Regulation of HIF-1α Stability through S-nitrosylation (original) (raw)
. Author manuscript; available in PMC: 2010 Jul 17.
Abstract
Hypoxia inducible factor 1 (HIF-1) is a master transcriptional factor. Under normal oxygen tension, HIF-1 activity is usually suppressed due to the rapid, oxygen-dependent degradation of one of its two subunits, HIF-1α. Here we report that normoxic HIF-1 activity can be up-regulated through NO-mediated S-nitrosylation and stabilization of HIF-1α. In murine tumors, exposure to ionizing radiation stimulated the generation of NO in tumor-associated macrophages. As a result, the HIF-1α protein is S-nitrosylated at cysteine 533 (through ‘biotin-switch’ assay) in the oxygen-dependent degradation domain, which prevents its destruction. Importantly, this mechanism appears to be independent of the prolyl hydroxylase-based pathway that is involved in oxygen-dependent regulation of HIF-1α. Selective disruption of this S-nitrosylation significantly attenuated both radiation-induced and macrophage-induced activation of HIF-1α. This interaction between NO and HIF-1 sheds new lights on their involvement in tumor response to treatment as well as mammalian inflammation process in general.
Keywords: HIF-1α regulation, S-nitrosylation, radiation induced HIF-1 activity, HIF-1 and inflammatory response, inducible nitric oxide synthase
Introduction
Hypoxia inducible factor 1 (HIF-1) is a master transcriptional regulator that plays important roles in development, physiology, and many pathological processes(Melillo, 2004; Semenza, 2002, 2003; Semenza et al., 2000). Originally cloned as a transcriptional factor activated under abnormally low oxygen conditions (Wang and Semenza, 1993a, b), its roles in cancer biology are increasingly being recognized. More than 60 genes have been identified as direct targets of HIF-1α (Semenza, 2003). Among these are genes involved in angiogenesis, metabolic adaptation, apoptosis induction/resistance, and invasion/metastasis.
HIF-1 is a heterodimeric protein that consists of the constitutively expressed HIF-1β/ARNT subunit and the highly regulated HIF-1α subunit(Wang and Semenza, 1995). The overall activity of HIF-1 is determined by intracellular HIF-1α level. In the past decade, a tremendous amount of insights on HIF-1α regulation has been gained. The most significant advance has been in the area of HIF-1α regulation by oxygen tension, which involves the ubiquitin-proteasome pathway. Under normoxic conditions, HIF-1α is hydroxylated by oxygen-activated prolyl hydroxylases(PHDs) at proline residues 402 and 564 in the oxygen-dependent degradation (ODD) domain (Ivan et al., 2001; Jaakkola et al., 2001). This hydroxylation drives HIF-1α towards ubiquitylation by E3 ubiquitin protein ligase, which is part of the von Hippel-Lindau (VHL) tumor suppressor protein complex (Maxwell et al., 2001; Maxwell et al., 1999; Pause et al., 1999). Ubiquitylated HIF-1α is then degraded by proteasome rapidly. Under hypoxic conditions, the enzymatic activities of PHDs are significantly reduced because oxygen is a necessary substrate for the hydroxylase activities. As a result, HIF-1α accumulates and HIF-1 activity is up-regulated.
In addition to hydroxylation of the proline residues in the ODD domain by PHDs, hydroxylation of the asparagine residue 803, which is located in the C-terminal transactivation domain, by the FIH-1 (factor inhibiting HIF-1) protein, regulates the activity of HIF-1α by preventing its interaction with its co-activators p300 and CBP(Lando et al., 2002a; Lando et al., 2002b).
Furthermore, several other posttranslational modifications of HIF-1α have been identified to regulate HIF-1 activities. For instance, acetylation of a lysine residue (lys532) has been shown to enhance the binding of HIF-1α to VHL and its subsequent degradation(Jeong et al., 2002). The ARD1 acetyl transferase catalyzes this acetylation. By contrast, nitrosylation of Cys800 of HIF-1α can stimulate the transcriptional activity of HIF-1 through increased binding affinity to CREB/p300(Yasinska and Sumbayev, 2003). In tissue cultured cells, exogenously added nitric oxide has been found to increase HIF-1α through the inhibition of prolyl hydroxylase in vitro(Metzen et al., 2003). In vivo, it was reported very recently that NO plays an important role in activating HIF-1 in macrophages during bacterial infection(Peyssonnaux et al., 2005).
In solid tumors, regulation of HIF-1α can be mediated through a multitude of mechanisms. Hypoxia is a common feature of the solid tumor microenvironment thanks to rapid proliferation of the tumor cells and the poor functions of newly formed tumor vasculature. In the majority of solid tumors, hypoxia plays important roles in up-regulating the expression levels of _HIF-1_α(Harris, 2002). In fact, hypoxia induced HIF-1 activation and subsequent VEGF expression has been recognized as the major driving force for tumor angiogenesis in solid tumors(Harris, 2002; Maltepe et al., 1997). A significant amount of effort is now being devoted to the development of HIF-1 inhibitors as anti-cancer drugs(Giaccia et al., 2003; Rapisarda et al., 2002; Sutphin et al., 2004).
In addition to tumor microenvironmental conditions and genetic alterations in tumor cells, it was shown recently that HIF-1 activity can also be modified by exposure to radiotherapy(Moeller et al., 2004; Moeller et al., 2005). Ionizing radiation exposure appears to activate HIF-1 in a hypoxia-independent manner. This activation is mediated by a post-transcriptional mechanism that involves the release of pre-stored HIF-1α-encoding mRNAs in “stress granules” located in the cytoplasm(Moeller et al., 2004). The triggering signals were identified to be free radical species induced by exposure to ionizing radiation. This was an important discovery since it has major implications for tumor therapy. It indicates that tumors respond to therapy by activating HIF-1, which mediates the expression of VEGF and other factors that protect tumor vasculature against cytotoxic therapy, thereby increasing overall tumor survival. Consistent with this hypothesis are data indicating that combined radiotherapy and HIF-1 inhibition appeared to have synergistic anti-tumor effects(Moeller et al., 2004).
Because of the importance of understanding HIF-1α regulation during tumor treatment, especially in the context of recent interests in the use of anti-angiogenic therapy in combination with conventional cyto-toxic therapies(Garcia-Barros et al., 2003; Wachsberger et al., 2003), we decided to further investigate the regulation of HIF-1α during cancer treatment. By use of a novel, non-invasive reporter system, we identified nitric oxide as a major regulator of HIF-1 activities during cancer treatment through direct nitrosylation of a single cysteine residue (Cys 533, mouse sequence) in the oxygen-dependent domain of HIF-1α. This is a novel mechanism of HIF-1α regulation that appears to be independent of the prolyl hydroxylase (PHD)-based pathway. Since HIF-1α is known to be involved in a variety of physiological and pathological processes such as cancer, cardiovascular diseases, and inflammatory responses, the identification of this mechanism may facilitate further understanding in these areas.
