Force-dependent polymorphism in type IV pili reveals hidden epitopes (original) (raw)
Abstract
Through evolution, nature has produced exquisite nanometric structures, with features unrealized in the most advanced man-made devices. Type IV pili (Tfp) represent such a structure: 6-nm-wide retractable filamentous appendages found in many bacteria, including human pathogens. Whereas the structure of Neisseria gonorrhoeae Tfp has been defined by conventional structural techniques, it remains difficult to explain the wide spectrum of functions associated with Tfp. Here we uncover a previously undescribed force-induced quaternary structure of the N. gonorrhoeae Tfp. By using a combination of optical and magnetic tweezers, atomic force microscopy, and molecular combing to apply forces on purified Tfp, we demonstrate that Tfp subjected to approximately 100 pN of force will transition into a new conformation. The new structure is roughly 3 times longer and 40% narrower than the original structure. Upon release of the force, the Tfp fiber regains its original form, indicating a reversible transition. Equally important, we show that the force-induced conformation exposes hidden epitopes previously buried in the Tfp fiber. We postulate that this transition provides a means for N. gonorrhoeae to maintain attachment to its host while withstanding intermittent forces encountered in the environment. Our findings demonstrate the need to reassess our understanding of Tfp dynamics and functions. They could also explain the structural diversity of other helical polymers while presenting a unique mechanism for polymer elongation and exemplifying the extreme structural plasticity of biological polymers.
Keywords: force polymorphism, alternate immunogenic properties
Helical protein filaments are found throughout nature (e.g., filamentous actin, microtubules, flagella, and pili) and serve a multitude of functions. Structural modifications in the protein monomer can lead to changes at the macromolecular level, influencing the assembly, disassembly, and function of these filaments (1, 2). Whereas studies on force-induced secondary and tertiary structural changes are well documented (3–5), quaternary structural changes on well-defined macromolecular assemblies have received less attention (6–8). The full structural diversity of helical protein filaments has proven difficult to assess because the variety of their molecular arrangements have often eluded observation and exceeded expectations (9, 10). A large number of these polymers have mechanical and structural roles in biology and thus may be subject to physical stress. Structural changes linked to mechanical stress have mainly been studied in bacterial flagella and P-pili, but such changes may be more common than previously thought (11–14).
Type IV pili (Tfp) are retractable helical filamentous appendages found in many bacteria, including human pathogens (15, 16). Despite recent findings regarding the Neisseria gonorrhoeae Tfp quaternary structure (16, 17), it remains difficult to explain the wide spectrum of functions associated with Tfp (18), including: twitching motility (19), DNA uptake (20), human cell infectivity (21, 22), and immunogenic properties (23). Because the Tfp retraction motor is one of the strongest molecular motor known to date (24) and certain pilin monomers are thought to be affected by force (25), we hypothesized that force could extend the repertoire of Tfp structures and functions. Here we use the N. gonorrhoeae Tfp for exploring force-induced structural changes in helical filaments.
Results and Discussion
N. gonorrhoea Tfp Undergoes Reversible Force-Induced Polymorphism.
We have previously shown, by using optical tweezers bead assays, that a single N. gonorrhoeae Tfp can sustain forces in the range of 100 pN (24). A typical Tfp retraction event consists of a transient tensile force (lasting up to a few seconds) with a subsequent and rapid release of force (24, 26). This abrupt release of force has been interpreted as a breakage event (24), a severing of the connection between the Tfp and the bead in the laser trap. Closer examination of recordings from those experiments (19) revealed bead return speeds too slow to be compatible with a free release/breakage event. (A small back-of-the-envelope calculation of a free release in the optical tweezers leads to a speed of at least 10,000 μm/s to compare with the speed of around 5 μm/s measured.) Rather, it suggested the persistence of a Tfp tether between the bacterium and the force apparatus (19). On the other hand, it is interesting to note that the speed of these elongations (5 μm/s) is 5–10 times greater than the Tfp elongation caused by polymerization previously recorded (0.5–1 μm/s) (27, 28).
