Protein turnover differences between neurons and other cells (original) (raw)
. Author manuscript; available in PMC: 2011 Jan 1.
Published in final edited form as: Autophagy. 2009 Oct;5(7):1037–1038. doi: 10.4161/auto.5.7.9291
Abstract
In a recent study, we investigated the relationship between formation of an inclusion body (IB) and activity of the ubiquitin-proteasome system (UPS) in a primary neuron model of Huntington disease. We applied single-cell longitudinal acquisition and analysis to simultaneously monitor mutant huntingtin, which causes Huntington disease, IB formation, UPS function, and neuronal toxicity. We found that proteasome inhibition is toxic to striatal neurons in a dose-dependent fashion. The UPS is more impaired in neurons that go on to form IBs than in those that do not; however, after IBs form, UPS function improves. Our findings suggest that IBs are a protective cellular response to mutant protein mediated, in part, by improving intracellular protein degradation. The study also revealed some surprising differences in the ways that neurons regulate protein turnover compared with non-neuronal cells, which we discuss further in this article.
Keywords: Huntington disease, autophagy, neurodegeneration, rapamycin, everolimus, LC3
To determine if concurrent changes in autophagy affected our measurement of UPS activity, we examined the activity of the autophagic pathway after treatment with the UPS inhibitor epoxomicin. We found that levels of LC3-II, which indicate the extent of autophagy, were unchanged in primary striatal neurons, suggesting no upregulation of autophagy. However, consistent with previous reports, proteasome inhibition in HEK293 cells led to LC3-II accumulation. We concluded that autophagy regulation in neurons might be different than in other cell types.
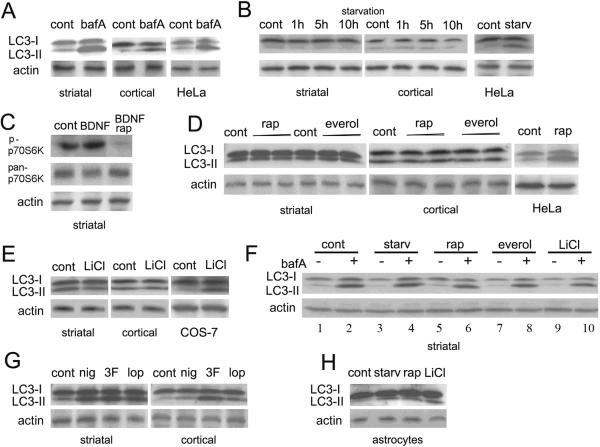
Although implicated in neurodegeneration, autophagy has been characterized mostly in yeast and mammalian non-neuronal cells, and the few studies in neurons often disagree. We sought to determine if common autophagy enhancers would stimulate autophagy in cultured primary striatal and cortical neurons. First, we treated primary neurons with bafilomycin A1 to block the fusion of autophagosomes with lysosomes. LC3-II levels increased in primary rat neurons and HeLa cells, indicating autophagosome accumulation (Fig. 1A). These results suggest that autophagy is constitutively active in neurons and that fusion of autophagosomes to lysosomes is similar in all cells.
Figure 1.
Common inducers of autophagy in non-neuronal cells fail to stimulate autophagy in primary neurons. Relative intensities of LC3-I and LC3-II bands reflect levels of autophagy. (A) Bafilomycin A1 (bafA; 4 h, 1 nM) induced LC3-II accumulation in striatal and cortical neurons and in HeLa cells. (cont), control untreated cells. (B) Striatal and cortical neurons were incubated in Hanks' solution. Starvation (2 h in Hanks' solution) induced autophagy in HeLa cells but not neurons. Longer incubations gave similar results. (C) Pretreatment with rapamycin (2 μM) blocked BDNF-induced phosphorylation of p70S6K in striatal neurons. (D) Striatal and cortical neurons incubated in medium with the mTOR inhibitors 2 μM rapamycin (rap) or everolimus (everol) for 48 h. Shorter or longer incubations and higher and lower concentrations (2 nM to 20 μM) gave similar results (not shown). Rapamycin (2 μM, 24 h) induced autophagy in HeLa cells. (E) Lithium chloride (LiCl, 10 mM) induced autophagy in COS-7 cells but not neurons. (F) Autophagy inducers did not increase the flux through the autophagic pathway. In striatal neurons, inhibition of lysosomal degradation with bafilomycin A1 (overnight) led to LC3-II accumulation (compare lanes 1 and 2). Starvation of these treated cells did not increase LC3-II levels (compare lanes 2, 3, and 4). Incubation of these treated cells with 2 μM rapamycin (compare 2, 5, 6), 2 μM everolimus (compare 2, 7, 8), or 10 mM LiCl (compare 2, 9, 10) did not increase LC3-II levels. (G) Striatal and cortical neurons were incubated in medium with niguldipine (nig, 4 μM), trifluoperazine (3F, 8 μM), or loperamide (lop, 5 μM) (overnight). Note LC3-II accumulation. (H) Starvation (starv, Hanks' solution, 8 h), rapamycin (rap, 2 μM, 24 h), and lithium chloride (LiCl, 10 mM) induced autophagy in astrocytes.