Results
Nitric oxide-sensitive induction of HIF-1α stabilization/activation by radiation in tumors
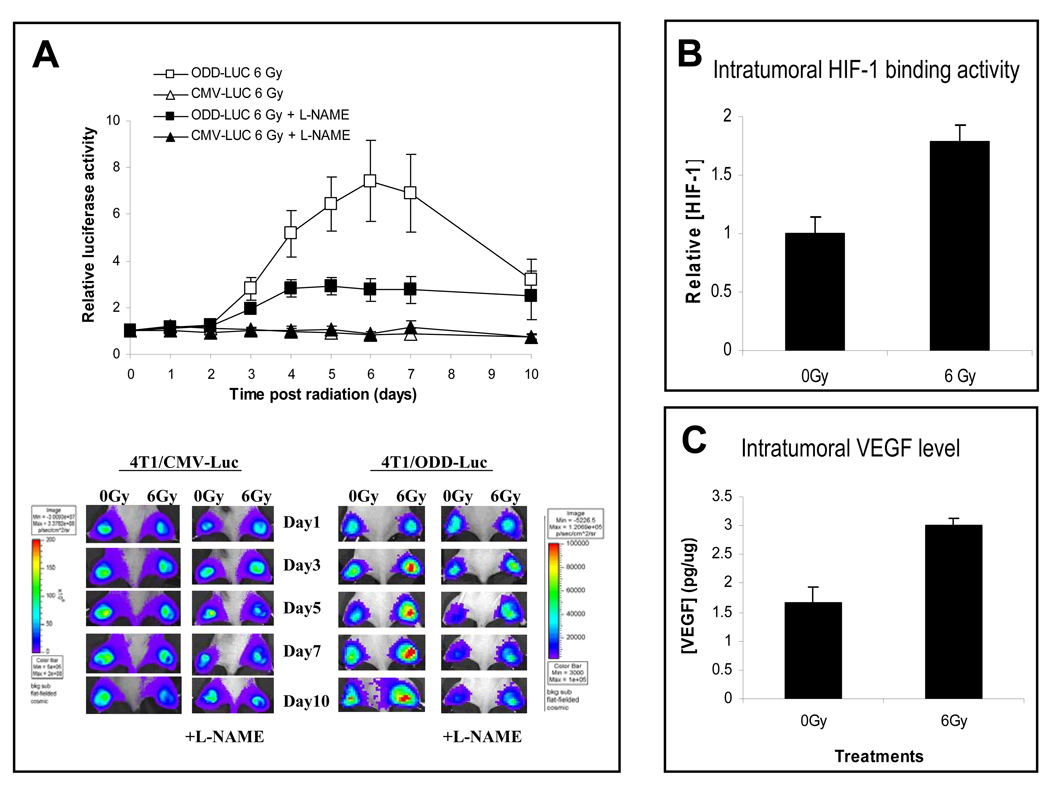
In order to observe HIF-1α regulation after treatment response, 4T1 murine breast tumor cells stably transduced with the ODD-luc reporter gene (supplementary Figures S1) were implanted subcutaneously into mice. After the tumors reach 6–8 mm in diameter, they were irradiated and followed for ODD-luc expression by use of the Xenogen IVIS bioluminescence imaging system. From day 3 after irradiation, the level of HIF-1α, as determined by ODD-luc, appeared to increase linearly over 3 days. It peaked at around 6 days and fall back to background levels after day 10 (Figure 1A). The differences between the irradiated and sham-irradiated groups were highly significant from day 3 (p<0.001). More importantly, radiation-induced stabilization of ODD-luc was accompanied by increases in HIF-1 promoter binding activities to the corresponding HRE binding element (Figure 1B) and up-regulation of a downstream target gene, vascular endothelial growth factor (Figure 1 C). These results indicate that radiation induce a persistently increasing level of HIF-1α expression and activities. While radiation has been shown to activate HIF-1α in our previous studies(Moeller et al., 2004), the pattern of in vivo induction similar to the one reported here has never been observed previously, mainly because of the lack of experimental approaches to detect HIF-1α accurately over an extended period of time.
Figure 1.
Nitric oxide is a key regulator of radiation-induced HIF-1α activation.
(A) 4T1-ODD-luc or 4T1-luc tumors were established in the hind legs of nude mice and irradiated (at day 0) with or without the administration of L-NAME (at day −1). ODD-luc level were then monitored post irradiation. Tumors with the CMV-luc reporter were used as controls. Notice the significant inhibitions of ODD-luc expression by the use of L-NAME. Eight animals were used in each group and the error bars indicate standard error of the mean. P<0.05 from day 4 (two-way ANOVA).
(B) Radiation-induced activation of endogenous HIF-1 binding activity to hypoxia responsive element (HRE) was measured by ELISA in tumors irradiated 5 days earlier. In each group average results from 4 tumor samples were shown (P<0.05, Student’s t test). Error bars represent standard deviation.
(C) Radiation induced increase in intratumoral VEGF level as measured by ELISA. In each group, average results from 4 tumors were shown (P<0.05, Student’s t test). Error bars represent standard deviation.
Of special significance are our data indicating that nitric oxide (NO) is a major factor for radiation-induced HIF-1α activation (Figure 1A). The administration of L-NAME, a potent inhibitor of nitric oxide synthases(NOS), effectively attenuated radiation-induced HIF-1α stabilization in tumors, as shown by the loss of ODD-luc signal. As NOS is the major source of NO in vivo, these results indicate that NO plays a pivotal role in radiation-induced HIF-1α stabilization. Control experiments indicated that NO produced by NOS did not influence constitutively expressed luciferase activities, confirming the role of NO in regulating ODD (and hence HIF-1α) stability (Figure 1A). Additional experiments in vitro indicated that the addition of exogenous NO-generating compound GSNO to ODD-luc transduced cells increased ODD-luc expression significantly (supplementary Figures S2A & B). It also causes an increase in endogenous HIF-1α level(supplementary Figure S2C), confirming previous reports(Metzen et al., 2003; Sandau et al., 2001).
The observed radiation-induced intratumoral regulation of HIF-1α by NO should be a hypoxia-independent phenomenon since previous studies have indicated no significant changes(Brizel et al., 1996) in the level of hypoxia in tumors following radiation. Indeed, it has been shown that in many cases intratumoral oxygen tension actually increases after irradiation due to tumor cell death and reduced cell proliferation that reduce oxygen consumption in tumors. Measurements of 4T1 tumors after irradiation indicated a similar scenario (Moeller et al., 2004).
Inducible nitric oxide synthase as the major source of NO in radiation-induced HIF-1α activation
What is the source of the NO that stimulates HIF in irradiated tumors in vivo? Of the three NOS isoforms, inducible NO synthase (iNOS) is the most likely candidate because, unlike neuronal and endothelial NOS, which are constitutively activated in healthy tissues, it is exclusively expressed and activated in pathological tissues such as tumors, where it can produce high micromolar levels of NO. Moreover, tumors usually contain significant number of macrophages(Colombo and Mantovani, 2005; Lewis and Murdoch, 2005), which express/activate their iNOS as part of their immunoeffector activities and thus can provide a ready source of NO. To pinpoint the source of NO, we carried out two series of experiments.
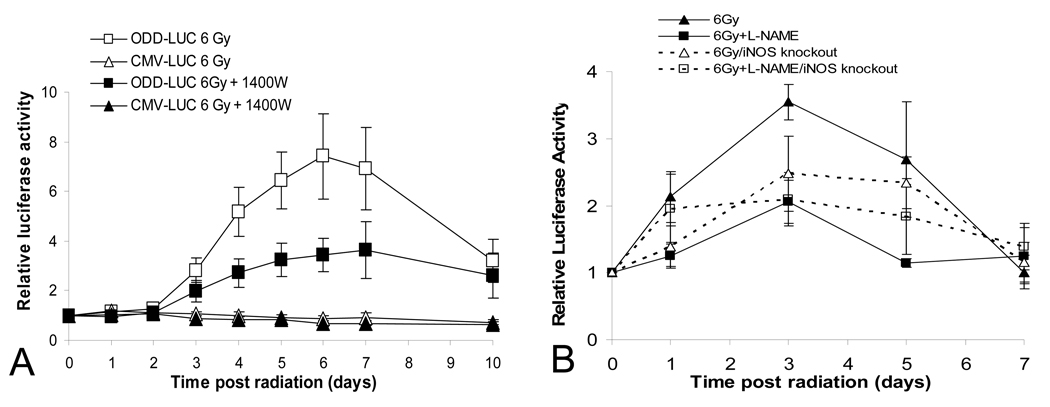
In the first series of experiments, the iNOS-specific inhibitor, 1400W(Alderton et al., 2001; Thomsen et al., 1997), was used to examine radiation-induced HIF-1α induction in ODD-luc transduced 4T1 tumors. The results indicate that 1400W attenuated radiation-induced ODD-luc in 4T1 as potently as the general NOS inhibitor L-NAME (Figure 1A&Figure 2A). This observation strongly argues that iNOS is the main mediator of radiation-induced HIF-1α stabilization.
