-α secretion by microglia and macrophages in vivo. Mice were i.v. injected with siTNF or control siLuc complexed with RVG-9dR and 24 hours later, treated with LPS. TNF-α mRNA levels in the immunomagnetically isolated CD11b+ splenic macrophages and brain microglial cells were determined 1 and 24 hours after LPS injection. At 1 hour after LPS injection, TNF-α mRNA level in the macrophages obtained from LPS injected and an irrelevant siRNA (siLuc)-treated mice increased 24-fold compared to that of macrophages from mice not treated LPS (Figure 4c, upper panel). Strikingly, TNF-α mRNA levels in macrophages from siTNF-treated mice was reduced by nearly 90% compared to that of siLuc-treated mice macrophages. TNF-α mRNA levels of macrophages decreased to basal level in all animals 24 hours after LPS injection. In contrast to the splenic macrophages, TNF-α mRNA levels in the brain microglia cells did not increase significantly in any group of animals at 1 hour after LPS injection. However, 24 hours after LPS injection TNF-α mRNA levels in siLuc-treated mice microglia increased 15-fold compared to that in normal mice microglia without LPS treatment (Figure 4c, lower panel). TNF-α mRNA expression in microglia from siTNF-treated mice was reduced ~65% compared to that of microglia from siLuc-treated mice. Correspondingly, microglial activation as revealed by CD86 expression was also significantly lower in siTNF-treated mice compared to siLuc-treated mice (Figure 4d). Although peripheral macrophage-released TNF-α is also known to stimulate microglial cells to secrete more TNF-α,6 this is unlikely to be the sole reason for TNF-α silencing in microglia cells because our results shown in Figure 2 clearly demonstrated siRNA delivery and gene silencing in microglia cells in vivo. However, to confirm siRNA delivery to microglia cells in this series of experiments, we also tested for the physical presence of TNF siRNA in isolated microglia cells by quantitative RT-PCR. As shown in Figure 4e, TNF siRNA could be detected in microglia cells from siTNF-, but not in the control siLuc-treated mice. Collectively these results show that TNF-α can be suppressed by RVG-9dR-peptide mediated siRNA delivery in macrophages and microglial cells in vitro as well as in vivo.
RVG-9dR mediated siTNF delivery protects from LPS-induced neuronal cell death in mice brains
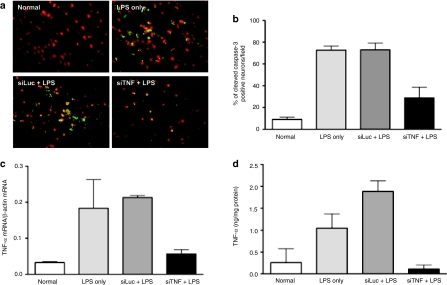
To discern the effects of TNF-α silencing on LPS-induced neuronal death, we measured the number of neurons undergoing apoptosis. Mice were treated with siTNF or the control siLuc siRNA complexed with RVG-9dR and 24 hours later, LPS was administered as described earlier. Brain sections, obtained 24 hours after LPS treatment were stained with the neuronal marker Nissl and the cell death marker, cleaved caspase-3. Few cleaved caspase-3 positive cells were found in normal mouse brain without LPS treatment (Figure 5a,b). In the LPS-injected group without siRNA treatment, the number of cleaved caspase-3 positive cells was significantly increased by eightfold as compared with that in normal mouse brain. The number of apoptotic cells was significantly reduced by 62% in siTNF-treated group, when compared with that in the non-siRNA-treated group (Figure 5a,b). However, this protective effect was not observed in siLuc-treated group and the number of apoptotic cells was similar to that of non-siRNA-treated group. From the same animals, we could also observe reduced TNF-α production only in siTNF-treated group both at the mRNA and protein levels (Figure 5c,d). Thus, RVG-9dR mediated siTNF delivery suppresses LPS-induced neuronal apoptosis.
Figure 5.
