Jaw muscularization requires Dlx expression by cranial neural crest cells (original) (raw)
Abstract
The origin of active predation in vertebrates is associated with the rise of three major, uniquely derived developmental characteristics of the head: (i) migratory cranial neural crest cells (CNCCs) giving rise to most skeletal skull elements; (ii) expression of Dlx genes by CNCCs in the _Hox_-free first pharyngeal arch (PA1); and (iii) muscularization of PA1 derivatives. Here we show that these three innovations are tightly linked. Expression of Dlx genes by CNCCs is not only necessary for head skeletogenesis, but also for the determination, differentiation, and patterning of cephalic myogenic mesoderm leading to masticatory muscle formation. In particular, inactivation of Dlx5 and Dlx6 in the mouse results in loss of jaw muscles. As Dlx5/6 are not expressed by the myogenic mesoderm, our findings imply an instructive role for _Dlx5/6_-positive CNCCs in muscle formation. The defect in muscularization does not result from the loss of mandibular identity observed in Dlx5/6−/− mice because masticatory muscles are still present in EdnRA−/− mutants, which display a similar jaw transformation. The genesis of jaws and their muscularization should therefore be seen as an integrated _Dlx_-dependent developmental process at the origin of the vertebrate head. The role of Dlx genes in defining gnathostome jaw identity could, therefore, be secondary to a more primitive function in the genesis of the oral skeletomuscular system.
Keywords: craniofacial development, Dlx5-Dlx6, myogenesis, mesoderm, evolution
During chordate evolution, innovations specific to vertebrates permit the apparition of the “new head” (1), a neomorphic unit endowed with a muscularized oral structure that allows the transition from filter feeding to active predation. The considerable selective advantage of predatory capacity in vertebrates goes together with the presence of _Hox_-negative skeletogenic cranial neural crest cells (CNCCs) migrating in the cephalic region (2, 3). The CNCC-derived cephalic skeletal elements need to associate with contractile muscles to give rise to a functional mouth. The question of how oral skeletal and muscular morphogenesis is coordinated is, therefore, central for understanding the origin of active predation during vertebrate evolution.
Craniofacial shapes are amazingly diverse among different vertebrate groups and also within individual species (4, 5). In all cases, however, vertebrates are characterized by a muscularized functional mouth, suggesting a tight link between skeletal elements derived from the CNCCs and craniofacial muscles of mesodermal origin. The possible interaction between CNCCs and the mesoderm in orchestrating craniofacial myogenesis was suggested several years ago (6). Only recently, however, has it been demonstrated that CCNCs can regulate muscle progenitors in the head and participate in shaping the cranial musculoskeletal architecture in vertebrate embryos (7–9).
Head muscles have various embryonic origins: in tetrapods, the tongue derives from the rostral migration of anterior somitic mesoderm; other skeletal muscles derive from cranial paraxial mesoderm (CPM) (10, 11) and from lateral splanchnic mesoderm (SpM) (12). Remarkably, the development of cephalic and trunk muscles depends on different regulatory pathways (13–16). In the head, bone morphogenetic proteins (BMPs) and canonical Wnt signaling molecules act to repress skeletal muscle differentiation. By contrast, the same BMP and Wnt ligands are required to stimulate myogenesis in the trunk. Myogenic differentiation of the CPM in vitro is permitted, therefore, by CNCC production of BMP and Wnt inhibitors (Noggin, Gremlin, Frzb) (17). Both the head skeleton and the head musculature are therefore different from their truncal counterpart, reinforcing the notion that the “new head” has been a recent addition to a primitive body plan (1).
CNCCs and mesodermal cells colonizing the first pharyngeal arch (PA1) and more anterior structures do not express Hox genes (18); their patterning depends on the expression of non_Hox_ homeobox genes including Distal-less (Dlx) transcription factors (19). In particular, Dlx5 and Dlx6 are necessary and sufficient to specify PA1 skeletal maxillo-mandibular identity in gnathostomes: in their absence mandibular skeletal structures are transformed into maxillary elements (20, 21), and their activation in the maxillary arch leads to embryos with four opposing mandibles (22). Dlx5/6 expression is restricted to CNCCs (23) and is not observed in the myogenic mesoderm (Fig. S1). Early Dlx5/6 activation in PA1 depends on endothelin 1 (Edn1) signaling from the endoderm: mice lacking Edn1 or its receptor A (EdnRA) present a skeletal transformation reminiscent of that observed in Dlx5/6 double mutants (24, 25).
Given the key role of Dlx5 and Dlx6 in determining PA1 identity, we used Dlx5/6 and EdnRA mutant mice to analyze how CNCCs control head muscle morphogenesis. We show that craniofacial myogenesis depends on Dlx5/6 expression by CNCCs, but is not directly related to the PA1 maxillo-mandibular identity. The results suggest that Dlx genes might have had a central role in coordinating the development of the oral skeleto-muscular apparatus at the origin of active predation in vertebrates.