Figure 2.
The critical role of iNOS in radiation induced HIF-1α activation.
(A) Effect of an iNOS specific inhibitor. Subcutaneous tumors were established in the hind legs of nude mice through the use of 4T1-ODD-luc cells and irradiated with or without the administration of 1400W, an (iNOS) inhibitor. ODD-luc level was then monitored daily post irradiation. Eight animals were used in each group. Significant inhibition of radiation-induced HIF-1 activation was observed in the group treated with 1400W(P<0.001 from day 4, two-way ANOVA).
(B) Effect of genetic disruption of the iNOS gene in host animal on HIF-1α activation. Tumors were established from B16F10-ODD-luc cells in syngeneic wild type or iNOS−/− C57BL/6 mice. In some groups, mice received L-NAME one day before tumor irradiation (6 Gy) at day 0. Luciferase activities were determined every other day. Eight animals were used in each group and the error bar represents the standard error of the mean. In wild type C57BL/6 mice (solid lines), the difference between L-NAME treated and non-treated groups are statistically different (P<0.01 on days 1, 3 & 5, two-way ANOVA test). In iNOS−/− mice (broken lines), the difference between L-NAME treated and non-treated groups are not significant (P>0.05 at all time points, two-way ANOVA).
In the second series of experiments, C57BL/6 mice with targeted disruption of the iNOS gene (iNOS−/−) were implanted with syngeneic B16F10 melanoma cells stably transduced with ODD-luc gene. The tumors were then irradiated and observed for HIF-1α activation. A significant attenuation of radiation-induced ODD-luc induction in the tumors grown in iNOS−/− mice was observed compared to wild-type controls. While radiation up-regulated ODD-luc expression in the iNOS-deficient mice, ODD-luc levels in iNOS−/− animals and in wild type mice treated with L-NAME were of similar amplitudes (Figure 2B), indicating that L-NAME-suppressed HIF-1α activation in the wild type mice is attributable to the inhibition of iNOS.
Macrophages as the major source of iNOS and NO in radiation-induced HIF-1α activation
Previous studies have indicated that macrophages are a rich source of NO and that the tumor microenvironment is abundantly populated with macrophages. In light of this information and our aforementioned results, we hypothesized that tumor associated macrophages may play a significant role in radiation-induced HIF-1α induction. This would also be consistent with previous findings that tumor-associated macrophages play important roles in regulating tumor angiogenesis, at least partially through NO release (Leek et al., 2000; Leek et al., 2002; Varney et al., 2002).
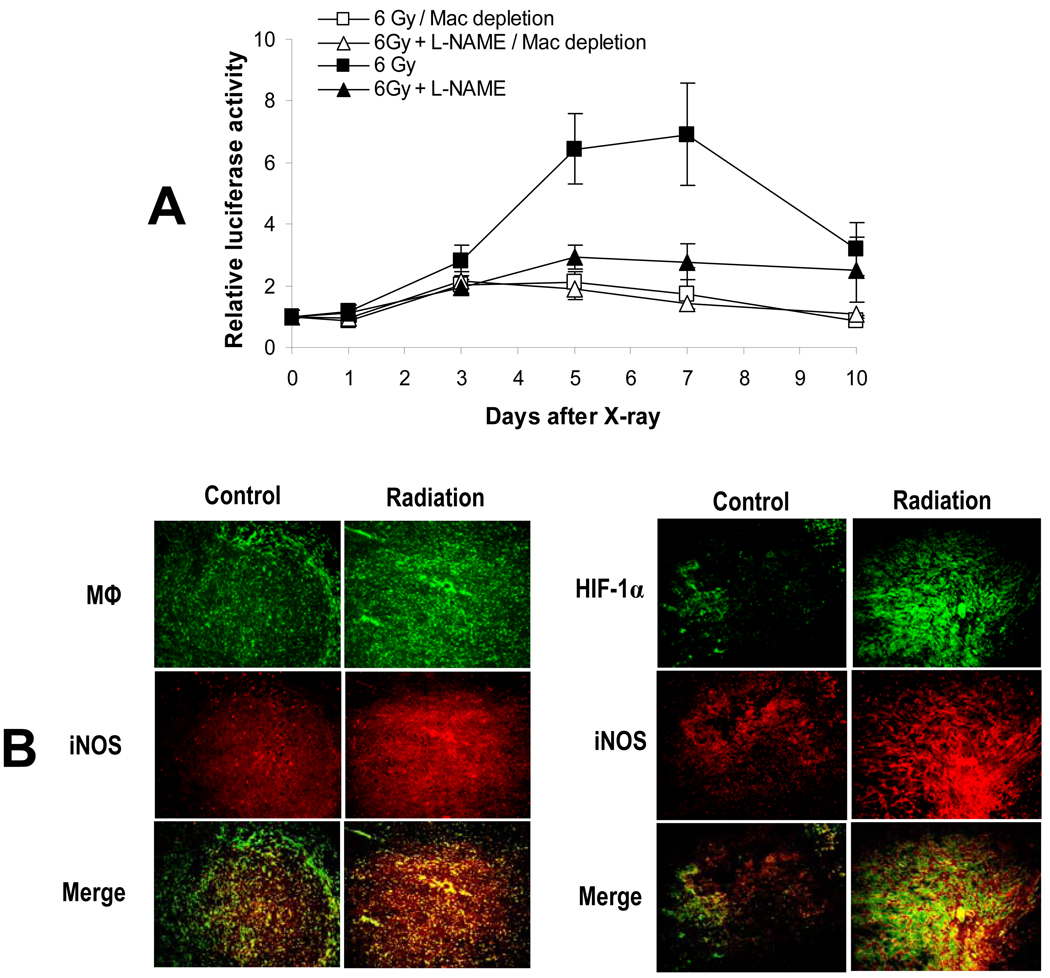
In order to investigate the potential involvement of macrophages in HIF-1α activation, we measured radiation-induced ODD-luc activation in mice that had been chemically depleted of macrophages through the use of carrageenan(Goldmann et al., 2004; Muller et al., 2005). The results were very similar to those obtained in iNOS−/− mice (Figure 2B). We observed a significant reduction in radiation-induced ODD-luc activation and the loss of L-NAME-mediated inhibition of ODD-luc activation in tumors in mice with macrophage depletion (Figure 3A). These results clearly establish that iNOS in tumor-associated macrophages is the main source for the NO that is involved in radiation-induced HIF-1α activation. Immunohistochemistry analysis further confirmed that irradiation of the tumor increased the number of tumor-associated macrophages with activated the iNOS gene (Figure 3B, left panel). The analysis also confirmed that activated iNOS gene expression is accompanied by concomitant HIF-1α activation in tumors (Figure 3B, right panel).
Figure 3.
The role of macrophages in radiation induced HIF-1α activation.
(A) Tumors were established from 4T1-ODD-luc cells in nude mice. In some mice, macrophages were depleted by injection of carrageenan. Selected groups of mice also received L-NAME one day before irradiation (6 Gy). Luciferase expression was determined every other day. Eight mice were included in each group. The error bar represents the standard error of the mean.
(B) Immunohistochemistry analysis of HIF-1α., iNOS, and macrophages in tumors. Mice with irradiated 4T1 tumors were sacrificed and their tumors excised 5 days after localized 6-Gy or sham irradiation of tumors. Shown in the left panel are representative results from co-staining of CD68 (a marker for macrophages (Mφ)) and iNOS. Co-staining of HIF-1α and iNOS were shown on the right panel. In each case, merged pictures are provided. Orange color in both panels represents co-localization.
The molecular mechanism of HIF-1α activation by NO
The aforementioned experiments provide strong evidence that NO generated by tumor-associated macrophages plays critical roles in radiation-induced HIF-1α activation. However, we still do not understand the exact molecular mechanism of how NO induces stabilization of HIF-1α. We explored two ways NO can influence HIF-1α: the inactivation of upstream prolyl hydroxulases and/or the direct modification of the ODD domain. Others have shown that NO can inhibit the activity of the prolyl hydroxylases(PHDs), which can results in the stabilization of HIF-1α(Metzen et al., 2003). Although our own data support the PHD-associated mechanism (data not shown), this mechanism appear to play only a minor role in NO-induced HIF-1α activation.
