Paraspeckles (original) (raw)
Abstract
Paraspeckles are a relatively new class of subnuclear bodies found in the interchromatin space of mammalian cells. They are RNA-protein structures formed by the interaction between a long nonprotein-coding RNA species, NEAT1/Men ε/β, and members of the DBHS (Drosophila Behavior Human Splicing) family of proteins: P54NRB/NONO, PSPC1, and PSF/SFPQ. Paraspeckles are critical to the control of gene expression through the nuclear retention of RNA containing double-stranded RNA regions that have been subject to adenosine-to-inosine editing. Through this mechanism paraspeckles and their components may ultimately have a role in controlling gene expression during many cellular processes including differentiation, viral infection, and stress responses.
Nuclear structures formed by a long noncoding RNA and DBHS proteins are thought to control gene expression by retaining mRNAs that have undergone adenosine-to-inosine editing in the nucleus.
DISCOVERY OF PARASPECKLES
The cell nucleus is a large and complex cellular organelle with an intricate internal organization that is still not fully characterized. One feature of nuclear organization is the presence of distinct subnuclear bodies, each of which contain specific nuclear proteins and nucleic acids (Platani and Lamond 2004). Most subnuclear bodies reside in the interchromatin space, including Cajal bodies, PML bodies, and nuclear speckles, enriched in splicing factors (Lamond and Spector 2003).
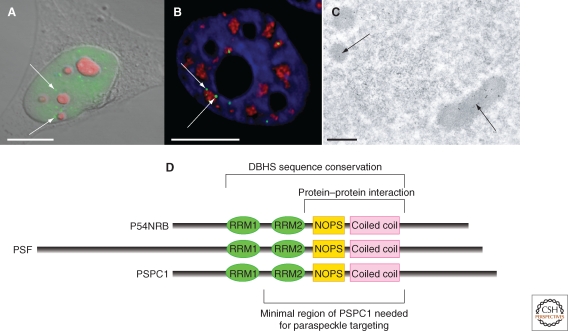
Paraspeckles are one of the most recent subnuclear bodies identified, discovered in 2002 as part of a study to better understand the full biological role of the nucleolus. In a mass spectrometry based proteomic analysis of purified human nucleoli, 271 proteins were identified, ∼30% of which were novel (Andersen et al. 2002). A follow up analysis on one of these newly identified novel proteins, showed that it was not enriched in nucleoli, but instead was found diffusely distributed within the nucleoplasm as well as concentrated in ∼5–20 subnuclear foci (Fox et al. 2002). Colocalization studies showed that these foci neither coincided, nor directly overlapped, with markers for any previously known subnuclear structure. The foci were thus named “paraspeckles” because they were observed in the interchromatin space near to, yet distinct from, nuclear speckles (Fig. 1). The novel protein that localized to these structures was subsequently named “Paraspeckle Protein 1” (PSPC1).
Figure 1.
Paraspeckles seen with fluorescent and electron microscopy. (A) Combined DIC and fluorescence micrograph of a HeLa cell stained with anti-PSPC1 to show paraspeckles (green) and B23 nucleolar marker (red). (B) Fluorescence micrograph of a section through a HeLa cell stained with anti-PSPC1 (green), anti-SC35 (red), and DAPI (blue) to show the relationship between paraspeckles and nuclear speckles. (C) Transmission electron micrograph of sections of HeLa cells immuno-gold labelled with anti-PSPC1: image kindly provided by Sylvie Souquere and Gerard Pierron (Villejuif, France). Scale bars in A–B, 10 µm, scale bar in C, 0.5 µm. (D) The DBHS protein family showing domain structure and indicating regions involved in paraspeckle biology.
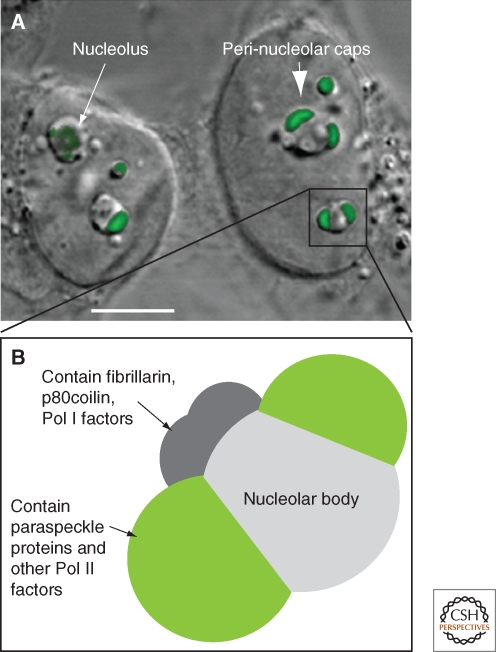
Considering that PSPC1 was first identified in a proteomic screen for nucleolar factors, it was initially surprising that localization studies did not detect it accumulated in nucleoli. However, exploring the dynamic nature of PSPC1 revealed its nucleolar relationship. When cells were treated with drugs that inhibit RNA Polymerase II (Pol II) transcription, PSPC1 relocalized to perinucleolar cap structures (Fox et al. 2002 and Fig. 2). Perinucleolar enrichment of PSPC1 was also observed in newly divided cells that had not recommenced transcription following cell division (Fox et al. 2005). Thus PSPC1 is found in paraspeckles in transcriptionally active cells, and perinucleolar caps in cells that are not actively transcribing Pol II genes. Further, photodynamic studies showed that under normal conditions PSPC1 molecules continually traffic through nucleoli, despite their steady-state enrichment within paraspeckles, explaining their presence in the nucleolar proteome (Fox et al. 2002).
Figure 2.
Perinucleolar caps observed with RNA Pol II transcription inhibition. (A) Combined DIC and fluorescence micrograph of HeLa cells following 4 h treatment with Actinomycin D to inhibit RNA Pol II transcription. Nucleolar morphology changes under these conditions, to create a nucleolar body and a number of perinucleolar caps (arrow). Cells were transfected with a plasmid expressing YFP-PSPC1 (green), that localizes to perinucleolar caps under these conditions (large arrow); scale bar, 5 µm. (B) Perinucleolar caps form upon inhibition of RNA Pol II transcription, see text and (Shav-tal et al. 2005).
