Expansion of human NK-22 cells with IL-7, IL-2, and IL-1β reveals intrinsic functional plasticity (original) (raw)
Abstract
Natural killer-22 (NK-22) cells are a human NK cell subset situated in mucosal-associated lymphoid tissues that specialize in IL-22 secretion in response to IL-23. Here we investigated the cytokine requirements for NK-22 cell expansion. IL-7 maintained the sur-vival of NK-22 cells and IL-22 production in response to IL-23 but was insufficient to induce robust expansion. Proliferation of NK-22 cells was increased markedly by adding either IL-1β or IL-2 to IL-7 and was even stronger in the presence of IL-1β plus IL-2. In contrast to IL-7, continuous culture in IL-1β and IL-2 modified NK-22 cytokine profiles. IL-1β promoted constitutive IL-22 secretion rather than acute IL-22 production in response to IL-23 and induced IL-17 in some cells. IL-2 reduced secretion of IL-22 and IL-17, increasing production of IFN-γ and leukemia inhibitory factor. Functional deviation toward IFN-γ production also was induced by continuous culture in IL-23. These results demonstrate the functional plasticity of NK-22 cells, which may allow flexible responses to different pathogens. Finally, we found that NK-22 cells released the B-cell survival factor, B-cell activating factor belonging to the TNF family (BAFF), suggesting a potential role of NK-22 cells in promoting B-cell–mediated mucosal immunity.
Keywords: B-cell activating factor belonging to the TNF family, IL-22, mucosal immunity, natural killer cell
We recently identified a subset of natural killer (NK)-like cells in human tonsils, Peyer's patches, and appendix, which express CD56 and the receptor NKp44. These cells specialize in the secretion of IL-22 in response to IL-23; therefore we designated them as “NK-22” cells (1, 2). NK-22 cells also produce IL-26 and the leukemia inhibitory factor (LIF). All these cytokines play an important role in the defense of mucosal barriers against pathogens (3, 4). NK-22 cells have remarkable similarities with T-helper (TH) 17 T cells: Both cell types secrete IL-22, express the chemokine receptor CCR6, migrate in response to CCL20, and express the transcription factors retinoic-acid–related orphan recep-tor γt (RORγt) and aryl hydrocarbon receptor. The latter is required for production of IL-22 (5). However, in contrast to TH17 cells, NK-22 cells isolated from the tonsils do not produce IL-17.
The developmental pathway that generates human NK-22 cells is unclear. In lymphoid tissues, classical NK cells develop through five distinct stages (6); stage 3 NK cells produce IL-22 (7), whereas stage 4 and 5 NK cells produce IFN-γ. Thus, it is possible that NK-22 cells correspond to stage 3 NK cells. However, NK-22 cells also are similar to human lymphoid tissue-inducer cells (LTi), which are present in the lymphoid aggregates of fetal mesentery, and to LTi-like cells, which are found in tonsils. Thus, NK-22 cells also may develop from LTi and LTi-like cells (8, 9).
The murine equivalent of NK-22 cells has been identified in gut-associated lymphoid tissues as NKp46+CD127+RORγt+ cells (1, 10–12). Their development depends on the common gamma chain (γc) of cytokine receptors, IL-7, RORγt, and the intestinal microbiota (10, 11, 13) but does not require IL-15 or the β chain of IL-2 and IL-15 receptors (CD122), which are essential for the development of classical NK cells. Thus, in the mouse IL-22–producing NKp46+CD127+RORγt+ cells and classical NK cells appear to develop through different pathways (13).
In this study we investigated the cytokines that are required for the expansion of NK-22 cells isolated from human tonsils. We found that IL-7, IL-2, and IL-1β promoted survival and proliferation of NK-22 cells but induced different functional profiles, indicating that NK-22 cells are functionally flexible and may contribute to immune responses in the gut by different mechanisms. Additionally, we observed that NK-22 cells release the B-cell survival factor, B-cell activating factor belonging to the TNF family (BAFF), suggesting a potential role for NK-22 cells in promoting B-cell–mediated immunity in the mucosae.
Results
Human CD56+NKp44+ NK Cells Are Functionally Heterogeneous.
We previously defined NK-22 cells in human tonsils and Peyer's patches based on the expression of CD56 and NKp44 and the capacity to produce IL-22 in response to IL-23 (1). To refine the phenotype of NK-22 cells, we isolated CD56+ cells from tonsils, stimulated them with IL-23, and analyzed them by multicolor flow cytometry for intracellular content of IL-22 and expression of NKp44 as well as various cell-surface markers, such as CCR6, the αE integrin CD103, and the ectonucleotidase CD39. All IL-22–producing cells expressed NKp44 and the chemokine receptor CCR6; in contrast, none expressed CD103 (Fig. S1). Some but not all IL-22–producing cells expressed CD39. Thus, CD56+NKp44+ cells include at least two functionally distinct subsets: CCR6+CD103− cells, which secrete IL-22 in response to IL-23, and CD103+ cells, which do not produce IL-22 and have an undefined function. The presence of these subsets, together with the classical CD56+NKp44− NK cells, reveals a considerable heterogeneity of NK cells in mucosal-associated lymphoid tissues. Importantly, the refined phenotype of NK-22 cells as CD56+NKp44+CCR6+CD103− cells allows the isolation of these cells with little or no contamination by other NK cell subsets, facilitating their functional characterization.
Human NK-22 Cells Proliferate in Response to IL-2 Plus IL-7.