Results
Inactivation of Either Dlx5/6 or EdnRA in the Mouse Results in Head Muscle Malformations.
Histo-anatomical analysis of embryonic day 18.5 (E18.5) EdnRA−/− and Dlx5/6−/− mutant heads shows that the skeletal malformations are associated with severe muscle defects (Fig. 1, Fig S2, and Table S1). In Dlx5/6−/− double-mutant mice, all PA1-derived masticatory muscles and PA2-derived muscles are absent and replaced by undifferentiated reticular connective tissue (Fig. 1 C and C′ and Fig. S2_G_). This observation contrasts sharply with the persistence of transformed jaw skeletal elements that are therefore devoid of any muscular connection. Oculo-motor muscles are present, but their morphology is altered in parallel with eye malformations (Fig. 1_C_). More posterior muscles are also present, but their morphology is altered and individual muscle masses are not identifiable (Fig. 1 D and F).
Fig. 1.
Head muscle phenotypes observed in EdnRA−/− and Dlx5/6−/− mutant mice. Mallory trichromic staining on frontal (A_–_C) and parasaggital (D_–_F) sections of E18.5 wild-type, EdnRA−/−, and Dlx5/6−/− mice. The EdnRA−/− and Dlx5/6−/− mutants show a duplicated maxillary bone (mx*) associated with head muscle defects. In the EdnRA−/− mutant, the masseter muscle (mm) is present but has an abnormal morphology and orientation (_B_–_B_′ and E); all masseter components pass through the infraorbital foramen of the transformed lower jaw (black arrowhead in B). The pterygoid and temporal muscles are indistinguishable and form a single muscular mass (E). In the Dlx5/6−/− mutant, the masseter muscle is replaced by connective tissue [see the masseter region (mr) in C and _C_′]. In the two mutants, the tongue is considerably reduced, the muscles fibers are totally disorganized (B, _B_′′, C, _C_′′, _E_′, and _F_′), and sublingual muscles are either absent or replaced by a few muscle fibers (_B_′′′ and _C_′′′). dg, digastric muscle; dnt, dentary bone; e, eye; jg, jugal bone; lpt, lateral pterygoid muscle; mh, mylohoid muscle; mm, masseter muscle; mr, masseter region; mx, maxillary bone; mx*, duplicated maxillary bone; pt, pterygoid muscle; sbr, sublingual region; tg, tongue; tp, temporal muscle; um, upper molar. (Scale bar: 500 μm in A_–_C; 700 μm in D_–_F and _D_′–_F_′; 100 μm for other panels.)
In EdnRA−/− mutants, masticatory muscles are present, but their morphology is perturbed (Fig. 1 B, B′, and E; Fig. S2_D_). However, they can often be unequivocally identified through their attachment to specific skeletal elements. In wild-type fetuses, the superficial and lateral masseter is inserted in the lateral aspect of the mandible (Fig. 1_D_), whereas the deep masseter passes through the infraorbital foramen to insert into the medial aspect of the mandible. In EdnRA−/−, the situation is different; all components of the masseter pass through the infraorbital foramen of the transformed lower jaw (see black arrowhead Fig. 1_B_) and have a large insertion in the medial aspect of this bone. In EdnRA−/−, the pterygoid and temporal muscles are indistinguishable and form a single muscular mass (Fig. 1_E_). PA2-derived muscles are reduced whereas oculo-motor muscles are normal.
In both EdnRA−/− and Dlx5/6−/− mice, intrinsic muscles of the tongue and sublingual muscles are severely affected: the genioglossus and the geniohyoid are absent, and other intrinsic muscles of the tongue are reduced and disorganized, thus making the tongue a vestigial medially located structure (Fig. 1 B, B′′, C, _C_′′, _E_′, and _F_′ and Fig. S2 B, E, and H). The mylohyoid and digastric muscles are absent and are replaced by reticular connective tissue with only a few identifiable muscles fibers (Fig. 1 B′′′ and _C_′′′ and Fig. S2 C, F, and I). To understand the ontogeny of these muscular defects, we then analyzed the myogenic developmental process in EdnRA−/− and Dlx5/6−/− mutant embryos.
Craniofacial Myogenesis Is Initiated in the Absence of Dlx5 and Dlx6 During Early Development.