RVG-9dR-mediated delivery of siTNF protects mice from LPS-induced neuronal apoptosis. Mice were injected i.v. with siLuc or siTNF complexed to RVG-9dR and 24 hours later administered LPS. Twenty-four hours after LPS treatment, neuronal apoptosis was monitored by staining of brain sections with Nissl1 and cleaved caspase-3 antibody. Representative staining in untreated and siRNA treated mice is shown in a and cumulative data from six mice in two independent experiments is shown in b. In (a), red staining indicates Nissl1 and green staining indicates cleaved caspase B staining. In (b), cleaved caspase-3 positive apoptotic neurons were counted from fluorescence microscope images. (c,d) Twenty-four hours after LPS injection, TNF-α mRNA levels were measured from isolated brain cells by (c) qRT-PCR and TNF-α protein levels in brain homogenates tested by ELISA. (d) Error bars indicate SD. ELISA, enzyme-linked immunosorbent assay; LPS, lipopolysaccharide; qRT-PCR, quantitative reverse transcription-PCR; RVG, rabies virus glycoprotein; TNF-α, tumor necrosis factor-α.
Discussion
Due to the relatively large size (compared to small molecule drugs) and anionic charge of siRNAs, delivery to the target cells in vivo remains the major constraint for its therapeutic use. In the present study, we have developed a new nonviral method for systemic delivery of siRNA to microglia and macrophages and shown the practical utility of this approach by preventing LPS-induced neuronal apoptosis in the mouse brain.
Macrophages and microglia play a critical role in the initiation and maintenance of neuroinflammation by releasing proinflammatory cytokines that can lead to tissue destruction. In fact, elevated levels of TNF are seen in a large number of neurological disorders including multiple sclerosis, Alzheimer's disease, and Parkinson's disease. Manipulation of TNF and TNF receptor pathway in several animal models of disease also suggest an important role for TNF in neurological diseases (reviewed in ref. 14). Thus, targeting TNF action in the brain may be an attractive therapeutic strategy that might slow the progression or attenuate the severity of the disease. RNA interference provides a novel method to silence-specific gene expression and is under clinical trial for several diseases.26 siRNA has also been used to suppress TNF and in fact, siRNA complexed with the cationic lipid TransIT TKO transfection reagent effectively silenced TNF in vivo and rescued mice from the lethal effects of LPS.27 Similarly, intraperitoneal administration of TNF siRNA complexed to TransIT TKO also silenced TNF in macrophages and enhanced herpes simplex virus encephalitis in mice.28 However, the lipid-based TransIT transfection reagent that was used in those studies to deliver siRNA is not ideal for human therapy and moreover, will not be useful for siRNA delivery to microglial cells. We have previously shown that RVG-9dR peptide can transport siRNA into the CNS and is therefore suited for silencing genes in the brain cells. Because as shown here, AchR is also expressed by macrophages and microglial cells, this system provides a powerful way for dual targeting of macrophages and microglia cells. Such dual targeting appears to be important for TNF-related CNS diseases because of the crosstalk between peripheral macrophages and CNS microglia cells. As mentioned earlier, a short outburst of TNF from peripheral macrophages can lead to chronic activation of microglia and once primed, microglia cells respond more vigorously to the peripheral macrophage-released TNF-α and therefore perpetuate neuroinflammation.6 Thus, by preventing both this initial damage by peripheral macrophages and the reactive microgliosis at the same time, RVG-9dR-mediated siRNA delivery is likely to prevent the self-propelling and vicious cycle of neuronal damage. Moreover, peripheral macrophages can also cross the blood–brain barrier to enter the brain in certain disease situations and suppressing TNF-α in peripheral macrophages would also prevent CNS inflammation induced by this. It is theoretically possible that uptake of siRNA by other AchR-expressing cells (neuronal cells and neuromuscular junction) might limit delivery to macrophages and conversely uptake by macrophages might limit delivery to neuronal cells. However, our experiments suggest that at a clinically acceptable dose of 2.5 mg/kg, effective siRNA delivery and gene silencing occurs in macrophages as well as microglia cells.
