mir-200c Regulates Induction of Apoptosis through CD95 by Targeting FAP-1 (original) (raw)
. Author manuscript; available in PMC: 2011 Jun 25.
SUMMARY
Tumor progression shares many characteristics with the process of epithelial-to-mesenchymal transition (EMT). Cells that have undergone an EMT are known to have an increased resistance to apoptosis. CD95/Fas is an apoptosis-inducing receptor expressed on many tissues and tumor cells. During tumor progression CD95 is frequently downregulated, and tumor cells lose apoptosis sensitivity. miR-200 microRNAs repress both the EMT-inducing ZEB1 and ZEB2 transcription factors. We now demonstrate that miR-200c sensitizes cells to apoptosis mediated by CD95. We have identified the apoptosis inhibitor FAP-1 as a target for miR-200c. FAP-1 was demonstrated to be responsible for the reduced sensitivity to CD95-mediated apoptosis in cells with inhibited miR-200. The identification of FAP-1 as a miR-200c target provides a molecular mechanism to explain both the downregulation of CD95 expression and the reduction in sensitivity of cells to CD95-mediated apoptosis that is observed in the context of reduced miR-200 expression during tumor progression.
INTRODUCTION
Micro(mi)RNAs are small, noncoding RNAs, 19–24 nucleotides in length, that negatively regulate expression of multiple genes either by inducing translational silencing or by causing degradation of the mRNA of the targeted gene (Bartel, 2009). It has been firmly established that miRNAs regulate many key cellular processes such as cell growth, differentiation, and death (Schickel et al., 2008). Recently, we and others identified the miR-200 family of miRNAs (miR-200a, 200b, 200c, 141, and 429) as both fundamental markers and powerful regulators of the process of epithelial-mesenchymal-transition (EMT) (Gregory et al., 2008; Park et al., 2008). miR-200 maintains the epithelial phenotype of tissues by suppressing expression of the EMT-inducing transcription factors, ZEB1 and ZEB2 (Christoffersen et al., 2007; Gregory et al., 2008; Hurteau et al., 2007; Park et al., 2008). The repression of E-cadherin by ZEB1 and ZEB2 is characterized by a full scale shift in phenotype indicative of EMT. A key step in the progression of carcinomas is the loss of an epithelial phenotype and the acquisition of mesenchymal characteristics in a manner highly reminiscent of EMT. The process of EMT is reversible, and a mesenchymal-epithelial-transition (MET) is found during metastases when tumor cells may become partially re-epithelialized (Savagner, 2001). Consistent with miR-200 being involved in the process of EMT and MET, it has been reported for a number of human cancers that early during malignant transformation members of the miR-200 family are downregulated, but are subsequently upregulated in advanced stages of cancer (Peter, 2009).
Tumor progression is a multi-step process during which cancer cells undergo a number of changes. These changes include acquisition of mutations in tumor suppressor pathways and in oncogenes (Hanahan and Weinberg, 2000). In addition, cells become more and more resistant to apoptosis, and as a result, refractory to therapy. One apoptosis pathway that is affected by these changes is the pathway activated by the death receptor, CD95 (Fas/APO-1). It is well established that cancer cells lose sensitivity to CD95-mediated apoptosis either through acquisition of mutations in CD95, downregulation of CD95, or upregulation of antiapoptotic proteins (Peter et al., 2007; Peter et al., 2005). CD95 induces apoptosis by forming a death-inducing signaling complex (DISC) at the receptor that contains FADD, caspase-8/10, and the caspase-8 regulator, c-FLIP (Peter and Krammer, 2003). An apoptosis inhibitor that affects CD95 signaling by regulating surface expression of CD95 is Fas-associated phosphatase-1 (FAP-1, also known as PTPN13, PTPL1, or PTP-BAS) (Sato et al., 1995). FAP-1 has been linked to resistance to apoptosis mediated by CD95 in many human cancers, and an inverse correlation between sensitivity to CD95-mediated apoptosis and the expression of FAP-1 is seen in several tumor types (Elnemr et al., 2001; Foehr et al., 2005; Ivanov et al., 2003; Lee et al., 2001; Lee et al., 1999; Li et al., 2000; Meinhold-Heerlein et al., 2001; Myc et al., 1999; Nakai et al., 2000; Ungefroren et al., 2001; Wieckowski et al., 2007).
We previously reported that among the 60 cell lines maintained by the National Cancer Institute (NCI60) cell lines representing early stages of cancer progression (Type II tumor cells, which die through a mitochondria-dependent pathway) are more sensitive to CD95 ligand when compared to cell lines that have characteristics of later stages (Type I tumor cells, which die without mitochondrial involvement). This provided a model for the changes in CD95 signaling that occur during tumor progression (Algeciras-Schimnich et al., 2003). We subsequently identified miR-200 to be more highly expressed in early/epithelial cancer cells (Park et al., 2008). We now report that miR-200 increases apoptosis sensitivity of cancer cells through targeting the apoptosis inhibitor, FAP-1. Our data provide a mechanistic explanation for how sensitivity of cancer cells to CD95-mediated apoptosis is lost early during neoplastic transformation.