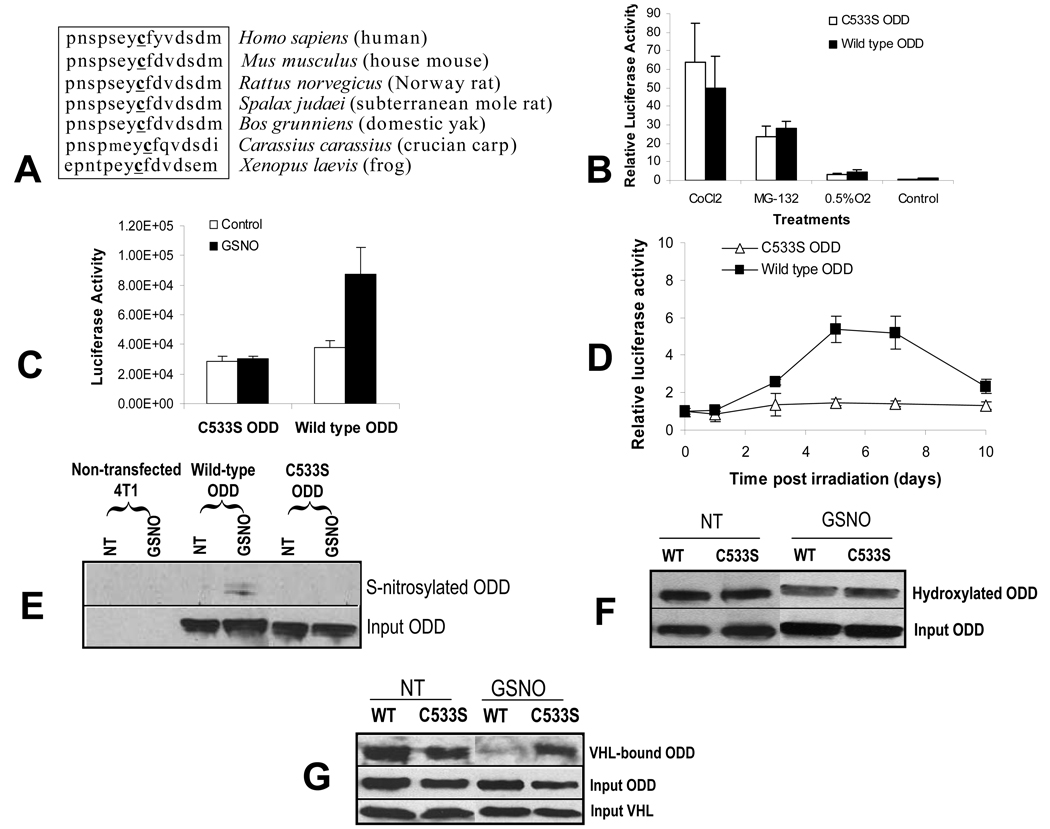
We hypothesized that NO may stabilize the HIF-1α through _S_-nitrosylation of the ODD domain during radiotherapy. To test this hypothesis, we generated a mutant (C533→S) involving the only Cys residue in the murine HIF-1α ODD domain, Cys533. This residue corresponds to Cys520 (which also is the only Cys in the human ODD domain) in human HIF-1α and is conserved among a wide spectrum of vertebrate species that included human, mouse, rat, frog, etc (Figure 4A). The replacement of the cysteine by a serine was chosen because the only difference between these amino acids is that the thiol (–SH) group of cysteine is replaced by the hydroxyl (-OH) group of serine. The likelihood that the point mutation will alter the 3-D structure of ODD is therefore the smallest compared with other potential substitutions. The [C533S] ODD domain was then fused with the luciferase reporter gene, transfected into 4T1 tumor cells, and examined for its activation in comparison with wild type ODD-luc in vitro and in vivo.
Figure 4.
Prevention of normoxic HIF-1α degradation though S-nitrosylation of cysteine 533.
(A) Amino acid sequence conservation across different species for murine HIF-1α Cys 533.
(B) The effects of various stimuli (0.5% O2 for 24 hrs, 10 µM MG132, a proteasome in inhibitor, for 12 hrs, and CoCl2 at 240 µM for 12 hrs) on the activation of wild type ODD-luc and [C533S]-ODD-luc in 4T1 cells. The data shown are the results of triplicate experiments. The error bars represent standard deviation. In all treatment groups, P>0.05 between wild type and mutant ODD-luc expression levels (Student’s t test).
(C) Wild type ODD-luc or [C533S] ODD-luc transduced 4T1 cells were treated with GSNO (1mM) and measured for luciferase activities. Note the significant attenuation of luc expression in [C533S]-ODD-luc transduced cells (P<0.01, Student’s t test). Each data point is the results of triplicate experiments and the error bars represent standard error. The unit for light output shown is p/sec/CM2/Sr.
(D) Tumors were established from 4T1 cells transduced with wild type or [C533S]-ODD-luc and irradiated (6Gy). Luciferase expression was then monitored every other day. Note the significant attenuation of luciferase expression in [C533S]-ODD-luc-transduced 4T1 tumors (P<0.01 from day 5, two-way ANOVA). Each group has five animals and the error bar represents standard error of the mean.
(E) S-nitrosylation of C533 in the ODD domain. 4T1 cells transduced with wild type ODD or [C533S]-ODD (both with a myc-tag at the 5’ end for western blot detection) were exposed to GSNO and then lysed. S-nitrosylation of ODD was determined through the biotin switch assay(Jaffrey and Snyder, 2001). Please note the clear nitrosylation signal for wild-type ODD after GSNO treatment and the absence of it in [C533S] ODD with or without GSNO treatment.
(F) Lack of effect of C533 nitrosylation on proline hydroxylation in the ODD domain of HIF-1α. 4T tumor cells were transduced with pCMV-ODD-mycTag. Where indicated, the transfected cells were exposed to 1 mM GSNO for 8 hours. Western blot analyses of the lysates were then conduced for the total amount of ODD (though the use of anti-mycTag antibody) and the hydroxylated ODD (through the use of an antibody directed against the hydroxylated HIF-1α). No significant difference in the amount of hydroxylated ODD was observed between the wild type (WT) and the [C533S) mutant in non-treated (NT) or GSNO-treated cells.
(G) Absence of binding between nitrosylated ODD and VHL. 4T1 tumor ells were transduced with CMV-ODD-mycTag CMV-[C533S]-ODD-mycTag, or CMV-HA-VHL. Where indicated, _ODD_-transfected cells were exposed to 1 mM GSNO for 8 hours. The lysate of _ODD_-transfected cells was admixed with lysate of cells expressing HA-VHL. Mixed lysates were immunoprecipitated with anti-HA antibody to pull down the VHL protein. The immunoprecipitate was then immunoblotted with antibody against mycTag to detect ODD bound to VHL. Total tagged ODD (Input ODD) and VHL (Input VHL) were detected using Western blot with antibodies against mycTag and HA-tag, respectively.
In vitro, the background expression level of mutant [C533S]-ODD-luc was very low, similar to wild type (Figure 4B). However, when [C533S]-ODD-luc was subjected to hypoxia, proteasome inhibition, or CoCl2 exposure, significant inductions, similar to wild-type, were observed (Figure 4B), indicating that the mutation did not cause any gross structural perturbation that would disrupt normal processing by upstream PHD- and downstream VHL- and proteasome-pathways. However, when the mutant [C533S]-ODD-luc transduced cells were exposed to the NO donor GSNO, induced ODD-luc expression was almost absent, in sharp contrast to wild-type ODD-luc transduced cells, which had significant GSNO induction (Figure 4C). Similar results were obtained when the cell were exposed to another chemical NO donor, SIN-1.
