Stealth Nanoparticles: High Density but Sheddable PEG is a Key for Tumor Targeting (original) (raw)
. Author manuscript; available in PMC: 2011 Aug 1.
Abstract
There are three major requirements for a nanoparticle to deliver its payload to the tumor. First, the particles need to be stable in the circulation without releasing the drug prematurely. Second, the nanoparticles accumulate in the tumor efficiently. Third, the drug is released locally in the tumor tissue or inside the tumor cells. A variety of approaches have been developed to fulfill these requirements. In this perspective, we will discuss them together with some examples.
1. Pharmacokinetics, biodistribution and toxicity of the nanoparticles
1.1. Stealth nanoparticles grafted with poly(ethylene glycol)
Poly(ethylene glycol) (PEG) was first introduced in the early ’90s to modify the surface of liposomes for improved pharmacokinetics (PK) after intravenous (i.v.) administration. This hydrophilic and inert polymer imparts a steric barrier to the surface of nanoparticles and minimizes their protein binding (also termed opsonization). Binding of plasma proteins is the primary mechanism for the reticuloendothelial system (RES) to recognize the circulating nanoparticles, causing a major loss of the injected dose (ID) (>50%) within a few hours after i.v. injection. Macrophages, such as the Kupffer cells in the liver, recognize the opsonized nanoparticles via the scavenger receptor. Liver, spleen and bone marrow are the major RES organs for nanoparticle clearance. PEGylation technology has been widely used for improving the PK of a variety of nanoparticles. PEGylated nanoparticles are often referred as “stealth” nanoparticles, because they escape the surveillance of RES better than the control nanoparticles. Nanoparticles with a mean size around 100–200 nm are attractive for tumor targeting because of the unique feature known as the enhanced permeability and retention (EPR) effect, for which the particles accumulate in the tissues with leaky blood vessels (i.e., tumor and inflamed tissues) after i.v. administration. The lack of draining lymphatic system in the tumor also contributes to the significant retention of nanoparticles that penetrate through the angiogenic vasculature from the systemic circulation. The enlarged capillary gaps (>400 nm) in the tumor vasculature allow transport of nanoparticles. However, the amount of blood circulates through the tumor is usually far less than that of the RES organs. Only the nanoparticles that are not rapidly cleared from the circulation will have a chance to encounter the leaky tumor vasculature. Thus, the longer the nanoparticles circulate, the higher the chance is for them to accumulate in the tumor. Producing stealth nanoparticles with a reduced RES uptake and a prolonged circulation half-life is an important prerequisite for enhanced tumor targeting. In other words, RES competes with tumor for the uptake of the stealth nanoparticles. Theoretically, a perfectly stealth nanoparticle should not be opsonized at all and should stay in the circulation until it encounters and penetrates a leaky vasculature. In reality, however, PEGylated nanoparticles are far from perfect for two reasons. First, the nanoparticles are not sufficiently PEGylated such that opsonization still occurs and the RES uptake of the nanoparticles is still substantial. On the other hand, PEGylation seriously hinders the uptake of the nanoparticles by the tumor cells once they extravasate. For example, the stealth liposomes (Doxil®) shows little cellular uptake when incubated with tumor cells for 24 h. Either case is not welcome. So, what could be the solution?
1.2. PEG configuration on the particle surface and its influence on pharmacokinetics
It is important that the grafted PEG sufficiently covers the surface of nanoparticles in order to efficiently prevent opsonization. Theoretically, for a 100 nm liposomal particle modified with DSPE-PEG2000, PEG is arranged in the mushroom conformation with <4 mol% DSPE-PEG2000; in the transition configuration in the presence of a 4–8 mol% modification; and in the brush mode with >8 mol% PEGylation. The brush mode is the ideal configuration that ensures complete coverage of the surfaces of the nanoparticles and provides full protection. However, it is difficult to prepare stable, PEGylated liposomes with a brush conformation while maintaining the integrity of lipid membrane. This is because the PEGylated amphiphiles used to coat liposomes are detergent-like molecules. Stealth liposomal formulations (e.g. Doxil®) often contain ~5 mol% graft of PEG. These partially stealthy formulations are still susceptible for protein binding and RES uptake as the dose accumulating in the liver and the spleen is very significant.