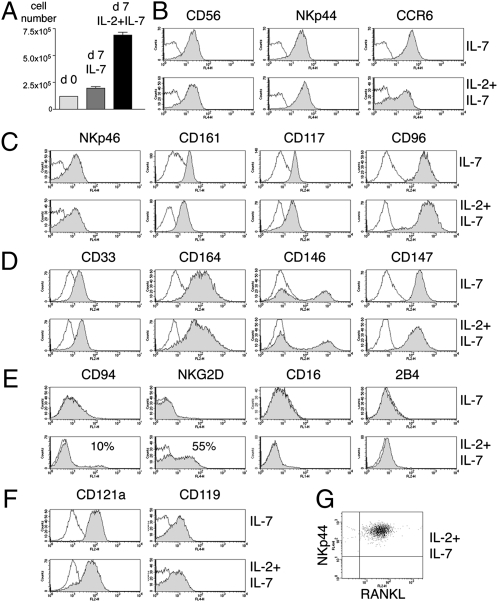
We next investigated the cytokine requirements for the survival and expansion of NK-22 cells in vitro. In mouse, the development of NKp46+CD127+RORγt+IL-22+ cells requires the common γ chain of cytokine receptors (γc) and IL-7 but not the β chain of IL-2/IL-15 receptors (CD122) or IL-15 (10, 13). Consistent with mouse data, we found that primary NK-22 cells express the IL-7 receptor α chain (CD127) (Fig. S2), and therefore we hypothesized that IL-7 might be sufficient to maintain and/or expand NK-22 cells in vitro. To test this hypothesis, we sorted CD56+NKp44+CCR6+CD103− cells from tonsils (Fig. S3) and cultured them in IL-7. After 1 week, we observed only a modest (2-fold) increase in number, suggesting that IL-7 is sufficient for cell survival but is a weak inducer of proliferation (Fig. 1_A_). To facilitate a robust expansion of NK-22 cells, we supplemented IL-7 with IL-2, which is commonly used to expand classical NK cells in vitro. In these conditions we observed a 7- to 10-fold increase in cell number within a week (Fig. 1_A_), which was caused by augmented proliferation, as assessed by BrdU incorporation assay (Fig. S4). Thus, IL-2 plus IL-7 promotes robust NK-22 cell expansion.
Fig. 1.
Impact of IL-7 and IL-2 on proliferation and phenotype of NK-22 cells. CD56+NKp44+CCR6+CD103− cells were sorted from tonsils and cultured in IL-7 alone or in IL-2 plus IL-7. After 7 days of culture cells were analyzed for cell numbers (A). After 10–15 days of culture, we measured cell surface expression of CD56, NKp44, and CCR6 (B); NKp46, CD161, CD117, and CD96 (C); CD33, CD164, CD146, and CD147 (D); CD94, NKG2D, CD16, and 2B4 (E); the receptors for IL-1 (CD121a) and IFN-γ (CD119) (F); and RANKL (presented in two-color analysis versus NKp44) (G). Empty and filled profiles represent staining with control and specific antibodies, respectively. Data shown here were obtained from one tonsil specimen representative of three.
Phenotypic analysis of NK-22 cells cultured in IL-7 or IL-2 plus IL-7 confirmed the expression of CD56, NKp44, and CCR6 (Fig. 1_B_). Cells also expressed high amounts of CD117 (c-Kit), NKp46, CD161 (NKRP1-A), and CD96 (Fig. 1_C_). Additionally, we observed the unprecedented expression of CD33, CD146, CD164, and CD147 (Fig. 1_D_). Many of these molecules have been shown to mediate adhesion (14, 15). Cells cultured in IL-2 plus IL-7 expressed more NKG2D and CD94 than cells cultured in IL-7 alone (Fig. 1_E_), whereas other NK cell receptors, such as CD16, 2B4, and KIRs, were undetectable in both culture conditions.
We previously reported the expression of the receptor for IL-1 (CD121a) in the transcriptome of primary NK-22 cells (1). We confirmed CD121a expression at the protein level on primary NK-22 cells (Fig. S2) and showed that it is maintained after culture in IL-7 with or without IL-2 (Fig. 1_F_). Cultured NK-22 cells also expressed the receptor for IFN-γ (CD119) (Fig. 1_F_). Thus, NK-22 cells can respond to IL-1β and IFN-γ. Moreover, cultured NK-22 cells expressed receptor activator for nuclear factor κ B ligand (RANKL) (Fig. 1_G_), which may facilitate the interaction of NK-22 cells with antigen-presenting cells and/or epithelial cells that express RANK (16). Overall, we conclude that the phenotype of NK-22 cells cultured in IL-7 or IL-2 plus IL-7 closely resembles that of primary NK-22 cells, although IL-2 appears to increase the frequency of cells expressing NKG2D and CD94.
IL-2 Deviates the Polarization of NK-22 Cells Toward the Production of IFN-γ.