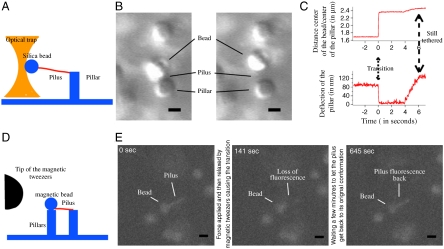
We hypothesized that the force release profile is the signature of a structural change in the Tfp filament itself. We therefore explored the effect of force on purified N. gonorrhoeae Tfp filaments to assure that we would not measure the properties of the attachment of the Tfp to the bacterial wall or be hindered by the elongation and retraction cycles of the Tfp. In our initial experiments, a Tfp was tethered between a silica bead and an elastic hydrogel pillar (26). Force was applied to the Tfp by using optical tweezers to pull on the bead (Fig. 1A and Movie S1). Low forces (typically 10–20 pN) applied to the bead were transmitted through the Tfp to the pillar, causing a deflection of the elastic pillar. Higher forces (typically around 50 pN) applied for extended periods of time resulted in a sudden release of force, returning the pillar to its resting state and increasing the distance between the bead and the pillar (Fig. 1 B and C). After the pillar returned to its resting state, we progressively increased the distance of the bead from the pillar. We observed a second displacement event that confirmed the persistence of the Tfp tether and verified that the loss of force was not caused by a breakage event. Importantly, the Tfp tether was now longer than the original unperturbed fiber (Fig. 1C). Application of force on the transitioned Tfp during the second displacement led to an increase in the bead/pillar distance that suggests elastic properties of the transitioned Tfp.
Fig. 1.
N. gonorrhoea Tfp undergoes reversible force-induced polymorphism. (A) Schematic of the optical tweezers experimental design. (B) Movie frames illustrating the position of the elastic pillar and silica bead before and after pilus transition. (Scale bar: 1 μm.) (C) Time course of the distance between the center of the bead and the center of the pillar and of the deflection of the pillar (time 0 = time of the Tfp transition). The displacement of the pillar represents a force of 45 pN. (D) Schematic of the magnetic tweezers experimental design. (E) Successive fluorescent images of a fluorescently labeled Tfp before transition (Left), in transition (Center), and after recovery (Right). (Scale bar: 1 μm.)
Next, the force that had been applied to the bead/Tfp was removed for over 5 min and then reapplied. Upon reapplication of small forces, the initial distance between the bead and the pillar was restored. With increased forces, we again detected the extension of the Tfp, demonstrating the reversibility of this structural transition (Movie S1). To visualize the structural change in Tfp during the transition, we conducted similar experiments with Tfp stained with carboxytetramethylrhodamine (TAMRA) succinimidyl ester (see Materials and Methods for details). By using magnetic tweezers (29), we applied forces to a magnetic bead attached to a labeled Tfp filament linking two elastic pillars in tandem (Fig. 1D and Movie S2). In this experimental setup, one pillar was in contact with the magnetic bead and the second pillar to part of the Tfp attached to the bead. This configuration enabled us to follow the fluorescence of Tfp without being hindered by the effects of Brownian motion. Contacts between the bead, the pillars, and the Tfp were confirmed by applying tensile forces to the bead and monitoring for the deflection of both pillars. Lower forces (typically 20–30 pN) thus resulted in the displacement of both pillars whereas the Tfp fluorescent signal remained constant. Upon application of higher forces (around 100 pN), the pillar attached to the Tfp returned to its resting state with a concomitant decline in Tfp fluorescence (Fig. 1E and Movie S2). This decrease in fluorescence was consistent with an increase in Tfp fiber length. In addition, the longer stretch-transitioned Tfp became subject to Brownian motion. As with unlabeled Tfp, this transition was reversible: Relaxation of the force for a few minutes led to the restoration of both the initial fluorescence and the mechanical contact between the two pillars (Movie S2). Finally, the entire process could be repeated by reapplying high forces to the Tfp. These results confirm the existence of a previously undescribed, force-induced quaternary structure that can be adopted by N. gonorrhoeae Tfp.
Atomic Force Microscopy (AFM) Characterization of the Force-Induced Tfp Transition.