The idea of introducing brushed PEG onto nanoparticles for further improved PK was suggested by the Discher group (1). They have shown that the polymersomes with brushed PEG could be formed by hydrating a polymer monomer that had enhanced hydrophobic interaction in a self-assembling manner. The polymersomes displayed a circulation half-life of 15–30 h in the rat. Recently, our lab has demonstrated that the post-inserted PEG was arranged in high enough density (~10.6 mol%) for the brush conformation onto the LPD (liposome-polycation-DNA) nanoparticles in which the lipid bilayer was stabilized by charge-charge interaction (2). These stealthy LPD nanoparticles exhibited reduced liver uptake (12±5.7% ID at 4 h) and delivered siRNA efficiently into the tumor tissue (32.5±12.3% ID at 4 h). Applying another force to antagonize the repulsion among the PEG chains; e.g., charge-charge interaction from the supported bilayer of LPD or hydrophobic interaction among the monomers of the polymersomes, is the key to maintain a high mol% of PEG arranged in the brush mode.
1.3. Toxicity of the long circulating PEGylated nanoparticles
Prolonged systemic circulation provides the particles with an increased chance to extravasate into the target tissue, but it also leads to enhanced drug exposure to other tissues which may cause new side effects. The cardiotoxicity of doxorubicin is greatly reduced by formulating the drug into the stealth liposomes (Doxil®); however, with its much extended half-life (~80 h in adults) this formulation introduces a new side effect (hand-foot syndrome and mucositis) that limits the maximum tolerated dose (MTD). Moreover, repeated injections of stealth liposomes into mouse provoked the immune system to generate PEG-specific IgM, which significantly decreased the half-life of the subsequently injected liposomes. A similar observation was reported later on the nucleic acid containing nanoparticles, such as stabilized plasmid-lipid particles (SPLP), stabilized nucleic acid lipid particles (SNALP) and PEGylated lipoplex (3). It is hypothesized that the PEG antibody was generated because of the prolonged contact of the PEGylated nanoparticles with the immune cells which were also activated by the nucleic acid in the nanoparticles. The induction of PEG antibody was significantly enhanced when the CpG containing nucleic acid was used (3), which further strengthens the hypothesis. The lipid chain of the PEGylated lipid in the SPLP/SNALP formulation was shortened to make it more “diffusible” from the particles for minimizing the direct contact of the activated immune cells with PEG. This approach significantly reduced the immunostimulatory effect of the subsequently injected nanoparticles and maintained comparable gene delivery activity after multiple injections. Although data from other groups have suggested that the ABC (accelerated blood clearance) phenomenon might not be PEG specific and is dose dependent, the anti-PEG mechanism is still of concern to the field of PEGylated nanomedicine. However, IgM may not be able to opsonize the nanoparticles if there is a good PEG brush grafted on the surface. This hypothesis should be experimentally tested.
These results suggest that there is a fine line between long circulation and systemic overexposure of the nanoparticles, which are likely to cause side effects including drug related and immuno-toxicity. An ideal drug carrier is the one that delivers a drug efficiently (in both time and dose) to the target tissue, and it needs to be eliminated from the blood in a reasonable period of time for minimal side effects.
2. Drug release from the PEGylated nanoparticles
Encapsulated drugs are to be released from the nanoparticles at the target site in order to be bioactive. A variety of stimulus-responsive nanoparticle formulations for enhancing local drug release, and hence the therapeutics effect, have been designed. For delivering a biopharmaceutical agent (nucleic acid, peptide and protein), releasing the payload intracellularly is a requirement since the drug is not membrane permeable. Usually a targeting ligand that recognizes a cell surface receptor is attached to the distal end of PEG to trigger cellular internalization of the nanoparticles. Most of the nanoparticles are destroyed in the endosome/lysosome and only a small fraction escapes with an unknown mechanism. Three models have been proposed to describe the possible mechanism of the PEGylated lipid based nanoparticles fleeing from the endosome/lysosome, including the ion-pairing model, the proton sponge model and the charge-charge destabilization model. For any of these models to work, the stealth nanoparticles may have to undergo de-PEGylation to destabilize or disrupt the endosomal membrane. Thus, it is favored to design the nanoparticles composed of sheddable/diffusible PEG.