PARASPECKLE CHARACTERIZATION
Paraspeckles are small, irregularly sized, and unevenly distributed subnuclear bodies. Depending on the cell type, paraspeckles number between 5 and 20 foci per nucleus (for example, HeLa contain 13–17 foci/nucleus and NIH3T3 5–10 foci/nucleus, Clemson et al. 2009). EM studies and fluorescent images show a paraspeckle size range of 0.5–1 µm in diameter, and they have an irregular, sausagelike shape (Cardinale et al. 2007 and Fig. 1C). Transmission EM of cells labeled with paraspeckle markers show labeling of distinct nuclear structures, rich in RNA (Prasanth et al. 2005; Cardinale et al. 2007, Fig. 1C). These EM structures labeled with paraspeckle markers correspond, at least partly, to the Interchromatin Granule Associated Zones (IGAZ – Visa et al. 1993). IGAZ are electron dense fibrillar regions closely aligned to interchromatin granules (nuclear speckles) with unknown function. IGAZ are reported to contain both U1 RNA and coilin, although there is no evidence of colocalization between paraspeckles and either of these molecules at the level of fluorescence (Fox et al. 2002).
Thus far, paraspeckles are only evident in mammalian nuclei. Within mammalian tissues and cells, paraspeckles are wide-spread: The majority of mouse and human cell lines and tissues examined contain paraspeckles, including transformed and primary cell lines, embryonic fibroblasts, and tumorigenic biopsies (Fox et al. 2002; Prasanth et al. 2005; Clemson et al. 2009; Sasaki et al. 2009; Sunwoo et al. 2009 and unpublished data). Interestingly, human embryonic stem cells (hESC) are so far the only mammalian cell type that are reported not to contain paraspeckles (Chen and Carmichael, 2009). Orthologs of the core paraspeckle protein components are found in other vertebrate and invertebrate species, however, a noncoding RNA (ncRNA) NEAT1, which is essential for paraspeckle formation (see below) is specific to mammals, likely explaining the restriction of paraspeckles to this class.
Paraspeckles are observed within the interchromatin space, sandwiched between larger nuclear speckles and chromatin. Current evidence suggests paraspeckles do not directly overlap with sites of active transcription, as they do not contain newly made pulse-labeled Br-UTP containing transcripts, however paraspeckles may still form in association with some active genes (see below) (Fox et al. 2002; Xie et al. 2006). Quantitation of staining on ultrathin sections of labeled cells shows that paraspeckles contain inactive RNA Polymerase II (Pol II), whereas the newly made RNA and active RNA Pol II reside on the edge of paraspeckles (Xie et al. 2006).
The functional relationship between paraspeckles and the nucleolus is yet to be fully elucidated, but the observed cycling of PSPC1 between paraspeckles and nucleoli, and the localization of paraspeckle proteins to perinucleolar caps when RNA Pol II transcription is inhibited, suggests that it may mediate some form of regulatory cross talk. Many other proteins involved in Pol II transcription are also observed within perinucleolar caps in cells where Pol II transcription is inactive (Shav-Tal et al. 2005). These proteins appear to segregate into distinct cap structures: large “dark” perinucleolar caps containing the paraspeckle proteins, as well as several other RNA-binding proteins; and a number of smaller “light” caps containing fibrillarin, coilin, and RNA Pol I components (Fig. 2) (Shav-Tal et al. 2005).
PARASPECKLE COMPONENTS
A small number of protein and RNA components are known to be enriched in paraspeckles (Table 1). Most of the paraspeckle proteins have roles in Pol II transcription and/or RNA processing; including the members of the DBHS protein family, several transcription factors, a cotranscriptional splicing factor and a 3′ RNA cleavage factor.
Table 1.
Paraspeckle components
| Name | Synonyms | Comments | Paraspeckle localization reference |
|---|---|---|---|
| Proteins | |||
| P54NRB | NONO, NMT55, NRB54 | DBHS; required for paraspeckle integrity in HeLa | Fox et al. 2002 |
| PSF | SFPQ | DBHS; required for paraspeckle integrity in HeLa | Prasanth et al. 2005 |
| PSPC1 | PSP1 | DBHS | Fox et al. 2002 |
| CoAAa | PSP2, RBM14, SIP, SYTIP1 | Transcriptional/splicing coregulator | Fox et al. 2002 |
| CFIM68 | CPSF6, HPBRII-4 | Cleavage factor. Also found in nuclear speckles | Dettwiler et al. 2004 |
| SOX9b | SRA1 | Developmental transcription factor | Hata et al. 2008 |
| WTXa | Wilms tumor, tumor suppressor | Rivera et al. 2009 | |
| WT1(+KTS)a | WAGR | Wilms tumor, partial colocalization with paraspeckles | Dutton et al. 2006 |
| BCL11Aa, b | CTIP1, ZNF856 | Zinc finger transcription factor | Liu et al. 2006 |
| RNA Pol II | Also found associated with chromatin and nuclear speckles | Xie et al. 2006 | |
| RNA | |||
| NEAT1 | Men ε/β_, VINC-1_ | Long noncoding RNA found in mammals; required for paraspeckle integrity in HeLa | Clemson et al. 2009; Sunwoo et al. 2009; Sasaki et al. 2009 |
| Ctn | Mouse-specific, alternative transcript produced from mCAT2 locus | Prasanth et al. 2005 |
There are two specific paraspeckle RNA components, each belong to different classes: Ctn is regulated within paraspeckles, and is implicated in the control of gene expression by RNA nuclear retention, whereas the abundant nuclear ncRNA, NEAT1, is an architectural paraspeckle component essential for their formation and maintenance.
DBHS Protein Family
The core protein components of paraspeckles are the three mammalian members of the DBHS protein family: PSF/SFPQ, NONO/P54NRB, and PSPC1. Both endogenous and tagged forms of these proteins are found localized within the nucleoplasm as well as paraspeckles in mammalian cells. Of the three DBHS proteins, PSPC1 has most often been used as the marker for paraspeckles, as it has a lower background nucleoplasmic signal in many cell types than P54NRB and PSF. The three proteins have 50% sequence identity within two amino-terminal RNA binding motifs and a carboxy-terminal coiled coil domain (Fig. 1D). The DBHS proteins appear to play a key role in the structural integrity of paraspeckles, because knockdown of the abundant proteins P54NRB or PSF in HeLa cells results in a loss of paraspeckles (Sasaki et al. 2009). Within HeLa cells, PSPC1 is much less abundant than PSF and P54NRB (Fox et al. 2005), and knockdown of PSPC1 does not affect paraspeckles in these cells (Sasaki et al. 2009). Reciprocal protein–protein interactions have been reported between members of this family, and it is likely in vivo they are found as either homo or heterodimers (Myojin et al. 2004; Fox et al. 2005). PSPC1 and P54NRB interact via their coiled-coil domains, and, given the sequence similarity, it is likely that PSF also interacts through this domain. Similarly, Hrp65, the Chironomus tentans DBHS homolog homodimerizes through its coiled-coil domain (Kiesler et al. 2003).
