Distribution of α1, α4, γ2, and δ subunits of GABAA receptors in hippocampal granule cells (original) (raw)
. Author manuscript; available in PMC: 2010 Jun 27.
Abstract
GABAA receptors are pentamers composed of subunits derived from the α, β, γ, δ, θ, ε, and π gene families. α1, α4, γ2, and δ subunits are expressed in the dentate gyrus of the hippocampus, but their subcellular distribution has not been described. Hippocampal sections were double-labeled for the α1, α4, γ2, and δ subunits and GAD65 or gephyrin, and their subcellular distribution on dentate granule cells was studied by means of confocal laser scanning microscopy (CLSM). The synaptic versus extrasynaptic localization of these subunits was inferred by quantitative analysis of the frequency of colocalization of various subunits with synaptic markers in high-resolution images. GAD65 immunoreactive clusters colocalized with 26.24±0.86% of the α1 subunit immunoreactive clusters and 32.35±1.49% of the γ2 subunit clusters. In contrast, only 1.58±0.13% of the α4 subunit immunoreactive clusters and 1.92±0.15% of the δ subunit clusters colocalized with the presynaptic marker GAD65. These findings were confirmed by studying colocalization with immunoreactivity of a postsynaptic marker, gephyrin, which colocalized with 27.61±0.16% of the α1 subunit immunoreactive clusters and 23.45±0.32% of the γ2 subunit immunoreactive clusters. In contrast, only 1.90±0.13% of the α4 subunit immunoreactive clusters and 1.76±0.10% of the δ subunit clusters colocalized with gephyrin. These studies demonstrate that a subset of α1 and γ2 subunit clusters colocalize with synaptic markers in hippocampal dentate granule cells. Furthermore, all four subunits, α1, α4, γ2, and δ, are present in the extrasynaptic locations.
Keywords: Subunit isoform, Synapse, Quantification, Gephyrin, GAD, Cluster
1. Introduction
The GABAA receptor gene family includes seven major classes of subunits with multiple subtypes: six types of α subunit, four types of β subunit, three types of γ subunit, one type of δ, one type each of θ, ε, and π subunit [2,27]. It is known that certain subunits cluster on postsynaptic membranes and mediate fast synaptic inhibition in the brain. For example, electron microscopic examination of GABAergic synapses in cerebellum and hippocampus reveals aggregates of α1, β2/3, and γ2 subunits at GABAergic synapses [16,19]. Conversely, post-embedding immunogold electron microscopic studies of cerebellar granule cells have demonstrated that α6 and δ subunits are expressed in the extrasynaptic membrane [11,19].
In addition to cerebellar granule cells, dentate granule cells in the hippocampus also express the δ subunit mRNA and polypeptide [3,32]. The α6 subunit is not expressed in these neurons and the δ subunit is believed to combine with the α4 subunit to form functional receptors [34]. A recent study of mouse dentate granule cells demonstrated that GABAA receptors containing the δ subunit are expressed in the perisynaptic region [35]. In addition to the α4 and δ subunits, dentate granule cells express α1 and γ2 subunits. Previous studies suggest that the α1 subunit is expressed at synapses [16]. Recent electrophysiological studies in hippocampal dentate granule cells suggest the existence of synaptic and extrasynaptic GABAA receptors in these neurons [14,33]. These studies suggested that GABAA receptors with distinct subunit compositions are present in either synaptic or extrasynaptic locations. Taken together, these studies suggest that in dentate granule cells, the distribution of α1, γ2 subunit-containing receptors is distinct from that of receptors containing α4, δ subunits; receptors containing α1, γ2 subunits are expressed at synapses, and α4, δ subunits are expressed at extrasynaptic membrane.
While the distribution of α1, α4, γ2, and δ subunits in dentate gyrus has been described using light microscopy, few studies have compared the distribution of these subunits using high-resolution confocal laser scanning microscopy (CLSM) [29]. Furthermore, the colocalization of these subunits with GABAergic synaptic markers, GAD65 and gephyrin, in hippocampal dentate granule cells has not been assessed. We used double-label fluorescence immunohistochemistry of the hippocampus visualized with CLSM, to study the distribution of α1, α4, γ2, and δ subunits in hippocampal dentate granule cells. High-resolution images of granule cell layer in sections double-labeled for various subunits and synaptic markers GAD65 or gephyrin were analyzed quantitatively.
2. Experimental procedures
2.1. Animal and tissue preparation and immunohistochemistry
Experiments were performed using 11 male adult Sprague–Dawley rats weighing 250–350 g. Six animals were used to study the colocalization of GABAA receptor subunits with GAD; and five animals were used to study colocalization with gephyrin. Animals were housed at 22 °C, two per cage, on a standard light–dark schedule with free access to food and water. Animals were handled according to NIH Animal Care and Use Guidelines and a protocol approved by the University of Virginia Animal Care and Use Committee.
Animals were injected with a lethal dose of pentobarbitone sodium (220 mg/kg, i.p.) and perfused through the ascending aorta with 50–100 ml 0.9% NaCl, followed by 300 ml 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4); for the gephyrin study, 2% paraformaldehyde was used [28]. The brains were removed and post-fixed in the same fixative for 1 h at 4 °C. After overnight incubation in 30% sucrose in 0.1 M PB for cryoprotection, the brains were frozen by immersion in −70 °C isopentane and sectioned on a cryostat. Coronal sections (40 μm) containing dorsal hippocampus were processed for free floating immunohistochemistry. Sections from each animal were collected in seven vials in sequence, for control, α1, α4, γ2, and δ subunits immunohistochemistry, GAD and gephyrin. Following three washes in 0.1 M PB and preincubation with blocking solution containing 5% normal goat serum (Jackson Immunoresearch Laboratories, West Grove, PA) and 0.1% Triton X-100 in 0.1 M phosphate-buffered saline (PBS, pH 7.4) for 1 h, tissue sections were incubated with the primary antibodies at 4 °C for 72 h on a shaker. The primary and secondary antibodies were diluted in PBS containing 2% normal goat serum, 0.2% bovine serum albumin (Jackson Immunoresearch Laboratories). Subsequently, the sections were incubated in secondary antibodies of goat anti-rabbit conjugated with Alexa fluor 488 and goat anti-mouse conjugated with Alexa fluor 594 (concentration used: 5μg/ml, Molecular Probes, Eugene, OR) for 1 h on a shaker at room temperature in darkness. Sections were then mounted on slides with Gel/Mount* (Foster City, CA) and air dried, the edge of each coverslip was sealed with clear nail polish, and slides were stored at −20 °C. Controls in which the primary antibody was omitted provided only very weak nonspecific staining.