3. A key to open the barriers: PEGylated LPD as an example
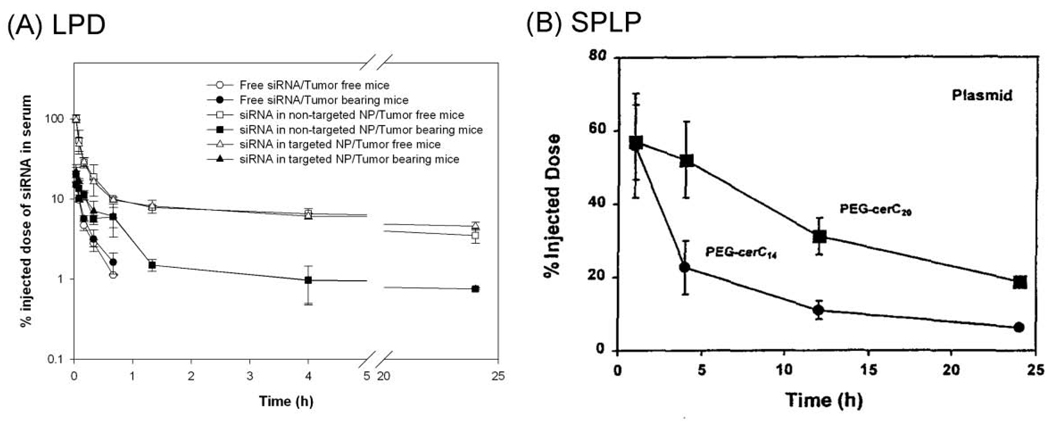
The PEGylated LPD consists of a compact core of nucleic acid-protamine complex coated with a supported lipid bilayer (DOTAP: cholesterol = 1:1) with ~10.6 mol% of PEG inserted onto the outer leaflet of the lipid membrane (2). The mean diameter was around 120 nm and according to the theoretical calculation the PEG was arranged in the brush mode at the nanoparticle surface, which provided complete shielding as evidenced by the neutral surface charge and the abolishment of the liver sinusoidal uptake in an isolated and perfused liver model (2). The nanoparticles delivered ~10% of the injected dose to the tumor after 30 min of i.v. injection and ~30% of the dose at 4 h with a relatively low RES uptake (5–15% of the administered dose) (2). It is of notice that the nanoparticles did not display a much prolonged circulation half-life as compared with other stealth liposomal formulations (2, 4), but exhibited accelerated clearance from the blood in the tumor-bearing mice compared to the tumor free mice (5). As can be seen in Figure 1A, PEGylated LPD displayed a bi-phasic PK profile with a relatively fast distribution phase in the tumor free mouse model. On the other hand, the SPLP composed of PEG-ceramide (C20) showed one-compartmental biodistribution (Figure 1B), which is typical for a stealth formulation. However, when the SPLP were prepared with the diffusible PEG-ceramide (C14), the PK profile resembles that of the PEGylated LPD, suggesting the PEG was sheddable in both formulations. It is of interest to notice that the PEGylated LPD showed significantly increased elimination from the circulation in the tumor-bearing mice than in the tumor free mice, indicating that the tumor was one of the primary tissues the particles distributed to.
Figure 1.
Comparison of the pharmacokinetic profiles of the PEGylated LPD (A) and the SPLP (B) in animal models. Non-targeted NP = PEGylated LPD and targeted NP = PEGylated LPD with surface decoration of a targeting ligand, anisamide. Data are reproduced from (4–5) with permission.
As shown in Figure 2, we proposed that the LPD nanoparticles with brushed PEG on the surface exhibits little initial RES uptake, rendering much increased passive targeting to the tumor with leaky vessels. As suggested by the theory of the first pass effect, the initial period of time after drug administration is crucial and the PEGylated LPD seizes the timing. DSPE-PEG2000 is likely to diffuse out of the lipid membrane composed of DOTAP (Tg = 0°C) at the body temperature. When the PEG content is reduced to <4 mol%, particle opsonization and RES uptake increase, causing enhanced blood clearance as evidenced by the PK data showing a rapid distribution phase (Figure 1A). The relatively low immunotoxicity of the PEGylated LPD might benefit from this much faster elimination from the blood compared to the conventional stealth nanoparticles (6–7). The introduction of the tumor creates a new compartment for the distribution, which results in further enhanced clearance of the particles from the blood. This phenomenon is likely to be tumor model dependent, favoring the tumors with increased permeability in their vasculature. To deliver the payload intracellularly, a targeting ligand is attached to the distal end of the PEG-lipid. After penetrating into the tumor tissue, the nanoparticles are internalized via ligand induced endocytosis into the endosome/lysosome. The shedding of the PEG-lipid from the particles continues, which eventually exposes the positive charges of the particles and allows the charge-charge interaction with the endosomal membrane, causing membrane fusion and the release of the nucleic acid-protamine complex into the cytosol. The shedded PEG-lipid might also insert into the endosomal membrane, which destabilizes the lipid bilayer and facilitates the release of the payload. This proposed mechanism suggests the importance of grafting brushed PEG onto the nanoparticles as well as controlling the kinetics of de-PEGylation. The brushed PEG grants improved stability for the nanoparticles to evade the RES for the initial period of time after i.v. injection that allows the nanoparticles to extravasate into the tumor. The PEG needs to come off from the nanoparticles to avoid systemic overexposure and to facilitate the dug release. The PEG shedding kinetics is yet to be studied. It is anticipated that once the PEGylation is reduced to 4 mol% (mushroom configuration), the particles will display much enhanced RES uptake and blood clearance. The initial high level of PEGylation (~10.6 mol%) gives the LPD nanoparticles sufficient time for tumor targeting before the level falls below 4 mol%.