RNA-binding domains, as well as protein–protein interaction domains are required for members of the DBHS family to be targeted to paraspeckles. The minimal fragment of PSPC1 that is targeted to paraspeckles contains at least one RRM motif, in addition to its coiled-coil domain (Fox et al. 2005 and Fig. 1D). In contrast, neither RNA recognition motif (RRM) is required for PSPC1 to be targeted to perinucleolar caps on transcription inhibition. Within the DBHS family, the specificity of RRMs for paraspeckle targeting varies, as, unlike PSPC1, which can use either one of its two RRMs to be targeted to paraspeckles, only the second RRM of PSF is sufficient to target PSF to subnuclear foci (Dye and Patton 2001). These findings raise the possibility that subtle variations in affinities for different RNA targets may allow each DBHS protein to target different RNA species to paraspeckles.
DBHS Family Member Functions
Members of the DBHS family of proteins have been shown to bind both double- and single-stranded DNA and RNA and have been copurified with numerous different complexes, leading to the label of “multifunctional nuclear proteins” for this protein family (reviewed in Shav-Tal and Zipori 2002). The paraspeckle connection to the known DBHS functions has not been addressed in most cases.
The DBHS family proteins are involved in many aspects of RNA production and processing including transcription initiation, transcriptional termination, and splicing (Patton et al. 1993; Yang et al. 1993; Peng et al. 2002; Kameoka et al. 2004; Kaneko et al. 2007; Ito et al. 2008). Beyond RNA production, PSF and P54NRB also play a role in RNA surveillance: Binding and retaining hyper-adenosine-to-inosine (A-to-I) edited RNA within the nucleus (Zhang and Carmichael 2001). Nuclear A-to-I edited RNA is generated when double-stranded RNA-dependent adenosine deaminases target long (optimally 100bp) double-stranded RNA and randomly convert up to 50% of the adenosines to inosines. In what is thought to have originated as an antiviral mechanism, the resulting inosine-containing-RNA can be retained in the nucleus, rather than being exported to the cytoplasm (Zhang and Carmichael 2001).
DBHS proteins may also have roles in the cytoplasm. PSF and P54NRB were isolated as part of an RNA-transport granule in dendrites (Kanai et al. 2004) and both proteins have CRM1-independent nuclear export signals, in addition to nuclear retention motifs, however only P54NRB actively shuttles into the cytoplasm (Zolotukhin et al. 2003).
With roles in constitutive processes such as splicing and transcription, the functional implications for the DBHS proteins are wide-ranging. One interesting example implicates P54NRB in the control of mammalian circadian rhythms. P54NRB is required for circadian rhythm maintenance via association with the PERIOD-1 protein, an essential component of negative feedback in mammalian circadian rhythm (Brown et al. 2005). Similarly in Drosophila melanogaster, mutants of the DBHS protein, NonA, are nearly arrhythmic, indicating a conserved role in circadian rhythm regulation for this protein family (Brown et al. 2005).
Other Paraspeckle Proteins
The current definition of a paraspeckle protein is one that colocalizes in subnuclear foci with DBHS proteins, however, the localization pattern is not always identical. For example, the cleavage factor CFIm is observed in paraspeckles, but is in addition found within nuclear speckles (Dettwiler et al. 2004), and RNA Pol II is partially localized to paraspeckles and also to chromatin and nuclear speckles (Xie et al. 2006). It should be noted that several of the proteins reported to localize in paraspeckles have only been examined following overexpression, namely BCL11A, WTX, WT1(+KTS), and COAA (Fox et al. 2002; Dutton et al. 2006; Liu et al. 2006; Rivera et al. 2009), therefore these proteins cannot be considered bona fide paraspeckle components until the localization of the endogenous factors has been examined.
Additional paraspeckle proteins identified to date are either transcription factors or transcriptional coregulators. CoAA (PSP2/RBM14) is a transcriptional coactivator that regulates steroid receptor-mediated transcription and alternative RNA splicing (Iwasaki et al. 2001; Auboeuf et al. 2004). An alternatively spliced isoform of CoAA, termed CoAM, acts as a dominant negative antagonistic corepressor of CoAA. Interestingly, there is evidence that PSF and P54NRB act to influence which splice variant is generated from the CoAA gene (Yang et al. 2007). RBM4, the mammalian homolog of the Drosophila melanogaster Lark protein (an essential component of circadian rhythm regulation and early development in Drosophila), forms a fusion with CoAA, via putative trans-splicing events (Brooks et al. 2009). This finding suggests another possible link between paraspeckles and proteins involved in the control of circadian rhythms. Another transcriptional coregulator and tumor suppressor, WTX, colocalizes with P54NRB in paraspeckles and coactivates WT1 transcription (Rivera et al. 2009). Interestingly, an isoform of WT1 also colocalizes with some, but not all, paraspeckles (Dutton et al. 2006).
Sox9 is a transcription factor that plays an essential role in bone formation and interacts with P54NRB (Hata et al. 2008). Whilst SOX9 is reported to be a paraspeckle component, its overexpression results in altered subnuclear localization of P54NRB and PSPC1, suggesting that it may instead be re-targeting these proteins out of paraspeckles to different subnuclear sites (Hata et al. 2008). BCL11A, a transcription factor involved in B cell lymphoma/leukemia, colocalizes with PSPC1 and P54NRB in subnuclear foci (Liu et al. 2006). Again, these foci do not resemble paraspeckles—suggesting the proteins have been directed out of paraspeckles to new complexes. Supporting this argument, in contrast to paraspeckles observed with PSPC1 and P54NRB, RNAase treatment of BCL11A foci does not lead to their dissolution, instead, DNAase treatment results in a diffuse BCL11A signal (Liu et al. 2006).
Mammalian cleavage factor I (CF Im) is an essential factor that is required for the first step in pre-mRNA 3′ end processing, and localizes to both paraspeckles and nuclear speckles (Dettwiler et al. 2004; Cardinale et al. 2007). The largest subunit of the CF Im heterodimer contains an amino-terminal RRM and a carboxy-terminal charged arginine- and serine-rich “RS” domain, common to splicing factors. Interestingly, the RS domain is sufficient for nuclear speckle localization, whereas both the RS and RRM domains are required for paraspeckle targeting (Cardinale et al. 2007). Because the RRM domain of CF Im has been reported to mediate protein–protein interactions (Dettwiler et al. 2004), it is likely CF Im is targeted to these two compartments via interaction with different subsets of proteins.
PARASPECKLE RNAs
When we first identified paraspeckles, although only protein components were known, we proposed that their function would most likely be linked to some aspect of RNA, such as processing or transport, because several lines of evidence indicated that paraspeckles were RNA-protein bodies. First, paraspeckles were disrupted when cells were treated with RNAse (Fox et al. 2005; Prasanth et al. 2005); second, all of the major paraspeckle proteins contained RNA binding motifs and had previously described functions in RNA processing; thirdly, PSPC1 required its RNA binding domain for paraspeckle targeting (Fox et al. 2005). Finally, it was shown that paraspeckles disassemble without active RNA Pol II transcription and subsequently reassemble when transcription is restored.
