Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells (original) (raw)
. Author manuscript; available in PMC: 2011 Jun 15.
Published in final edited form as: Cancer Cell. 2010 Jun 15;17(6):584–596. doi: 10.1016/j.ccr.2010.05.015
Abstract
SUMMARY
We report a Jak2V617F knock-in mouse myeloproliferative neoplasm (MPN) model resembling human polycythemia vera (PV). The MPN is serially transplantable and we demonstrate that the hematopoietic stem cell (HSC) compartment has the unique capacity for disease initiation but does not have a significant selective competitive advantage over wild type HSCs. In contrast, myeloid progenitor populations are expanded and skewed towards the erythroid lineage, but cannot transplant the disease. Treatment with a JAK2 kinase inhibitor ameliorated the MPN phenotype, but did not eliminate the disease-initiating population. These findings provide insights into the consequences of JAK2 activation on HSC differentiation and function and have the potential to inform therapeutic approaches to JAK2V617F positive MPN.
SIGNIFICANCE
The JAK2V617F mutation is a promising candidate for molecularly targeted therapy in MPN. Early data from JAK2 inhibitor clinical trials have called into question the capacity of these compounds to alter the natural history of JAK2V617F mediated MPN. Determining the effect of JAK2 inhibitors on the disease-initiating population requires a model in which the JAK2V617F allele is expressed at physiological levels in hematopoietic stem and progenitor cells, as it is in humans. Our model demonstrates that JAK2V617F causes expansion of erythroid progenitors but that only the HSC compartment can initiate disease in a transplanted mouse. We further demonstrate that the HSC compartment, the definitive target for curative therapy of JAK2V617F mediated MPN, is resistant to treatment with a JAK2 inhibitor.
INTRODUCTION
The JAK2V617F mutation is the most common molecular abnormality in BCR-ABL negative MPN, and is present in approximately 95% of patients with PV, and in approximately 50% of patients with essential thrombocythemia (ET) and primary myelofibrosis (PMF) (Baxter et al., 2005). JAK2V617F is present at low frequency in other myeloid malignancies and is not observed at all in lymphoid neoplasms (Steensma et al., 2005), (Levine et al., 2005a). This acquired point mutation in the JAK2 gene results in a valine to phenylalanine substitution at position 617 and constitutive activation of JAK2 kinase signaling (James et al., 2005), (Kralovics et al., 2005), (Baxter et al., 2005), (Levine et al., 2005b). Overexpression of JAK2V617F confers interleukin-3 (IL-3) independence to Ba/F3 cells that co-express a homodimeric Type I cytokine receptor, such as the erythropoietin receptor (EpoR) (Lu et al., 2005). Transplantation of _JAK2V617F_–overexpressing hematopoietic cells into mice is sufficient to re-capitulate a PV disease phenotype (Wernig et al., 2006), (Lacout et al., 2006), (Zaleskas et al., 2006). In aggregate, JAK2 therefore represents an excellent therapeutic target in MPN patients.
To cure MPN in human patients, it may be necessary to eradicate all JAK2V617F mutated hematopoietic cells that have the capacity to self-renew and thus maintain disease. In this context, it is therefore imperative to understand the precise role and function of the JAK2V617F allele as it relates to hematopoietic stem and multipotent progenitor cells (HSPCs). In MPN patients, JAK2V617F is detectable in CD34+ CD38− hematopoietic stem cells (HSCs) (Jamieson et al., 2006) and in all mature cell lineages (Ishii et al., 2006), (Delhommeau et al., 2007). However, functional characterization of JAK2V617F HSPCs has been limited in existing retroviral and transgenic murine models (Wernig et al., 2006), (Lacout et al., 2006), (Zaleskas et al., 2006), (Tiedt et al., 2008), (Xing et al., 2008), (Shide et al., 2008) and has been described only in the non-obese diabetic-severe combined immunodeficient (NOD/SCID) murine model to date (James et al., 2008). While the retroviral JAK2V617F model has been informative (Wernig et al., 2006), (Lacout et al., 2006), (Zaleskas et al., 2006), it is subject to the problems inherent to retroviral-mediated transduction, including the identity of the transduced cells, with preferential transduction of mitotic progenitor cells relative to quiescent long term HSCs and the non-physiologic level of oncogene expression. These factors may ultimately affect the resultant biological and phenotypic outcome in these models (Ren, 2004). Transgenic model systems also have non-physiologic expression of the oncogene due to increased copy number and the use of exogenous promoters.
Over the course of a decade of treating chronic myelogenous leukemia (CML) with imatinib, it has become evident that cure is difficult to achieve as a consequence of a reservoir of disease initiating cells contained within the quiescent HSC compartment (Holyoake et al., 1999), (Graham et al., 2002), (Michor et al., 2005). Furthermore, preliminary results from small molecule JAK2 kinase inhibitor trials suggest that curative therapy of BCR-ABL negative MPN may prove even more difficult (Pardanani et al., 2009), (Verstovsek et al., 2009b). An accurate knowledge of the early changes that occur in the HSPC compartment directly as a result of acquisition of the JAK2V617F mutation is therefore critical in determining the curative potential of therapies that target this molecular event.