Figure 2.
Proposed mechanisms for the biodistribution and the drug release of the liganded and PEGylated LPD nanoparticles. A. The liganded and PEGylated LPD is introduced intravenously with brushed PEG that prevents opsonization at the initial period of time, allowing the nanoparticles to extravasate efficiently to the tumor with a leaky vasculature. B. The PEG-lipid gradually sheds from the LPD, leading to increased protein binding, RES uptake and accelerated clearance from the blood. C. After cellular internalization, the cationic lipid membrane interacts with the endosomal membrane via ion-pairing after de-PEGylation, causing membrane fusion and payload release.
4. Perspectives and future directions
An ideal delivery system carries the payload to the target site efficiently (>10% ID in 4 h) (i.e., potent) and is cleared from the systemic circulation in a short period of time (in 4–10 h) (i.e., safe). Schnitzer’s group (8) has developed a tissue-specific antibody that recognized a receptor in the caveolae in the luminal site of the lung endothelium. Approximately 80% of the antibody-conjugated gold nanoparticles (10–15 nm) were transcytosed into the lung in 30 min after i.v. injection, suggesting the circulation half-life for the targeted nanoparticles was only a few min. The research trend in drug delivery is no longer to develop a vehicle that is capable of circulating in the blood indefinitely but to employ a smart mechanism allowing efficient distribution to the diseased tissue and also with a rapid elimination from the circulation (90% ID elimination in 4–10 h). Another example is Rouslahti’s artificial platelet particles that bound specifically with the clotting proteins on the lumen of the angiogenic blood vessels and formed clots in the vessels in the tumor in a self-amplifying manner, resulting in significant tumor accumulation in a few hours (9). This efficient delivery is also anticipated to couple with a very short circulation half-life (a few min to 1 h). Although the artificial platelet particles did not truly overcome the endothelial barrier, they self-amplified the accumulation in the tumor blood vessels by active aggregation. A drug might be loaded inside the particles and be released locally in the tumor.
Different from the active targeting mechanism, the PEGylated LPD is proposed to utilize the full protection from the brushed PEG (initial PEGylation ~10.6 mol%) to gain a sufficient time window for passively extravasting to the tumor and then leaves the blood circulation by being cleared by the RES after the PEG coating is reduced to <4 mol%. Producing a nanoparticle with brushed PEG coating requires applying another mechanism to stabilize the formulation (e.g., charge-charge interaction or enhanced hydrophobic interaction) as described in the Section 2.2. According to this hypothesis, other types of nanoparticles with a brushed PEG coating can be developed. More importantly, the release kinetics of the PEG from the particles has to be carefully optimized in order to maintain the passive tumor targeting while reducing the risk of systemic over-exposure. The release kinetics can be controlled by using PEG-lipids with different lipid chain lengths or changing the lipid composition of the nanoparticle membrane. Finally, the drug release from the nanoparticles is critical for gaining therapeutic effects.
For a drug that is membrane permeable, it is desirable to release it locally in the tumor tissue for improved tumor penetration and intratumoral distribution. Ideally, the release kinetics should match with the pharmacokinetics of the nanoparticles to attain local drug release without significant loss of the dose in the circulation, which probably requires a pulse release mechanism after the nanoparticles have extravasated in the target tissue with a minimal dose in the circulation. This is very difficult to achieve if the drug release is solely dependent on the formulation design; however, in the presence of external (e.g., heat, and ultrasound) or internal (e.g., pH and enzyme) triggering mechanisms, local drug release (or pulse release) can be attained. Needham and Dewhirst have developed a thermo-sensitive liposomal formulation that released 100% of the encapsulated doxorubicin at the hyperthermia condition (42°C) within a few min but remained stable at 37°C. Recently, they have used MRI to monitor the drug release and distribution in the tumor after concomitant delivery of the thermo-sensitive formulation with hyperthermia and found that the intratumoral drug diffusion rate was increased by 5-fold and the released drug concentration was increased by 2-fold compared to the liposomal doxorubicin alone. The combination of the thermo-sensitive liposomal doxorubicin and local hyperthermia has been shown to eradicate the tumor in a mouse xenograft model. Intracellular delivery and drug release are to be used for membrane impermeable drugs via the ligand targeting approach followed by the endosomal release, which is the limiting step for biopharmaceutical agents, such as oligonucleotides, peptides and proteins as evidenced by several targeted toxins showing no killing activity without an endosomo-lytic domain. Endosomal release can be triggered by different mechanisms, including the pH sensitive drug release, the proton sponge effect and the ion-paring effect, which can be facilitated after PEG shedding as discussed earlier.