RESULTS AND DISCUSSION
We previously noted that cells with properties of early cancers (Type II cells) had an epithelial phenotype, whereas cells resembling more advanced cancer (Type I cells) had a more mesenchymal phenotype (Algeciras-Schimnich et al., 2003). Because cancer cells lose apoptosis sensitivity during tumor progression including sensitivity to CD95-mediated apoptosis we determined if changes in miR-200 levels affected CD95 signaling. miR-200c was introduced stably into CAKI-1 and HeyA8 cells, or transiently into ACHN cells. These three cell lines with different levels of sensitivity to CD95-mediated apoptosis possess mesenchymal features. Morphological changes consistent with acquisition of a more epithelial phenotype were noted in all three cell lines treated with miR-200c (Figure 1A). In addition, the miR-200c treated cells showed increased sensitivity to CD95-mediated apoptosis when treated with the agonistic anti-CD95 mAb, anti-APO-1 (Figure 1B and Figure S1). This effect was dramatically seen when CAKI-1 or ACHN cells transiently transfected with miR-200c were treated with anti-APO-1 (Figure 1B and Figure S1), or with anti-APO-1 plus protein A (to crosslink anti-APO-1), or with a leucine-zipper tagged CD95L (LzCD95L), which mimics membrane-bound CD95 ligand in its activity (Figure S1). This enhancement of CD95-mediated apoptosis was also seen when cells were treated with anti-APO-1 in the presence of cycloheximide, suggesting that miR-200 targets a preexisting protein that may affect the CD95 signaling pathway (Figure 1C and Figure S1). In a converse experiment, when the concentration of miR-200 was reduced in HCT116 cells (which has an epithelial phenotype and expresses high miR-200), using an inhibitor that targets all 5 miR-200 family members (LNA200, see (Park et al., 2008)), cells became more resistant to apoptosis induction (Figure 1B) (Algeciras-Schimnich et al., 2003; Park et al., 2008).
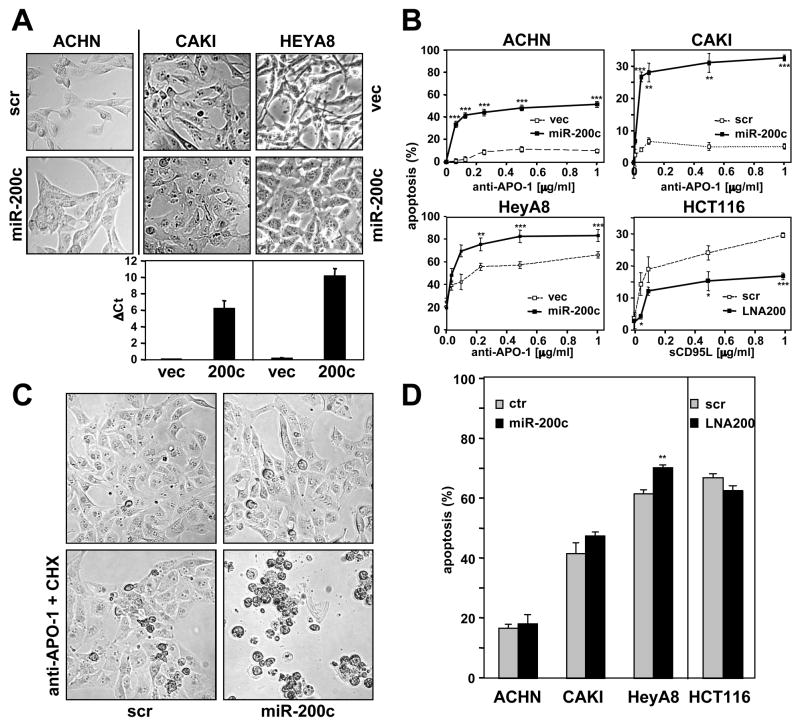
Figure 1. Altering Levels of miR-200 Changes Sensitivity of Cells to CD95-induced Apoptosis.
(A) Phase contrast pictures showing morphological changes of cells transfected with scrambled pre-miRNA (scr) and pre-miR-200c (3 times over 9 days) or cells stably infected with a retrovirus expressing scrambled oligo (vec) and miR-200c. Levels of mir-200c in retrovirus infected cells were quantified by real-time PCR. (B) Cells shown in A stimulated through CD95. HCT116 cells were transfected with LNA200 seven times prior to stimulation. Cells ectopically expressing miR-200c or cells treated with LNA200 were stimulated with different concentrations of anti-APO-1 for 20 hrs and apoptosis was quantified by MTS assay. (C) CAKI-1 cells transfected as in B were treated with 1 μg/ml anti-APO-1 and 1 μg/ml cycloheximide for 20 hrs. (D). Cells with increased or reduced levels of miR-200 were treated with 2 μg/ml of staurosporine for 20 hrs and cell death was quantified by MTS assay. Asterisks indicate p-values * p<0.05, ** p<0.01, *** p<0.001. Note: different CD95 stimuli were used in the assays shown in A-C because Type I cells are not sensitive to sCD95L, and Type II cells are not sensitive to non-crosslinked anti-APO-1 (Algeciras-Schimnich et al., 2003). The horizontal bars in B represent the mean. Values in graphs A, B and D represent the mean −/+ s.d. from three independent experiments.