Although naked siRNA is rapidly destroyed by serum nucleases, by using the appropriate delivery system, it is possible to improve the biological stability, targeted cell uptake, and the pharmacokinetics of siRNA.29 We have previously shown that siRNA binding to RVG-9dR peptide confers resistance to nuclease degradation and thus improves the biological stability of siRNA.22 In other studies, we have found that substituting chemically stabilized siRNA to naked siRNA for RVG-9dR binding did not enhance gene silencing activity in vivo, suggesting that RVG-9dR binding is enough for stability. Thus our delivery method obviates the need for introduction of expensive chemical modifications to the siRNA for in vivo use. In addition, since it allows targeted delivery to desired cell types, our method also enhances the bioavailability of siRNA. We have also previously shown that injection of RVG-9dR/siRNA complex in mice does not induce toxicities due to activation of interferon or innate immunity. Similarly the peptide also did not induce peptide-antibody responses and thus this system could be used for repeated siRNA administration.
Although silencing TNF-α provided substantial protection against LPS-induced apoptosis, the protection was not complete. This could be because TNF-α may not be the sole contributor to neurotoxicity. Because factors other than TNF-α are also involved in inflammatory reactions, silencing these pathways might be important. For example, in multiple sclerosis, activated macrophages secrete an increased amount of nitric oxide, which has neurotoxic effects.30 In addition, excessive oxidative stress is also thought to play a critical role during the pathogenesis of Parkinson's disease and much attention has been placed on nitric oxide as a key factor.31 Thus, a combination of siRNAs to silence several inflammatory mediators may be necessary to enhance neuronal protection. Because RVG-9dR provides a generic platform for siRNA delivery, multiple gene silencing in targeted cells could be achieved simply by mixing the different siRNAs with the peptide. Moreover, AchR α7 subunit is also expressed by human macrophages and our unpublished data suggests that RVG peptide also binds to the receptor human macrophages, making clinical translation relatively easy.
In summary, we have shown that RVG-9dR provides a tool for targeted siRNA delivery to both microglia and macrophages in vivo. These findings overcome a critical barrier of in vivo delivery, significantly enhancing the prospect of siRNA-based therapeutics for autoimmunity and neuroinflammation.
Materials and Methods
Peptides and siRNAs. RVG (YTIWMPENPRPGTPCDIFTNSRGKRASNG), scrambled RVG (WESYRTRAIPKCSPGTDPMINPFTRGNGN), RVG-9dR (YTIWMPENPRPGTPCDIFTNSRGKRASNGGGGRRRRRRRRR), and RV-MAT-9dR (MNLLRKIVKNRRDEDTQKSSPASAPLDGGGGRR RRRRRRR) peptides were synthesized and purified by high-performance liquid chromatography at the Tufts University Core Facility (Boston, MA). In RVG-9dR and RV-MAT-9dR peptides, the carboxy-terminal nine arginine residues were D-arginine. For some experiments, RVG and scrambled RVG peptides were conjugated with FITC at the amino terminus. siRNAs used in the studies included those targeting GFP (siGFP; 5′-GGC UACGUCCAGGAGCGCAdTdT-3′), firefly luciferase (siLuc; 5′-CUUACG CUGAGUACUUCGAdTdT-3′),22 mouse TNF-α (siTNF; 5′-GACAACCAACUAGUGGUGCdTdT-3′), and CyPB (siCyPB, 5′-UGUCUUGGUGCUCUCCACCdTdT-3′), were synthesized at Dharmacon (Lafayette, CO). For some experiments, siRNA with FITC label at the 3′ end of the sense strand was used.