In addition to the degree of PEGylation and kinetics of de-PEGylation, the chain length of the PEG block might have profound impact on the particle size, stability, activity and toxicity as demonstrated by Tang et al. (10). The data suggest that chain length of PEG that is coated onto the particle surface needs to be optimized for desirable effects. Similarly, different PEG structures (linear, branched, blocked, grafted or combination) could substantially influence those properties as well (11–12). Currently, covalent coating is still the method of choice for PEGylation; however, physical adsorption (i.e., via charge) is an attractive alternative to PEGylate nanoparticles. This method is not only more convenient but may also to offer a different mechanism and kinetics of de-PEGylation that will likely affect the activity and toxicity of the particles (11). In conclusion, PEGylation is required for nanoparticles to exhibit prolonged circulation half-life and de-PEGylation is needed to facilitate the clearance of the particles from the body or to enhance the drug release at the target site. A balance between PEGylation and de-PEGylation is needed to produce a nanoparticle formulation that is potent and safe.
Acknowledgements
We thank Yu-Cheng Tseng (UNC) for his assistance in composing Figure 2 and National Institute of Health for supporting the original work (CA129835).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Photos PJ, Bacakova L, Discher B, Bates FS, Discher DE. Polymer vesicles in vivo: correlations with PEG molecular weight. J Control Release. 2003;90:323–334. doi: 10.1016/s0168-3659(03)00201-3. [DOI] [PubMed] [Google Scholar]
- 2.Li SD, Huang L. Nanoparticles evading the reticuloendothelial system: role of the supported bilayer. Biochim Biophys Acta. 2009;1788:2259–2266. doi: 10.1016/j.bbamem.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tagami T, Nakamura K, Shimizu T, Yamazaki N, Ishida T, Kiwada H. CpG motifs in pDNA-sequences increase anti-PEG IgM production induced by PEG-coated pDNA-lipoplexes. J Control Release. 2010;142:160–166. doi: 10.1016/j.jconrel.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Monck MA, Mori A, Lee D, Tam P, Wheeler JJ, Cullis PR, Scherrer P. Stabilized plasmid-lipid particles: pharmacokinetics and plasmid delivery to distal tumors following intravenous injection. J Drug Target. 2000;7:439–452. doi: 10.3109/10611860009102218. [DOI] [PubMed] [Google Scholar]
- 5.Li SD, Chen YC, Hackett MJ, Huang L. Tumor-targeted Delivery of siRNA by Self-assembled Nanoparticles. Mol Ther. 2008;16:163–169. doi: 10.1038/sj.mt.6300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chono S, Li SD, Conwell CC, Huang L. An efficient and low immunostimulatory nanoparticle formulation for systemic siRNA delivery to the tumor. J Control Release. 2008;131:64–69. doi: 10.1016/j.jconrel.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li SD, Chono S, Huang L. Efficient gene silencing in metastatic tumor by siRNA formulated in surface-modified nanoparticles. J Control Release. 2008;126:77–84. doi: 10.1016/j.jconrel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh P, Borgstrom P, Witkiewicz H, Li Y, Borgstrom BJ, Chrastina A, Iwata K, Zinn KR, Baldwin R, Testa JE, Schnitzer JE. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat Biotechnol. 2007;25:327–337. doi: 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simberg D, Duza T, Park JH, Essler M, Pilch J, Zhang L, Derfus AM, Yang M, Hoffman RM, Bhatia S, Sailor MJ, Ruoslahti E. Biomimetic amplification of nanoparticle homing to tumors. Proc Natl Acad Sci U S A. 2007;104:932–936. doi: 10.1073/pnas.0610298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang R, Palumbo RN, Nagarajan L, Krogstad E, Wang C. Well-defined block copolymers for gene delivery to dendritic cells: probing the effect of polycation chain-length. J Control Release. 2010;142:229–237. doi: 10.1016/j.jconrel.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park JW, Mok H, Park TG. Physical adsorption of PEG grafted and blocked poly-L-lysine copolymers on adenovirus surface for enhanced gene transduction. J Control Release. 2010;142:238–244. doi: 10.1016/j.jconrel.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Park K. To PEGylate or not to PEGylate, that is not the question. J Control Release. 2010;142:147–148. doi: 10.1016/j.jconrel.2010.01.025. [DOI] [PubMed] [Google Scholar]