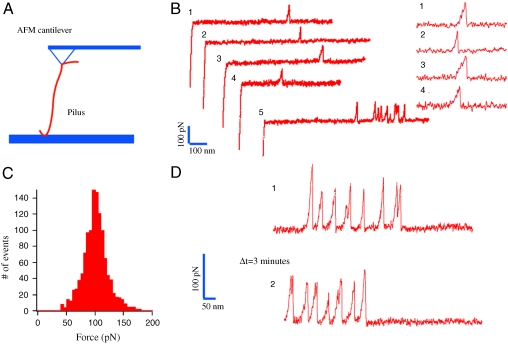
We next characterized this force-induced Tfp transition with greater temporal and spatial resolution by using AFM in the force spectroscopy mode (Fig. 2A) (5). Tfp were subjected to force-extension studies with the AFM while operating at physiologically relevant speeds (∼1 μm/s) (24). Force-extension experiments on single polyproteins have shown well-defined force peaks associated with the strength of the mechanical transition state and precise spacing in length because of the unraveling of the amino acids into a linear polypeptide chain for each monomer. This unraveling has previously been modeled by the worm-like chain model, which assumes an entropic elasticity. In the case of stretching of Tfp, we observed a qualitatively different force response potentially because of the supramolecular structural organization within each Tfp fiber. Namely, we identified long extensions with no measurable force (below the 10-pN resolution of our apparatus) and a sudden nonlinear increase in the force followed by a linear force response from ∼20 pN up to a well-defined transition at 100 ± 20 pN (Fig. 2 B and C). Beyond this transition, the force on the Tfp relaxed to zero (or at least below the 10-pN resolution of our AFM) and the Tfp continued to extend (Fig. 2B, traces 1–4). In rarer cases, further transitions occurred after the first one (Fig. 2B, trace 5). This pattern is in agreement with our optical and magnetic tweezers experiments in which we predominantly observe a single peak of force with little or no force following the transition. This characteristic force profile is compatible with a sudden transition to a longer configuration of the Tfp. We tested the reversibility of the transition by pulling multiple times on the same Tfp. We first confirmed that we had picked up a Tfp: The cantilever was moved away from the surface until one or more peaks were detected (the cantilever position was typically set between 0.5 and 1 μm away from the surface). From this initial point, the Tfp was further extended and force peaks were recorded (Fig. 2D, trace 1). The cantilever was then returned to its initial position. After 3 min, the Tfp was again extended. The presence of peaks in the subsequent force measurement confirmed the reversible nature of the conformational change (Fig. 2D, trace 2). Interestingly, extension away from the surface of “picked-up” Tfp led to more frequent observation of multiple peaks. These results suggest that the transition need not invoke the entire Tfp but can consist of multiple transitions of regions within the Tfp.
Fig. 2.
AFM characterization of the force-induced Tfp transition. (A) Schematic of the AFM experimental design. (B) Typical example of force-extension curves showing the transition occurring at ∼100 pN. Insets are zooms on the force peaks for four curves. (C) Force histogram of the force associated with the Tfp transition event averaging 100 ± 20 pN (standard deviation, n = 1,210). (D) The reversibility of the force-induced transition is demonstrated by the observation of multiple transition events in two consecutive trajectories separated by a time Δ_t_ = 3 min.
The AFM data presented here are similar to previous experiments performed on Pseudomonas aeruginosa Tfp (30). In those experiments, the Tfp tested were not purified and were still attached to the bacterium body leading to the hypothesis that the AFM data could be caused by a component of the bacterial wall. Nevertheless, the AFM force traces in that study showed predominantly a single peak of force, with rarer instances of multiple peaks in one trace, nearly identical to our findings with purified N. gonorrhoeae Tfp. These AFM data could not be explained with the known elastic properties of P. aeruginosa Tfp. A force-induced transition model of P. aeruginosa Tfp would provide an explanation for this apparent paradox and hint at a common nanomechanical behavior of Tfp across species. A force transition, which exhibits linear elasticity, could be assigned to a “nanospring” behavior, analogous to that reported in α-helical macrostructures of ankyrin repeats (31). This behavior is thought to originate from the stacking energy between these repeats, and, in the case of Tfp, it may originate from the stacking energy of the α-helices in the center of the fiber (17).
Beyond the existence of a force-induced transition between two structures of the Tfp, some particular features of the transition are of special interest. The ability of a single Tfp to undergo multiple transitions makes the Tfp a very good candidate model for the design of nanothreads with great adhesive strength (32). It also indicates that the Tfp fiber is able to buffer force fluctuations through changes in its length, potentially enabling N. gonorrhoeae to remain attached to its host in the presence of high transient hydrodynamic forces (such as those originating from urination or mucous flow) that would otherwise break those attachments. In this context, it is interesting to note that the stall force of the Tfp retraction motor is about 100 pN (24). The consistent nature of our observations on the transitional forces of Tfp along with the previously reported stall forces of the Tfp retractile motor seems to exemplify an adaptation or coevolution between the N. gonorrhoeae Tfp motor and the physical properties of the N. gonorrhoeae Tfp filament. This coevolution is reminiscent of the optimization of the mechanical properties of Escherichia coli type I fimbriae in order to maintain adhesion under different fluid flow conditions (8).