Two paraspeckle-associated RNA species have since been identified, each providing clues to paraspeckle formation and function.
Ctn RNA
The first RNA found that specifically localized to paraspeckles was termed Ctn, described by Spector and colleagues (Prasanth et al. 2005). Ctn is a mouse-specific, nuclear-enriched, spliced poly(A+) transcript that is generated from the mCAT2 gene locus. Ctn contains all of the coding exons of the CAT2 (cationic amino acid transporter 2) protein. However, compared with the canonical mCAT2, it is generated from a different promoter, and has a distal poly(A+) site resulting in a much longer 3′ untranslated region (UTR). RNA-FISH with _Ctn_-specific probes showed that it was nucleoplasmic as well as localizing to paraspeckles in several cell types. Why then is Ctn not subject to the usual nuclear export fate of most mRNAs? The answer lies in the long 3′ UTR of Ctn, which contains A-to-I edited stretches of RNA. The editing takes place on inverted repeat elements that form long dsRNA regions. Given that P54NRB was known to preferentially bind inosine-RNA and retain it in the nucleus (Zhang and Carmichael 2001), the hypothesis was that DBHS proteins would mediate the paraspeckle- and nuclear retention of Ctn. Consistent with this, both PSPC1 and P54NRB were shown to associate with Ctn in vivo (Prasanth et al. 2005).
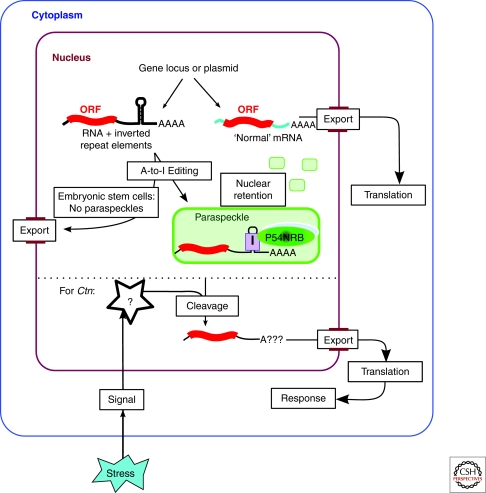
The nuclear retention of Ctn is linked to the control of gene expression of mCAT2: a protein in the nitric oxide response pathway, needed for wound healing and defense against infection. A variety of stress signals were shown to trigger the cleavage of the long 3′UTR from Ctn, resulting in lower nuclear levels of Ctn, with a concomitant rise in mCAT2 levels in the cytoplasm, and increased mCAT2 protein production. This gene expression control system results in a pulse of protein expression, and effectively provides the cell with a rapid increase in the production of protein upon the signal being received (Fig. 3).
Figure 3.
Gene expression by nuclear retention. RNA transcripts containing double-stranded RNA regions (formed by inverted repeat elements) are subject to A-to-I editing and retained in the nucleus and within paraspeckles. This mechanism has been shown for several endogenous genes, as well as exogenously expressed reporter genes (Prasanth et al. 2005; Chen et al. 2008; Chen and Carmichael, 2009). Nuclear retention of hyper-edited RNA is linked to the formation of paraspeckles, and does not occur efficiently in human embryonic stem cells that lack paraspeckles. In the case of Ctn, stress signals mediate cleavage of the 3′UTR and release of the RNA from the nucleus (Prasanth et al. 2005).
Although Ctn is mouse-specific, analysis of the human transcriptome has indicated that the control of gene expression by nuclear retention likely also occurs in humans: as many as 50% of all genes may produce transcripts with extended 3′ UTRs that use a distal poly(A+) site (Iseli et al. 2002). Moreover, the repeat elements that are A-to-I edited in Ctn are amongst the most abundant repetitive elements in mammalian genomes, and a large proportion of these elements are A-to-I edited in humans (Levanon et al. 2005; Chen and Carmichael 2008). A recent study found 333 genes in the human genome with inverted Alu repeat elements in their 3′ UTR (Alu being the most common family of human repeat elements) (Chen et al. 2008). Amplified inverted Alus from two of these genes were both capable of mediating RNA nuclear retention of a reporter gene (Chen et al. 2008). Moreover, as with Ctn, DBHS protein interaction plays a role in the retention of the reporter RNA, within subnuclear foci that partly overlap paraspeckles, as defined by exogenously expressed P54NRB (Chen et al. 2008). Additionally, because there is at least one other example of a nuclear-retained RNA that does not have inverted repeats (Kay et al. 2005), it is also likely that other RNA elements may mediate nuclear retention.
The exploration of the RNA nuclear retention mechanism for controlling gene expression has only just begun. One issue is the need for a large scale study of nuclear enriched coding and ncRNA transcripts to determine how prevalent nuclear retention is, and if all nuclear retained RNAs may be associated with paraspeckles or analogous structures. Another area that will be important to examine is the release of RNA from nuclear retention in paraspeckles. A recent bioinformatic analysis has begun to address this by determining that sequence databases contain hundreds of examples of mRNA transcripts in which the genome-encoded inverted repeats have been excised, an event presumably linked with release of these mRNAs from nuclear retention (Osenberg et al. 2009).
NEAT1/Men ε/β RNA
We have previously speculated that a paraspeckle-specific RNA species was required for paraspeckle formation, however Ctn could not fulfill this role: first, because it is mouse-specific and second, because knockdown of Ctn does not disrupt paraspeckles (Prasanth et al. 2005). In 2009, three different groups reported the discovery that NEAT1, a long nuclear ncRNA, is essential for paraspeckle structural integrity and formation (Clemson et al. 2009; Sasaki et al. 2009; Sunwoo et al. 2009).
It is now known, for mammals in particular, that the majority of the genome is transcribed, to generate both protein-coding and nonprotein coding RNA (Carninci et al. 2005). NcRNAs may arise from transcribed introns of protein-coding genes, or may be antisense to them, or may be transcribed from their own bona fide gene loci between protein-coding genes (Mercer et al. 2009). In recent years the explosion of information related to the identity and function of small ncRNAs has been evident, however, we are only beginning to understand the varying roles that long ncRNAs (>200nt) may be playing in the cell (Prasanth and Spector 2007).