We then determined if autophagy is induced similarly in neurons and non-neuronal cells. Others showed that GFP-LC3 transgenic mice exhibited no autophagy in the brain after starvation. But was this because the brain is protected from starvation? To ensure that neurons were deprived of nutrients, we eliminated contributions from glial cells (our cultures are 95% neurons) and homeostatic mechanisms outside the central nervous system. The pathways that mediate starvation-induced autophagy in neurons evidently differ from those in non-neuronal cells; starvation in Hank's balanced salt solution increased LC3-II levels in HeLa cells, but not in neurons (Fig. 1B).
We also assessed the effects of rapamycin. In neurons, rapamycin pretreatment blocked BDNF-induced phosphorylation of p70S6K (Fig. 1C), indicating mTOR inhibition. Next we determined if rapamycin or everolimus induced autophagy. As expected, rapamycin potently increased LC3-II levels in HeLa cells. However, neither chemical increased LC3-II levels in primary neurons (Fig. 1D). While surprising, this result is consistent with our observations in nutrient-deprived neurons. Even in non-neuronal cells, rapamycin effects are complex. Nanomolar concentrations completely inhibit mTOR activity, but only vast excesses induce autophagy in non-neuronal cells, suggesting rapamycin may act on additional cellular targets. In fly and mouse HD models, it attenuates mutant htt toxicity and promotes htt clearance. However, more experiments are needed to show that the benefits of rapamycin are due only to autophagy. In autophagy-deficient fibroblasts, for example, rapamycin apparently inhibits huntingtin aggregation by reducing protein synthesis.
Lithium chloride induces autophagy in non-neuronal cells by inhibiting inositol monophosphatase—a mechanism that is independent of mTOR. We found that lithium chloride effectively induced autophagy in control cells, but LC3-II did not accumulate in primary neurons (Fig. 1E), suggesting that neurons may differ somewhat from other cells in both mTOR-dependent and -independent mechanisms of autophagy.
Western blots show steady-state LC3-II levels but not the flux through the autophagic pathway. If autophagy basal rates are high in neurons, steady-state levels of autophagosomes and LC3-II might not change in neurons under autophagy-inducing conditions. To determine if flux through autophagy changes with drugs, we examined LC3-II levels with these drugs and bafilomycin A1, which blocks fusion of autophagosomes and lysosomes. We incubated primary neurons with bafilomycin A1, under starvation conditions, or with rapamycin, everolimus, or lithium chloride (Fig. 1F). Simultaneous application of bafilomycin A1 and drugs that induce autophagy in non-neuronal cells did not increase LC3-II accumulation in neurons over conditions in which only fusion is inhibited. Thus, starvation, rapamycin, everolimus, and lithium chloride do not significantly increase autophagic flux in primary neurons.
Since at least one study reports LC3-II accumulation in rapamycin-treated neurons, we thought perhaps our western blots might not detect LC3-II in neurons undergoing autophagy. We tested other small molecules that induce autophagy in non-neuronal cells. Niguldipine, trifluoroperazine, and loperamide robustly induced autophagy in primary neurons (Fig. 1G). Therefore, we conclude that the upregulation of autophagy, in principle, can be induced and detected in our system.
Is there another way to reconcile the different results? They might be affected by contaminating non-neuronal cells in the mixed primary cultures. To test this, we prepared pure primary cultures of astrocytes and starved them or treated them with rapamycin or lithium chloride. Like the cell lines, astrocytes responded by inducing autophagy (Fig. 1H).
These results raise the intriguing possibility that autophagy in neurons is regulated by mechanisms that differ, at least in part, from those in non-neuronal cells. Our results underscore the potential importance of using primary neurons to study the role of autophagy in neurodegeneration and the consideration of these potential differences in any efforts to target this pathway therapeutically.
Acknowledgments
This work was supported by R01 2NS039746 and 2R01 NS045191 from the National Institute of Neurological Disease and Stroke, P01 2AG022074 from the National Institute on Aging, the Taube-Koret Center for Huntington's Disease Research, and the J. David Gladstone Institutes (S.F.); a Milton Wexler Award and a fellowship from the Hereditary Disease Foundation (A.T.); NIH-NIGHMS UCSF Medical Scientist Training Program and a fellowship from the UC-wide adaptive biotechnology (GREAT) program (S.M.); and RR018928 from the National Center for Research Resources. We thank Dr. Walter Schuler (Novartis) for everolimus, helpful discussions, and bringing to our attention the difference in the doses of rapamycin that inhibit mTOR and those that are typically used to induce autophagy. We also thank Jayanta Debnath for LC3 antibodies and helpful discussions, Dr. Ana Maria Cuervo for helpful advice, and members of the Finkbeiner laboratory for helpful discussions. Kelley Nelson provided administrative assistance, and Gary C. Howard edited the manuscript.