Extended Tfp Conformation Reveals Hidden Epitopes.
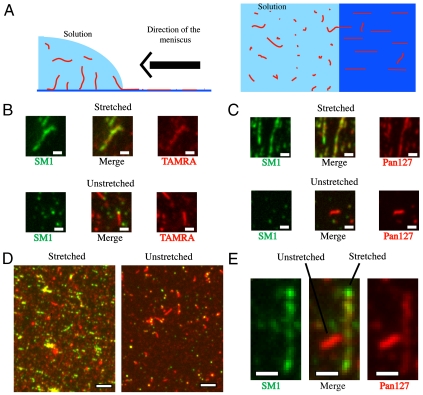
To further characterize the structural change of the Tfp, given the transient nature of the force-induced Tfp transition, we next locked the Tfp filament in its “stretched” or extended state. By using a modified version of molecular combing (33) (Fig. 3A and Movie S3), we were able to apply surface tension forces on Tfp. An estimate of the force thus applied on Tfp is available (33) (∼1 nN), and it is a force sufficient to cause the Tfp polymorphic transition. Indeed, after the action of the drying meniscus, the Tfp were irreversibly fixed on the cover glass, most of them in their stretched configuration. We determined whether the stretched Tfp fiber was recognized by the SM1 monoclonal antibody. The SM1 mAb recognizes the conserved epitope EYYLN in the pilin monomer. In the native Tfp fiber, the SM1 EYYLN epitope was predicted to be buried (17). We found that the SM1 mAb bound to only the exposed ends of unstretched Tfp filaments, as reported (34) (Fig. 3 B_–_D). In contrast, the SM1 mAb bound the length of stretched Tfp (Fig. 3 B_–_D). Thus, force-induced stretch exposed residues in Tfp that were hidden in the unstretched form.
Fig. 3.
Extended Tfp conformation reveals hidden epitopes. (A) A schematic of the molecular combing experimental design. (B) Close-up of fluorescent images of stretched or unstretched TAMRA-prestained purified Tfp. The green signal indicates the SM1 epitope; the red signal (TAMRA) labels the exterior of the Tfp fiber. (Scale bar: 1 μm.) (C) Close-up of fluorescent images of stretched or unstretched purified Tfp. The green signal indicates the SM1 epitope; the red signal (Pan127 antibody) labels the exterior of the Tfp fiber. (Scale bar: 1 μm.) (D) Fluorescent images of TAMRA-prestained purified Tfp processed by molecular combing. The red signal is against an exposed region of Tfp (TAMRA), whereas the green signal indicates the SM1 epitope. (Scale bar: 5 μm.) (E) Dual fluorescent images of the SM1 epitope (Green) and the exposed epitope Pan127 (Red) of purified Tfp processed by molecular combing. (Scale bar: 1 μm.)