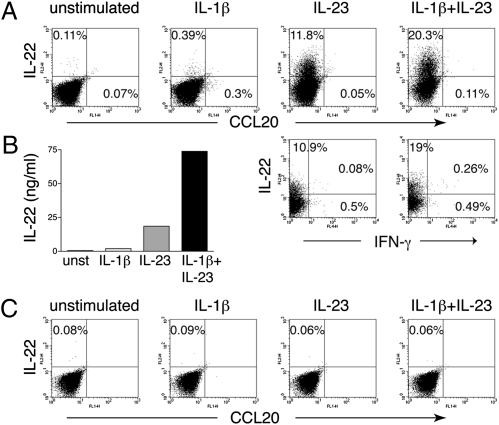
To examine the cytokine produced by NK-22 cells cultured in IL-7 or in IL-2 plus IL-7, we stimulated them with IL-23 and measured the intracellular content of IL-22 as well as IL-22 secreted in the culture supernatant. We also measured the intracellular content of IFN-γ, which is produced by conventional NK cells, and CCL20, a chemokine that is preferentially released by NK-22 cells (1). Given the expression of CD121a on NK-22 cells, we included IL-1β with or without IL-23 as stimuli. Moreover, in some experiments, we stimulated cells with phorbol 12-myristate 13-acetate (PMA)/ionomycin. NK-22 cells cultured in IL-7 alone produced IL-22 in response to IL-23 (Fig. 2 A and B); additionally, IL-1β strongly synergized with IL-23 in inducing IL-22 secretion, whereas IL-1β alone had no effect. NK-22 cells cultured in IL-7 alone produced no IFN-γ or CCL20 (Fig. 2_A_). In control experiments, peripheral blood NK cells produced no IL-22 in response to various stimuli (Fig. 2_C_).
Fig. 2.
NK-22 cells cultured in IL-7 maintain their capacity to produce IL-22 in response to IL-23. CD56+NKp44+CCR6+CD103− cells were sorted from tonsils and cultured in IL-7 for 10–15 days. Cells were left untreated or stimulated with IL-1β, IL-23, or both and were analyzed for (A) frequencies of IL-22–, CCL20-, and IFN-γ–producing cells (by intracellular staining) and (B) release of IL-22 in the supernatants 18 h after stimulation (by ELISA). Data presented here were obtained from one tonsil specimen representative of three. (C) For comparison, we measured intracellular IL-22 and CCL20 contents of peripheral blood NK cells in response to IL-1β, IL-23, or both.
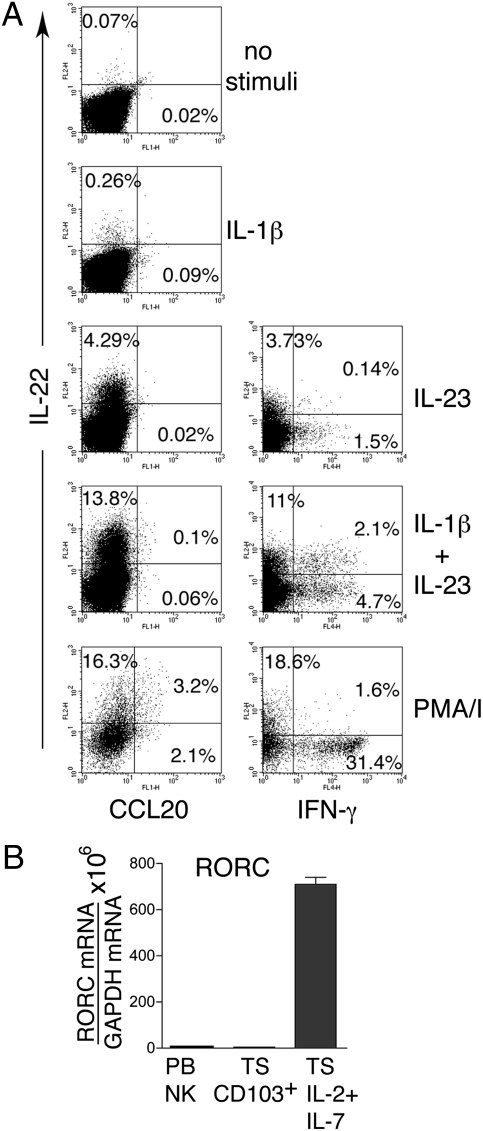
NK-22 cells cultured in IL-2 plus IL-7 showed a different polarization profile. The frequencies of IL-22–producing cells in response to IL-23, IL-1β plus IL-23, and PMA/I were reduced in comparison with those of cells grown in IL-7 alone (Fig. 3_A_). Moreover, a discrete subset of cells produced IFN-γ after stimulation with IL-23 plus IL-1β and PMA/ionomycin. Stimulation with IL-1β plus IL-23 also induced cells producing both IL-22 and IFN-γ (Fig. 3_A_). Despite the production of IFN-γ, NK-22 cells cultured in IL-2 plus IL-7 continued to express RORγt mRNA, which was undetectable in classical NK cells or CD103+ tonsil NK cells cultured in IL-2 (Fig. 3_B_). Finally, only cells stimulated with PMA/ionomycin made CCL20 at low frequency (Fig. 3_A_). Overall, these results suggest that NK-22 cells have functional plasticity. Although NK-22 cells in the presence of IL-7 mainly produce IL-22 in response to IL-23 with or without IL-1β, the addition of IL-2 reduces the frequency of IL-22–producing cells and increases that of IFN-γ–producing cells. However, NK-22 cells grown in IL-2 plus IL-7 remain distinct from classical NK cells, because they continue to express RORγt. The IL-2–induced deviation of NK-22 cells toward IFN-γ production mirrors the previously observed capacity of IL-2 to interfere in the development of TH17 T cells, facilitating the generation of IFN-γ–producing TH1 T cells (17).
Fig. 3.