siRNA transduction and gene silencing in vitro. Uptake of FITC-labeled siRNA and silencing of target gene expression were monitored in vitro using N9, Raw 264.7, GFP-expressing Raw 264.7 cells (transduced with the pLL3.7 lentiviral vector). Cells were plated in 12-well plates at 2.5 × 105 cells per well for 12–16 hours before transduction. RVG-9dR/siRNA complexes, prepared by incubating 100 pmol of indicated siRNA (100 µmol/l concentration for cell culture) with RVG-9dR peptide at a 10:1 peptide/siRNA ratio in serum-free medium for 15 minutes, were added to the wells in a final volume of 0.5 ml. After incubation for 4 hours at 37 °C the medium was replaced with 0.5 ml of fresh medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and the cells were cultured for a further 24 or 48 hours before being examined by flow cytometry or RT-PCR. Transfection with Lipofectamine 2000 (Invitrogen) was performed in accordance with the manufacturer's instructions. To test TNF-α gene silencing, Raw 264.7 and N9 cells were transduced with 100 pmol of siTNF (100 µmol/l concentration for cell culture) complexed to RVG-9dR peptide and treated with 5 µg/ml of LPS (Sigma, St Louis, MO) 24 hours later. TNF-α expression was analyzed by RT-PCR and ELISA 10 hours after LPS treatment.
Animal experiments for testing siRNA delivery and gene silencing. BALB/cJ, C57BL/6J, B6.129S7-Chrna7tm1Bay/J (AchR knockout mice), and B6; 129S-Tnftm1Gkl/J (TNF knockout mice) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and used at 6–8 weeks of age. All animal experiments had been approved by the Immune Disease Institute institutional review board and performed in the animal facility at the Immune Disease Institute. To test peptide uptake in vivo, 50 µg of RVG-FITC or scrambled RVG-FITC peptides in 0.2 ml of PBS were injected into tail veins of AchR knockout mice and wild-type mice; 1 hour later, single-cell suspensions of spleens, brains, and peripheral blood were treated with phycoerythrin-labeled CD11b antibody and analyzed by flow cytometry. To test LPS-induced neuronal cell death in mice, 5 mg/kg LPS was injected intraperitonealy into TNF knockout and wild-type mice. TNF-α production was monitored by ELISA in the serum 1 hour and 24 hours after LPS injection. Terminal dUTP nick-end labeling staining of brain sections was performed to assess neuronal cell death 24 hours after LPS injection using in situ cell death detection kit (Roche, Indianapolis, IN). Brains were embedded in OCT compound (Sakura Finetek, Torrance, CA) and frozen sectioned on a sliding microtome (Leica Instruments GmbH, Nussloch, Germany) at a thickness of 40 µm. Brain sections were stained in accordance with the manufacturer's protocol and counter-stained with 0.5 µg/ml 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). For all siRNA delivery experiments, peptide/siRNA complexes (at a peptide to siRNA molar ratio of 10:1) were prepared in 100–200 µl of 5% glucose and injected i.v. into the tail vein at 50 µg of siRNA (2.5 mg/kg for in vivo injection) per mouse per injection. To test siCyPB delivery, C57BL/6J mice were injected with peptide/siRNA complexes twice, 6 hours apart, and spleen and brain were harvested after a further 18 hours to analyze siRNA uptake and gene silencing. To test in vivo TNF-α silencing, BALB/cJ mice were i.v. injected twice with peptide/siRNA complexes, 6 hours apart. Twenty-four hours later, LPS (5 mg/kg) was intraperitonealy injected into mice and organs were harvested after a further 1 hour and 24 hours. Microglia and macrophages were immunomagnetically isolated from harvested brain and spleen using CD11b+ magnetic beads (Militenyi Biotec, Auburn, CA) and analyzed for TNF-α mRNA expression by RT-PCR. To isolate microglia, total CNS leukocytes were directly isolated from mice as described.32 Mice were transcardially perfused with ice-cold PBS. Brains were dissected and mechanically dissociated in PBS. Single-cell suspensions prepared and centrifuged over a 37%/70% discontinuous Percoll gradient (GE Healthcare, Piscataway, NJ) and immune cells isolated from the interface. Microglia were selectively purified from the CNS by CD11b+ magnetic beads as described.33 For testing protection against neuronal apoptosis, harvested brains were frozen sectioned and incubated for 24 hours at 4 °C with fluorescein-conjugated anti-cleaved caspase-3 antibodies (1:100 dilution; Cell Signaling Technology, Danvers, MA). Next, the sections were counter-stained with the nuclear Nissl counterstain, Neuro Trace Red Fluorescent Nissl Stain (1:200 dilution; Molecular Probes, Leiden, the Netherlands).