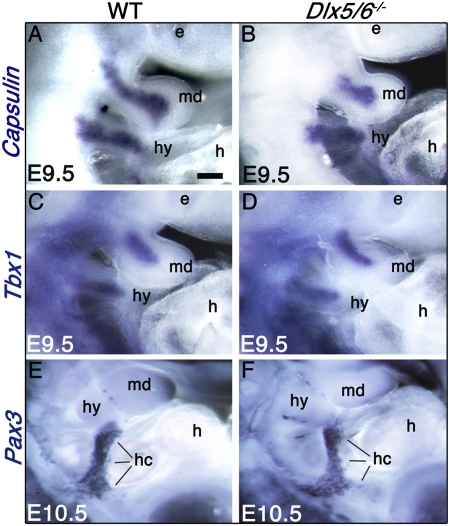
Craniofacial muscle formation initiates with the comigration of CNCCs and the myogenic mesoderm toward PA1 and PA2 (26). The initial stage of specification is characterized by the activation of Tbx1 and Capsulin specifically in head myogenic lineages (15, 27). In contrast, tongue muscles are generated from the rostral migration of myoblasts deriving from the first five somites. The first stages of craniofacial myogenesis are not affected in Dlx5/6−/− embryos. Although the shape of Dlx5/6−/− PA1 is already slightly altered at E9.5, the migration and initial specification of head myogenic mesoderm is taking place, as shown by the mesodermal activation of Tbx1 and Capsulin at E9.5 in PA1 and PA2 (Fig. 2 A_–_D). Furthermore, migration of somitic tongue muscle precursors along the hypoglossal cord is not altered, as shown by the expression pattern of Pax3 at E10.5 (Fig. 2 E_–_F).
Fig. 2.
Expression of early myogenic markers in Dlx5/6−/− embryos. Whole-mount in situ hybridization for Capsulin (A and B), Tbx1 (C and D), and Pax3 (E and F) on E9.5–E10.5 wild-type and Dlx5/6−/− embryos. In the mandibular and hyoid arches at E9.5, the expression of the early markers of myogenic specification, Capsulin and Tbx1 (A_–_D), is not affected by the Dlx5/6 inactivation. The persistence of Pax3 expression in the hypoglossal cord of E10.5 Dlx5/6−/− embryos (E and F) indicates that somitic myoblast migration during tongue development is not altered. e, eye; h, heart; hc, hypoglossal cord; hy, hyoid arch; md, mandibular arch. (Scale bar: 300 μm in A_–_D; 450 μm in E and F.)
Dlx5 and Dlx6 Expression by CNCCs Is Required for Determination, Differentiation, and Patterning of Craniofacial Muscles.
The initial stages of craniofacial muscle determination are characterized by expression of Myf5 (28). To follow the onset of myogenic determination, we used Myf5nLacZ/+ reporter embryos (29). At E10.5, Myf5-LacZ is expressed in the extraocular premuscle mass (peom) and in the mandibular arch myoblasts, which later develop into masticatory premuscle masses (pmm) at E11.5–E12.5 (Fig. 3 A, C, and E). In E10.5–E11.5 Dlx5/6−/−;Myf5nLacZ/+ embryos, Myf5-LacZ expression is strongly reduced in the ocular and masticatory region (Fig. 3 B and D) and is barely detectable at E12.5 (Fig. 3_F_), indicating a defect in myogenic determination.
Fig. 3.
β-Galactosidase staining on E10.5, E11.5, and E12.5 Myf5nLacZ/+ and Dlx5/6−/−;Myf5nLacZ/+ mutant embryos. In E10.5 Myf5nLacZ/+ embryos, Myf5-LacZ is expressed in the extraocular premuscle mass and in the mandibular and hyoid arches (A). At this stage, the expression of Myf5-LacZ is not perturbed by Dlx5/6 inactivation in the hyoid and extraocular region, but is reduced in the mandibular arch (B). At E11.5 and E12.5 in Myf5nLacZ/+ embryos, Myf5-LacZ is expressed in the extraocular and masticatory premuscle masses (C and E). This expression is dramatically reduced in the ocular and mandibular region of Dlx5/6−/−;Myf5nLacZ/+ mutant embryos (D and F). e, eye; hy, hyoid arch; md, mandibular arch; mx, maxillary bud; mx*, duplicated maxillary bud; peom, extraocular premuscle mass; pmm, masticatory premuscle mass. (Scale bar: 500 μm in A and B; 650 μm in C and D; 750 μm in E and F.)