NK-22 cells cultured in IL-2 plus IL-7 exhibit limited IL-22 production in response to IL-23 but produce IFN-γ. CD56+NKp44+CCR6+CD103− cells were sorted from tonsils and cultured in IL-2 plus IL-7 for 10–15 days. Cells were stimulated with IL-1β, IL-23, IL-1β plus IL-23, or PMA/ionomycin (PMA/I) and were analyzed for (A) production of IL-22, CCL20, and IFN-γ by intracellular staining and (B) expression of RORC mRNA by RT-PCR analysis. Data presented in A were obtained from the same tonsil specimen used for Figs. 2_A_ and 4 D and E to facilitate comparisons. PB NK, peripheral blood NK cells; TS, tonsil.
IL-1β Plus IL-7 Expands Human NK-22 Cells.
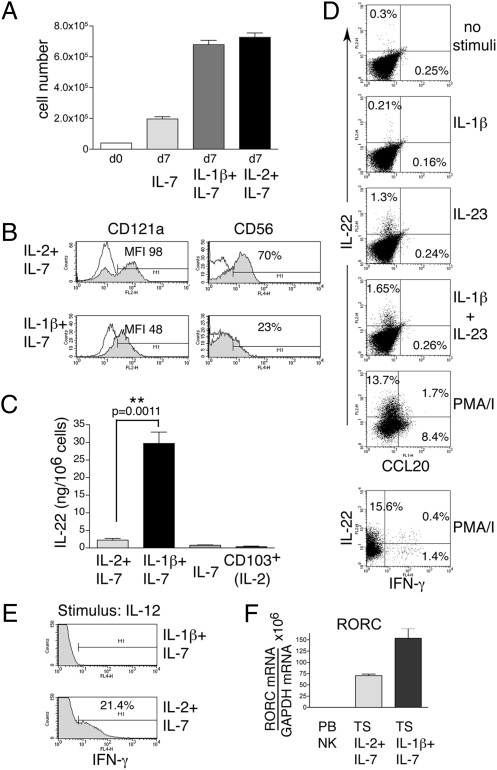
Given the expression of the receptor for IL-1 on both primary and cultured NK-22 cells (Fig. S2 and Fig. 1_F_), we attempted to culture primary NK-22 cells using IL-1β. Although IL-1β alone did not support NK-22 proliferation, IL-1β plus IL-7 expanded NK-22 cells as vigorously as IL-2 plus IL-7 (Fig. 4_A_ and Fig. S4). The cell-surface phenotype of cells expanded in IL-1β plus IL-7 was remarkably similar to that of primary NK-22 cells or NK-22 cells cultured in IL-2 plus IL-7. One exception was the reduced expression of CD121a, probably reflecting the engagement and down-regulation of CD121a by IL-1β present in the culture medium (Fig. 4_B_). Another noticeable exception was the reduced expression of CD56.
Fig. 4.
IL-1β plus IL-7 induces proliferation of NK-22 cells and constitutive production of IL-22. Identical numbers of CD56+NKp44+CCR6+CD103− cells sorted from tonsils were cultured in IL-7, IL-1β plus IL-7, or IL-2+IL-7. After 10 days of culture we analyzed (A) cell numbers; (B) expression of CD121a and CD56; (C) release of IL-22 in cell-culture supernatants by ELISA; (D) intracellular IL-22, IFN-γ, and CCL20 after stimulation with IL-1β, IL-23, IL-1β plus IL-23, and PMA/I by flow cytometry; (E) intracellular IFN-γ after stimulation with IL-12; (F) RORC mRNA by RT-PCR. Empty and filled profiles in B represent staining with control and specific antibodies, respectively. Data presented in D and E were obtained from the same tonsil specimen used for Figs. 2_A_ and 3_A_ to facilitate comparisons. PB NK, peripheral blood NK cells; TS, tonsil.
To characterize functionally NK-22 cells cultured in IL-1β plus IL-7, we investigated cytokine production by ELISA and intracellular staining. Supernatants of cells grown in IL-1β and IL-7 constitutively contained higher amounts of IL-22 than supernatants of cells cultured in IL-7 plus IL-2 or in IL-7 alone (Fig. 4_C_). However, only a few cells produced IL-22 when stimulated with IL-23 with or without IL-1β, as evidenced by intracellular staining (Fig. 4_D_). It is possible that culture in IL-1β promotes a continuous release of IL-22, decreasing the responsiveness of NK-22 cells to stimulation with IL-23 with or without IL-1β. Only PMA/ionomycin induced IL-22 in a significant subset of NK-22 cells (10–20% in different experiments) and CCL20 in some cells. The physiological stimuli that induce CCL20 in NK-22 cells remain unknown.
In contrast to cells cultured in IL-2 and IL-7, cells grown in IL-1β plus IL-7 did not produce IFN-γ in response to any of the stimuli used to elicit IL-22 (Fig. 4_D_). Additionally, no IFN-γ was produced in response to IL-12, whereas NK-22 cells cultured in IL-2 plus IL-7 were responsive to IL-12 and produced IFN-γ (Fig. 4_E_). Finally, cells grown in IL-1β and IL-7 exhibited high levels of expression of RORγt (Fig. 4_F_). We conclude that IL-1β together with IL-7 induces the proliferation of NK-22 cells and maintains their expression of RORγt and their capacity of producing IL-22.