2.2. Antibodies
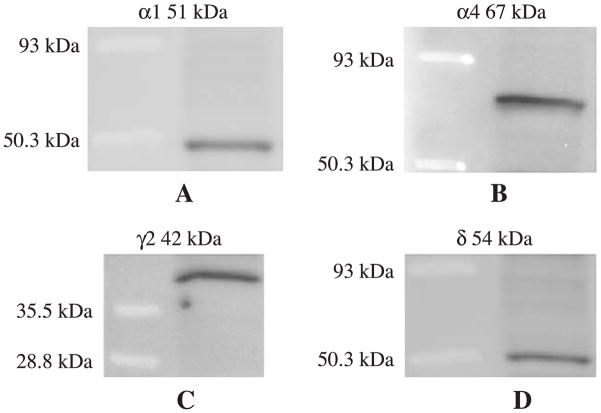
The antibody against the GABAA receptor subunit α1 (1–16) was obtained from Alomone labs (Israel). The antibody against α4 subunit (1–14) has been characterized previously [1,12,32]. The antibodies against the γ2 (1–33) and δ (1–44) subunit of the GABAA receptor have been characterized extensively in the past by immunoprecipitation, Western blot, and immunohistochemistry [11,19–22,32]. The antibodies against α1, α4, γ2 and δ were characterized by Western blot. Membrane proteins were collected from whole hippocampus, and Western blot was performed and visualized by chemiluminescence system. Each antibody recognized a single band (Fig. 1). The antibody anti-glutamate decarboxylase (GAD) was obtained from Chemicon (Clone GAD-6, MAB351R, Temecula, CA) and characterized [5,31,37]. Anti-gephyrin was obtained from BD Transduction Laboratories (clone 45, Franklin Lakes, NJ); this antibody has been characterized by Western blot. The different concentrations of primary antibodies were titrated to arrive at the final concentrations (1.5 μg/ml for α1; 2.0 μg/ml for γ2 and GAD; 2.5 μg/ml for gephyrin; 5 μg/ml for α4 and δ) used in this study.
Fig. 1.
Characterization of GABAA receptor subunit α1, γ2, α4, and δ by Western blot. (A) A single 51 kDa band of α1. (B) A 67 kDa band of α4. (C) A 42 kDa band of γ2 was seen. (D) A 54 kDa δ band was shown.
2.3. Image acquisition and analysis
Sections were studied on a Nikon PCM2000 confocal microscope system equipped with a Nikon TE-200 inverted epifluorescence microscope (Nikon, Melville, NY) with an Argon/HeNe laser. Images were acquired with an Orca-1 CCD Camera (Hamamatsu Photonics System, Middlesex, NY). Confocal and camera-based image acquisition and processing were driven by SimplePCI software (version 4.0.6, Compix, Cranberry Township, PA). Argonion and He/Ne lasers were used to visualize the fluorochromes, Alexa Fluor 488 and Alexa Fluor 594, respectively. Double-labeled sections were scanned by the simultaneous application of both excitation wavelengths. The intensity of each laser was optimized for the sections of each animal and each combination of antibodies. The parameters were kept constant for each animal. Controls included dual-channel recording of sections without antigen labeling and single antigen labeling to detect non-specific staining and bleed-through phenomena. All CLSM was performed at the W.M. Keck Center for Cellular Imaging at the University of Virginia.
Each hippocampal section was examined with a low-magnification objective lens (40×) in order to locate the dentate gyrus and the granule cell layer. In preliminary experiments, dentate gyrus sections were labeled with antibody against calbindin to identify granule cells; previous studies have shown that calbindin-like immunoreactivity was present in all dentate granule cells [30]. Sections were visualized using a Nikon plan Apo 40×/1.0 NA oil immersion objective; the molecular layer, granule cell layer, and hilar border were included in the images. For quantitative analysis of GABAA receptor immunoreactive clusters and their colocalization with GAD and gephyrin, high resolution digitized images (50 nm/pixel) were acquired using a Nikon plan Apo 100×/1.40 NA oil immersion objective, and an electronic zoom factor 2.3. All settings were kept constant throughout the analysis to yield unbiased measurements for each set of comparisons.
The immunoreactivity of GABAA receptor subunits and synaptic markers in dorsal hippocampal sections were analyzed with MetaMorph 6.03 software (Universal Imaging, PA, USA). The same threshold was applied to all images acquired from the sections of one brain in each experiment. Most of these antigens were revealed as individual clusters, which were considered as individual objects in binary images. The degree of colocalization was evaluated by comparing binary images for two antigens with a “logical and” function. An aggregation of 20 to 1000 pixels (0.05–2.5 μm2) of intensity at least twice the background intensity was considered a cluster. Preliminary analysis of the images in this study also demonstrated that clusters smaller than 0.05 μm2 rarely colocalized with synaptic markers. Data were analyzed using Prism 3.0 software (GraphPad Software, San Diego, CA). Unless specified otherwise, all values are reported as mean±S.E.M. The digital images selected for publication were processed in PhotoShop 6.0 (Adobe, San Jose, CA). For each antibody, a contrast/brightness setting was selected that yielded a high-resolution image for both bright and dim sections without exceeding a maximal pixel intensity of 255.