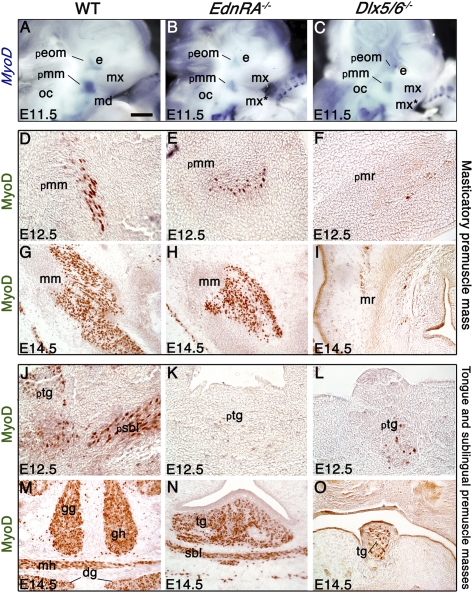
The myogenic determination factor MyoD is under the control of both the Myf5 and Tbx1 (28). In E11.5 wild-type embryos, MyoD is expressed in the extraocular and masticatory premuscle masses; this territory of MyoD expression is preserved, but reduced in both EdnRA−/− and Dlx5/6−/− embryos (Fig. 4 A_–_C).
Fig. 4.
Expression of MyoD in EdnRA−/− and Dlx5/6−/− mutant embryos. (A_–_C) MyoD whole-mount in situ hybridization on E11.5 wild-type (A), EdnRA−/− (B), and Dlx5/6−/− (C) embryos. MyoD expression in the masticatory premuscle mass (pmm) (A) is reduced in EdnRA−/− and Dlx5/6−/− mutants (B and C). (D_–_O) MyoD immunostaining on frontal sections in the masseter (D_–_I) and tongue (J_–_O) region of wild-type (D, G, J, and M), EdnRA−/− (E, H, K, and N), and Dlx5/6−/− embryos (F, I, L, and O) at E12.5 (D_–_F and J_–_L) and E14.5 (G_–_I and M_–_O). In EdnRA−/− mutants, the masticatory and masseter muscle mass (E and H) are MyoD positive, but are reduced and show an abnormal morphology. In the Dlx5/6−/− mutant, only a few cells express MyoD in the masseter region (F and I), resulting in an absence of masticatory muscle masses. In EdnRA−/− and Dlx5/6−/− mutants at E12.5, only a few cells express MyoD in the tongue and sublingual region (K and L). At E14.5, the myogenic marker is expressed in the vestigial tongue of the two mutants (N and O), the genioglossus and geniohyoid muscles are never present, and sublingual muscles are barely differentiated in EdnRA−/− (N) and are absent in Dlx5/6−/− mutants (O). dg, digastric muscle; e, eye; gg, genioglossus; gh, geniohyoid; md, mandibular bud; mh, mylohyoid; mm, masseter muscle; mr, masseter region; mx, maxillary bud; mx*, duplicated maxillary bud; oc, otic capsule; peom, extraocular premuscle mass; pmm, masticatory premuscle mass; pmr, masticatory premuscle region; psbl, sublingual premuscle mass; ptg, tongue premuscle mass; sbl, sublingual muscle; tg, tongue. (Scale bar: 400 μm in _A_–C; 70 μm in D_–_F and J_–_L; 150 μm in G_–_I and M_–_O.)
At later stages, in wild-type fetuses, the masticatory premuscle mass at E12.5 and the masseter muscle at E14.5 and E18.5 are MyoD (Fig. 4 D and G), Desmin (Figs. S3 A and D and S4_A_), and fast myosin heavy chain (fMHC) (Fig. S4_A_′) positive, indicating that myogenic differentiation is taking place. In EdnRA−/− mutants, the presumptive masseter is present and positive for the above-mentioned myogenic markers, but is clearly reduced at E12.5 and E14.5 (Fig. 4 E and H and Fig. S3 B and E) and shows a different morphology and orientation at E18.5 (Fig. S4 B and _B_′). In the masseter region of Dlx5/6−/− mutants, only a few cells express MyoD, Desmin, and fMHC; the few positive myoblasts do not fuse into myotubes, resulting in the absence of masticatory muscle masses (Fig. 4 F and I and Figs. S3 C and F and S4 C and _C_′). In EdnRA−/− and in Dlx5/6−/− mutants, we find expression of muscle determination and differentiation markers in the vestigial tongue from E12.5 to E18.5 (Fig. 4 J_–_O and Figs. S3 G_–_L and S4 D_–_F and _D_′–_F_′), the genioglossus muscle is never present, and sublingual muscles are barely differentiated (Fig. 4 K, L, N, and O and Fig. S3 H, I, K, and L).