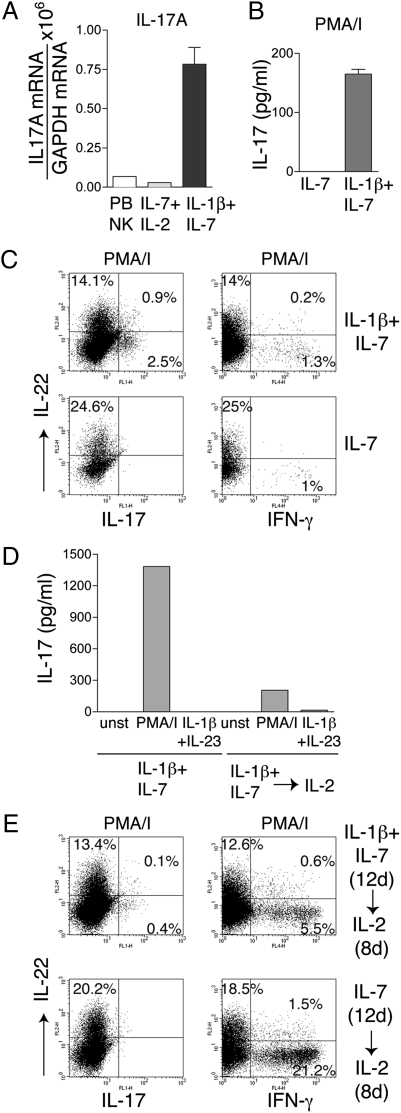
Some NK-22 Cells Grown in IL-1β Plus IL-7 Acquire the Capacity to Produce IL-17.
Because it recently was shown that IL-1β drives proliferation of TH17 T cells (18), we investigated whether NK-22 cells cultured in IL-1β and IL-7 also produce IL-17. We found that cells expressed IL-17 mRNA (Fig. 5_A_). Furthermore, stimulation of cells with PMA/ionomycin promoted the release of IL-17 in culture supernatant (Fig. 5_B_) and induced a small but measurable percentage of cells containing intracellular IL-17 (Fig. 5_C_). In contrast, stimulation with IL-23 or IL-23 plus IL-1β did not elicit IL-17 production. The physiological stimuli that trigger IL-17 production remain unknown. Cells maintained in IL-7 alone did not produce IL-17, indicating that IL-1β is necessary to induce IL-17 production.
Fig. 5.
NK-22 cells produce IL-17, IL-22, or IFN-γ depending on the cytokine milieu. CD56+NKp44+CCR6+CD103− cells were sorted from tonsils and cultured in IL-1β plus IL-7 or in IL-7 alone for 12 days. Cells then were divided into two aliquots: One aliquot was cultured in the original medium; IL-2 was added to the culture medium of the second aliquot. Both cultures were continued for 8 additional days. Cells were analyzed for (A) expression of IL-17 mRNA (by RT-PCR); (B and D) release of IL-17 in culture supernatants 8 h after stimulation with PMA/I or IL-1β plus IL-23 (by ELISA); (C and E) intracellular content of IL-17, IL-22, and IFN-γ after stimulation with PMA/I (by flow cytometry). Data presented here were obtained from one tonsil specimen representative of three. PB NK, peripheral blood NK cells.
Functional Polarization Induced by IL-1β Plus IL-7 Is Modified by IL-2 and IL-23.
We next determined the stability of the functional polarization induced by IL-1β plus IL-7. Highly purified tonsil NK-22 cells were cultured initially in IL-1β plus IL-7 or in IL-7 alone. After 12 days, we added IL-2 and continued the cultures for 8 additional days. As a result of this change in culture conditions, we observed a clear switch in cytokine production from IL-22 and IL-17 to IFN-γ both by ELISA and intracellular staining (Fig. 5 D and E). These results corroborate the plasticity of NK-22 cells, which can produce IL-22 and IL-17 or IFN-γ in response to IL-7 plus IL-1β or to IL-2, respectively.
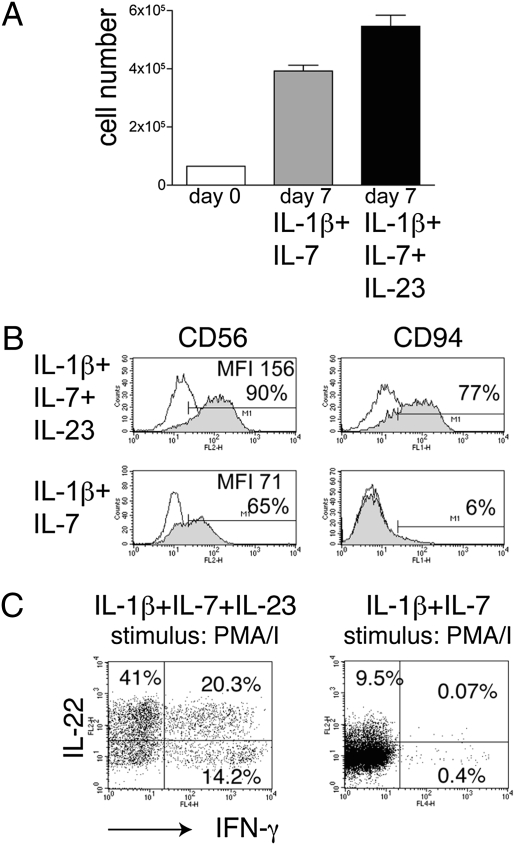
Because IL-23 has been shown to expand TH17 T cells and promote their terminal differentiation (19), we investigated whether we could expand IL-17–producing NK cells by adding IL-23 to IL-1β and IL-7 throughout the culture. IL-23 did not affect cell recovery (Fig. 6_A_). However, it induced up-regulation of CD94 and CD56 (Fig. 6_B_) and marked expansion of IL-22–producing cells (Fig. 6_C_). IL-17–producing cells were undetectable. In contrast, IL-23 increased the frequency of IFN-γ–producing cells as well as cells producing both IFN-γ and IL-22. These IFN-γ–producing cells were not responsive to IL-12. The ability of IL-23 to enhance the production of IL-22 and IFN-γ and to inhibit IL-17 further supports the functional flexibility of NK-22 cells.