The changes in apoptosis sensitivity accompanying altered miR-200 expression in most of these cell lines were limited to induction of the extrinsic apoptosis pathway and were not due to a general increase in apoptosis sensitivity, as indicated by the fact that staurosporine treatment, which activates the intrinsic apoptosis pathway, did not lead to significant changes in cell death in these cells (Figure 1D). In summary, in different cell lines sensitive to CD95-mediated apoptosis, changing miR-200 levels resulted in altered apoptosis sensitivity, thereby identifying miR-200 as a positive regulator of CD95-mediated apoptosis.
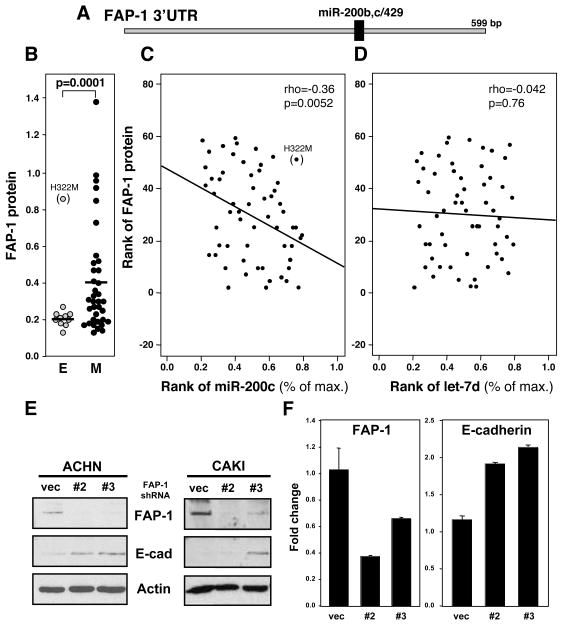
We searched the list of conserved genes predicted to be miR-200 targets using TargetScan 4.2 (targetscan.org) for known regulators of CD95-mediated apoptosis and identified FAP-1, a known inhibitor of CD95-mediated apoptosis, as a putative target. FAP-1 was a good candidate to cause the observed effects because it has been shown to directly regulate CD95-mediated apoptosis by binding to CD95 (Yanagisawa et al., 1997) and by regulating trafficking of CD95 to the cell surface (Foehr et al., 2005; Ivanov et al., 2003; Ivanov et al., 2006). The 3′UTR of FAP-1 mRNA contains a conserved miR-200bc/429 target sequence (Figure 2A). This result predicted that FAP-1 would be predominantly expressed in mesenchymal cells. This could be confirmed by comparing FAP-1 protein expression in the epithelial cell lines with its expression in the mesenchymal cell lines among the NCI60 cells we recently described (Park et al., 2008) (Figure 2B). The lung cancer cell line H322M was the only outlier among the epithelial cell lines. Sequence and PCR analyses demonstrated that the miR-200c seed match in the 3′UTR of FAP-1 mRNA in the H322M cell line is unmutated, and the 3′UTR is attached to the open reading frame of FAP-1 (data not shown), suggesting that an unknown mechanism prevents FAP-1 from being targeted by miR-200 in this cell line. Expanding the analysis to the entire set of NCI60 cells we found a significant correlation between FAP-1 expression and the expression of three classical markers of mesenchymal cells: Vimentin, Fibronectin 1 and N-Cadherin (Figure S2A and B). In addition, a cell line by cell line inverse correlation of expression of FAP-1 protein and miR-200c was detected (Figure 2C). No correlation was found between FAP-1 and let-7d (Figure 2D). The data strongly suggested that FAP-1 is a protein that is predominantly expressed in mesenchymal cells and which is under control of miR-200c. To determine if FAP-1 also plays a role in maintaining mesenchymal cells, we stably knocked down FAP-1 expression using lentiviral shRNAs, and monitored expression of a number of epithelial and mesenchymal markers. Two different FAP-1 specific shRNAs (#2 and #3) efficiently knocked down FAP-1 expression (Figure 2E). Interestingly, by reducing the expression of FAP-1 we noticed a modest but reproducible upregulation of E-cadherin mRNA and protein (Figure 2E and F) suggesting that FAP-1 is not only expressed in mesenchymal cells, but also contributes to the mesenchymal state. Consistent with FAP-1 being a downstream effector of miR-200, reduction in FAP-1 expression did not affect the expression of EMT regulators, ZEB1, ZEB2, Snail, or Twist, or of miR-200c (Figure S2C).
Figure 2. FAP-1 Is Expressed in Mesenchymal Cells.
(A) Schematic diagram of the 3′UTR of FAP-1 with the location of the putative miR-200b,c/429 target site. (B) Relative expression of FAP-1 protein in epithelial and mesenchymal cells among the NCI60 cells. (C,D) Correlation between expression level of miR-200c (% of max) (C) or let-7d (D) and FAP-1 protein in the NCI60 cells. Expression values were transformed to rank values in both axes, and red straight lines indicate linear regression for transformed rank values. Rho = Spearman Rank Correlation Coefficients between miRNAs and FAP-1 expression, and p values are given. (E) Detection of FAP-1 and E-cadherin by Western blot analysis of CAKI-1 and ACHN cells infected with lentiviruses expressing control shRNA or shRNAs for FAP-1. β-actin was detected to demonstrate equal loading. (F) Real-time PCR analysis of FAP-1 and E-cadherin in CAKI-1 cells expressing control shRNA or FAP-1 shRNAs relative to expression of GAPDH. Values in the graph in F represent the mean −/+ s.d. from three independent experiments.