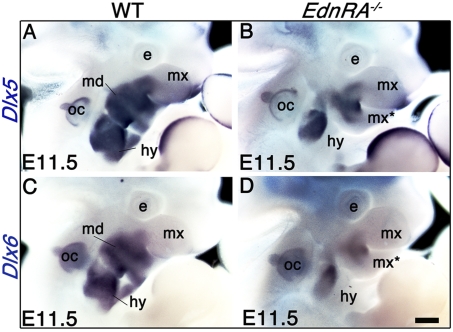
The presence of masticatory muscles in EdnRA−/− demonstrates that they can form even in the presence of the lower-to-upper jaw transformation that occurs in both EdnRA−/− and Dlx5/6−/− mutants. An explanation for the muscle development in EdnRA−/− embryos can be found by analyzing the Edn1-independent expression of Dlx5/6. Inactivation of EdnRA completely prevents the activation of Dlx5 and Dlx6 in E9.5 PA1 during CNCC specification. However, in the same mutant at E10.5, Dlx5/6 are expressed in an Edn1-independent territory in the proximal part of PA1 (25). Similarly, in E11.5 EdnRA−/− mutants, distal expression of Dlx5 and Dlx6 is lost in the arches, but is maintained in the proximal part of the mandibular and hyoid arches and in the maxillary bud (Fig. 5). Reflecting the mirror transformation of the lower into an upper jaw, at E11.5 the expression of Dlx5 and Dlx6 also displays mirror-like symmetry (Fig. 5 B and D). Remarkably, in EdnRA−/− mutants, the myogenic territory at the origin of masticatory muscles [defined by the expression of _Myf5_ (Fig. 3_C_) and MyoD (Fig. 4_A_)] is included within the Edn1-independent _Dlx5/6_-positive region, suggesting that the presence of Dlx5 and Dlx6 is at the origin of the myogenic differentiation observed in these mutants. Similarly, PA2-derived muscles present milder defects in EdnRA−/− than in _Dlx5/6_−/− mutants (Table S1). This might reflect the presence of an Edn1-independent _Dlx5/6_-positive region also within PA2 (Fig. 5). The masticatory muscular defects observed in EdnRA−/− and in Dlx5/6−/− mutants are summarized in Fig. 6.
Fig. 5.
Whole-mount in situ hybridization on E11.5 wild-type (A and C) and EdnRA−/− (B and D) embryos using Dlx5 (A and B) and Dlx6 (C and D) probes. In wild-type embryos (A and C), Dlx5 and Dlx6 are expressed in the mandibular and the hyoid arches and in the proximal part of the maxillary bud. In EdnRA−/− mutants, expression of Dlx5 and Dlx6 is lost in distal but not in proximal pharyngeal arches, including the duplicated maxillary bud (mx*), the hyoid arch (hy), and the maxillary bud (mx) (B and D). In these mutants, the pattern of Dlx5 and Dlx6 expression in the maxillary bud is a mirror image of that found in the duplicated maxillary bud. e, eye; hy, hyoid arch; md, mandibular arch, mx, maxillary bud; mx*, duplicated maxillary bud; oc, otic capsule. (Scale bar: 650 μm.)
Fig. 6.
Summary diagram. (Left) The territory of Dlx5/6 expression in E11.5 PA1 of the three mouse genotypes analyzed in this study. (Right) The skeleto-muscular defects observed in these mice are summarized. Muscles are shown as red, bones as green, and the tongue, which is a somite-derived muscle, as yellow. In both EdnRA−/− and Dlx5/6−/− mutants, lower jaw skeletal elements undergo a mirror image transformation into upper-jaw-like skeletal structures. Although in EdnRA−/− mutants the masseter muscle persists, no PA1-derived muscles are differentiated in Dlx5/6−/− mutants. dnt, dentary bone; md, mandibular bud; mm, masseter muscle; mx, maxillary bone or maxillary bud; mx*, duplicated maxillary bone or duplicated maxillary bud; pmx, premaxillary bone; sbl, sublingual muscles; tg, tongue.
In summary, our data show that Dlx5 and Dlx6 expression by CNCCs is necessary to maintain the myogenic program in the craniofacial mesoderm. In the absence of Dlx5/6, the myoblasts cannot initiate their differentiation. We reasoned that a possible mechanism could involve the derepression of BMP and Wnt signaling, which are known to prevent craniofacial myogenesis (17). Indeed, in a systematic quantitative PCR comparative analysis of microdissected E10.5 wild type and Dlx5/6−/− PA1s, we have shown that BMP7 and Wnt5a expression is increased two- and threefold, respectively, in the double-mutant mouse (30).
Discussion
In this study, we have shown that Dlx5/6 expression by CNCCs is necessary for CNCC–mesoderm interactions resulting in myogenic determination, differentiation, and patterning, but is not required for the early migration and specification of skeletal muscle progenitors. Our findings provide insight on the evolution of the “new head” (1), shedding light on the mechanism at the origin of the muscularized oral apparatus in vertebrates (Fig. 7).
Fig. 7.
Roles of Dlx genes during chordate evolution. The “new head” characterized by the simultaneous appearance of migratory skeletogenic CNCCs, _Hox_-negative _Dlx_-positive PA1 and PA1 muscularization may or may not include Myxynoidea (39). In this study, we show that CNCC expression of Dlx genes not only determines jaw identity in Gnathostomata, but also plays a role in organizing mouth muscularization in vertebrates. The expression of Dlx genes in PA1 CNCCs might have had a determining role in the transition between filter feeding and active predation. CNCCs, cranial neural crest cells; PA1, first pharyngeal arch.