Fig. 6.
Addition of IL-23 to IL-1β and IL-7 in the culture medium enhances NK-22 production of IL-22 and IFN-γ. CD56+NKp44+CCR6+CD103− cells were sorted from tonsils and cultured in IL-1β plus IL-7 or in IL-1β plus IL-7 plus IL-23 for 10 days. Cultures were analyzed for (A) cell numbers; (B) expression of CD56 and CD94; and (C) intracellular IL-2 and IFN-γ after stimulation with PMA/I. Empty and filled profiles in B represent staining with control and specific antibodies, respectively. Data shown here were obtained from one tonsil specimen representative of three.
IL-1β and IL-2 Synergize in Expanding NK-22 Cells.
Because both IL-2 and IL-1β induced NK-22 proliferation, we investigated whether these cytokines could cooperate in expanding NK-22 cells. Culture of NK-22 cells with IL-1β plus IL-2 induced a striking and sustained cell growth in comparison with culture with IL-1β plus IL-7, IL-2 plus IL-7, or IL-2 alone (Fig. S4). Cells produced IL-22 and/or IFN-γ (Fig. S5) but not IL-17 and expressed NKG2D (Fig. S6), similar to NK-22 cells cultured in IL-2 plus IL-7. Thus, IL-2 and IL-1β synergize in expanding NK-22 cells, although the presence of IL-2 deviates the functional profile of NK-22 cells toward that of classical NK cells.
IL-2 Promotes NK-22 Cell Secretion of Leukemia Inhibitory Factor.
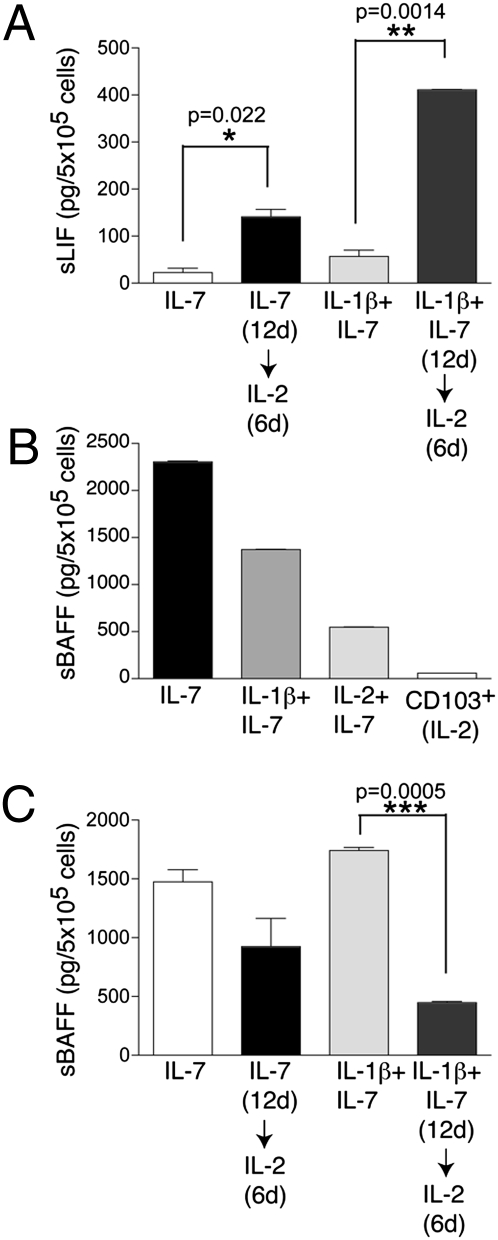
We previously reported that NK-22 cells produce LIF (1), a cytokine that has been reported to protect the mucosal barriers (20). To determine whether cultured NK-22 cells produce LIF, we measured LIF released in supernatants by ELISA. The amount of LIF secreted by NK-22 cells was modest when cells were cultured with IL-7 or IL-7 plus IL-1β but increased considerably when IL-2 was added to the medium (Fig. 7_A_). We conclude that secretion of LIF is mainly a feature of NK-22 cells exposed to IL-2.
Fig. 7.
Cultured NK-22 cells release LIF and BAFF. (A) CD56+NKp44+CCR6+CD103− cells were initially cultured in IL-7 or in IL-1β plus IL-7 for 12 days. Cells were divided into two aliquots: One was cultured in the original medium; IL-2 was added to the second aliquot. After 6 additional days of culture, LIF was quantified in the supernatants by ELISA. (B) Release of soluble BAFF by NK-22 cells in different culture conditions. Supernatants were tested 3 days after the last change of medium by ELISA. Note that CD103+ tonsil NK cells do not produce detectable amounts of BAFF. (C) Cells were cultured as described in A, and release of soluble BAFF in supernatants was quantified by ELISA. Data presented here were obtained from one tonsil specimen representative of three.