While we found a strong inverse correlation between miR-200c and FAP-1 expression in the NCI60 cells, the only way to confirm a miRNA target is to observe an inverse change in protein expression when the level of the miRNA is altered and to perform a luciferase reporter assay that compares wild-type (wt) and mutated (mut) 3′UTR target sites. First, we performed western blot analysis of FAP-1 expressing cell lines transiently transfected with pre-miR-200c, or stably expressing miR-200c (Figure 3A). In each case the activity of miR-200c was documented by upregulation of E-cadherin, and FAP-1 expression was reduced. Conversely, the inhibition of endogenous miR-200 in HCT116 cells with anti-miR LNA200 led to an increase in FAP-1 and a decrease in E-cadherin expression (Figure 3A), strongly suggesting that miR-200c directly targets FAP-1. Interestingly, FAP-1 mRNA levels did not change with the introduction of miR-200c or with the inhibition of miR-200 as determined by semi-quantitative RT-PCR (Figure 3B). Using quantitative real time PCR, a 20% reduction in FAP-1 mRNA in miR-200c transfected ACHN cells was detected, but no increase in FAP-1 mRNA was found in LNA200 treated HCT116 cells (data not shown) indicating that miR-200 regulates FAP-1 expression predominantly through translational silencing.
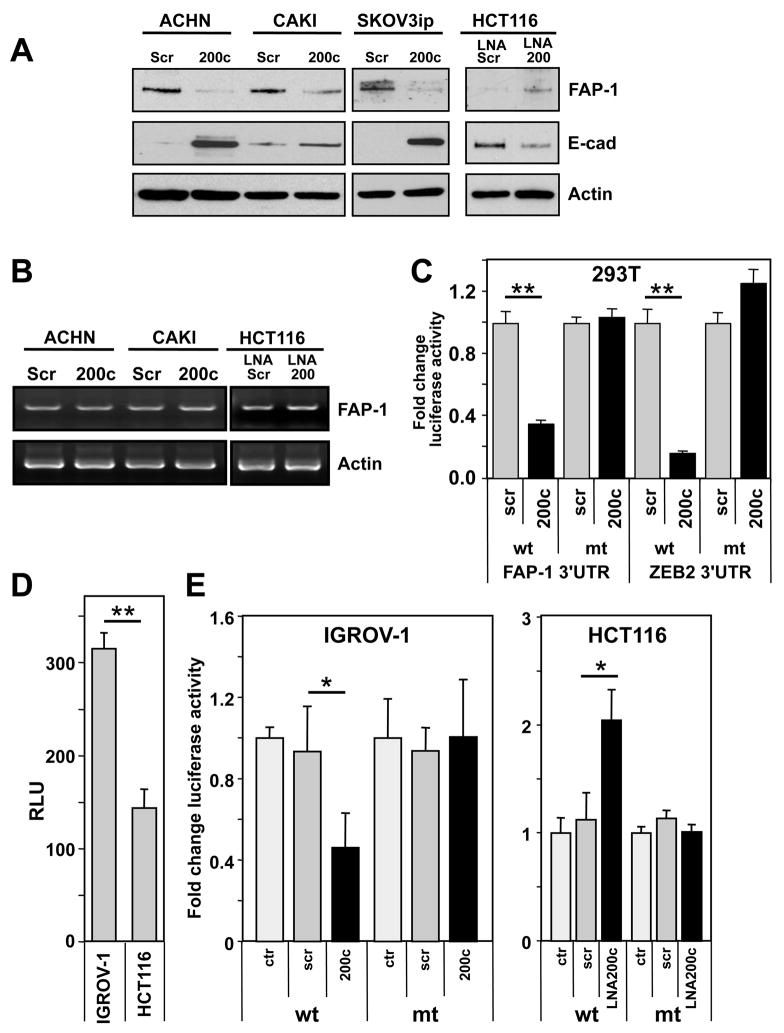
Figure 3. Identification of FAP-1 as a Target of miR-200c.
(A) ACHN, CAKI-1 and SKOV3ip cells were transfected with scrambled pre-miRNA or pre-miR-200c. HCT116 cells were serially treated seven times with LNA scrambled or LNA200. Expression of FAP-1 and E-cadherin was determined by Western blot analysis. (B) HCT116 cells were transfected as in A. FAP-1 mRNA level was determined by semi-quantitative RT-PCR. Actin mRNA was determined as a control. (C) 293T cells were transfected with reporter plasmids containing the 3′UTR of FAP-1 (psiCHECK 3′UTR FAP-1wt) or its corresponding mutant (psiCHECK 3′UTR FAP-1mut) together with either 1 pmol of pre-miRNA-scrambled or pre-miR-200c. Reporter plasmids with a fragment of the 3′UTR of ZEB2 (harboring sites 4, 5 and 6 of the seven mir-200 sites found in the full length) and its mutant (Park et al., 2008) were used as positive controls. Renilla luciferase activity was normalized to firefly luciferase activity. (D) IGROV-1 (miR-200 low) and HCT116 (miR-200 high) cells were transfected with reporter plasmid psiCHECK 3′UTR FAP-1. (E) IGROV-1 cells were transfected with psiCHECK 3′UTR FAP-1wt or its mutant together with 1 pmol of scrambled pre-miRNA, pre-miR-200a or pre-miR-200c. HCT116 cells were transfected with the same reporter plasmids together with 50 nM of LNA scrambled or LNA-200 oligos. * p<0.05, ** p<0.001. Values in C, D and E represent the mean −/+ s.d. from three independent experiments.