In vivo, background levels of mutant [C533S]-ODD-luc transduced tumors were similar to those observed in wild type ODD-luc transduced tumors (Figure 4D). However, the C533→S mutation significantly attenuated radiation-induced ODD-luc activation in vivo (Figure 4D, days 5,7 & 10), providing strong evidence that S-nitrosylation of the Cys533 residue in the HIF-1α protein plays a critical role in regulating the stabilization of HIF-1α after radiation therapy.
The direct proof for S-nitrosylation of HIF-1α at C533 came from our “biotin switch” experiment. In this experiment, chemical evidence for the nitrosylation of the ODD domain was sought following a published protocol(Jaffrey and Snyder, 2001). In the absence of GSNO treatment, neither wild-type ODD nor [C533S]-ODD was S-nitrosylated (Figure 4E). Upon GSNO treatment, S-nitrosylation was clearly observed in wild- type ODD upon GSNO treatment, but completely absent in [C533S]-ODD(Figure 4E), demonstrating that C533 can be S-nitrosylated in the cellular environment in the presence of NO. Although there remains some controversy regarding the validity of “biotin switch” method for assaying S-nitrosylation, the results here strongly agrees with previous reports that HIF-1α can be nitrosylated(Gaston et al., 2003; Palmer et al., 2000).
How does _S_-nitrosylation of Cys533 affect the stability of HIF-1α? One possibility is that nitrosylation affected the ability of the prolyl hydroxylase to hydroxylate key proline residues in the ODD domain, which in turn prevents its subsequent degradation. However, this scenario is not supported when NO-treated 4T1-ODD cells were examined for the extent of ODD hydroxylation. While our results indicated that NO-exposure caused clear increases in the relative amount of hydroxylated vs total ODD levels, nitrosylation of the Cys533 had no effect on PHD-mediated hydroxylation of the prolines in the ODD domain. This is evidenced by the fact that the same amount of the hydroxylation was observed in both wild type and C533 mutant ODD proteins (Figure 4F). This indicates that nitrosylation-mediated HIF-1α stabilization is mostly independent of hydroxylation of the proline residues in the ODD domain.
Another possibility is that nitrosylation at Cys533 renders the HIF-1α protein resistant to degradation by preventing the binding of HIF-1α by VHL. To examine this possibility, we tested the effects of NO and of C533S on the binding of ODD with VHL in _ODD_-transfected tumor cells. Co-immunoprecipitation results revealed that the strong binding of wild-type ODD with VHL in the absence of NO was mostly abolished in cells exposed to GSNO (Figure 4G). Strikingly, this regulation was completely lost in cells expressing [C533S]-ODD, whose binding with VHL was not affected at all, consistent with the continuous degradation of mutated ODD in the presence of NO (Figure 4C & D). Taken together, the in vivo and in vitro results (Figure 4A–G) provide strong evidence that NO-mediated stabilization of HIF-1α is largely mediated by S-nitrosylation of the Cys533 in the ODD domain, which leads to the inhibition of binding between the ODD domain and VHL. The revelation of this mechanism is highly significant in that it appears to be an alternative pathway independent of prolyl hydroxylase-mediated HIF-1α degradation pathway, which is the predominant pathway for oxygen-mediated regulation of HIF-1α.
The critical role of S-nitrosylation in macrophage-mediated trans-regulation of HIF-1α
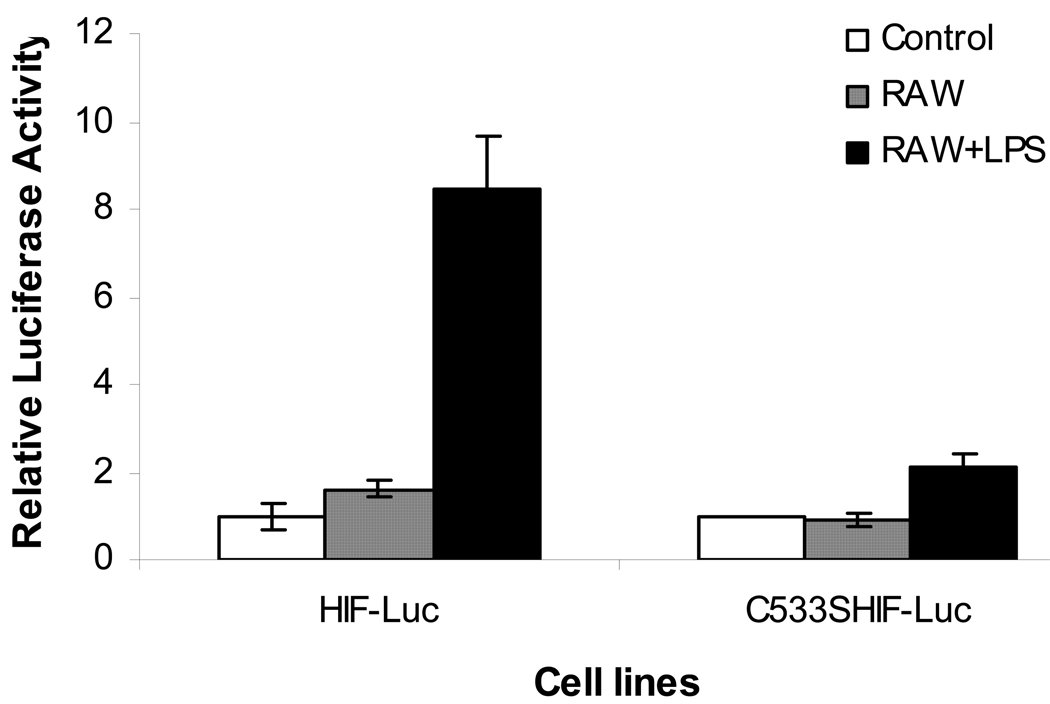
As the iNOS enzyme mainly functions within macrophages and iNOS transcription is activated by a variety of signals including necrotic cell bodies and microbe infections, we reasoned that activated macrophages should be able to regulate HIF-1α level in trans. To examine this possibility, we co-cultured macrophage cells (RAW264.7) and 4T1 cells transduced with a full-length HIF-1-luc reporter. The level of the reporter gene was then monitored in the presence or absence of bacteria-derived imunostimulatory agent liposaccharide (LPS) (Figure 5). It is clear that the presence of LPS caused a significant induction of the HIF-1α level. Most interestingly, this induction is absent in the mutant C533S reporter (also full length), which again confirms the critical role of C533 S-nitrosylation in macrophage-mediated HIF-1α activation. An interesting aspect of these data is that this HIF-1 activation mechanism may be involved in processes other than tumor responses to therapy. Because macrophage activation is involved in many other biological processes such as wound healing and inflammatory response, our discovery of the S-nitrosylation-mediated HIF-1 activation may be involved in those processes as well. Indeed, our data is consistent with recently published data that suggest the intimate involvement of HIF-1 in inflammatory responses(Cramer and Johnson, 2003; Peyssonnaux et al., 2005). S-nitrosylation may be the key hypoxia-independent mechanism involved in the “positive loop” of mutual regulation between HIF-1 and iNOS.
Figure 5.
The effect of C533S mutation on activated macrophage-mediated HIF-1α stabilization by use of a full-length HIF-1α-luciferase fusion reporter gene. 4T1 cells stably transduced with either the wild-type full-length HIF-Luc reporter gene or its mutant version C533S HIF-luc gene were cultured in 24-well plates to 50% confluence (1.0×105 cell per well). About 1.5× 10 5 RAW 264.7 (mouse macrophage) were added after 24 hours. Two hrs later, 10 µg/ml of LPS and 0.1 ng/ml of IFN-γ were added to the medium. Twelve hours later, cells were imaged for luciferase activity in the Xenogen IVIS200 system. It is clear that the addition of LPS, which activated the wild type HIF-1α-luc fusion reporter significantly (p<0.005, t test), had minimal effect minimal effect on the C533S mutant HIF-1α fusion reporter.