NK-22 Cells Release Soluble BAFF.
Transcriptional profiling of primary tonsil NK-22 cells versus conventional NK cells had shown previously that NK-22 cells express the B-cell survival and proliferation factor BAFF (1). However, we could not detect BAFF protein on the cell surface of primary NK-22 cells. Because BAFF is cleaved from the cell surface and released in the extracellular medium as a soluble protein (21), it was possible that BAFF might be cleaved rapidly from primary NK-22 cells and released in the lymphoid tissue. We tested this hypothesis by measuring BAFF in the supernatants of NK-22 cells cultured in vitro with different cytokine combinations. As controls, we tested the supernatants of NKp44+CD103+ tonsil NK cells cultured in IL-2. NK-22 cells cultured in IL-7 or in IL-1β plus IL-7 constitutively released very large amounts of BAFF in the absence of any stimulation (Fig. 7_B_). Cells cultured in IL-2 plus IL-7 showed a reduced release of BAFF, although the amount of BAFF was still remarkable compared with that released by NKp44+CD103+ cells (Fig. 7_B_) or peripheral blood NK cells. These results suggest that NK-22 cells constitutively release BAFF and that IL-2 partially decreases this capacity.
To corroborate that IL-2 inhibits BAFF release, we measured BAFF in the culture supernatant of NK-22 cells that were cultured first with IL-7 or IL-7 plus IL-1β and then were switched to medium containing IL-2. Release of BAFF was markedly reduced after IL-2 was added to the culture medium (Fig. 7_C_). Interestingly, we found that ≈50% of cultured NK-22 cells expressed CXCR5 (Fig. S7). This chemokine receptor drives cells to migrate toward the B-cell area of lymphoid aggregates (22). Altogether, the release of soluble BAFF and the expression of CXCR5 suggest that NK-22 cells may contribute to B-cell–mediated mucosal immunity.
Discussion
This study demonstrates that IL-7, IL-2, and IL-1β have different impacts on the proliferation of human NK-22 cells. IL-7 is sufficient to mediate the survival of NK-22 cells but not their expansion. IL-2 promotes strong proliferation of NK-22 cells. IL-1β alone has little impact on NK-22 cell proliferation but promotes strong expansion of NK-22 cells together with IL-7 and an even stronger expansion with IL-2. Interestingly, it was shown recently that IL-1β drives a T-cell receptor-independent proliferation of TH17 T cells (18). Thus, our demonstration that NK-22 cells require IL-1β for expansion further underscores the remarkable similarities between TH17 and NK-22 cells. It should be noted that IL-1β also synergizes with IL-23 in stimulating NK-22 cells to produce IL-22 or IFN-γ, depending on whether NK-22 cells are cultured in IL-7 or IL-2 plus IL-7, respectively. Thus, IL-1β signaling appears to have a crucial role in triggering cytokine secretion in NK-22 cells.
Another important conclusion of our study is that NK-22 cells maintain a certain degree of plasticity that allows different functional polarizations. Cells exposed to IL-7 alone most faithfully recapitulate the polarization of primary NK-22 cells, particularly the selective production of IL-22 in response to IL-23. Cells cultured with IL-1β plus IL-7 also produce IL-22; however, they secrete IL-22 constitutively rather than in response to stimuli. Moreover, some of the cells cultured with IL-1β plus IL-7 produce IL-17. Thus, IL-1β modifies the polarization of primary NK-22 cells, inducing the capacity to produce IL-17. Although we did not detect any IL-17 production in primary NK-22 cells, this feature was observed in fetal and adult LTi-like cells cultured in vitro, which closely resemble our NK-22 cells in phenotype (8). We envision that there may be yet undefined pathological conditions in which dendritic cells or other cells release large amounts of IL-1β, inducing IL-17 secretion by NK-22 cells.
Remarkably, culture of NK-22 cells with IL-2–containing medium induced the appearance of cells producing IFN-γ and expressing the NK cell cytotoxicity receptors NKG2D and CD94. Moreover, NK-22 cells cultured with IL-2 plus IL-7 became responsive to IL-12. Because NK-22 cells cultured in vitro with IL-2 plus IL-7 retained the expression of RORγt transcript, we envision that the IFN-γ–producing cells derive, at least in part, from the functional deviation of NK-22 cells. Although the initial CD56+NKp44+CCR6+CD103− cells were fairly homogenous, we cannot exclude the possibility that some of the IFN-γ–producing cells may derive from the expansion of another NK cell subset included within the original population. Consistent with the func-tional plasticity of NK-22 cells, a recent study showed that LTi-like cells isolated from human tonsils and cultured in IL-2 secrete IFN-γ (9). Interestingly, our results show that IL-2 also may be an important factor in promoting the secretion of LIF, a cytokine that has been shown to protect epithelial cells against bacterial infections (20). The functional deviation of NK-22 cells toward IFN-γ production is reminiscent of that of TH17 T cells, which also can convert into IFN-γ–producing TH1 T cells (23–26). Further supporting the functional flexibility of NK-22 cells, we found that continuous presence of IL-23 in addition to IL-1β and IL-7 in the culture medium enhanced NK-22 cell production of IFN-γ and IL-22 and suppressed IL-17. These effects of IL-23 on NK-22 cell polarization are remarkably similar to those observed when IL-23 is used to culture TH17 cells in the absence of TGF-β (24).