Analysis of cellular siRNA levels. Mice were given two administrations, 6 hours apart, of RVG-9R/siRNA (50 µg siRNA/injection, intravenous). Twenty-four hours after administration, microglia and macrophages were isolated from brain and spleen with CD11b+ magnetic beads, and small RNAs were extracted with the miRNeasy mini kit (Qiagen, Valencia, CA). The small RNAs were poly(A) tailed by A-plus poly(A) polymerase tailing kit (Epicentre, Madison, WI) and subjected to 3′RACE RT-PCR-based real-time PCR with RT-oligo dT and siRNA specific or small noncoding RNA U6B-specific primers as described.34
Quantitative RT-PCR. Total RNA was isolated from the cells or brain tissue by using an RNeasy mini kit (Qiagen). The RNA was reverse transcribed with the SuperScript III First Strand synthesis kit and random hexamers (Invitrogen) in accordance with the manufacturer's protocol. RT-PCR was performed on 2 µl of complementary DNA with SYBR Premix Ex Taq Polymerase (Takara Bio, Otsu, Japan) and ABI prism 7000 according to the manufacturer's instructions. Amplification conditions were as follows: 40 cycles of denaturation at 95 °C for 30 seconds, annealing at 55 °C for 30 seconds, and extension at 72 °C for 30 seconds with a Bio-Rad iCycler (Bio-Rad, Philadelphia, PA). Primers used were: mouse TNF-α (forward, 5′-CTACTCCCAGGTTCTCTTCAA-3′ and reverse, 5′-GCAGAGAGGAGGTTGACTTTC-3′), mouse β-actin (forward, 5′-AGAGGGAAATCGTGCGTGAC-3′ and reverse, 5′-CAATAGTGATGACCTGGCCGT3′). Relative TNF-α and CyPB mRNA expression was normalized with β-actin mRNA and calculated using the ΔCt method.
Measurement of TNFα-production. Brains were removed after euthanization and homogenized in 500 µl of sterile PBS. Supernatants were collected by centrifugation at 12,000 r.p.m. for 5 minutes at 4 °C and used for analysis. Cell culture supernatants or serum samples were also collected by centrifugation at 12,000 r.p.m. for 5 minutes at 4 °C to remove any particulates. Each sample was assayed by TNF-α ELISA kit (R&D Systems, Minneapolis, MN) according to the manufacturer's procedures. Briefly, samples were added to a 96-well plate precoated with affinity-purified polyclonal antibodies specific for mouse TNF-α. An enzyme-linked polyclonal antibody specific for mouse TNF-α was added to the wells and left to react for 2 hours, followed by a final wash to remove any unbound antibody-enzyme reagent. The intensity of the color detected at 450 nm (correction wave length 570 nm) was measured after addition of a substrate solution.
Flow cytometry. Flow cytometry of cell surface antigens was performed as described.35 The following monoclonal antibodies were used: rabbit polyclonal anti-nicotinic AchR α7 (Abcam, Cambridge, MA), FITC-conjugated anti-rabbit IgG (Abcam), anti-CD86-Biotin (eBioscience, San Diego, CA), and Streptavidin-APC (BD Biosciences, Franklin Lakes, NJ). Data were acquired and analyzed on FACScan with CellQuest (Becton Dickinson, Franklin Lakes, NJ) or FlowJo (TreeStar, Ashland, OR) software.