The functional importance of NO-mediated HIF-1α activation during cancer therapy
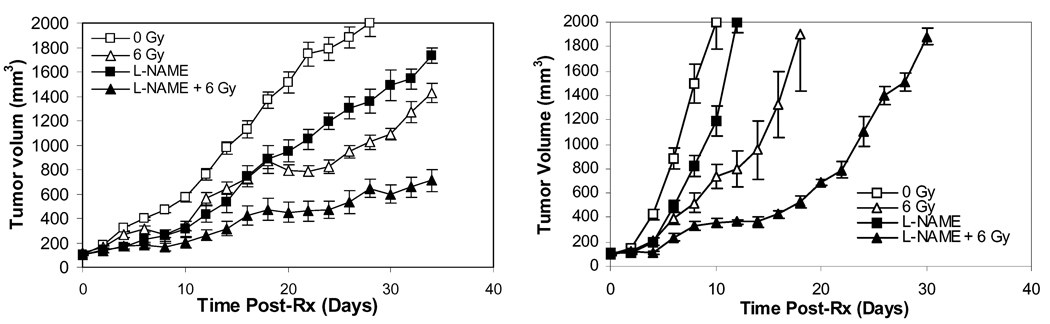
As HIF-1α has been shown to be a key tumor survival factor during cancer therapy, we postulated that the inhibition of HIF-1α activation through the prevention of NO production would have anti-tumor efficacy. To examine this hypothesis, we carried out tumor growth delay experiments with co-administration of radiotherapy (3×6Gy) and L-NAME, in two aggressive tumor models, 4T1 (murine mammary adenocarcinoma) and B16F10 (murine melanoma), as shown in Figure 6. In both models, the inhibition of NO production by L-NAME significantly enhanced the therapeutic efficacy of radiotherapy. Additional results with B16F10 grown in iNOS−/− mice indicated both the non-treated control and radiation-treated group showed significantly slower tumor growth rate than their wild type counterparts (supplementary Figure S3). Taken together, these results suggest that NO-mediated HIF-1α activation indeed plays a critical role in overall tumor response to radiotherapy, consistent with previous reports that the survival of tumor vasculature is key to tumor survival during radiotherapy(Garcia-Barros et al., 2003; Moeller et al., 2004). They further suggest that NOS inhibitors may be used as therapeutic agents to enhance the efficacy of conventional cancer treatments.
Figure 6.
Enhanced anti-tumor efficacy of radiotherapy in combination with L-NAME. B16F10 and 4T1 tumors were established in syngeneic C57BL/6 and Balb/C mice, respectively and irradiated with 3 fractions of X-rays at 6Gy/fraction (irradiation every other day). In some of the groups, L-NAME was administered in the drinking water one day before irradiation. Tumor sizes were monitored every other day. At least 6 animals were used in each treatment groups. Tumor sizes were then plotted against time for each tumor type. The error bars represent the standard error of the mean.
(A) 4T1 tumor growth delay.
(B) B16F10 melanoma growth delay.
Discussion
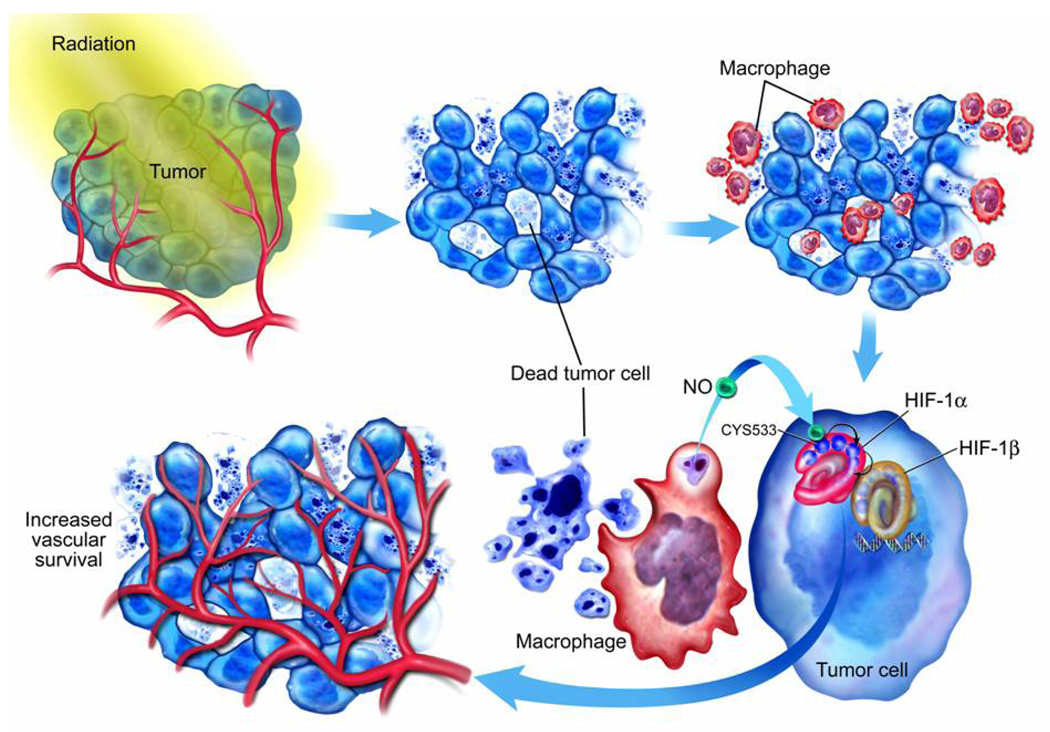
Understanding HIF-1 regulation during cancer treatment can provide important insights into how tumors respond to therapy. This is because HIF-1 has been shown to be a key tumor survival factor after cancer therapy(Moeller et al., 2004; Zhang et al., 2004). Our discovery of HIF-1α up-regulation through NO generated from tumor-associated macrophages is important in two aspects: recognizing the tumor-associated macrophages (TAMs) as a major regulator of HIF-1 during cancer therapy and the identification of S-nitrosylation of C533 (human equivalent C520) as a key mechanism for NO-mediated HIF-1α stabilization (Figure 7). The first establishes TAM as a pivotal mediator of tumor angiogenic activities after radiotherapy while the latter unveils a novel mechanism for HIF-1α regulation that is independent of the prolyl hydroxylase pathway. This mechanism have implications beyond cancer biology since both nitric oxide and HIF-1 are involved in a wide range of normal physiological and pathological conditions such as wound healing, inflammatory response, hypoxia/ischemia injury, etc.
Figure 7.
A diagram summarizing radiotherapy induced HIF-1α stabilization through nitrosylation of C533 by macrophage-derived nitric oxide.
What is the rationale for mammalian organisms to have a NO-mediated pathway for HIF-1 activation? A key may be found in the immune system. When facing infection from foreign organisms, during wound healing or inflammatory responses, the immunoeffectors cells, especially the macrophages, play the frontline defensive roles such as phagocytosing/killing foreign organisms or secreting factors that promote tissue regeneration. Nitric oxide is generated in ample quantities in the activated macrophages to carry out these functions. On the other hand, HIF-1α is shown to be a key mediator of the body’s immune response against foreign organisms with particular importance in myeloid cell mediated inflammation(Cramer et al., 2003). Of special interest is the fact that HIF-1 has also been known to enhance iNOS gene expression in a variety of cell types (Jung et al., 2000; Matrone et al., 2004; Melillo et al., 1997). Therefore, it is possible that activated iNOS and HIF-1 forms an amplification loop during wound healing or inflammation. In consistent with this hypothesis is a recent report that indicate HIF-1 and iNOS do appear to regulate each other positively under normoxic conditions during bacterial infections(Peyssonnaux et al., 2005). This amplification loop may be a key mechanism during inflammatory response. Indeed, our data (Figure 5) confirming the role of Cys533 nitrosylation in macrophage-mediated HIF-1α stabilization lend strong support for this point. It also underscores new opportunities of drug development afforded by the relationship between NO and HIF-1α for various inflammatory diseases and wound healing.