3. Results
3.1. Distribution of α1 and γ2 subunits
The distribution of GABAA receptor α1 subunit immunoreactivity in hippocampal dentate gyrus of adult rats was studied using CLSM. Interneurons at the hilar margin of the dentate gyrus were more intensely stained by anti-α1 subunit antibody than neurons in the granule cell layer (Fig. 2A, arrowhead). Previous studies have demonstrated that a subset of GABAergic interneurons expresses the α1 subunit at a higher level than the principal neurons [8,10]. Only moderately intense immunoreactivity was found in the granule cell layer, which consisted of punctate staining outlining the soma of granule cells, and dendrites passing through the granule cell layer (Fig. 2A). The molecular layer was more intensely stained than the granule cell layer, with distinct clusters of α1 subunit immunoreactivity extending along dendrites of granule cells (Fig. 2A).
Fig. 2.
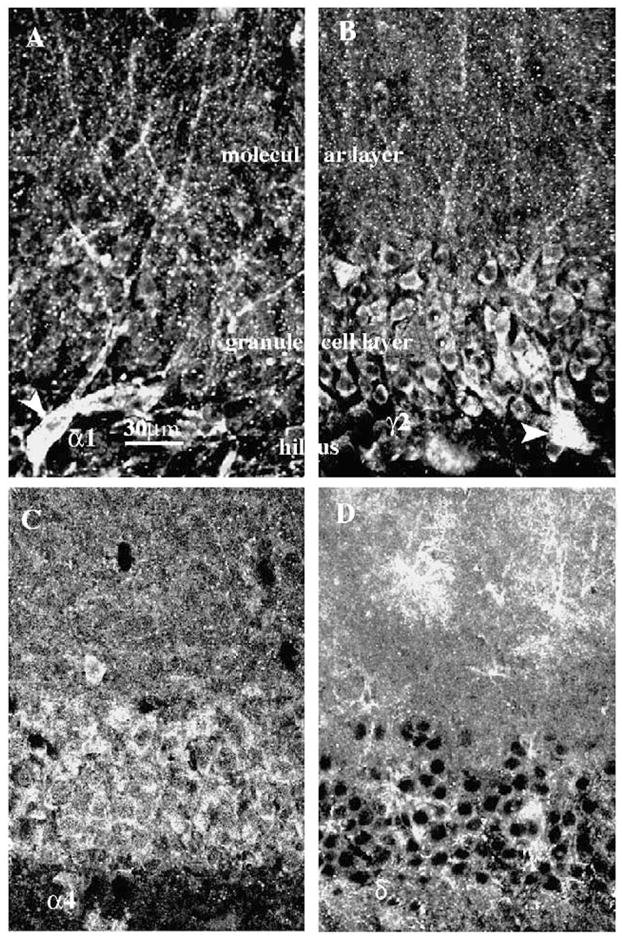
Distribution of GABAA receptor α1, γ2, α4, and δ subunit immunoreactivity in hippocampal dentate granule cells acquired by CLSM, 40× objective lens, scale bar =30 μm (shown in A). Sections were labeled with GABAA subunit isoform-specific antibodies and immunoreactivity was assessed in the form of clusters that outlined dentate granule cell soma. The immunoreactivity of α1 (A) and γ2 (B) subunits was similar, the somata and dendrites of interneurons near the hilar border were strongly labeled (A, B; arrowhead); fewer clusters were found in granule cell somata, molecular, and granule cell layers. The immunoreactivity of α4 (C) and δ (D) subunits was more diffuse and clusters were less obvious. Some interneurons in the molecular layer stained intensely for the δ subunit. Intense staining of the δ subunit was found in the molecular layer.
The distribution of the γ2 subunit immunoreactivity was similar to that of the α1 subunit (Fig. 2B). At the hilar border of the granule cell layer, the γ2 subunit immunoreactivity was present in somata of interneurons (Fig. 2B, arrowhead). The granule cell layer was stained moderately with γ2 subunit immunoreactivity distributed circumferentially around granule cells; clusters of γ2 subunit immunoreactivity were present along the dendrites in the molecular layer, too (Fig. 2B).
3.2. Distribution of α4 and γ subunit
The distribution of α4 subunit immunoreactivity in the dentate gyrus was quite distinct from that of the α1 and γ2 subunits (Fig. 2C). It was distributed diffusely over the dentate granule cell layer and the molecular layer. The immunoreactivity was more intense in the granule cell layer and the inner molecular layer, and less intense in the outer molecular layer. Neither distinct clusters of immunoreactivity nor outlines of cell body dendrites of granule cells were obvious at this magnification.
δ subunit immunoreactivity appeared to outline dentate granule cell bodies (Fig. 2D). Some clusters of the δ subunit were present but these were far sparser than that seen for the α1 and γ2 subunits. In contrast to the α4 subunit, the δ subunit immunoreactivity was more intense in the molecular layer than in the granule cell layer. Some interneurons in the molecular layer were intensely immunoreactive for the δ subunit but not for the α4 subunit. A detailed characterization of δ subunit expression in the interneurons was not performed.
3.3. Distribution of GAD65 and gephyrin
We wished to describe the colocalization of GABAA receptor subunit clusters with the synaptic markers, GAD65 and gephyrin. Hippocampal sections were stained for GAD65, the synthetic enzyme for GABA, which is known to be concentrated in the synaptic terminals of GABAergic neurons [4,23,26]. Punctate GAD65 immunostaining formed a dense network in the dentate granule cell layer and inner molecular layer, and less staining in the outer molecular layer of the dentate gyrus.
We tested whether gephyrin, which is present at GABAergic synapses [6,13,25], was also present in the dentate granule cell layer. Hippocampal sections were stained for gephyrin and the dentate gyrus was studied by CLSM. The most intense gephyrin staining occurred in the granule cell layer and in the cell bodies of interneurons in the hilus (Fig. 3B). Less intense staining was present in the molecular layer. In the cell soma of the dentate granule cells, there was diffuse low-intensity immunostaining and more intense punctate staining for gephyrin. This suggested that both pre- and postsynaptic markers of GABAergic synapses were present in the granule cell layer and molecular layer.