Vertebrate heads share several similar morphological features: multiple brain divisions, pharyngeal arches, migrating CNCCs, sensory placodes, head muscles deriving from unsegmented cephalic medoderm (31), and a cartilaginous endoskeleton deriving from pharyngeal CNCCs (32).
Although the primordial role of Dlx genes in chordates has been the control of appendage development (33), in vertebrates, including gnatostomes and agnathans, these genes are also expressed in the PAs. Particularly, the expression of Dlx genes in the PA1 by _Hox_-negative CNCCs (2) is central for the development of oral cartilages (19, 34). Gnathostomes and agnathans differ, however, in the number and pattern of expression of Dlx genes in the head. In lampreys, the four Dlx genes are uniformly expressed by PA1 CNCCs. In gnathostomes, the increased number of PA1 Dlx genes goes hand-in-hand with the appearance of a dorso-ventral patterning of the branchiomeres at the origin of jaws (20, 32, 34, 35).
Here we show that Dlx expression endowed migratory CNCCs with the new function of coordinating cranial skeletogenesis and myogenesis (Fig. 7). It may be wondered whether the role of Dlx genes as organizers of a muscularized mouth in vertebrates is distinct from their function in maxillo-mandibular jaw specification in gnathostomes. Although in Dlx5/6−/− mice all masticatory muscles are lost, they are still present in _EdnRA_−/− mutants. Because these two mutants present a similar lower jaw transformation, we deduce that the capacity of Dlx genes to specify jaw identity is distinct from their role in coordinating oral muscularization. Remarkably, in EdnRA mutant mice, the masticatory muscles are adapted to the new skeletal morphology reflecting the mirror transformation of the jaws. Dlx genes might have had a central role in the harmonious coordination of the oral skeletal and muscular morphogenesis necessary to support the rich craniofacial shape diversity noted in vertebrates. Moreover, it appears that head mesodermal development in lampreys might correspond to the ancestral state of the gene regulatory mechanism at the origin of the vertebrate head (31).
We conclude that the role of Dlx genes in defining gnathostome jaw identity might be secondary to a more primitive function in the genesis of the oral skeletomuscular system in vertebrates. Expression of Dlx genes in the cephalic region therefore might have had a determining role in the transition between filter feeding and active predation.
Materials and Methods
Mice.
EdnRA and Dlx5;Dlx6 mutant mice were genotyped by PCR using allele-specific primers as reported (20, 36). Embryos were fixed in PBS (Sigma) 4% paraformaldehyde (E9.5–E12.5) for 1 d or in Bouin's fixative solution (75% saturated picric acid, 20% formol 40%, 5% acetic acid) for 5 d (E14.5 and E18.5). E18.5 fetuses were decalcified for 2 d with Jenkin's solution (50 mL, 2 d, three changes, 4% hydrochloric acid 37%, 3% acetic acid, 10% chloroform, 10% dH2O, 73% absolute ethanol). Fixed embryos and fetuses were embedded in paraffin and sectioned at 8–12 μm. Serial sections of E18.5 fetuses were subjected to Mallory trichromic staining (22) for anatomical analysis.
Dlx5/6+/−;Myf5nLacZ/+ heterozygous parents, obtained by crossing Myf5nLacZ/+ transgenic males (29) with heterozygous Dlx5/6+/− females, were crossed with Dlx5/6+/− mice to obtain Myf5nLacZ/+, Dlx5/6+/−;Myf5nLacZ/+, and Dlx5/6−/−;Myf5nLacZ/+ embryos, which were then fixed in PBS 4% PFA and stained to reveal LacZ expression.
Immunohistochemistry.
Immunohistochemistry was performed on deparaffinized sections using standard protocols of the Dako Envision kit and the Dako ARK kit. Polyclonal rabbit primary antibodies (anti-MyoD; Santa Cruz C-20:sc-304, 1/1200) and anti-Desmin (Abcam AB15200, 1/800) or mouse monoclonal primary antibody (anti-fMHC; Sigma MY-32, 1/100) were diluted in PBS, 1% BSA (Sigma).
Whole-Mount in Situ Hybridization.
Whole-mount in situ hybridization was performed with digoxigenin-labeled RNA probes corresponding to the antisense sequence of murine Tbx1, Capsulin, Pax3, MyoD, Dlx5, and Dlx6 (all previously reported in refs. 29, 37, and 38), on E9.5–E11.5 embryos adapting the procedure described by Tajbakhsh (29).