In terms of cancer therapy, the recognition that NO mediated S-nitrosylation of Cys 533 can up-regulate HIF-1 activity during radiation or chemotherapy has important implications as well. This is because quite a few studies have indicated that HIF-1 plays critical roles for tumor growth and survival during cancer therapy(Moeller et al., 2004; Yeo et al., 2003). One very interesting class of novel targeted anti-cancer agents that may be very relevant to our results here are the Hsp90 inhibitors. One of the Hsp90 inhibitors, 17-AAG, showed great promise as an anti-tumor agent that can sensitize radiotherapy(Bisht et al., 2003; Dote et al., 2006)in a variety of tumor models and are now in Phase I&II clinical trials(Neckers and Ivy, 2003; Neckers and Neckers, 2002). One of the main secondary targets of this drug is HIF-1α, which is destabilized in cells exposed to 17-AAG(Isaacs et al., 2004). Of further interest is the finding that 17-AAG can mediate the destabilization of HIF-1α independent of the ubiquitin-proteasome system and abrogate NO-mediated HIF-1α stabiliztion(Thomas et al., 2004), emphasizing the complicated nature of HIF-1α regulation by multiple mechanisms.
The recognition of the role of NO in the up-regulation of HIF-1α during cancer therapy suggests a promising strategy to enhance current therapy: the use of NOS inhibitors in conjunction with conventional radiation and chemotherapy modalities. Our results combining NOS inhibitor L-NAME and radiotherapy (Figure 6) lent strong support for this approach.
Although our study was primarily conducted in tumors that were exposed to ionizing radiation, the same NO-mediated HIF-1 activation pathway may operate in other normal cells/tissues. The potential biological roles of this NO-mediated HIF-1 activation in additional cell types clearly warrant further studies.
In summary, the results presented in this study establish the importance of nitric oxide-mediated S-nitrosylation in regulating the stability of HIF-1α. They indicate that S-nitrosylation of Cys533 (murine equivalent of human Cys520) in HIF-1α is directly responsible for radiation-induced HIF-1α stabilization in tumors. They also suggest that modulating HIF-1α activation through NOS inhibitors may be a promising strategy for therapeutic development in a variety of diseases such as cancer and inflammatory diseases where it has been established that both NO and HIF-1α play prominent roles.
Methods
Cell lines and tissue culture
The 4T1 murine mammary adenocarcinoma cell line and B16F10 murine melanoma cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The two cell lines were cultured in Dulbeccos’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum.
Reagents
Please see supplementary data section for more information.
Plasmids and cloning procedures
The HIF-1α bioluminescence reporter (ODD-luc) construct was created by fusing PCR product of ODD domain of HIF-1α (Genbank Accession# U59496) to the 5’ end of firefly luciferase reporter gene. Along with this construct, a luciferase expression vector in which luciferase gene was driven by the CMV promoter was used as a control.
C533→S ODD mutation was achieved by in vitro site-directed mutagenesis. ODD fragments with or without the mutation were cloned into pCMV-3Tag-4B Epitop Tagging Mammalian Expression vector (Stratagene, La Jolla, CA). The full length mouse VHL gene (Genbank Accession# S76748) was cloned from mouse tissue and tagged with HA tag by PCR. All constructs were sequence-verified. Details are available upon request.
A full-length HIF-1α-luc fusion reporter and its C533→S mutant counterpart were also constructed using similar approaches.
SiRNA
SiRNA sequences targeted to VHL gene were designed by using an Internet-based program available at the website of Ambion Inc.( Austin, TX). A retroviral siRNA expression vector (pSilencer 5.1-U6 Retro from Ambion Inc) was used to stably introduce the following siRNA sequence targeted to VHL gene to 4T1 cells: AACATCACATTGCCAGTGTAT. pSilencer 5.1-U6 Scrambled siRNA (Ambion Inc ) was used as a negative control.
Imaging luciferase activity
Luciferase expression/activity was detected and quantified as relative light units (RLUs) by using the Xenogen IVIS™ imaging system and associated Living Image® software (Xenogen, Alameda, CA).
For in vitro observations, cells transfected with luciferase reporter gene constructs were grown in the 12-well or 24-well cell culture plates. After cells reached 80% confluence, they were treated with the different chemicals or were cultured in the hypoxic chamber (Sheldon Manufacturing, Inc., Cornelius, OR). At the times indicated, luciferin (150 µg/ml) was added and the plates were imaged for luciferase expression.
For in vivo experiments, treated tumor-bearing mice received an i.p. injection of luciferin (150 mg/kg) during isofluorane anesthesia. Repeated images of luciferase expression/activity were acquired following manufacturer’s specified procedures.
Animal experiments
Please see supplementary data section for the details of animal experiments.
ELISA and western blot analysis
The majority of reagents used for ELISA and western blot analyses were obtained commercially with the exception of the antibody against hydroxylated HIF-1α, which was a gift from Prof. Peter Ratcliff of Oxford University. Please see supplementary data section for additional details.
Immunohistochemical stainings
Please see supplementary data section for details.
In vitro interaction assay for ODD and VHL Protein
Please see supplementary data for the details of the experiments.
Biotin switch assay
Biotin switch assay was performed as described (Jaffrey and Snyder, 2001). Please see supplementary data for the details of the experiments.
Macrophage depletion experiments
Macrophage depletion was achieved in mice as shown elsewhere (Muller et al., 2005). Briefly, nude mice received repeated i.p. injections of 2 mg carrageenan at 6, 3 and 1 day before s.c. injection of 4T1 tumor cells, after which mice were injected once per week until the end of the experiment.
Statistics
Student’s t test, one-way and two-way ANOVA were used where indicated. In growth delay experiments, days for tumors to reach 5× of initial volume were used for comparing different treatment groups. P<0.05 was considered to be statistically significant.
Supplementary Material
01
Acknowledgement
This study was supported in part by grant EB001882 from US National Institute of Bioimaging and Bioengineering, grant CA81512 from the US National Cancer Institute, a grant from the Komen Foundation for Breast Cancer Research, and a grant from the US Department of Defense (DAMD17-02-0052) to C-Y. Li. P. Sonveaux is a fellow of the Belgian American Educational Foundation (BAEF), the ‘Fonds National de la Recherche Scientifique’ (FNRS), and received additional support from the ‘Fonds Spéciaux de la Recherche’ at UCL. We thank Prof. Peter Ratcliff of Oxford University for kindly providing the antibody against hydroxylated HIF-1α.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Data
Supplementary information includes more details of material and methods and eight additional figures.