Fig. 3.
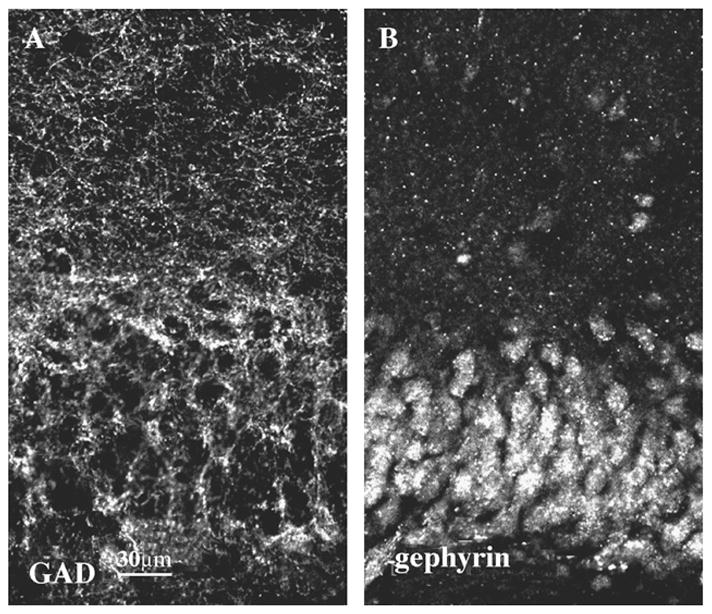
Distribution of GAD65 (A) and gephyrin (B) immunoreactivity in the dentate gyrus, scale bar =30 μm. There was intense punctate GAD65 staining in the granule cell layer and in the inner molecular layer, which formed a net of clusters (A). In the somata of dentate granule cells, there was diffuse low intensity gephyrin immunoreactivity and more intense punctate staining (B).
3.4. Colocalization of α1, γ2 subunit clusters with synaptic markers in high-resolution images
High magnification images of granule cells in hippocampal sections stained for α1 subunit immunoreactivity were examined (Fig. 4A,G). Clusters of α1 subunit immunoreactivity appeared to outline the cell somata of dentate granule cells and were of various sizes (0.05 to 1.0 μm2). There were more small clusters than large clusters. Similar to the α1 subunit clusters, the γ2 subunit clusters were distributed in the periphery of granule cell soma and both small and large clusters were present (Fig. 4D,J). Similar to the α1 subunit immunoreactive clusters, small γ2 subunit clusters were more common than larger clusters.
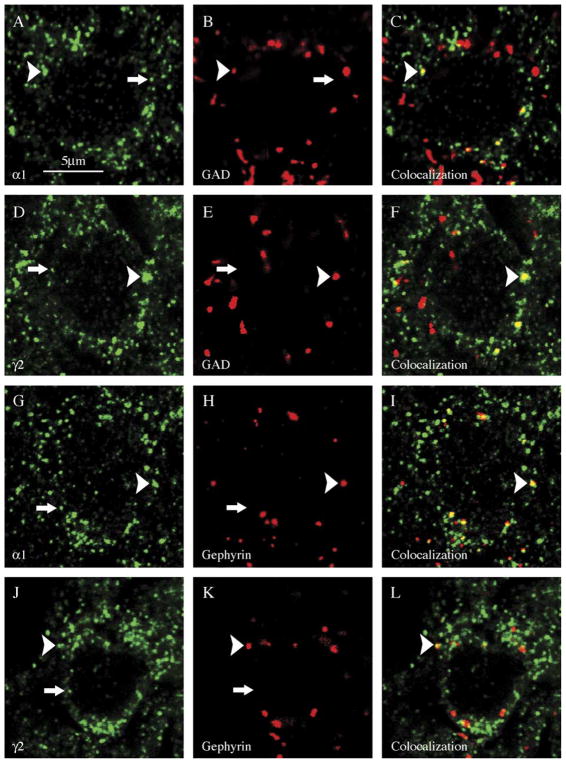
Fig. 4.
Large clusters of α1 or γ2 GABAA receptor subunits frequently colocalized with synaptic makers (arrowhead), whereas most small clusters did not (arrow). Hippocampal dentate granule cells were double-labeled with the presynaptic marker GAD (B, E) or the postsynaptic marker gephyrin (H, K) and GABAA subunit isoform-specific antibodies to α1 (A, G) or γ2 (D, J). Merged images of the markers and subunits are shown in C, F, I, and L. GABAA subunits were visualized by secondary antibody conjugated with Alexa Fluor 488 (green) and synaptic markers with Alexa Fluor 594 (red). Scale bar =5 μm.
In the same sections, the distribution of GAD65 clusters was studied. Clusters of GAD65 immunoreactivity outlined the somata of granule cells. The GAD65 clusters were larger in size and fewer than the α1 and γ2 subunit clusters. The median size of GAD65 clusters was 0.15 μm2 and the mean size was 0.212 μm2. In high magnification images, the distribution of gephyrin immunoreactivity in dentate granule cell somata was similar to that of GAD65. Gephyrin formed large clusters that outlined somata of dentate granule cells. Previous studies have described similar punctate distribution of gephyrin in dentate granule cells [28].