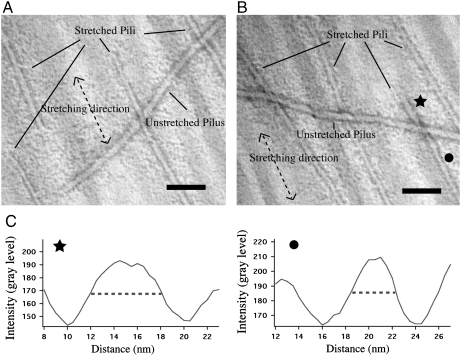
To estimate the fractional length change upon stretch, Tfp were stained with the SM1 mAb in combination with either TAMRA or pAB127 (Pan127), a polyclonal antibody that recognized native purified Tfp. As predicted, the TAMRA and Pan127 signals were observed along the length of the stretched and unstretched Tfp fiber. By using TAMRA or Pan127, we established a correspondence between fluorescent signals and Tfp lengths. Signals per unit of length of stretched and unstretched Tfp fibers revealed that stretched fibers were approximately 3 times longer (having 3 times less fluorescence) than unstretched fibers [550 ± 25 arbitrary units (a.u.) vs. 1,800 ± 100 a.u. for TAMRA fluorescence signal and 2,500 ± 100 a.u. vs. 7,300 ± 250 a.u. for Pan127 fluorescence signal; standard errors and _n_ = 60 in all cases; see Fig. S1_A_ and Materials and Methods for details]. In any given sample, not all Tfp were stretched; unstretched fibers were used as an internal control (Fig. 3E). Molecular combing was also used to stretch Tfp on EM slot grids. Images of stretched samples also revealed a mixture of extended and nonextended Tfp (Fig. 4 A and B). The stretched Tfp were approximately 40% narrower in diameter than unstretched fibers (3.5 ± 0.7 nm vs. 6.0 ± 0.7 nm for evaluation “by eye” of the diameter and 3.9 ± 0.6 nm vs. 6.2 ± 0.5 nm for evaluation via plot profile; standard deviations are indicated for 100 measurements over 20 different micrographs in each case; see Fig. S1_B_ and Materials and Methods for details), indicating that they had only about 1/3 of the mass per length of the unstretched fibers (Fig. 4 A and B). Rare instances of partially transitioned Tfp (the fraction that has not undergone transition corresponds to less than 1% of the total length of observed pili) can be found (Fig. 4 B and C).
Fig. 4.
Stretched Tfp are 40% narrower than unstretched Tfp. (A) Electron micrograph of purified Tfp processed by molecular combing. Note the smaller diameter of the stretched Tfp. Direction of stretch indicated by the double-headed arrow. (Scale bar: 50 nm.) (B) Electron micrograph of purified Tfp processed by molecular combing. Note the smaller diameter of the stretched Tfp. Direction of stretch indicated by the double-headed arrow. (Scale bar: 50 nm.) (C) Plots of averaged intensities across a line drawn perpendicularly to the direction of a stretched Tfp at the level of either the star or the circle in B. Those plots, along with B, present an example of partially transitioned Tfp.
Conclusion
In summary, we have established that N. gonorrhoeae Tfp can undergo a reversible force-induced transition into a unique quaternary structure that is longer and narrower than the resting state not subjected to force (Fig. S2 and Movie S4). In its extended form, hidden epitopes in the fiber become exposed. Our findings open an unforeseen chapter for the study of the dynamics of the Tfp structure as well as of its immunogenic properties. We predict that, besides explaining previously observed structural diversity in the Tfp (35), force-induced structural changes of N, gonorrhoeae Tfp will prove a useful model in understanding the structural diversity of other helical polymers (36, 37). Finally, we postulate that the exact structural origin of the present transition may, in the future, facilitate the design of useful biomimetic nanostructures.
Materials and Methods
Pili Preparation.
Purified Tfp were prepared in a similar manner as previously published (17). One or two plates of WT MS11 N. gonorrhoeae grown for 16–20 h on (GonoCoccal Broth) agar plates were resuspended in 1 mL of 50 mM CHES [2-(cyclohexylamino)ethanesulfonic acid from Sigma] pH 9.5. The suspension was vortexed for 2 min and the bacteria bodies were spun down at 18,000 × g for 5 min. The supernatant was collected and spun down at 100,000 × g for 1.5 h. The pellet was resuspended in 1 mL 50 mM CHES pH 9.5. In the case of prestained Tfp, the bacteria were resuspended in 1 mL of labelling buffer (50 mM KPO4. pH 8.0/5 mM MgCl2/25 μM EDTA), vortexed, and spun down at 18,000 × g for 5 min. About 40 μL of a 10 mg/mL solution of TAMRA succinimidyl ester in DMSO was added to the supernatant and allowed to react at room temperature for 1.5 h. The mixture was then spun down at 100,000 × g for 1.5 h. The pellet was washed 5 times with 50 mM CHES pH 9.5 and finally resuspended in 1 mL 50 mM CHES pH 9.5.
Optical Microscopy.
All the optical microscopy was performed on conventional inverted microscopes (either Olympus IX 81 or Zeiss Axiovert 100). The images were analyzed by using ImageJ software [National Institutes of Health (NIH)]. The measurements of the intensity per unit of length of the Tfp were performed by using a home-written plug-in on ImageJ following the procedure outlined in Fig. S1_A_: The fluorescence signal over a rectangle of set width and length along Tfp fiber was recorded. The fluorescence signal over a similar rectangle parallel to the first one and away from the Tfp was also measured to normalize for background noise. The fluorescence signal per unit of length of the Tfp was the difference between these two measurements. The measure was repeated for both stretched and unstretched Tfp.