References
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht KS, Bradbury CM, Mattson D, Kaushal A, Sowers A, Markovina S, Ortiz KL, Sieck LK, Isaacs JS, Brechbiel MW, et al. Geldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicity. Cancer research. 2003;63:8984–8995. [PubMed] [Google Scholar]
- Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Dodge RK, Charles HC, Samulski TV, Prosnitz LR, Dewhirst MW. Radiation therapy and hyperthermia improve the oxygenation of human soft tissue sarcomas. Cancer Res. 1996;56:5347–5350. [PubMed] [Google Scholar]
- Colombo MP, Mantovani A. Targeting myelomonocytic cells to revert inflammation-dependent cancer promotion. Cancer Res. 2005;65:9113–9116. doi: 10.1158/0008-5472.CAN-05-2714. [DOI] [PubMed] [Google Scholar]
- Cramer T, Johnson RS. A novel role for the hypoxia inducible transcription factor HIF-1alpha: critical regulation of inflammatory cell function. Cell Cycle. 2003;2:192–193. [PubMed] [Google Scholar]
- Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dote H, Burgan WE, Camphausen K, Tofilon PJ. Inhibition of hsp90 compromises the DNA damage response to radiation. Cancer research. 2006;66:9211–9220. doi: 10.1158/0008-5472.CAN-06-2181. [DOI] [PubMed] [Google Scholar]
- Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- Gaston BM, Carver J, Doctor A, Palmer LA. S-nitrosylation signaling in cell biology. Molecular interventions. 2003;3:253–263. doi: 10.1124/mi.3.5.253. [DOI] [PubMed] [Google Scholar]
- Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003;2:803–811. doi: 10.1038/nrd1199. [DOI] [PubMed] [Google Scholar]
- Goldmann O, Rohde M, Chhatwal GS, Medina E. Role of macrophages in host resistance to group A streptococci. Infect Immun. 2004;72:2956–2963. doi: 10.1128/IAI.72.5.2956-2963.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Isaacs JS, Jung YJ, Neckers L. Aryl hydrocarbon nuclear translocator (ARNT) promotes oxygen-independent stabilization of hypoxia-inducible factor-1alpha by modulating an Hsp90-dependent regulatory pathway. J Biol Chem. 2004;279:16128–16135. doi: 10.1074/jbc.M313342200. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jaffrey SR, Snyder SH. The biotin switch method for the detection of S-nitrosylated proteins. Sci STKE. 2001 doi: 10.1126/stke.2001.86.pl1. PL1. [DOI] [PubMed] [Google Scholar]
- Jeong JW, Bae MK, Ahn MY, Kim SH, Sohn TK, Bae MH, Yoo MA, Song EJ, Lee KJ, Kim KW. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002;111:709–720. doi: 10.1016/s0092-8674(02)01085-1. [DOI] [PubMed] [Google Scholar]
- Jung F, Palmer LA, Zhou N, Johns RA. Hypoxic regulation of inducible nitric oxide synthase via hypoxia inducible factor-1 in cardiac myocytes. Circ Res. 2000;86:319–325. doi: 10.1161/01.res.86.3.319. [DOI] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002a;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002b;295:858–861. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- Leek RD, Hunt NC, Landers RJ, Lewis CE, Royds JA, Harris AL. Macrophage infiltration is associated with VEGF and EGFR expression in breast cancer. J Pathol. 2000;190:430–436. doi: 10.1002/(SICI)1096-9896(200003)190:4<430::AID-PATH538>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Leek RD, Talks KL, Pezzella F, Turley H, Campo L, Brown NS, Bicknell R, Taylor M, Gatter KC, Harris AL. Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha) expression in tumor-infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in Human breast cancer. Cancer Res. 2002;62:1326–1329. [PubMed] [Google Scholar]
- Lewis C, Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol. 2005;167:627–635. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- Matrone C, Pignataro G, Molinaro P, Irace C, Scorziello A, Di Renzo GF, Annunziato L. HIF-1alpha reveals a binding activity to the promoter of iNOS gene after permanent middle cerebral artery occlusion. J Neurochem. 2004;90:368–378. doi: 10.1111/j.1471-4159.2004.02483.x. [DOI] [PubMed] [Google Scholar]
- Maxwell PH, Pugh CW, Ratcliffe PJ. The pVHL-hIF-1 system. A key mediator of oxygen homeostasis. Adv Exp Med Biol. 2001;502:365–376. [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Melillo G. HIF-1: a target for cancer, ischemia and inflammation--too good to be true? Cell Cycle. 2004;3:154–155. [PubMed] [Google Scholar]
- Melillo G, Taylor LS, Brooks A, Musso T, Cox GW, Varesio L. Functional requirement of the hypoxia-responsive element in the activation of the inducible nitric oxide synthase promoter by the iron chelator desferrioxamine. J Biol Chem. 1997;272:12236–12243. doi: 10.1074/jbc.272.18.12236. [DOI] [PubMed] [Google Scholar]
- Metzen E, Zhou J, Jelkmann W, Fandrey J, Brune B. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell. 2003;14:3470–3481. doi: 10.1091/mbc.E02-12-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Moeller BJ, Dreher MR, Rabbani ZN, Schroeder T, Cao Y, Li CY, Dewhirst MW. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005;8:99–110. doi: 10.1016/j.ccr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- Neckers L, Ivy SP. Heat shock protein 90. Current opinion in oncology. 2003;15:419–424. doi: 10.1097/00001622-200311000-00003. [DOI] [PubMed] [Google Scholar]
- Neckers L, Neckers K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutic agents. Expert opinion on emerging drugs. 2002;7:277–288. doi: 10.1517/14728214.7.2.277. [DOI] [PubMed] [Google Scholar]
- Palmer LA, Gaston B, Johns RA. Normoxic stabilization of hypoxia-inducible factor-1 expression and activity: redox-dependent effect of nitrogen oxides. Molecular pharmacology. 2000;58:1197–1203. doi: 10.1124/mol.58.6.1197. [DOI] [PubMed] [Google Scholar]
- Pause A, Peterson B, Schaffar G, Stearman R, Klausner RD. Studying interactions of four proteins in the yeast two-hybrid system: structural resemblance of the pVHL/elongin BC/hCUL-2 complex with the ubiquitin ligase complex SKP1/cullin/F-box protein. Proc Natl Acad Sci U S A. 1999;96:9533–9538. doi: 10.1073/pnas.96.17.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapisarda A, Uranchimeg B, Scudiero DA, Selby M, Sausville EA, Shoemaker RH, Melillo G. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res. 2002;62:4316–4324. [PubMed] [Google Scholar]
- Sandau KB, Fandrey J, Brune B. Accumulation of HIF-1alpha under the influence of nitric oxide. Blood. 2001;97:1009–1015. doi: 10.1182/blood.v97.4.1009. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med. 2002;8:S62–S67. doi: 10.1016/s1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Agani F, Feldser D, Iyer N, Kotch L, Laughner E, Yu A. Hypoxia, HIF-1, and the pathophysiology of common human diseases. Adv Exp Med Biol. 2000;475:123–130. doi: 10.1007/0-306-46825-5_12. [DOI] [PubMed] [Google Scholar]
- Sutphin PD, Chan DA, Giaccia AJ. Dead cells don't form tumors: HIF-dependent cytotoxins. Cell Cycle. 2004;3:160–163. [PubMed] [Google Scholar]
- Thomas DD, Espey MG, Ridnour LA, Hofseth LJ, Mancardi D, Harris CC, Wink DA. Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8894–8899. doi: 10.1073/pnas.0400453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen LL, Scott JM, Topley P, Knowles RG, Keerie AJ, Frend AJ. Selective inhibition of inducible nitric oxide synthase inhibits tumor growth in vivo: studies with 1400W, a novel inhibitor. Cancer Res. 1997;57:3300–3304. [PubMed] [Google Scholar]
- Varney ML, Olsen KJ, Mosley RL, Bucana CD, Talmadge JE, Singh RK. Monocyte/macrophage recruitment, activation and differentiation modulate interleukin-8 production: a paracrine role of tumor-associated macrophages in tumor angiogenesis. In Vivo. 2002;16:471–477. [PubMed] [Google Scholar]
- Wachsberger P, Burd R, Dicker AP. Tumor response to ionizing radiation combined with antiangiogenesis or vascular targeting agents: exploring mechanisms of interaction. Clin Cancer Res. 2003;9:1957–1971. [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J Biol Chem. 1993a;268:21513–21518. [PubMed] [Google Scholar]
- Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci U S A. 1993b;90:4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- Yasinska IM, Sumbayev VV. S-nitrosation of Cys-800 of HIF-1alpha protein activates its interaction with p300 and stimulates its transcriptional activity. FEBS Lett. 2003;549:105–109. doi: 10.1016/s0014-5793(03)00807-x. [DOI] [PubMed] [Google Scholar]
- Yeo EJ, Chun YS, Cho YS, Kim J, Lee JC, Kim MS, Park JW. YC-1: a potential anticancer drug targeting hypoxia-inducible factor 1. J Natl Cancer Inst. 2003;95:516–525. doi: 10.1093/jnci/95.7.516. [DOI] [PubMed] [Google Scholar]
- Zhang X, Kon T, Wang H, Li F, Huang Q, Rabbani ZN, Kirkpatrick JP, Vujaskovic Z, Dewhirst MW, Li CY. Enhancement of hypoxia-induced tumor cell death in vitro and radiation therapy in vivo by use of small interfering RNA targeted to hypoxia-inducible factor-1alpha. Cancer Res. 2004;64:8139–8142. doi: 10.1158/0008-5472.CAN-03-2301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01