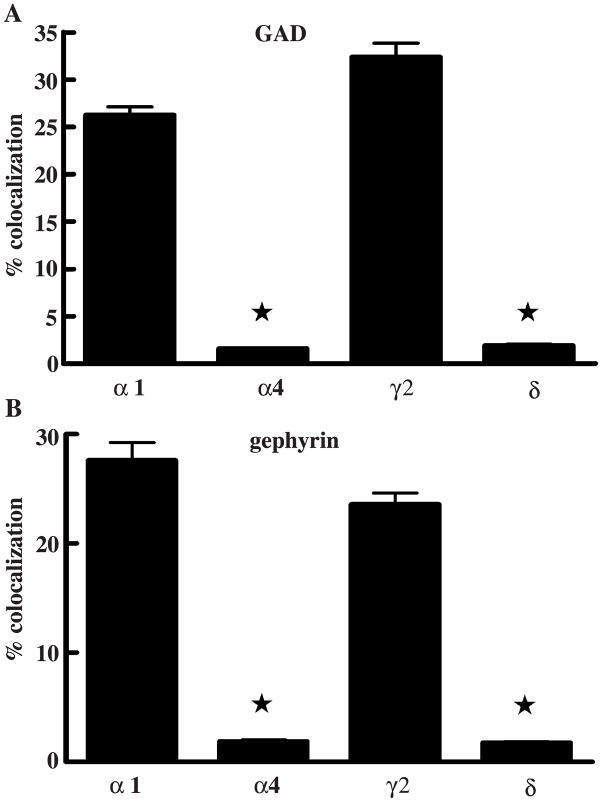
We examined colocalization of the immunoreactivity of GAD65 with clusters of α1 and γ2 subunit immunoreactivity (Fig. 5A). The degree of colocalization of GABAA receptor α1 subunit clusters was 26.24±0.86% (_n_=6). Similar to the α1 subunit, that of γ2 subunit clusters colocalized with GAD65was 32.35±1.49% (_n_=8). The majority of GABAA receptor subunits did not colocalize with the presynaptic marker GAD65 in hippocampal dentate granule cells of the adult rat. To further confirm the finding, double-labeling of gephyrin and the GABAA receptor subunits α1 or γ2 was studied (Fig. 5B). The degree of colocalization of the α1 subunit clusters with gephyrin was 27.61±1.64% (_n_=5) while that of γ2 subunit clusters was 23.45±0.32% (_n_=8).
Fig. 5.
Percent colocalizaton of different GABAA receptor subunits with the synaptic markers GAD65 and gephyrin is demonstrated. The percent fraction colocalization of GABAA receptor α1 or γ2 subunits with GAD65 was significantly higher than those of α4 or δ subunits, respectively (*p<0.0001, A). The percent fraction of colocalization of GABAA receptor α1 or γ2 with gephyrin are significantly higher than those of α4 or δ subunits, respectively (*p<0.0001, B).
3.5. Cluster size and colocalization with synaptic markers
We examined whether GABAA receptor clusters of different sizes had different associations with synaptic markers by analyzing the correlation of subunit cluster size with the degree of colocalization with GAD65. Large α1 subunit clusters commonly colocalized with GAD (arrowhead, Fig. 4A–C), whereas small clusters did not (arrow, Fig. 4A–C). There was a strong correlation between the degree of colocalization and α1 subunit cluster size (non-parametric Spearman correlation coefficient _r_=0.92, p<0.0001). Similar to the α1 subunit, most of the large γ2 subunit clusters colocalized with GAD (arrowhead in Fig. 4D–F), whereas small clusters did so rarely (arrow, Fig. 4D–F). The fraction of clusters colocalizing with GAD increased with the cluster size (nonparametric Spearman correlation coefficient _r_=0.88, p<0.0001).
The preferential colocalization of large clusters of α1 and γ2 subunit immunoreactivity was confirmed by analyzing colocalization with gephyrin. Gephyrin clusters colocalized with large α1 subunit clusters commonly (arrowhead, Fig. 4G–I), but did not colocalize with small ones (arrow, Fig. 4G–I). Similar to α1 subunit colocalization with gephyrin, large γ2 subunit clusters colocalized commonly with gephyrin (arrowhead in Fig. 4J–L), but small ones did so rarely (arrow, Fig. 4J–L). There was a linear correlation between the degree of colocalization and γ2 subunit cluster size (nonparametric Spearman correlation coefficient _r_=0.74, p<0.001). The mean area of α1 subunit clusters that colocalized with GAD65 (0.122±0.010 μm2) was similar to the size of α1 subunit clusters that colocalized with gephyrin (0.133±0.008 μm2, _p_=0.3876, grouped _t_-test). Similarly, the mean area of γ2 subunit clusters that colocalized with GAD65 (0.124±0.006 μm2) was similar to the size of γ2 subunit clusters that colocalized with gephyrin (0.128±0.008 μm2, _p_=0.694, _t_-test). In addition, all four measures of the cluster size that colocalized with the synaptic marker were compared using a two-way ANOVA test; and there was no significant difference between any two pairs of values. While the absence statistical difference does not prove similarly and the possibility of type II error remains, these comparisons suggested that large clusters of α1 and γ2 subunit immunoreactivity that colocalized with synaptic markers were a distinct subset of all clusters.
3.6. Relationship of α4 and δ subunit immunoreactivity to synaptic markers
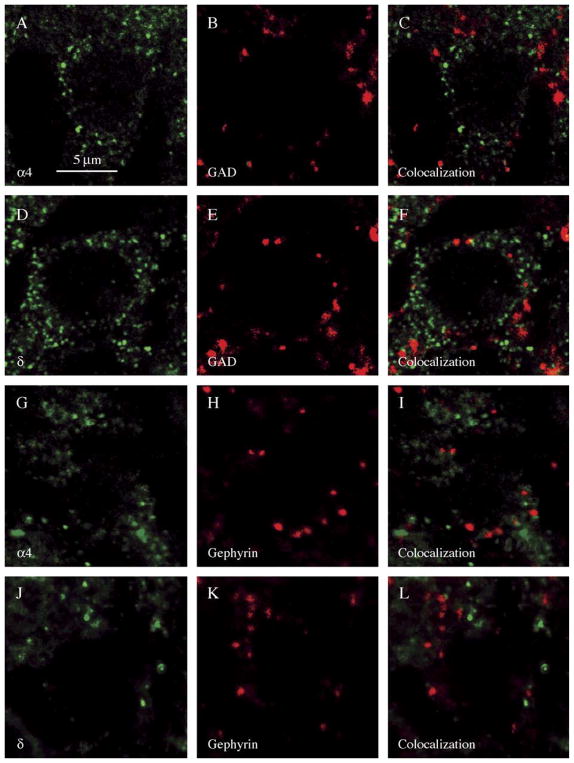
The distribution of α4 subunit clusters and GAD65 clusters in the granule cell layer was examined from double-labeled hippocampal sections. The α4 clusters were distributed circumferentially around the granule cell (Fig. 6A–C). The colocalization of α4 subunit clusters and GAD65 clusters was analyzed. Using the same criteria used to define colocalization of α1 and γ2 subunits with synaptic markers, we found that 1.58±0.13% (_n_=5) of all α4 subunit clusters colocalized with GAD65 clusters (Fig. 5A), which was significantly lower than the degree of colocalization of α1 subunit clusters (_n_=6), 26.24±0.86% (grouped _t_-test, p<0.0001).