To confirm that FAP-1 mRNA was a direct target for miR-200c, we transfected different cells with a luciferase fusion construct of either the wt 3′UTR of the FAP-1 mRNA or a version with a mutated seed match. In a first experiment 293T cells were cotransfected with one of these constructs and with miR-200c. A wt and mutant ZEB2-3′UTR-luciferase construct (Park et al., 2008) was used as a positive control (Figure 3C). In the presence of miR-200c, there was a greater than 60% decrease in luciferase activity with the FAP-1-3′UTRwt construct but no significant change with the FAP-1-3′UTRmut construct. To determine if endogenous miR-200 levels regulated FAP-1 expression, miR-200 low expressing IGROV-1 cells and miR-200 high expressing HCT116 cells were transfected with the wt 3′UTR reporter construct (Figure 3D). Luciferase activity was lower in the miR-200 high expressing cells, consistent with negative regulation of the FAP-1 3′UTR by miR-200. Introducing miR-200c into IGROV-1 cells decreased, and inhibiting miR-200 in HCT116 cells increased, luciferase activity of the wt but not of the mutant 3′UTR construct (Figure 3E). Therefore, FAP-1 is a target of miR-200c, and regulation of FAP-1 is due to translational repression and not mRNA degradation.
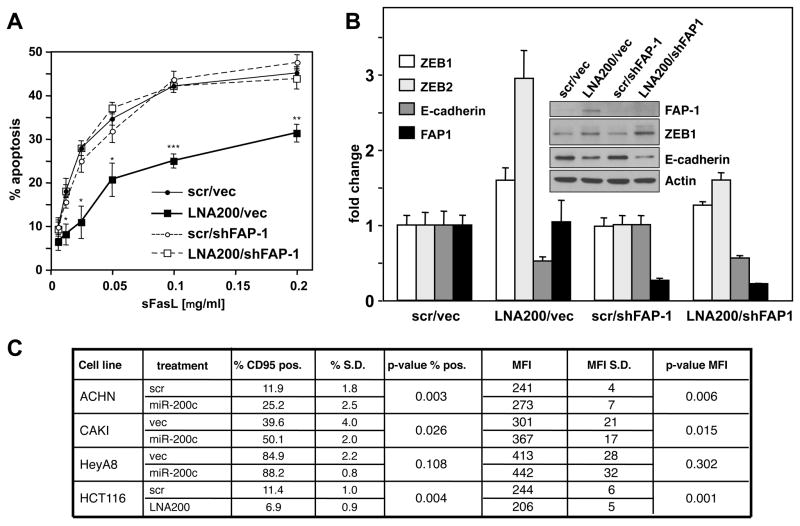
The fact that miR-200c is likely to regulate many targets prompted us to determine the relevance of FAP-1 for the resistance to CD95-mediated apoptosis. The only way to test this is to inhibit miR-200, which should cause upregulation of FAP-1 and increased resistance to CD95-mediated apoptosis. The subsequent knock down of FAP-1 should allow assessment of the effect and contribution of endogenous FAP-1 and endogenous miR-200 on sensitivity to CD95-mediated apoptosis. As was shown in Figure 2E efficient knock down of FAP-1 could be accomplished using lentivirus shRNAs. To determine the contribution of FAP-1 to the miR-200-induced changes in sensitivity to CD95-mediated apoptosis, HCT116 cells were transfected with LNA200 which resulted in an increase in FAP-1 protein expression, a reduction of sensitivity to CD95-mediated apoptosis, an increase in endogenous ZEB1 mRNA and protein and ZEB2 mRNA, and a reduction in E-cadherin mRNA and protein (Figure 4A and B). These same cells with knocked down FAP-1, however, regained the apoptosis sensitivity of the parental HCT116 cells demonstrating that the increase in FAP-1 protein that we observed after inhibition of miR-200 was responsible for the reduced sensitivity to CD95-mediated apoptosis. This indicated that the miR-200c effect on CD95 directed apoptosis is mediated by repression of FAP-1.
Figure 4. Targeting of FAP-1 by miR-200c Regulates Sensitivity to CD95-induced Apoptosis.
(A) HCT116 cells infected with control shRNA or FAP-1 shRNA#2 were serially treated seven times with scrambled LNA or LNA-200. Cells were stimulated with different concentration of sCD95L and apoptosis was measured by MTS assay. (B) Real-time PCR analysis of FAP-1, E-cadherin, ZEB1, and ZEB2 in HCT116 cells. The insert shows a Western blot analysis of the same experiment. (C) Flow cytometric analysis of surface CD95 expression of cells with increased or reduced miR-200. Scrambled oligo or miR-200c was introduced in ACHN, CAKI-1, HeyA8, and SF295 cells either transiently or stably. For HCT116 cells, cells were serially treated seven times with scrambled LNA or LNA-200. Percentage of CD95 positivity and mean fluorescence intensity (MFI) were determined. S.D., standard deviation. Values in A and B represent the mean −/+ s.d. from three independent experiments.