Statistical analysis. Statistical analysis was performed by one-way analysis of variance and comparisons among groups were performed by independent sample _t_-test or Bonferroni's multiple-comparison _t_-test. P < 0.05 was considered significant.
SUPPLEMENTARY MATERIALFigure S1. RVG-9dR allows siRNA delivery to AchR-expressing macrophage and microglial cell lines in vitro. (a) Raw 264.7 and N9 cell lines were tested for AchR α7 subunit expression before and after activation with LPS by flow cytometry. Blue, no LPS treatment; Red, after LPS treatment (upper panel). The cells were tested for uptake of a FITC-labeled siRNA complexed with RVG-9dR peptide (lower panel). Black lines indicate untreated cells. (b,c) Raw 264.7 cells stably expressing GFP were transduced with 100 pmol of siGFP complexed with RVG-9dR peptide or lipofectamine 2000, and silencing of GFP tested 24 h later using flow cytometry. Raw 264.7 cells not expressing GFP, irrelevant siRNA (siLuc) complexed to RVG-9dR, and siGFP alone without RVG-9dR served as controls. Representative histograms (b) and cumulative data of MFI change (from three independent experiments) (c) are shown. MFI, mean fluorescence intensity. Error bars indicate SD.Figure S2. Naked siRNA is not taken up by macrophages in vivo. Mice were either mock injected or injected iv with naked CyPB siRNA and CyPB gene silencing tested in isolated CD11b+ macrophages by qRT-PCR 48h later.
Supplementary Material
Figure S1.
RVG-9dR allows siRNA delivery to AchR-expressing macrophage and microglial cell lines in vitro. (a) Raw 264.7 and N9 cell lines were tested for AchR α7 subunit expression before and after activation with LPS by flow cytometry. Blue, no LPS treatment; Red, after LPS treatment (upper panel). The cells were tested for uptake of a FITC-labeled siRNA complexed with RVG-9dR peptide (lower panel). Black lines indicate untreated cells. (b,c) Raw 264.7 cells stably expressing GFP were transduced with 100 pmol of siGFP complexed with RVG-9dR peptide or lipofectamine 2000, and silencing of GFP tested 24 h later using flow cytometry. Raw 264.7 cells not expressing GFP, irrelevant siRNA (siLuc) complexed to RVG-9dR, and siGFP alone without RVG-9dR served as controls. Representative histograms (b) and cumulative data of MFI change (from three independent experiments) (c) are shown. MFI, mean fluorescence intensity. Error bars indicate SD.
Figure S2.
Naked siRNA is not taken up by macrophages in vivo. Mice were either mock injected or injected iv with naked CyPB siRNA and CyPB gene silencing tested in isolated CD11b+ macrophages by qRT-PCR 48h later.
Acknowledgments
This work was supported by NIH grant AI075419 to N.M. S.-S.K. was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD) (KRF-2006-352-D00070). S.-S. Kim performed research, analyzed data, and wrote the paper. C.Y. performed research, P.K. performed research, I.C. performed research, S.S. performed research, H.W. performed research, P.S. designed research, and N.M. designed research and wrote the paper.