Fig. 6.
Clusters of α4 and δ GABAA receptor subunits rarely colocalized with synaptic makers. Hippocampal dentate granule cells were double-labeled with the presynaptic marker GAD (B, E) or the postsynaptic marker gephyrin (H, K) and GABAA subunit isoform-specific antibodies to α4 (A, G) or δ (D, J). GABAA subunits were visualized by secondary antibody conjugated with Alexa Fluor 488 (green) and synaptic markers with Alexa Fluor 594 (red). Merged images of synaptic markers and subunits are shown in C, F, I, and L. Scale bar =5 μm.
The distribution of δ subunit clusters and GAD65 clusters in the granule cell layer was examined from double-labeled hippocampal sections. The δ subunit clusters were distributed circumferentially around the granule cell. The colocalization of δ subunit and GAD65 clusters was analyzed (Fig. 6D–F). Only 1.92±0.15% (_n_=7) of δ subunit clusters colocalized with GAD65 clusters (Fig. 5A), which was significantly lower than the degree of colocalization of γ2 clusters (_n_=8), 32.35±1.49% (_t_-test, p<0.0001).
These findings suggested that α4 and δ subunit clusters rarely colocalized with the synaptic marker GAD65. This was confirmed using the synaptic marker gephyrin. As demonstrated in Fig. 6G–I, α4 subunit clusters rarely colocalized with gephyrin clusters. The fraction of α4 subunit clusters that colocalized with gephyrin was 1.90±0.13% (_n_=7) (Fig. 5B), which was significantly less than the fraction of α1 clusters (_n_=5) colocalized with gephyrin 27.61±0.16% (_t_-test, p<0.0001). Similar to the α4 subunit clusters, δ subunit clusters rarely colocalized with gephyrin clusters (Fig. 6J–L). The fraction of δ subunit clusters colocalized with gephyrin was 1.76±0.10% (_n_=8) (Fig. 5B). In contrast, 23.45±0.32% (_n_=8) of γ2 subunit clusters colocalized with gephyrin. The difference was significant (_t_-test, p<0.0001).
4. Discussion
The distribution of GABAA receptor α1, α4, γ2, and δ subunits and synaptic markers GAD65 and gephyrin in rat hippocampal dentate gyrus was described using high-resolution CLSM. Quantitative analysis of high-resolution images of the granule cell layer in sections double-labeled for various subunits and the synaptic markers revealed that a distinct fraction of α1 and γ2 immunoreactivity colocalized with synaptic markers, which was in the form of large clusters. In contrast, the α4 and δ subunit immunoreactivity rarely colocalized with the synaptic markers. This study further suggested that α1 and γ2 subunits are also expressed in extrasynaptic locations in hippocampal dentate granule cells.
4.1. Subcellular distribution of α1 and γ2 subunits
The cellular distribution of the α1 subunit in the dentate gyrus was similar to that described in detail in the past [8,10,16,29,32]. The distribution of the γ2 subunit was similar to that of the α1 subunit and it is possible that the two antibodies detected similar sets of GABAA receptors on granule cells. The γ2 subunit commonly co-assembles with the α1 subunit to form the BZ type I benzodiazepine site in GABAA receptors [36]. The α1 subunit has been localized to GABAergic synapses in dentate granule cells [16] and the γ2 subunit is essential for clustering of GABAA receptors at synapses [9]. While the distribution of α1 and γ2 subunits was similar, the current study did not determine whether these subunits co-assemble into functional receptors.
Quantitative analysis revealed that large clusters of α1 and γ2 subunits detected by CLSM are present at synapses. The mean area of α1 clusters that colocalized with the presynaptic marker GAD65 was similar to that colocalized with postsynaptic marker gephyrin, and similar to the mean area of γ2 subunit clusters that colocalized with GAD65 and gephyrin, ranging from 0.12 to 0.13 μm2 in size. The size of synaptic clusters of GABAA receptors determined by the current study was within the range of that determined by electron microscopy. Post-embedding immunogold electron microscopic studies of cerebellar interneurons suggested that the area of GABAergic synapses is 0.152 μm2 and the size distribution is skewed [17]. In hippocampal dentate granule cells, quantitative immunogold studies estimated the area of GABAergic synapses on the cell soma to be 0.044 μm2, and in the axon initial segment, 0.042 μm2 [18]. Cluster size is not the only criterion for making the distinction between synaptic and extrasynaptic receptors because not all large clusters of α1 and γ2 subunits colocalized with the synaptic markers, and α4 and δ subunit immunoreactivity did not colocalize with the synaptic markers, regardless of the cluster size. These studies suggest that the most reliable criterion for detecting synaptic clusters of GABAA receptors when using CLSM is to determine colocalization with GAD65 and gephyrin clusters. Recent studies using double-label immunohistochemistry combined with CLSM have also demonstrated that large clusters of α1 and γ2-subunit colocalize with synaptic markers. In one study, gephyrin was found to colocalize extensively with GABAA receptor clusters larger than 0.1 μm2 [25]. These results are similar to those of our study, which found extensive colocalization of gephyrin with large GABAA receptor clusters. While this and other studies have found colocalization of gephyrin with GABAA receptors, this colocalization does not arise from direct binding of two molecules. While gephyrin is involved in GABAA receptor clustering it does not directly bind to the receptor protein [24].