Optical Tweezers.
The optical tweezers system consisted of a neodymium-doped yttrium aluminum garnet-neodymium (Nd:YAG) laser (2 W) mounted on a Zeiss Axiovert 100. Purified Tfp were put into contact overnight with 1.5 μm carboxylated silica beads at a concentration allowing on average a single Tfp to bind per bead. The beads were then allowed to settle onto a bed of hydrogel micropillars prepared as presented elsewhere (26). The coverslip was then scanned in order to find the following condition: a bead still agitated by Brownian motion but remaining in the vicinity of a pillar (Fig. 1A). We verified the connection between the bead and the pillar by applying force on the bead by using optical tweezers.
Magnetic Tweezers.
The magnetic tweezers setup has been presented elsewhere (29) and was mounted on an Olympus IX81 inverted scope. TAMRA stained purified Tfp were put into contact overnight with 1 μm MyOne carboxylated magnetic beads (Invitrogen) at a concentration allowing on average a single Tfp to bind per bead. The beads were then allowed to settle onto a bed of hydrogel micropillars prepared as presented elsewhere (26). The surface was then scanned for the following configuration: a bead, with one Tfp attached to it, stuck to one pillar with the Tfp attached to a neighboring pillar (Fig. 1D). We could apply forces on the magnetic bead by using the magnetic tweezers.
AFM.
The details of the custom-made AFM have been described elsewhere (5). Each cantilever used in our experiments (Si3N4 Veeco MLCT-AUHW) was individually calibrated by using the equipartition theorem, giving rise to spring constants between ∼20 and ∼100 pN nm-1. We worked at a diluted concentration of purified Tfp to prevent multiple Tfp from binding at the same time. No force peak has been recorded when a Tfp preparation from a nonpiliated strain was used. The force-extension curves were recorded and analyzed by using IGOR 6.0 software (Wavemetrics).
Molecular Combing.
Cleaned cover glasses were incubated in a solution of purified Tfp in 50 mM CHES pH 9.5 at a dilution between 1/1,000 and 1/10,000 from an original purified Tfp solution for 15 min at the bottom of the well of a six-well plate. After incubation, cover glasses were maintained vertically, with the bottom end placed on a lint-free Kimwipes tissue to remove excess liquid and allow slow drainage by gravity and capillarity action. The receding meniscus on the cover glass exerted forces on Tfp, causing most of them to be stretched. A number of Tfp remained in their native state likely because of a prior close contact with the cover glass, therefore resisting the combing action. The samples processed by combing were referred to as “stretched” samples. “Unstretched” samples were obtained by incubating cover glass with the same diluted solution of Tfp for 15 min, followed by three washes with no drying of the cover glass.
Immunostaining.
Either stretched or unstretched samples on cover glass were fixed with 3.7% formaldehyde in PBS, blocked with a solution of 0.2% fish gelatin in PBS, and incubated with either a monoclonal antibody against the SM1 domain of pilin or the polyclonal antibody pAb127 (Pan127) generated against purified Tfp, or a combination of both antibodies. Samples incubated with the SM1 antibody were stained with an Alexa 488-conjugated secondary anti-mouse antibody. Samples incubated with the Pan127 antibody were stained with an Alexa 568-conjugated secondary anti-rabbit antibody.
EM.
A 1 × 2 mm formvar/carbon coated slot grid (Electron Microscopy Sciences) was allowed to float on top of 10 μL of a solution of purified Tfp for 5 min. The grid was then held vertically with tweezers and drained from the bottom end with a lint-free Kimwipes tissue to remove excess liquid and allow slow drainage by gravity and capillary action. The grid was then floated on top of a 10-μL droplet of a solution of 3.7% formaldehyde in PBS to fix the sample. The grid was then floated on top of a 10-μL droplet of a 2% solution of uranyl acetate for negative staining. The excess stain solution was removed and the sample imaged by using a JEOL transmission electron microscope. The images were analyzed by using ImageJ software (NIH). The diameter of the Tfp was either evaluated by eye and measured with a line selection or a plot of averaged intensities across a 50-pixel-wide line perpendicular to the direction of the fiber was created and the diameter was the width at midheight of the resulting peak.