FAP-1 has been reported to directly bind CD95 rendering cells resistant to CD95-mediated apoptosis (Sato et al., 1995; Yanagisawa et al., 1997), likely by dephosphorylating CD95 on Tyr291—a site important for the receptor to internalize and to signal apoptosis (Foehr et al., 2005; Ivanov et al., 2003; Ivanov et al., 2006; Lee et al., 2006; Spanos et al., 2008; Zhu et al., 2008). We previously demonstrated that strong DISC formation requires that CD95 be internalized (Lee et al., 2006). To test whether miR-200 affects sensitivity to CD95-mediated apoptosis by either inhibiting or promoting formation of the DISC, we analyzed CAKI-1 cells, stably expressing miR-200c, and HCT116 cells, transfected with LNA200 (Figure S3A and B). In CAKI-I cells DISC formation is prominent because it predominantly forms after internalization of CD95 on endosomes (Lee et al., 2006). In contrast, in HCT116 cells, which do not efficiently internalize CD95, DISC formation is not detectable by Western blotting (Algeciras-Schimnich et al., 2003). Neither elevation nor reduction of miR-200 levels had a significant impact on DISC formation in either of the two cell lines, suggesting that FAP-1 does not act by interfering with DISC formation. While this result may seem to be in contradiction to the observed change in sensitivity to CD95-mediated apoptosis it is consistent with out previous analyses that demonstrated that the efficiency of the formation of the DISC does not correlate with the sensitivity of cells to CD95-mediated apoptosis (Algeciras-Schimnich et al., 2003).
A careful analysis of CD95 surface expression using flow cytometry revealed that in three out of four cell lines expressing exogenous miR-200c, surface CD95 levels were significantly increased (Figure 4C). In HeyA8 cells we also observed a trend that was not statistically significant probably because these cells already express very high levels of CD95. In contrast, in LNA200-treated HCT116 cells CD95 surface levels were reduced. The data are consistent with miR-200c targeting FAP-1, which then affects CD95 surface expression. The change in CD95 surface expression did not translate into a change in the formation of the DISC, which is consistent with the fact that even high CD95-expressing epithelial cells barely form a DISC (Algeciras-Schimnich et al., 2003; Lee et al., 2006).
Because regulation of CD95-mediated apoptosis sensitivity by miR-200 may occur downstream of the formation of the CD95 DISC, we tested whether miR-200c expression would sensitize cells to apoptosis mediated by other death receptors (Figure S3C). When miR-200c was highly overexpressed, CAKI-1 and ACHN cells were found to be more sensitive to TRAIL-induced apoptosis, and CAKI-1 cells were also more sensitive to TNFα-induced apoptosis suggesting that the activity of miR-200c may not be limited to CD95 signaling. However, when endogenous miR-200 was inhibited in HCT116 cells, which rendered these cells less sensitive to CD95-mediated apoptosis, apoptosis sensitivity to either TRAIL or TNFα was not significantly affected (Figure S3D). This was confirmed in cells stably expressing a lentiviral antimiR against miR-200 (data not shown).
In summary, we report that miR-200c affects sensitivity to CD95-mediated apoptosis, and have identified and validated FAP-1 as a miR-200 target which regulates CD95-mediated apoptotic signaling in tumor cells. We have determined that EMT induction and miR-200c affect sensitivity of tumor cells to CD95 mediated apoptosis. Loss of apoptosis sensitivity is one of the hallmarks of cancer (Hanahan and Weinberg, 2000), and miR-200, which is lost during cancer progression, was expected to regulate this. Our data provide a molecular mechanism that explains how miR-200c regulates sensitivity of tumor cells to CD95-mediated apoptosis. Loss of miR-200 causes an increase in FAP-1 expression and a reduction in CD95 surface expression. Although we did not find an effect of miR-200 on sensitivity of cells to staurosporine, a classical activator of the intrinsic apoptosis pathway, loss of miR-200 has recently been reported to render tumor cells resistant to chemotherapeutic drugs including tubulin-disrupting compounds such as paclitaxel. Class III β-tubulin (TUBB3) was identified as another miR-200 target regulating this activity (Cochrane et al., 2009). Therefore, the EMT regulator miR-200 likely regulates apoptosis sensitivity of cancer cells in multiple ways. Future studies will be aimed at identifying other components of the apoptosis machinery that are targeted by miR-200.
EXPERIMENTAL PROCEDURES
Details regarding cell lines and reagents, cell death assays, Western Blotting, PCR, immunostaining, CD95 DISC analysis, and data analysis are provided in the Supplemental Data.
Generation of FAP-1 Luciferase Construct
The entire 649bp FAP-1 3′UTR with 5′ Xho1 and 3′ Not1 sites was synthesized as wild-type (wt) or miR-200c seed mutant (mut) by Integrated DNA Technologies, and was cloned into a pUC57 amp resistant vector. The miR-200c binding site (5′-ccagtatttaagc-3′) remained intact in the wt FAP-1-3′UTR. This site was change to 5′-cctgaaattaagc-3′ in the mutant FAP-1-3′UTR. Following digestion of the pUC57 plasmid with Xho 1 and Not 1, wt and mutant FAP-1-3′UTRs were ligated into the psiCHECK-2 vector, generating psiCHECK 3′UTR FAP-1wt and psiCHECK 3′UTR FAP-1mut.