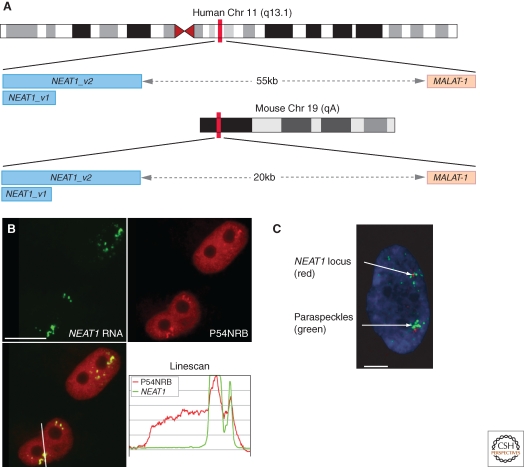
In 2007 a study aimed at identifying nuclear ncRNA species described two abundant, ubiquitously expressed Nuclear Enriched Autosomal noncoding Transcripts termed NEAT1 (also known as Menε/β or VINC-1*) and _NEAT2 (_also known as MALAT-1) (Hutchinson et al. 2007). RNA-FISH showed that MALAT-1 localized to nuclear speckles, whereas NEAT1 was observed within subnuclear foci found near nuclear speckles, shown to be paraspeckles. Both NEAT1 and MALAT-1 ncRNAs are produced by RNA Pol II, independently of protein-coding genes, and their genes are conserved syntenically a short distance apart in mammalian genomes (Fig. 4A). Two variant transcripts of NEAT1 are transcribed, NEAT1_ v1 and NEAT1_v2 (previously referred to as Menε and Menβ), these share approx 3–4 kb of sequence at the 5′ end that precisely delineates NEAT1_v1, with the longer isoform, NEAT1_v2, containing an additional approximately 20kb of RNA (Fig. 4A). Both NEAT1_v2 RNA, as well as MALAT-1 RNA, are cleaved very close to their 3′ ends to produce an unusual small tRNA-like molecule (Wilusz et al. 2008; Sunwoo et al. 2009). Interestingly, bioinformatic analysis, although not exhaustive, has not found any other ncRNAs with this unusual 3′ end, suggesting it could be specific to this duo of long nuclear ncRNAs (Wilusz et al. 2008).
Figure 4.
Paraspeckles contain NEAT1 ncRNA and form near to the NEAT1 gene. (A) NEAT1 and MALAT-1 gene loci on human chromosome 11 q13.1 and mouse chromosome 19qA. Two transcripts are produced from the NEAT1 gene, 3.7kb and 23kb in humans, and 3 kb and approximately 20 kb in mouse. (B) RNA-FISH with probes to NEAT1 ncRNA (green) and immunofluorescence against P54NRB (red) colocalizing in paraspeckles. The line scan is taken over the line indicated in the merged image (lower panels). (C) A HeLa cell in interphase with combined NEAT1 RNA-FISH to mark paraspeckles (green) and chromosome 11 q13.1 DNA-FISH (red). Panels B and C are reproduced from (Clemson et al. 2009) with permission. Scale bar in B, 10 µm, in panel C, 5 µm.
NEAT1 is found within paraspeckles in a variety of different cell lines in both mouse and human cells (Clemson et al. 2009; Sasaki et al. 2009; Sunwoo et al. 2009). An essential role for NEAT1 in paraspeckle integrity was shown by knockdown of NEAT1 in cultured cells, resulting in the loss of paraspeckles, as judged by loss of DBHS protein localization to subnuclear foci (Clemson et al. 2009; Sasaki et al. 2009; Sunwoo et al. 2009). Moreover, paraspeckles do not re-form following reversible transcription inhibition in the presence of the NEAT1 knockdown, suggesting NEAT1 is also required for paraspeckle formation (Clemson et al. 2009; Sasaki et al. 2009; Sunwoo et al. 2009). Further evidence suggests that NEAT1 is the nucleating factor for paraspeckles, at least in NIH3T3 cells, as stable overexpression of NEAT1_v1 results in an increase in paraspeckle number (Clemson et al. 2009). NEAT1 ncRNA is not only needed for paraspeckle integrity, but there is evidence that paraspeckles form near to the NEAT1 gene itself. Combined DNA and RNA FISH showed that cells in early G1 have the first paraspeckles forming close to the NEAT1 gene, and in interphase, clusters of paraspeckles are observed close to the NEAT1 gene locus (Clemson et al. 2009).
As with Ctn, it appears that interaction with DBHS proteins plays a role in NEAT1 paraspeckle localization, as coimmunoprecipitation experiments with each of the DBHS proteins pulled out NEAT1 to varying levels of enrichment (Clemson et al. 2009; Sasaki et al. 2009; Sunwoo et al. 2009). The precise NEAT1 RNA binding site for the DBHS proteins has not been established, although recombinant DBHS proteins can bind the NEAT1_v1 in vitro (Clemson et al. 2009), and paraspeckles can persist solely in the presence of NEAT1_v1 (Sunwoo et al. 2009), other evidence suggests that the final 10kb of human NEAT1_v2 may contain the necessary sequence for paraspeckle formation in vivo (Sasaki et al. 2009). Interestingly, in contrast to Ctn, NEAT1 is not A-to-I edited, indicating that the molecular interactions of the DBHS proteins to each RNA may differ. Moreover, the NEAT1 genes in mouse and human vary greatly in sequence, suggesting that likely RNA structure, rather than primary sequence, is conserved.
Changes in NEAT1 RNA levels can inform about paraspeckle function, because NEAT1 is linked to controlling the formation of paraspeckles. A relevant example of this is the recent finding that human embryonic stem cells have little or no detectable NEAT1 RNA and do not show paraspeckles (Chen and Carmichael, 2009). However, hESCs do express all three DBHS proteins, and hESCs also express mRNAs containing A-to-I edited inverted repeats. Critically, within hESCs these A-to-I edited RNAs are not exclusively retained in the nucleus, instead they also appear in the cytoplasm. When the hESCs are induced to differentiate, _NEAT_1 is expressed and paraspeckles appear. Importantly, the appearance of paraspeckles is linked to an increase in the efficiency of nuclear retention of the A-to-I edited mRNAs, suggesting that it is NEAT1 forming the paraspeckle structures themselves that are critical for mediating the nuclear retention mechanism. This study highlights the importance of organising the DBHS proteins into paraspeckles for RNA nuclear retention, but also reveals a clear link between the formation of paraspeckles and cellular differentiation. A role for paraspeckles in differentiation was first suggested by Sunwoo et al. (2009), who found that NEAT1 was up-regulated when a cultured myoblast cell-line was induced to differentiate into myotubes, with a corresponding increase in paraspeckle size. Another example of varying NEAT1 RNA levels was reported in an earlier study that found levels of VINC-1 RNA (subsequently found to correspond to NEAT1) increased in the central nervous system of mice infected with Japanese encephalitis or rabies viruses (Saha et al. 2006). Although changes in paraspeckles in this experimental model are yet to be evaluated, these viruses may trigger an increase in paraspeckle size and number, raising the possibility that certain viruses may use paraspeckles for their processing, or that paraspeckles are part of a viral defense mechanism.