Supplementary Material
Supporting Information
Acknowledgments.
The authors thank Simon Moore for critical reading of the manuscript and the rest of the Sheetz lab members for their technical support. N.B. and M.S. acknowledge the award of NIH Grant AI079030. J.B. holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 2.Oda T, Iwasa M, Aihara T, Maeda Y, Narita A. The nature of the globular- to fibrous-actin transition. Nature. 2009;457(7228):441–445. doi: 10.1038/nature07685. [DOI] [PubMed] [Google Scholar]
- 3.del Rio A, et al. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323(5914):638–641. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zlatanova J, Lindsay SM, Leuba SH. Single molecule force spectroscopy in biology using the atomic force microscope. Prog Biophys Mol Bio. 2000;74(1–2):37–61. doi: 10.1016/s0079-6107(00)00014-6. [DOI] [PubMed] [Google Scholar]
- 5.Oberhauser AF, Marszalek PE, Erickson HP, Fernandez JM. The molecular elasticity of the extracellular matrix protein tenascin. Nature. 1998;393(6681):181–185. doi: 10.1038/30270. [DOI] [PubMed] [Google Scholar]
- 6.Miller E, Garcia T, Hultgren S, Oberhauser AF. The mechanical properties of E. coli type 1 pili measured by atomic force microscopy techniques. Biophys J. 2006;91(10):3848–3856. doi: 10.1529/biophysj.106.088989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson M, Axner O, Almqvist F, Uhlin BE, Fällman E. Physical properties of biopolymers assessed by optical tweezers: Analysis of folding and refolding of bacterial pili. ChemPhysChem. 2008;9(2):221–235. doi: 10.1002/cphc.200700389. [DOI] [PubMed] [Google Scholar]
- 8.Forero M, Yakovenko O, Sokurenko EV, Thomas WE, Vogel V. Uncoiling mechanics of Escherichia coli type I fimbriae are optimized for catch bonds. PLoS Biol. 2006;4(9):e298. doi: 10.1371/journal.pbio.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galkin VE, et al. Divergence of quaternary structures among bacterial flagellar filaments. Science. 2008;320(5874):382–385. doi: 10.1126/science.1155307. [DOI] [PubMed] [Google Scholar]
- 10.Kueh HY, Mitchison TJ. Structural plasticity in actin and tubulin polymer dynamics. Science. 2009;325(5943):960–963. doi: 10.1126/science.1168823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esther Bullit, Makowski L. Structural polymorphism of bacterial adhesion pili. Nature. 1995;373:164–167. doi: 10.1038/373164a0. [DOI] [PubMed] [Google Scholar]
- 12.Hotani H. Micro-video study of moving bacterial flagellar filaments: III Cyclic transformation induced by mechanical force. J Mol Biol. 1982;156(4):791–806. doi: 10.1016/0022-2836(82)90142-5. [DOI] [PubMed] [Google Scholar]
- 13.Brown AEX, Litvinov RI, Discher DE, Purohit PK, Weisel JW. Multiscale mechanics of fibrin polymer: Gel stretching with protein unfolding and loss of water. Science. 2009;325(5941):741–744. doi: 10.1126/science.1172484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeRosier DJ, Tilney LG, Bonder EM, Frankl P. A change in twist of actin provides the force for the extension of the acrosomal process in Limulus sperm: The false-discharge reaction. J Cell Biol. 1982;93(2):324–337. doi: 10.1083/jcb.93.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattick JS. Type IV pili and twitching motility. Annu Rev Microbiol. 2002;56(1):289–314. doi: 10.1146/annurev.micro.56.012302.160938. [DOI] [PubMed] [Google Scholar]
- 16.Hansen JK, Forest KT. Type IV pilin structures: Insights on shared architecture, fiber assembly, receptor binding and type II secretion. J Mol Microbiol Biotechnol. 2006;11(3–5):192–207. doi: 10.1159/000094054. [DOI] [PubMed] [Google Scholar]