Supplementary Material
Supporting Information
Acknowledgments
We thank Professor Margaret Buckingham (Institut Pasteur, Paris) for the donation of plasmids and Myf5LacZ/+ transgenic male mice and Dr. Eldad Tzahor (Weizmann Institute of Science, Rehovot, Israel) for the donation of Tbx1 plasmids. We thank Anastasia Fontaine, Brice Bellessort, Vanessa Garnier, and Damien Habert for their excellent technical assistance. Particular thanks go to Professor Barbara Demeneix and Dr. Yorick Gitton for their very interesting discussion. This work has been supported in part by grants-in-aid by Association Française contre les Myopathies, the Agence Nationale pour la Recherche, projects Gendactyl and DrOS, and European Community Crescendo LSHM-CT-2005-018652. É.H. is a recipient of a doctoral fellowship from the Gendactyl project supported by the Agence National pour la Recherche; K.B. is a recipient of a doctoral fellowship from the French Government (Ministère de la Recherche et des Enseignements Supérieurs).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Glenn Northcutt R. The new head hypothesis revisited. J Exp Zool B Mol Dev Evol. 2005;304:274–297. doi: 10.1002/jez.b.21063. [DOI] [PubMed] [Google Scholar]
- 2.Takio Y, et al. Evolutionary biology: Lamprey Hox genes and the evolution of jaws. Nature. 2004;429 doi: 10.1038/nature02616. 1 p following 262. [DOI] [PubMed] [Google Scholar]
- 3.Horigome N, et al. Development of cephalic neural crest cells in embryos of Lampetra japonica, with special reference to the evolution of the jaw. Dev Biol. 1999;207:287–308. doi: 10.1006/dbio.1998.9175. [DOI] [PubMed] [Google Scholar]
- 4.Helms JA, Cordero D, Tapadia MD. New insights into craniofacial morphogenesis. Development. 2005;132:851–861. doi: 10.1242/dev.01705. [DOI] [PubMed] [Google Scholar]
- 5.Helms JA, Amasha RR, Leucht P. Bone voyage: An expedition into the molecular and cellular parameters affecting bone graft fate. Bone. 2007;41:479–485. doi: 10.1016/j.bone.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Noden DM, Trainor PA. Relations and interactions between cranial mesoderm and neural crest populations. J Anat. 2005;207:575–601. doi: 10.1111/j.1469-7580.2005.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinon A, et al. Cranial neural crest cells regulate head muscle patterning and differentiation during vertebrate embryogenesis. Development. 2007;134:3065–3075. doi: 10.1242/dev.002501. [DOI] [PubMed] [Google Scholar]
- 8.Grenier J, Teillet MA, Grifone R, Kelly RG, Duprez D. Relationship between neural crest cells and cranial mesoderm during head muscle development. PLoS One. 2009;4:e4381. doi: 10.1371/journal.pone.0004381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tokita M, Schneider RA. Developmental origins of species-specific muscle pattern. Dev Biol. 2009;331:311–325. doi: 10.1016/j.ydbio.2009.05.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noden DM. The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat. 1983;168:257–276. doi: 10.1002/aja.1001680302. [DOI] [PubMed] [Google Scholar]
- 11.Trainor PA, Tan SS, Tam PP. Cranial paraxial mesoderm: regionalisation of cell fate and impact on craniofacial development in mouse embryos. Development. 1994;120:2397–2408. doi: 10.1242/dev.120.9.2397. [DOI] [PubMed] [Google Scholar]
- 12.Nathan E, et al. The contribution of Islet1-expressing splanchnic mesoderm cells to distinct branchiomeric muscles reveals significant heterogeneity in head muscle development. Development. 2008;135:647–657. doi: 10.1242/dev.007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mootoosamy RC, Dietrich S. Distinct regulatory cascades for head and trunk myogenesis. Development. 2002;129:573–583. doi: 10.1242/dev.129.3.573. [DOI] [PubMed] [Google Scholar]
- 14.Hadchouel J, et al. Modular long-range regulation of Myf5 reveals unexpected heterogeneity between skeletal muscles in the mouse embryo. Development. 2000;127:4455–4467. doi: 10.1242/dev.127.20.4455. [DOI] [PubMed] [Google Scholar]
- 15.Lu JR, et al. Control of facial muscle development by MyoR and capsulin. Science. 2002;298:2378–2381. doi: 10.1126/science.1078273. [DOI] [PubMed] [Google Scholar]
- 16.Kelly RG, Jerome-Majewska LA, Papaioannou VE. The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Hum Mol Genet. 2004;13:2829–2840. doi: 10.1093/hmg/ddh304. [DOI] [PubMed] [Google Scholar]