Whether NK-22 cells can be classified as NK cells has been matter of debate (27). Our data on RORγt expression in NK-22 cells and conventional NK cells suggest that they may be developmentally distinct cell subsets. However, in the presence of IL-2, NK-22 cells and conventional NK cells exhibit similar functions, such as IFN-γ production. It is likely that in certain pathological conditions both NK-22 and classical NK cells converge in promoting IFN-γ–mediated immune responses and, probably, cytotoxicity, despite deriving from distinct developmental pathways.
NK-22 cells have been proposed to contribute to gut innate defense through the secretion of IL-22, IL-26, and LIF (1). This study also indicates that NK-22 cells may contribute to mucosal adaptive immunity. NK-22 cells release large amounts of soluble BAFF, particularly when grown in IL-7 and in IL-7 plus IL-1β, and express CXCR5, which can direct NK-22 cells toward the B-cell areas of lymphoid aggregates. Thus, NK-22 cells may con-tribute to adaptive responses, promoting B-cell survival in lymphoid aggregates and enhancing the generation of protective antibodies, such as mucosal IgA.
Materials and Methods
Cell Preparation and Sorting.
Single-cell suspensions of tonsil cells were prepared as previously described (1). CD56+ cells were enriched by microbeads magnetic isolation (Miltenyi Biotec). Cells were stained with anti-CD56, -NKp44, -CCR6, and -CD103, and sorted on a FACSAria II (BD Biosciances).
Cell Culture, Cytokines, Flow Cytometry, and Quantification of mRNA.
Sorted cells (2–4 × 104/well) were plated in 96 U-bottom plates in RPMI medium supplemented with 10% FCS (HyClone), GlutaMAX I, kanamycin, sodium pyruvate, nonessential amino acids (all from Invitrogen), and 10−5 M β-mercaptoethanol. IL-7 (50 ng/mL; Peprotech), IL-1β (50 ng/mL; Peprotech), IL-23 (50 ng/mL; R&D), and IL-2 (1,000 U/mL; Roche) were added for long-term culture or acute stimulation. BrdU incorporation was measured by the APC BrdU Flow Kit (BD). Human ELISA IL-22 construction kit was purchased from Antigenix America. ELISAs for BAFF and LIF were purchased from R&D. A detailed description of the antibodies used for flow cytometry is given in the SI Text. RORC, IL-17A, and GAPDH mRNA levels were quantified by real-time PCR, using the procedure and primers described in the SI Text.
Supplementary Material
Supporting Information
Acknowledgments
We thank Todd Fehniger, Tom Hannan, Melissa Swiecki, and Susan Gilfillan for critically reviewing the manuscript and the Cell Sorting Facility of the Department of Pathology and Immunology, Washington University School of Medicine, for excellent cell sorting. This work was supported by Grant A1067854 from the National Institute of Allergy and Infectious Diseases Center for HIV/AIDS Vaccine Immunology.
Footnotes
The authors declare no conflict of interest.
References
- 1.Cella M, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Aujla SJ, Kolls JK. IL-22: A critical mediator in mucosal host defense. J Mol Med. 2009;87:451–454. doi: 10.1007/s00109-009-0448-1. [DOI] [PubMed] [Google Scholar]
- 4.Zenewicz LA, Flavell RA. IL-22 and inflammation: Leukin’ through a glass onion. Eur J Immunol. 2008;38:3265–3268. doi: 10.1002/eji.200838655. [DOI] [PubMed] [Google Scholar]
- 5.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 6.Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev. 2006;214:56–72. doi: 10.1111/j.1600-065X.2006.00451.x. [DOI] [PubMed] [Google Scholar]
- 7.Hughes T, et al. Stage 3 immature human natural killer cells found in secondary lymphoid tissue constitutively and selectively express the TH 17 cytokine interleukin-22. Blood. 2009;113:4008–4010. doi: 10.1182/blood-2008-12-192443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cupedo T, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 9.Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med. 2010;207:281–290. doi: 10.1084/jem.20091509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh-Takayama N, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Sanos SL, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10:83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luci C, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 13.Satoh-Takayama N, et al. IL-7 and IL-15 independently program the differ-entiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J Exp Med. 2010;207:273–280. doi: 10.1084/jem.20092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu XL, et al. Crystal structure of HAb18G/CD147: Implications for immunoglobulin superfamily homophilic adhesion. J Biol Chem. 2008;283:18056–18065. doi: 10.1074/jbc.M802694200. [DOI] [PubMed] [Google Scholar]
- 15.Guezguez B, et al. Dual role of melanoma cell adhesion molecule (MCAM)/CD146 in lymphocyte endothelium interaction: MCAM/CD146 promotes rolling via microvilli induction in lymphocyte and is an endothelial adhesion receptor. J Immunol. 2007;179:6673–6685. doi: 10.4049/jimmunol.179.10.6673. [DOI] [PubMed] [Google Scholar]
- 16.Knoop KA, et al. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol. 2009;183:5738–5747. doi: 10.4049/jimmunol.0901563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurence A, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGeachy MJ, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinton LJ, et al. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2008;38:699–706. doi: 10.1165/rcmb.2007-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 22.Müller G, Höpken UE, Lipp M. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol Rev. 2003;195:117–135. doi: 10.1034/j.1600-065x.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 23.Bending D, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bluestone JA, Mackay CR, O'Shea JJ, Stockinger B. The functional plasticity of T cell subsets. Nat Rev Immunol. 2009;9:811–816. doi: 10.1038/nri2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: New players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9:229–234. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information