CONCLUSIONS
Paraspeckle Formation and Function
The model for paraspeckle formation begins with the production of NEAT1 transcripts in daughter nuclei following cell division. Once transcribed, NEAT1 molecules form complexes with DBHS proteins, generally before the RNA has had a chance to diffuse far away from its gene locus (Fig. 5). The finished paraspeckle likely consists of multiple copies of NEAT1 RNA-DBHS protein complexes, which form a structural scaffold that is nevertheless dynamic, in that individual DBHS protein molecules in paraspeckles can exchange with a pool of DBHS proteins in the nucleoplasm. It is possible that both the known oligomerization propensity of the DBHS proteins, as well as possible RNA-RNA intramolecular interactions in NEAT1 ncRNA both contribute to the paraspeckle structural lattice. Without the production of NEAT1 RNA, paraspeckles fail to form, explaining why paraspeckles are not observed when all RNA Pol II transcription is inhibited, or in cell types that do not express NEAT1. Conversely, without abundant DBHS proteins, paraspeckles are also not observed. Throughout interphase, some paraspeckles are observed closely associated with the NEAT1 gene, however, it is not known if these are exclusively newly formed paraspeckles, nor is it known what mechanism may be holding the paraspeckles close to the locus.
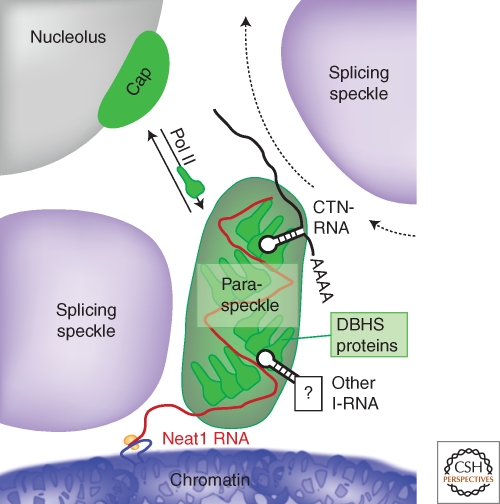
Figure 5.
Model of paraspeckle formation. A paraspeckle forms near the NEAT1 gene locus within the interchromatin space, abutting a nuclear speckle. The paraspeckle is formed via interactions between the NEAT1 RNA and DBHS proteins. Additional RNA species regulated within paraspeckles and elsewhere in the nucleus, such as A-to-I edited mRNA, are likely recruited via interaction with DBHS proteins, and traffic through the paraspeckle. Under steady-state conditions the DBHS proteins are dynamic, and exchange between paraspeckles, the nucleoplasm and the nucleolus. When RNA Pol II transcription is inhibited, DBHS proteins accumulate at perinucleolar caps.
A major function of paraspeckles likely relates to their other known RNA component, namely the mRNA containing dsRNA structures, generally formed by inverted repeats, and A-to-I edited. The RNA nuclear retention mechanism may be involved in many cellular processes such as stress responses, viral infection and circadian rhythm maintenance. Perhaps most significantly, given the link between paraspeckles and two different models of differentiation, it is likely that paraspeckles are playing a part in the reprogramming of a cell that takes place with differentiation, possibly by altering the expression of key proteins via RNA nuclear retention. The corollary of this is that the absence of both NEAT1 and paraspeckles can potentially be used as a marker for pluripotency (Chen and Carmichael 2009).
As yet, the roles of non-DBHS proteins known to localize to paraspeckles have not been addressed within the context of paraspeckle function. Some proteins could be involved in the control of gene expression through nuclear retention, for example, CFIm may be the cleavage factor mediating release of RNA, whereas others proteins with roles in transcription may be involved in recruiting paraspeckles to certain active gene loci.
Nuclear RNA and Disease
As yet there are no examples of diseases caused by the absence or presence of paraspeckles, however, there are several RNA dominant diseases associated with the production of toxic nuclear RNAs (Osborne and Thornton 2006). An example is myotonic muscular dystrophy, in which RNA transcribed from mutated genes with expanded CTG-repeats is retained in the nucleus within subnuclear foci. The repeats form RNA hairpins that are bound by the muscleblind-like family of proteins. The toxicity arises as the muscleblind-like proteins are effectively sequestered in the foci and are no longer able to carry out their roles as modulators of alternative splicing (O’Rourke and Swanson 2009). These CUG-repeat foci do not colocalize with paraspeckles in patient-derived cells (Clemson et al. 2009). However, the parallels to paraspeckles are striking: specific RNA-protein interactions, retention of RNA in the interchromosome space and splicing functions of the proteins. These parallels suggest that the cell has common themes in nuclear retention of RNA that are apparent in both normal cell function and disease.
Paraspeckles as a Paradigm for a Class of Subnuclear Bodies
Given that the complexity of the mammalian transcriptome is only just beginning to be explored, including the full extent of long ncRNA production, it is exciting to speculate that paraspeckles will not be the only example of a subnuclear structure formed around a long ncRNA. Indeed, there already exist examples of long ncRNA localizing to unique subnuclear foci, although in these cases no corresponding protein partners are known (Royo et al. 2007; Sone et al. 2007). One interesting possibility is that the IGAZ (structures labeled in the EM by paraspeckle markers) may not be solely composed of paraspeckles, but may also include other subnuclear bodies akin to paraspeckles, each containing distinct structural ncRNAs, specific RNA-binding proteins and having different species of RNA regulated/retained within them. The lack of identified marker ncRNAs and proteins for these bodies may have so far prevented their detection and characterization. Further studies on long ncRNAs and their subcellular localization will in future provide a fuller picture of these structures and their roles in the cell.
CONCLUDING REMARKS
Paraspeckles were identified as recently as 2002, making them one of the ‘youngest’ nuclear structures known. However, already in the short time since their discovery, much has been learned about their composition, formation and function. Paraspeckles are the first, but likely not the last example of a subnuclear body that forms dependent on a long ncRNA, whose function, at least at this point, appears to be to form this subcellular structure. Paraspeckles are also critical for a novel mechanism for controlling gene expression, i.e., the nuclear retention of otherwise translation-competent RNA. We suggest that many key molecules may be regulated in this manner and discovering their identity and functions will be of great interest in the years to come.
ACKNOWLEDGMENTS
We thank all those authors who have agreed for their published work to appear in figures within this review. We would also like to thank Charles Bond (University of WA, Australia), Sam Swift and Silvana van Koningsbruggen (University of Dundee, UK) for help generating figures and Sylvie Souquere and Gerard Pierron, (Villejuif, France) for providing TEM images. AIL is a Wellcome Trust Principal Research Fellow. AHF is funded by the National Health & Medical Research Council, Australia.