Transfections and Luciferase Assays
Transient miRNA expressing cells were generated by following the siPORT™ NeoFX™ manufacturer’s protocol using 150,000 cells and 50 nM of the appropriate miRNA or scrambled control. All pre-miRNAs were supplied from Applied Biosystems. Serial transiently transfected cells were completed following the same transfection protocol repeated every 72 hours. HCT116 cells were serially transfected with locked nucleic acid (LNA) based inhibitor to miR-200 (LNA200), (Park et al., 2008)) or with scrambled LNA control oligonucleotide (Exiqon) every 3 days for up to 21 days (7 transfections) following the siPORT™ NeoFX™ protocol for 150,000 cells. In some experiments, HCT116 cells were infected with lentivirus PLKO-control shRNA or PLKO-FAP-1 shRNA (Sigma Aldrich) and were selected with medium containing 2 μg/ml puromycin for 2 weeks. After selection cells were serially transfected seven times with scrambled LNA or LNA-200 oligonucleotides. Luciferase assays were performed on cells seeded in a 48-well plate (2.5 × 104 cells/well) one day prior to transfection. Cells were transfected with either psiCHECK 3′UTR FAP-1wt or psiCHECK 3′UTR FAP-1mut, along with either Pre-miR-200c or negative control #2 precursor miRNA, using Lipofectamine 2000 (Invitrogen). After 48 hrs cells were lysed and Renilla Luciferase activity was determined according to the manufacturer’s instructions (Promega). All experiments were performed in triplicate and normalized to the activity of the internal control Luciferase gene contained within the psiCHECK-2 vector.
Supplementary Material
01
Acknowledgments
We would like to thank Drs. Susan Holbeck and Graham Brock for providing the NCI60 cells and the miR-200c retroviral vector, respectively. We are grateful to Dr. You-Jia Hua for performing statistical analyses. This work was supported by NIH grant R01 GM61712.
Footnotes
Supplemental data include 3 figures, Supplemental Experimental Procedures, and Supplemental References, and can be found online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Algeciras-Schimnich A, Pietras EM, Barnhart BC, Legembre P, Vijayan S, Holbeck SL, Peter ME. Two CD95 tumor classes with different sensitivities to antitumor drugs. Proc Natl Acad Sci U S A. 2003;100:11445–11450. doi: 10.1073/pnas.2034995100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen NR, Silahtaroglu A, Orom UA, Kauppinen S, Lund AH. miR-200b mediates post-transcriptional repression of ZFHX1B. Rna. 2007;13:1172–1178. doi: 10.1261/rna.586807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane DR, Spoelstra NS, Howe EN, Nordeen SK, Richer JK. MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol Cancer Ther. 2009 doi: 10.1158/1535-7163.MCT-08-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elnemr A, Ohta T, Yachie A, Kayahara M, Kitagawa H, Fujimura T, Ninomiya I, Fushida S, Nishimura GI, Shimizu K, Miwa K. Human pancreatic cancer cells disable function of Fas receptors at several levels in Fas signal transduction pathway. Int J Oncol. 2001;18:311–316. doi: 10.3892/ijo.18.2.311. [DOI] [PubMed] [Google Scholar]
- Foehr ED, Lorente G, Vincent V, Nikolich K, Urfer R. FAS associated phosphatase (FAP-1) blocks apoptosis of astrocytomas through dephosphorylation of FAS. J Neurooncol. 2005;74:241–248. doi: 10.1007/s11060-004-7202-x. [DOI] [PubMed] [Google Scholar]
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hurteau GJ, Carlson JA, Spivack SD, Brock GJ. Overexpression of the MicroRNA hsa-miR-200c Leads to Reduced Expression of Transcription Factor 8 and Increased Expression of E-Cadherin. Cancer Res. 2007;67:7972–7976. doi: 10.1158/0008-5472.CAN-07-1058. [DOI] [PubMed] [Google Scholar]
- Ivanov VN, Lopez Bergami P, Maulit G, Sato TA, Sassoon D, Ronai Z. FAP-1 association with Fas (Apo-1) inhibits Fas expression on the cell surface. Mol Cell Biol. 2003;23:3623–3635. doi: 10.1128/MCB.23.10.3623-3635.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VN, Ronai Z, Hei TK. Opposite roles of FAP-1 and dynamin in the regulation of Fas (CD95) translocation to the cell surface and susceptibility to Fas ligand-mediated apoptosis. J Biol Chem. 2006;281:1840–1852. doi: 10.1074/jbc.M509866200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Feig C, Tchikov V, Schickel R, Hallas C, Schutze S, Peter ME, Chan AC. The role of receptor internalization in CD95 signaling. EMBO J. 2006;25:1009–1023. doi: 10.1038/sj.emboj.7601016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Shin MS, Lee HS, Bae JH, Lee HK, Kim HS, Kim SY, Jang JJ, Joo M, Kang YK, et al. Expression of Fas and Fas-related molecules in human hepatocellular carcinoma. Hum Pathol. 2001;32:250–256. doi: 10.1053/hupa.2001.22769. [DOI] [PubMed] [Google Scholar]