Larger clusters may have colocalized more often with synaptic markers than smaller clusters because of their size. However, frequency of occurrence of specific clusters would also impact on likelihood of random colocalization. In fact, small clusters were much more common than larger clusters. We did not construct a mathematical model to test the impact of cluster size and frequency of colocalization, qualitatively it is possible that these two effects cancel each other. In addition, low degree of colocalization of α4 and δ subunits with synaptic markers suggests the colocalization event is not purely random.
A substantial fraction of α1 and γ2 subunit clusters did not colocalize with synaptic markers. These aggregates of immunoreacitvity were smaller than those that colocalized with synaptic markers. Previous electron microscopic studies of cat and rat dentate granule cells have reported extrasynaptic GABAA receptors containing α1 and β2/3 subunits [16]. A quantitative immunogold study compared the relative density of α1 and β2/3 subunits in synaptic and extrasynaptic membrane of cerebellar granule cells [15] and found that these subunits are concentrated in synapses but are present also in the extrasynaptic membrane. Studies using double-label immunohistochemistry combined with CLSM have also demonstrated that many clusters of α1 and γ2 subunits do not colocalize with synaptic markers. In one study [7], colocalization of α2 or γ2 subunit clusters with gephyrin was evaluated in the inferior olivary nucleus; 22% of α2 subunit clusters and 36% of γ2 subunit clusters did not colocalize with gephyrin clusters.
4.2. Distribution of α4 and δ subunits
Quantitative evaluation of high-resolution images revealed that α4 and δ immunoreactivity rarely colocalized with the synaptic markers GAD65 and gephyrin. This colocalization degree was far lower than that of α1 and γ2 subunits suggesting that α4 and δ subunit containing receptors were not present at synapses. The colocalization of the α4 or δ subunit with synaptic markers has not been investigated by means of CLSM in the past but a recent ultrastructural study demonstrated that immunogold-labeled δ subunits were located in the perisynaptic region in the molecular layer of dentate granule cells [35]. Furthermore, electrophysiological studies demonstrate that the properties of tonic inhibition are similar to the properties of GABAA receptors containing α4 and δ subunits. Other ultrastructural studies in cerebellar granule cells have demonstrated that the δ subunit is located on the extrasynaptic membrane.
GABAergic synapses have been studied by means of electron microscopy in the past but only a limited number of GABAA receptor subunits, synapses, and cell types have been studied with this technique. Recently, CLSM has been used to study the spatial distribution of GABAA receptors and gephyrin [25,29]. CLSM offers the advantage that a large number of neurons can be sampled using immunohistochemical techniques. However, the use of CLSM also has certain limitations. The resolution of confocal microscopy is insufficient to distinguish between the receptors expressed on the cell membrane from those expressed in the intracellular compartments. In addition, hippocampal sections were treated with a detergent to improve antibody penetration, further preventing the separation of surface receptors from intracellular receptors.
In summary, these studies demonstrate that a subset of GABAA receptor α1 and γ2 subunit clusters colocalize with synaptic markers in hippocampal dentate granule cells. Furthermore, all four subunits, α1, α4, γ2, and δ, are present in the extrasynaptic locations. CLSM combined with double label immunohistochemistry offers a method for localizing neurotransmitter receptors to synapses.
Acknowledgments
Public Health Service Grants from NINDS (RO1 NS40337, and RO1NS044370) and a grant from the Epilepsy Foundation through the generous support of the American Epilepsy Society and the Milken Family Foundation supported this work. We thank Dr. A Periasamy and the staff of the Keck Center for Cellular Imaging for their assistance with confocal laser microscopy. We thank Drs. Kevin Kelly and Howard Goodkin for reviewing the manuscript.
References
- 1.Bencsits E, Ebert V, Tretter V, Sieghart W. A significant part of native gamma-aminobutyric acid A receptors containing alpha 4 subunits do not contain gamma or delta subunits. J Biol Chem. 1999;274:19613–19616. doi: 10.1074/jbc.274.28.19613. [DOI] [PubMed] [Google Scholar]
- 2.Bonnert TP, McKernan RM, Farrar S, Le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Brown N, Wafford KA, Whiting PJ. Theta, a novel gamma-aminobutyric acid type A receptor subunit. Proc Natl Acad Sci U S A. 1999;96:9891–9896. doi: 10.1073/pnas.96.17.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective Changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med. 1998;4:1166–1172. doi: 10.1038/2661. [DOI] [PubMed] [Google Scholar]
- 4.Brunig I, Suter A, Knuesel I, Luscher B, Fritschy JM. GABAergic terminals are required for postsynaptic clustering of dystrophin but not of GABAA receptors and gephyrin. J Neurosci. 2002;22:4805–4813. doi: 10.1523/JNEUROSCI.22-12-04805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang YC, Gottlieb DI. Characterization of the proteins purified with monoclonal antibodies to glutamic acid decarboxylase. J Neurosci. 1988;8:2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig AM, Banker G, Chang W, McGrath ME, Serpin-skaya AS. Clustering of gephyrin at GABAergic but not glutamatergic synapses in cultured rat hippocampal neurons. J Neurosci. 1996;16:3166–3177. doi: 10.1523/JNEUROSCI.16-10-03166.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devor A, Fritschy JM, Yarom Y. Spatial distribution and subunit composition of GABAA receptors in the inferior olivary nucleus. J Neurophysiol. 2001;85:1686–1696. doi: 10.1152/jn.2001.85.4.1686. [DOI] [PubMed] [Google Scholar]