*
As of 27/7/09 the official HUGO gene nomenclature symbol for this ncRNA is NEAT1 and the HUGO gene name is “Nuclear ParaspEckle Assembly Transcript 1 (nonprotein coding).” HUGO does not have jurisdiction on nomenclature of transcript variants, however, in line with the HUGO recommendation, we propose the use of NEAT1_v1 for the 3.7kb variant and NEAT1_v2 for the 23kb variant.
Editors: Tom Misteli and David Spector
REFERENCES
- Andersen JS, Lyon CE, Fox AH, Leung AKL, Lam YW, Steen H, Mann M, Lamond AI 2002. Directed proteomic analysis of the human nucleolus. Current Biology 12:1–11 [DOI] [PubMed] [Google Scholar]
- Auboeuf D, Dowhan DH, Li X, Larkin K, Ko L, Berget SM, O’Malley BW 2004. CoAA, a nuclear receptor coactivator protein at the interface of transcriptional coactivation and RNA splicing. Mol Cell Biol 24:442–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks YS, Wang G, Yang Z, Smith KK, Bieberich E, Ko L 2009. Functional pre-mRNA Trans-splicing of coactivator CoAA and corepressor RBM4 during stem/progenitor cell differentiation. J Biol Chem 284:18033–18046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Ripperger J, Kadener S, Fleury-Olela F, Vilbois F, Rosbash M, Schibler U 2005. PERIOD1-associated proteins modulate the negative limb of the mammalian circadian oscillator. Science 308:693–696 [DOI] [PubMed] [Google Scholar]
- Cardinale S, Cisterna B, Bonetti P, Aringhieri C, Biggiogera M, Barabino SM 2007. Subnuclear localization and dynamics of the Pre-mRNA 3’ end processing factor mammalian cleavage factor I 68-kDa subunit. Mol Biol Cell 18:1282–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, Oyama R, Ravasi T, Lenhard B, Wells C, et al. 2005. The transcriptional landscape of the mammalian genome. Science 309:1559–1563 [DOI] [PubMed] [Google Scholar]
- Chen LL, Carmichael GG 2008. Gene regulation by SINES and inosines: Biological consequences of A-to-I editing of Alu element inverted repeats. Cell Cycle 7:3294–3301 [DOI] [PubMed] [Google Scholar]
- Chen LL, Carmichael GG 2009. Altered nuclear retention of mRNAs containing inverted repeats in human embryonic stem cells: Functional role of a nuclear noncoding RNA. Mol Cell DOI: 101016/jmolcel200906027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, DeCerbo JN, Carmichael GG 2008. Alu element-mediated gene silencing. EMBO J 27:1694–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemson CM, Hutchinson JN, Sara SA, Ensminger AW, Fox AH, Chess A, Lawrence JB 2009. An architectural role for a nuclear noncoding RNA: NEAT1 RNA is essential for the structure of paraspeckles. Mol Cell 33:717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer T, Cremer M, Dietzel S, Muller S, Solovei I, Fakan S 2006. Chromosome territories–a functional nuclear landscape. Curr Opin Cell Biol 18:307–316 [DOI] [PubMed] [Google Scholar]
- Dettwiler S, Aringhieri C, Cardinale S, Keller W, Barabino SM 2004. Distinct sequence motifs within the 68-kDa subunit of cleavage factor Im mediate RNA binding, protein-protein interactions, and subcellular localization. J Biol Chem 279:35788–35797 [DOI] [PubMed] [Google Scholar]
- Dutton JR, Lahiri D, Ward A 2006. Different isoforms of the Wilms’ tumour protein WT1 have distinct patterns of distribution and trafficking within the nucleus. Cell Prolif 39:519–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye BT, Patton JG 2001. An RNA recognition motif (RRM) is required for the localization of PTB-associated splicing factor (PSF) to subnuclear speckles. Exp Cell Res 263:131–144 [DOI] [PubMed] [Google Scholar]
- Fox AH, Bond CS, Lamond AI 2005. P54nrb forms a heterodimer with PSP1 that localizes to paraspeckles in an RNA-dependent manner. Mol Biol Cell 16:5304–5315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AH, Lam YW, Leung AK, Lyon CE, Andersen J, Mann M, Lamond AI 2002. Paraspeckles: A novel nuclear domain. Curr Biol 12:13–25 [DOI] [PubMed] [Google Scholar]
- Hata K, Nishimura R, Muramatsu S, Matsuda A, Matsubara T, Amano K, Ikeda F, Harley VR, Yoneda T 2008. Paraspeckle protein p54nrb links Sox9-mediated transcription with RNA processing during chondrogenesis in mice. J Clin Invest 118:3098–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JN, Ensminger AW, Clemson CM, Lynch CR, Lawrence JB, Chess A 2007. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics 8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseli C, Stevenson BJ, de Souza SJ, Samaia HB, Camargo AA, Buetow KH, Strausberg RL, Simpson AJ, Bucher P, Jongeneel CV 2002. Long-range heterogeneity at the 3’ ends of human mRNAs. Genome Res 12:1068–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Watanabe H, Yamamichi N, Kondo S, Tando T, Haraguchi T, Mizutani T, Sakurai K, Fujita S, Izumi T, et al. 2008. Brm transactivates the telomerase reverse transcriptase (TERT) gene and modulates the splicing patterns of its transcripts in concert with p54(nrb). Biochem J 411:201–209 [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Chin WW, Ko L 2001. Identification and characterization of RRM-containing coactivator activator (CoAA) as TRBP-interacting protein, and its splice variant as a coactivator modulator (CoAM). J Biol Chem 276:33375–33383 [DOI] [PubMed] [Google Scholar]
- Kameoka S, Duque P, Konarska MM 2004. p54(nrb) associates with the 5’ splice site within large transcription/splicing complexes. EMBO J 23:1782–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Dohmae N, Hirokawa N 2004. Kinesin transports RNA: Isolation and characterization of an RNA-transporting granule. Neuron 43:513–525 [DOI] [PubMed] [Google Scholar]
- Kaneko S, Rozenblatt-Rosen O, Meyerson M, Manley JL 2007. The multifunctional protein p54nrb/PSF recruits the exonuclease XRN2 to facilitate pre-mRNA 3’ processing and transcription termination. Genes Dev 21:1779–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay RA, Ellis IR, Jones SJ, Perrier S, Florence MM, Schor AM, Schor SL 2005. The expression of migration stimulating factor, a potent oncofetal cytokine, is uniquely controlled by 3’-untranslated region-dependent nuclear sequestration of its precursor messenger RNA. Cancer Res 65:10742–10749 [DOI] [PubMed] [Google Scholar]