- 17.Tzahor E, et al. Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev. 2003;17:3087–3099. doi: 10.1101/gad.1154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Couly G, Grapin-Botton A, Coltey P, Ruhin B, Le Douarin NM. Determination of the identity of the derivatives of the cephalic neural crest: Incompatibility between Hox gene expression and lower jaw development. Development. 1998;125:3445–3459. doi: 10.1242/dev.125.17.3445. [DOI] [PubMed] [Google Scholar]
- 19.Depew MJ, Simpson CA, Morasso M, Rubenstein JL. Reassessing the Dlx code: The genetic regulation of branchial arch skeletal pattern and development. J Anat. 2005;207:501–561. doi: 10.1111/j.1469-7580.2005.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beverdam A, et al. Jaw transformation with gain of symmetry after Dlx5/Dlx6 inactivation: mirror of the past? Genesis. 2002;34:221–227. doi: 10.1002/gene.10156. [DOI] [PubMed] [Google Scholar]
- 21.Depew MJ, Lufkin T, Rubenstein JL. Specification of jaw subdivisions by Dlx genes. Science. 2002;298:381–385. doi: 10.1126/science.1075703. [DOI] [PubMed] [Google Scholar]
- 22.Sato T, et al. An endothelin-1 switch specifies maxillomandibular identity. Proc Natl Acad Sci USA. 2008;105:18806–18811. doi: 10.1073/pnas.0807345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruest LB, Hammer RE, Yanagisawa M, Clouthier DE. Dlx5/6-enhancer directed expression of Cre recombinase in the pharyngeal arches and brain. Genesis. 2003;37:188–194. doi: 10.1002/gene.10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruest LB, Xiang X, Lim KC, Levi G, Clouthier DE. Endothelin-A receptor-dependent and -independent signaling pathways in establishing mandibular identity. Development. 2004;131:4413–4423. doi: 10.1242/dev.01291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozeki H, Kurihara Y, Tonami K, Watatani S, Kurihara H. Endothelin-1 regulates the dorsoventral branchial arch patterning in mice. Mech Dev. 2004;121:387–395. doi: 10.1016/j.mod.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Trainor PA, Tam PP. Cranial paraxial mesoderm and neural crest cells of the mouse embryo: Co-distribution in the craniofacial mesenchyme but distinct segregation in branchial arches. Development. 1995;121:2569–2582. doi: 10.1242/dev.121.8.2569. [DOI] [PubMed] [Google Scholar]
- 27.Dastjerdi A, et al. Tbx1 regulation of myogenic differentiation in the limb and cranial mesoderm. Dev Dyn. 2007;236:353–363. doi: 10.1002/dvdy.21010. [DOI] [PubMed] [Google Scholar]
- 28.Sambasivan R, et al. Distinct regulatory cascades govern extraocular and pharyngeal arch muscle progenitor cell fates. Dev Cell. 2009;16:810–821. doi: 10.1016/j.devcel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- 30.Vieux-Rochas M, et al. Spatio-temporal dynamics of gene expression of the Edn1-Dlx5/6 pathway during development of the lower jaw. Genesis. 2010 doi: 10.1002/dvg.20625. 10.1002/dvg.20625. [DOI] [PubMed] [Google Scholar]
- 31.Kusakabe R, Kuratani S. Evolutionary perspectives from development of mesodermal components in the lamprey. Dev Dyn. 2007;236:2410–2420. doi: 10.1002/dvdy.21177. [DOI] [PubMed] [Google Scholar]
- 32.Kimmel CB, Miller CT, Keynes RJ. Neural crest patterning and the evolution of the jaw. J Anat. 2001;199:105–120. doi: 10.1046/j.1469-7580.2001.19910105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panganiban G, et al. The origin and evolution of animal appendages. Proc Natl Acad Sci USA. 1997;94:5162–5166. doi: 10.1073/pnas.94.10.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neidert AH, Virupannavar V, Hooker GW, Langeland JA. Lamprey Dlx genes and early vertebrate evolution. Proc Natl Acad Sci USA. 2001;98:1665–1670. doi: 10.1073/pnas.98.4.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham A. Jaw development: Chinless wonders. Curr Biol. 2002;12:R810–R812. doi: 10.1016/s0960-9822(02)01315-5. [DOI] [PubMed] [Google Scholar]
- 36.Kurihara Y, et al. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368:703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- 37.Perera M, et al. Defective neuronogenesis in the absence of Dlx5. Mol Cell Neurosci. 2004;25:153–161. doi: 10.1016/j.mcn.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Tirosh-Finkel L, Elhanany H, Rinon A, Tzahor E. Mesoderm progenitor cells of common origin contribute to the head musculature and the cardiac outflow tract. Development. 2006;133:1943–1953. doi: 10.1242/dev.02365. [DOI] [PubMed] [Google Scholar]
- 39.Nicholls H. Evolution: Mouth to mouth. Nature. 2009;461:164–166. doi: 10.1038/461164a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information