REFERENCES
- Jahoor A, Patel R, Bryan A, Do C, Krier J, Watters C, et al. Peroxisome proliferator-activated receptors mediate host cell proinflammatory responses to Pseudomonas aeruginosa autoinducer. J Bacteriol. 2008;190:4408–4415. doi: 10.1128/JB.01444-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu Y, Zhang H, Chen G, Wang K., and , Xiao X. KLF4 promotes the expression, translocation, and releas eof HMGB1 in RAW264.7 macrophages in response to LPS. Shock. 2008;30:260–266. doi: 10.1097/shk.0b013e318162bef7. [DOI] [PubMed] [Google Scholar]
- Czapski GA, Cakala M, Chalimoniuk M, Gajkowska B., and , Strosznajder JB. Role of nitric oxide in the brain during lipopolysaccharide-evoked systemic inflammation. J Neurosci Res. 2007;85:1694–1703. doi: 10.1002/jnr.21294. [DOI] [PubMed] [Google Scholar]
- Perry VH, Newman TA., and , Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Emerit J, Edeas M., and , Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer EG, Klegeris A., and , McGeer PL.2005Inflammation, the complement system and the diseases of aging Neurobiol Aging 26(suppl 1): 94–97. [DOI] [PubMed] [Google Scholar]
- Polazzi E., and , Contestabile A. Reciprocal interactions between microglia and neurons: from survival to neuropathology. Rev Neurosci. 2002;13:221–242. doi: 10.1515/revneuro.2002.13.3.221. [DOI] [PubMed] [Google Scholar]
- Layé S, Parnet P, Goujon E., and , Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Brain Res Mol Brain Res. 1994;27:157–162. doi: 10.1016/0169-328x(94)90197-x. [DOI] [PubMed] [Google Scholar]
- Pitossi F, del Rey A, Kabiersch A., and , Besedovsky H. Induction of cytokine transcripts in the central nervous system and pituitary following peripheral administration of endotoxin to mice. J Neurosci Res. 1997;48:287–298. doi: 10.1002/(sici)1097-4547(19970515)48:4<287::aid-jnr1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Sawada M, Kondo N, Suzumura A., and , Marunouchi T. Production of tumor necrosis factor-α by microglia and astrocytes in culture. Brain Res. 1989;491:394–397. doi: 10.1016/0006-8993(89)90078-4. [DOI] [PubMed] [Google Scholar]
- Rivest S, Lacroix S, Vallières L, Nadeau S, Zhang J., and , Laflamme N. How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med. 2000;223:22–38. doi: 10.1046/j.1525-1373.2000.22304.x. [DOI] [PubMed] [Google Scholar]
- Lorenz HM., and , Kalden JR.2002Perspectives for TNF-α-targeting therapies Arthritis Res 4(suppl 3): S17–S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK., and , Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Ziring D, Desai S, Kim S, Wong M, Korin Y, et al. TNFα blockade in human diseases: an overview of efficacy and safety. Clin Immunol. 2008;126:13–30. doi: 10.1016/j.clim.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirman I, Whelan RL., and , Nielsen OH. Infliximab: mechanism of action beyond TNF-α neutralization in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2004;16:639–641. doi: 10.1097/01.meg.0000108345.41221.c2. [DOI] [PubMed] [Google Scholar]
- Charles P, Elliott MJ, Davis D, Potter A, Kalden JR, Antoni C, et al. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-α therapy in rheumatoid arthritis. J Immunol. 1999;163:1521–1528. [PubMed] [Google Scholar]
- Howard KA, Paludan SR, Behlke MA, Besenbacher F, Deleuran B., and , Kjems J. Chitosan/siRNA nanoparticle-mediated TNF-α knockdown in peritoneal macrophages for anti-inflammatory treatment in a murine arthritis model. Mol Ther. 2009;17:162–168. doi: 10.1038/mt.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury M, Louis-Plence P, Escriou V, Noel D, Largeau C, Cantos C, et al. Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor α in experimental arthritis. Arthritis Rheum. 2006;54:1867–1877. doi: 10.1002/art.21876. [DOI] [PubMed] [Google Scholar]
- Peng X, Tao K, Cheng T, Zhu J., and , Zhang X. Efficient Inhibition of wear debris-induced inflammation by locally delivered siRNA. Biochem Biophys Res Commun. 2008;377:532–537. doi: 10.1016/j.bbrc.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Inoue A, Takahashi KA, Mazda O, Arai Y, Saito M, Kishida T, et al. Comparison of anti-rheumatic effects of local RNAi-based therapy in collagen induced arthritis rats using various cytokine genes as molecular targets. Mod Rheumatol. 2009;19:125–133. doi: 10.1007/s10165-008-0131-3. [DOI] [PubMed] [Google Scholar]