- Kiesler E, Miralles F, Ostlund Farrants AK, Visa N 2003. The Hrp65 self-interaction is mediated by an evolutionarily conserved domain and is required for nuclear import of Hrp65 isoforms that lack a nuclear localization signal. J Cell Sci 116:3949–3956 [DOI] [PubMed] [Google Scholar]
- Lamond AI Spector DL 2003. Nuclear speckles: A model for nuclear organelles. Nat Rev Mol Cell Biol 4:605–612 [DOI] [PubMed] [Google Scholar]
- Levanon K, Eisenberg E, Rechavi G, Levanon EY 2005. Letter from the editor: Adenosine-to-inosine RNA editing in Alu repeats in the human genome. EMBO Rep 6:831–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Ippolito GC, Wall JK, Niu T, Probst L, Lee BS, Pulford K, Banham AH, Stockwin L, Shaffer AL, et al. 2006. Functional studies of BCL11A: Characterization of the conserved BCL11A-XL splice variant and its interaction with BCL6 in nuclear paraspeckles of germinal center B cells. Mol Cancer 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer TR, Dinger ME, Mattick JS 2009. Long non-coding RNAs: Insights into functions. Nat Rev Genet 10:155–159 [DOI] [PubMed] [Google Scholar]
- Myojin R, Kuwahara S, Yasaki T, Matsunaga T, Sakurai T, Kimura M, Uesugi S, Kurihara Y 2004. Expression and functional significance of mouse paraspeckle protein 1 on spermatogenesis. Biol Reprod 71:926–932 [DOI] [PubMed] [Google Scholar]
- O’Rourke JR Swanson MS 2009. Mechanisms of RNA-mediated disease. J Biol Chem 284:7419–7423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne RJ Thornton CA 2006. RNA-dominant diseases. Hum Mol Genet 15 Spec No 2:R162–169 [DOI] [PubMed] [Google Scholar]
- Osenberg S, Dominissini D, Rechavi G, Eisenberg E 2009. Widespread cleavage of A-to-I hyperediting substrates. RNA 15:1632–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JG, Porro EB, Galceran J, Tempst P, Nadal-Ginard B 1993. Cloning and characterization of PSF, a novel pre-mRNA splicing factor. Genes Dev 7:393–406 [DOI] [PubMed] [Google Scholar]
- Peng R, Dye BT, Perez I, Barnard DC, Thompson AB, Patton JG 2002. PSF and p54nrb bind a conserved stem in U5 snRNA. RNA 8:1334–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platani M, Lamond AI 2004. Nuclear organisation and subnuclear bodies. Prog Mol Subcell Biol 35:1–22 [DOI] [PubMed] [Google Scholar]
- Prasanth KV, Prasanth SG, Xuan Z, Hearn S, Freier SM, Bennett CF, Zhang MQ, Spector DL 2005. Regulating gene expression through RNA nuclear retention. Cell 123:249–263 [DOI] [PubMed] [Google Scholar]
- Prasanth KV, Spector DL 2007. Eukaryotic regulatory RNAs: An answer to the ‘genome complexity’ conundrum. Genes Dev 21:11–42 [DOI] [PubMed] [Google Scholar]
- Rivera MN, Kim WJ, Wells J, Stone A, Burger A, Coffman EJ, Zhang J, Haber DA 2009. The tumor suppressor WTX shuttles to the nucleus and modulates WT1 activity. Proc Natl Acad Sci 106:8338–8343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royo H, Basyuk E, Marty V, Marques M, Bertrand E, Cavaille J 2007. Bsr, a nuclear-retained RNA with monoallelic expression. Mol Biol Cell 18:2817–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Murthy S, Rangarajan PN 2006. Identification and characterization of a virus-inducible non-coding RNA in mouse brain. J Gen Virol 87:1991–1995 [DOI] [PubMed] [Google Scholar]
- Sasaki YT, Ideue T, Sano M, Mituyama T, Hirose T 2009. MENε/β noncoding RNAs are essential for structural integrity of nuclear paraspeckles. Proc Natl Acad Sci 106:2525–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shav-Tal Y Zipori D 2002. PSF and p54(nrb)/NonO–multi-functional nuclear proteins. FEBS Lett 531:109–114 [DOI] [PubMed] [Google Scholar]
- Shav-Tal Y, Blechman J, Darzacq X, Montagna C, Dye BT, Patton JG, Singer RH, Zipori D 2005. Dynamic sorting of nuclear components into distinct nucleolar caps during transcriptional inhibition. Mol Biol Cell 16:2395–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone M, Hayashi T, Tarui H, Agata K, Takeichi M, Nakagawa S 2007. The mRNA-like noncoding RNA Gomafu constitutes a novel nuclear domain in a subset of neurons. J Cell Sci 120:2498–2506 [DOI] [PubMed] [Google Scholar]
- Sunwoo H, Dinger ME, Wilusz JE, Amaral PP, Mattick JS, Spector DL 2009. MEN ε/β nuclear-retained non-coding RNAs are up-regulated upon muscle differentiation and are essential components of paraspeckles. Genome Res 19:347–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visa N, Puvion-Dutilleul F, Bachellerie JP, Puvion E 1993. Intranuclear distribution of U1 and U2 snRNAs visualized by high resolution in situ hybridization: Revelation of a novel compartment containing U1 but not U2 snRNA in HeLa cells. Eur J Cell Biol 60:308–321 [PubMed] [Google Scholar]
- Wilusz JE, Freier SM, Spector DL 2008. 3’ end processing of a long nuclear-retained noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell 135:919–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie SQ, Martin S, Guillot PV, Bentley DL, Pombo A 2006. Splicing speckles are not reservoirs of RNA polymerase II, but contain an inactive form, phosphorylated on serine2 residues of the C-terminal domain. Mol Biol Cell 17:1723–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YS, Hanke JH, Carayannopoulos L, Craft CM, Capra JD, Tucker PW 1993. NonO, a non-POU-domain-containing, octamer-binding protein, is the mammalian homolog of Drosophila nonAdiss. Mol Cell Biol 13:5593–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Sui Y, Xiong S, Liour SS, Phillips AC, Ko L 2007. Switched alternative splicing of oncogene CoAA during embryonal carcinoma stem cell differentiation. Nucleic Acids Res 35:1919–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z Carmichael GG 2001. The fate of dsRNA in the nucleus: A p54(nrb)-containing complex mediates the nuclear retention of promiscuously A-to-I edited RNAs. Cell 106:465–475 [DOI] [PubMed] [Google Scholar]
- Zolotukhin AS, Michalowski D, Bear J, Smulevitch SV, Traish AM, Peng R, Patton J, Shatsky IN, Felber BK 2003. PSF acts through the human immunodeficiency virus type 1 mRNA instability elements to regulate virus expression. Mol Cell Biol 23:6618–6630 [DOI] [PMC free article] [PubMed] [Google Scholar]