- 17.Craig L, et al. Type IV pilus structure by cryo-electron microscopy and crystallography: Implications for pilus assembly and functions. Mol Cell. 2006;23(5):651–662. doi: 10.1016/j.molcel.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Craig L, Li J. Type IV pili: Paradoxes in form and function. Curr Opin Struct Biol. 2008;18(2):267–277. doi: 10.1016/j.sbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merz AJ, So M, Sheetz MP. Pilus retraction powers bacterial twitching motility. Nature. 2000;407(6800):98–102. doi: 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- 20.Chen I, Christie PJ, Dubnau D. The ins and outs of DNA transfer in bacteria. Science. 2005;310(5753):1456–1460. doi: 10.1126/science.1114021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merz AJ, So M. Interactions of pathogenic Neisseriae with epithelial cell membranes. Annu Revi Cell Dev Biol. 2000;16(1):423–457. doi: 10.1146/annurev.cellbio.16.1.423. [DOI] [PubMed] [Google Scholar]
- 22.Howie HL, Glogauer M, So M. The N. gonorrhoeae type IV pilus stimulates mechanosensitive pathways and cytoprotection through a pilT-dependent mechanism. PLoS Biol. 2005;3(4):e100. doi: 10.1371/journal.pbio.0030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tramont EC, Boslego JW. Pilus vaccines. Vaccine. 1985;3(1):3–10. doi: 10.1016/0264-410x(85)90003-9. [DOI] [PubMed] [Google Scholar]
- 24.Maier B, Potter L, So M, Seifert HS, Sheetz MP. Single pilus motor forces exceed 100 pN. Proc Natl Acad Sci USA. 2002;99(25):16012–16017. doi: 10.1073/pnas.242523299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helaine S, Deyer DH, Nassif X, Pelicic V, Forest KT. 3D structure/function analysis of PilX reveals how minor pilins can modulate the virulence properties of type IV pili. Proc Natl Acad Sci USA. 2007;104(40):15888–15893. doi: 10.1073/pnas.0707581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biais N, Ladoux B, Higashi D, So M, Sheetz M. Cooperative retraction of bundled type IV pili enables nanonewton force generation. PLoS Biol. 2008;6(4):e87. doi: 10.1371/journal.pbio.0060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maier B, Koomey M, Sheetz MP. A force-dependent switch reverses type IV pilus retraction. Proc Natl Acad Sci USA. 2004;101(30):10961–10966. doi: 10.1073/pnas.0402305101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clausen M, Koomey M, Maier B. Dynamics of type IV pili is controlled by switching between multiple states. Biophys J. 2009;96(3):1169–1177. doi: 10.1016/j.bpj.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanase M, Biais N, Sheetz M, YuLi Wang, Dennis ED. Methods in Cell Biology. Vol 83. New York: Academic; 2007. Magnetic tweezers in cell biology; pp. 473–493. [DOI] [PubMed] [Google Scholar]
- 30.Touhami A, Jericho MH, Boyd JM, Beveridge TJ. Nanoscale characterization and determination of adhesion forces of Pseudomonas aeruginosa pili by using atomic force microscopy. J Bacteriol. 2006;188(2):370–377. doi: 10.1128/JB.188.2.370-377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee G, et al. Nanospring behaviour of ankyrin repeats. Nature. 2006;440(7081):246–249. doi: 10.1038/nature04437. [DOI] [PubMed] [Google Scholar]
- 32.Smith BL, et al. Molecular mechanistic origin of the toughness of natural adhesives, fibres and composites. Nature. 1999;399(6738):761–763. [Google Scholar]
- 33.Bensimon A, et al. Alignment and sensitive detection of DNA by a moving interface. Science. 1994;265(5181):2096–2098. doi: 10.1126/science.7522347. [DOI] [PubMed] [Google Scholar]
- 34.Forest KT, et al. Assembly and antigenicity of the Neisseria gonorrhoeae pilus mapped with antibodies. Infect Immun. 1996;64(2):644–652. doi: 10.1128/iai.64.2.644-652.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swanson J, Kraus SJ, Gotschlich EC. Studies on gonococcus infection: I. Pili and zones of adhesion: Their relation to gonococcal growth patterns. J Exp Med. 1971;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang YA, Yu X, Silverman PM, Harris RL, Egelman EH. The structure of F-pili. J Mol Biol. 2009;385(1):22–29. doi: 10.1016/j.jmb.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang YA, Yu X, Ng SYM, Jarrell KF, Egelman EH. The structure of an archaeal pilus. J Mol Biol. 2008;381(2):456–466. doi: 10.1016/j.jmb.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information