- Lee SH, Shin MS, Lee JY, Park WS, Kim SY, Jang JJ, Dong SM, Na EY, Kim CS, Kim SH, Yoo NJ. In vivo expression of soluble Fas and FAP-1: possible mechanisms of Fas resistance in human hepatoblastomas. J Pathol. 1999;188:207–212. doi: 10.1002/(SICI)1096-9896(199906)188:2<207::AID-PATH337>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Li Y, Kanki H, Hachiya T, Ohyama T, Irie S, Tang G, Mukai J, Sato T. Negative regulation of Fas-mediated apoptosis by FAP-1 in human cancer cells. Int J Cancer. 2000;87:473–479. doi: 10.1002/1097-0215(20000815)87:4<473::aid-ijc3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Meinhold-Heerlein I, Stenner-Liewen F, Liewen H, Kitada S, Krajewska M, Krajewski S, Zapata JM, Monks A, Scudiero DA, Bauknecht T, Reed JC. Expression and potential role of Fas-associated phosphatase-1 in ovarian cancer. Am J Pathol. 2001;158:1335–1344. doi: 10.1016/S0002-9440(10)64084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myc A, Arscott PL, Bretz JD, Thompson NW, Baker JR., Jr Characterization of FAP-1 expression and function in thyroid follicular cells. Endocrinology. 1999;140:5431–5434. doi: 10.1210/endo.140.11.7241. [DOI] [PubMed] [Google Scholar]
- Nakai Y, Irie S, Sato TA. Identification of IkappaBalpha as a substrate of Fas-associated phosphatase-1. Eur J Biochem. 2000;267:7170–7175. doi: 10.1046/j.1432-1327.2000.01818.x. [DOI] [PubMed] [Google Scholar]
- Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors, ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter ME. Let-7 and miR-200: guardians against pluripotency and cancer progression. Cell Cycle. 2009;8:843–852. doi: 10.4161/cc.8.6.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter ME, Budd RC, Desbarats J, Hedrick SM, Hueber AO, Newell MK, Owen LB, Pope RM, Tschopp J, Wajant H, et al. The CD95 receptor: apoptosis revisited. Cell. 2007;129:447–450. doi: 10.1016/j.cell.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- Peter ME, Legembre P, Barnhart BC. Does CD95 have tumor promoting activities? Biochim Biophys Acta. 2005;1755:25–36. doi: 10.1016/j.bbcan.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Sato T, Irie S, Kitada S, Reed JC. FAP-1: a protein tyrosine phosphatase that associates with Fas. Science. 1995;268:411–415. doi: 10.1126/science.7536343. [DOI] [PubMed] [Google Scholar]
- Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays. 2001;23:912–923. doi: 10.1002/bies.1132. [DOI] [PubMed] [Google Scholar]
- Schickel R, Boyerinas B, Park SM, Peter ME. MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene. 2008;27:5959–5974. doi: 10.1038/onc.2008.274. [DOI] [PubMed] [Google Scholar]
- Spanos WC, Hoover A, Harris GF, Wu S, Strand GL, Anderson ME, Klingelhutz AJ, Hendriks W, Bossler AD, Lee JH. The PDZ binding motif of human papillomavirus type 16 E6 induces PTPN13 loss, which allows anchorage-independent growth and synergizes with ras for invasive growth. J Virol. 2008;82:2493–2500. doi: 10.1128/JVI.02188-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungefroren H, Kruse ML, Trauzold A, Roeschmann S, Roeder C, Arlt A, Henne-Bruns D, Kalthoff H. FAP-1 in pancreatic cancer cells: functional and mechanistic studies on its inhibitory role in CD95-mediated apoptosis. J Cell Sci. 2001;114:2735–2746. doi: 10.1242/jcs.114.15.2735. [DOI] [PubMed] [Google Scholar]
- Wieckowski E, Atarashi Y, Stanson J, Sato TA, Whiteside TL. FAP-1-mediated activation of NF-kappaB induces resistance of head and neck cancer to Fas-induced apoptosis. J Cell Biochem. 2007;100:16–28. doi: 10.1002/jcb.20922. [DOI] [PubMed] [Google Scholar]
- Yanagisawa J, Takahashi M, Kanki H, Yano-Yanagisawa H, Tazunoki T, Sawa E, Nishitoba T, Kamishohara M, Kobayashi E, Kataoka S, Sato T. The molecular interaction of Fas and FAP-1. A tripeptide blocker of human Fas interaction with FAP-1 promotes Fas-induced apoptosis. J Biol Chem. 1997;272:8539–8545. doi: 10.1074/jbc.272.13.8539. [DOI] [PubMed] [Google Scholar]
- Zhu JH, Chen R, Yi W, Cantin GT, Fearns C, Yang Y, Yates JR, 3rd, Lee JD. Protein tyrosine phosphatase PTPN13 negatively regulates Her2/ErbB2 malignant signaling. Oncogene. 2008;27:2525–2531. doi: 10.1038/sj.onc.1210922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01