- Kumar P, Wu H, McBride JL, Jung KE, Kim MH, Davidson BL, et al. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. shRNA and siRNA delivery to the brain. Adv Drug Deliv Rev. 2007;59:141–152. doi: 10.1016/j.addr.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ. Blood-brain barrier transport of non-viral gene and RNAi therapeutics. Pharm Res. 2007;24:1772–1787. doi: 10.1007/s11095-007-9321-5. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, et al. Cholinergic modulation of microglial activation by α7 nicotinic receptors. J Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- Castanotto D., and , Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457:426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen DR, Leirdal M., and , Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol. 2003;327:761–766. doi: 10.1016/s0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- Lundberg P, Welander PV, Edwards CK, 3rd, van Rooijen N., and , Cantin E. Tumor necrosis factor (TNF) protects resistant C57BL/6 mice against herpes simplex virus-induced encephalitis independently of signaling via TNF receptor 1 or 2. J Virol. 2007;81:1451–1460. doi: 10.1128/JVI.02243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar S., and , Benter IF. Nonviral delivery of synthetic siRNAs in vivo. J Clin Invest. 2007;117:3623–3632. doi: 10.1172/JCI33494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misko TP, Trotter JL., and , Cross AH. Mediation of inflammation by encephalitogenic cells: interferon gamma induction of nitric oxide synthase and cyclooxygenase 2. J Neuroimmunol. 1995;61:195–204. doi: 10.1016/0165-5728(95)00091-f. [DOI] [PubMed] [Google Scholar]
- Xing B, Xin T, Hunter RL., and , Bing G. Pioglitazone inhibition of lipopolysaccharide-induced nitric oxide synthase is associated with altered activity of p38 MAP kinase and PI3K/Akt. J Neuroinflammation. 2008;5:4. doi: 10.1186/1742-2094-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick JD, Schwender S, Imrich H, Dörries R, Butcher GW., and , ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc Natl Acad Sci USA. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Chen A, Zheng Y, Kosaras B, Tsiftsoglou SA, Vartanian TK, et al. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc Natl Acad Sci USA. 2008;105:17913–17918. doi: 10.1073/pnas.0804610105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Neilson JR, Kumar P, Manocha M, Shankar P, Sharp PA, et al. miRNA profiling of naïve, effector and memory CD8 T cells. PLoS ONE. 2007;2:e1020. doi: 10.1371/journal.pone.0001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Ban HS, Kim SS, Wu H, Pearson T, Greiner DL, et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized mice. Cell. 2008;134:577–586. doi: 10.1016/j.cell.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
RVG-9dR allows siRNA delivery to AchR-expressing macrophage and microglial cell lines in vitro. (a) Raw 264.7 and N9 cell lines were tested for AchR α7 subunit expression before and after activation with LPS by flow cytometry. Blue, no LPS treatment; Red, after LPS treatment (upper panel). The cells were tested for uptake of a FITC-labeled siRNA complexed with RVG-9dR peptide (lower panel). Black lines indicate untreated cells. (b,c) Raw 264.7 cells stably expressing GFP were transduced with 100 pmol of siGFP complexed with RVG-9dR peptide or lipofectamine 2000, and silencing of GFP tested 24 h later using flow cytometry. Raw 264.7 cells not expressing GFP, irrelevant siRNA (siLuc) complexed to RVG-9dR, and siGFP alone without RVG-9dR served as controls. Representative histograms (b) and cumulative data of MFI change (from three independent experiments) (c) are shown. MFI, mean fluorescence intensity. Error bars indicate SD.
Figure S2.
Naked siRNA is not taken up by macrophages in vivo. Mice were either mock injected or injected iv with naked CyPB siRNA and CyPB gene silencing tested in isolated CD11b+ macrophages by qRT-PCR 48h later.