- 8.Esclapez M, Chang DK, Houser CR. Subpopulations of GABA neurons in the dentate gyrus express high levels of the alpha 1 subunit of the GABAA receptor. Hippocampus. 1996;6:225–238. doi: 10.1002/(SICI)1098-1063(1996)6:3<225::AID-HIPO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 9.Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the gamma 2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- 10.Gao B, Fritschy JM. Selective allocation of GABAA receptors containing the alpha 1 subunit to neurochemically distinct subpopulations of rat hippocampal interneurons. Eur J Neurosci. 1994;6:837–853. doi: 10.1111/j.1460-9568.1994.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 11.Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Makela R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD, Sieghart W, Somogyi P, Smith AJ, Wisden W. Ligand-gated ion channel subunit partnerships: GABAA receptor alpha6 subunit gene inactivation inhibits delta subunit expression. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kern W, Sieghart W. Polyclonal antibodies directed against an epitope specific for the alpha 4-subunit of GABAA receptors identify a 67-kDa protein in rat brain membranes. J Neurochem. 1994;62:764–769. doi: 10.1046/j.1471-4159.1994.62020764.x. [DOI] [PubMed] [Google Scholar]
- 13.Levi S, Chesnoy-Marchais D, Sieghart W, Triller A. Synaptic control of glycine and GABAA receptors and gephyrin expression in cultured motoneurons. J Neurosci. 1999;19:7434–7449. doi: 10.1523/JNEUROSCI.19-17-07434.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- 15.Nusser Z, Roberts JD, Baude A, Richards JG, Somogyi P. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. J Neurosci. 1995;15:2948–2960. doi: 10.1523/JNEUROSCI.15-04-02948.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nusser Z, Roberts JDB, Baude A, Richards JG, Sieghart W, Somogyi P. Immunocytochemical localization of the α1 and β2/3 subunits of the GABAA receptor in relation to specific GABAergic synapses in the dentate gyrus. Eur J Neurosci. 1995;7:630–646. doi: 10.1111/j.1460-9568.1995.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 17.Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABAA receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- 18.Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of GABAA receptors underlies potentiation of hippocampal synapses. Nature. 1998;395:172–177. doi: 10.1038/25999. [DOI] [PubMed] [Google Scholar]
- 19.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nusser Z, Ahmad Z, Tretter V, Fuchs K, Wisden W, Sieghart W, Somogyi P. Alterations in the expression of GABAA receptor subunits in cerebellar granule cells after the disruption of the alpha6 subunit gene. Eur J Neurosci. 1999;11:1685–1697. doi: 10.1046/j.1460-9568.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- 21.Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, Houser CR. GABAA receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- 22.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101(4):815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 23.Rao A, Cha EM, Craig AM. Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons. J Neurosci. 2000;20:8344–8353. doi: 10.1523/JNEUROSCI.20-22-08344.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sassoe-Pognetto M, Fritschy JM. Mini-review: gephyrin, a major postsynaptic protein of GABAergic synapses. Eur J Neurosci. 2000;12:2205–2210. doi: 10.1046/j.1460-9568.2000.00106.x. [DOI] [PubMed] [Google Scholar]
- 25.Sassoe-Pognetto M, Panzanelli P, Sieghart W, Fritschy JM. Colocalization of multiple GABAA receptor subtypes with gephyrin at postsynaptic sites. J Comp Neurol. 2000;420:481–498. doi: 10.1002/(sici)1096-9861(20000515)420:4<481::aid-cne6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Scotti AL, Reuter H. Synaptic and extrasynaptic gamma-aminobutyric acid type A receptor clusters in rat hippocampal cultures during development. Proc Natl Acad Sci U S A. 2001;98:3489–3494. doi: 10.1073/pnas.061028798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 28.Simburger E, Plaschke M, Kirsch J, Nitsch R. Distribution of the receptor-anchoring protein gephyrin in the rat dentate gyrus and changes following entorhinal cortex lesion. Cereb Cortex. 2000;10:422–432. doi: 10.1093/cercor/10.4.422. [DOI] [PubMed] [Google Scholar]
- 29.Simburger E, Plaschke M, Fritschy JM, Nitsch R. Localization of two major GABAA receptor subunits in the dentate gyrus of the rat and cell type-specific up-regulation following entorhinal cortex lesion. Neuroscience. 2001;102:789–803. doi: 10.1016/s0306-4522(00)00505-4. [DOI] [PubMed] [Google Scholar]
- 30.Sloviter RS. Calcium-binding protein (calbindin-D28k) and parvalbumin immunocytochemistry: localization in the rat hippocampus with specific reference to the selective vulnerability of hippocampal neurons to seizure activity. J Comp Neurol. 1989;280:183–196. doi: 10.1002/cne.902800203. [DOI] [PubMed] [Google Scholar]
- 31.Sloviter RS, Dichter MA, Rachinsky TL, Dean E, Goodman JH, Sollas AL, Martin DL. Basal expression and induction of glutamate decarboxylase and GABA in excitatory granule cells of the rat and monkey hippocampal dentate gyrus. J Comp Neurol. 1996;373:593–618. doi: 10.1002/(SICI)1096-9861(19960930)373:4<593::AID-CNE8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 32.Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. GABAA receptor subunits in the rat hippocampus I: immunocytochemical distribution of 13 subunits. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- 33.Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABAA conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of alpha4 and delta subunits of the gamma-aminobutyric acidA receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- 35.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whiting PJ, Wafford KA, McKernan RM. Pharmacologic subtypes of GABAA receptors based on subunit composition. In: Martin DL, Olsen RW, editors. GABA in the Nervous System: the View at 50 years. Lippincott Williams & Wilkins; Philadelphia: 2002. pp. 113–126. [Google Scholar]
- 37.Winer JA, Larue DT. Anatomy of glutamic acid decarboxylase immunoreactive neurons and axons in the rat medial geniculate body. J Comp Neurol. 1988;278:47–68. doi: 10.1002/cne.902780104. [DOI] [PubMed] [Google Scholar]