We describe a Jak2V617F knock-in model in which expression of Jak2V617F is under the control of the endogenous murine Jak2 promoter; provide detailed analysis of the effects of the Jak2V617F allele on hematopoietic stem and progenitor cells and evaluate the impact of a small molecule JAK2 inhibitor on the HSPC compartment.
RESULTS
Expression of Jak2V617F from its endogenous promoter results in a lethal MPN resembling human polycythemia vera (PV)
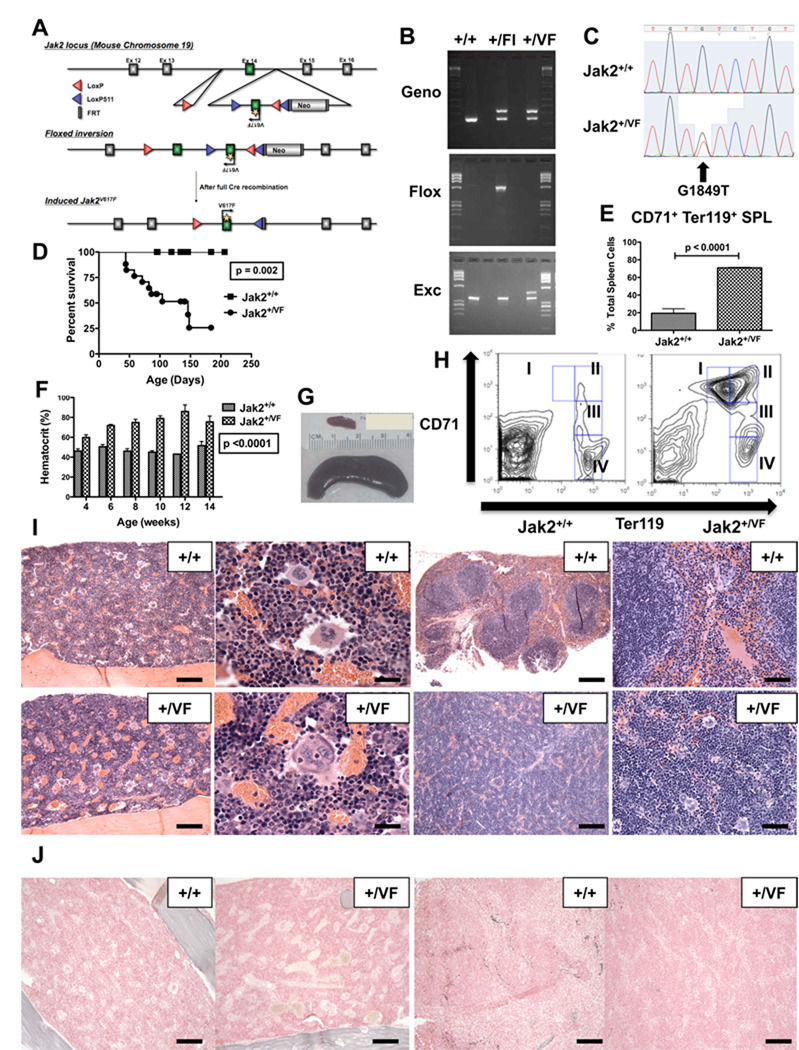
We generated a Jak2V617F conditional knock-in (KI) allele by gene targeting in mouse embryonic stem cells (Figure 1A). The resulting chimeric animals were crossed with wild-type (WT) C57Bl/6 mice to generate floxed heterozygous Jak2V617F mice. Floxed heterozygous Jak2V617F animals were then crossed with E2ACre transgenic mice to induce germline Jak2V617F expression during early mouse embryogenesis (Lakso et al., 1996). Inversion of the floxed knock-in allele and excision of the wild type exon were confirmed by PCR (Figure 1B) and expression of the G1849T mutation (that results in a valine to phenylalanine substitution at amino acid position 617, Jak2V617F), was confirmed by cDNA sequencing (Figure 1C). These heterozygous Jak2V617F germline expressing animals (Jak2+/VF) developed a lethal MPN that was 100% penetrant with a median survival of 146 days (Figure 1D). The MPN was characterized by an elevated hematocrit (HCT) (Figure 1F), splenomegaly (Figure 1G, Figure S1A) and prominent splenic extra-medullary erythropoiesis (Figure 1E). Flow-cytometric analysis showed increased CD71+ Ter119+ erythroid precursors in Jak2+/VF bone marrow (BM) (Figure S1B) and spleen (Figure 1H). Histopathology demonstrated marked erythroid and mild megakaryocytic hyperplasia within the Jak2+/VF splenic red pulp with overall effacement of the normal splenic architecture (Figure 1I). The Jak2+/VF BM showed a milder increase in erythroid elements compared to the spleen, but demonstrated megakaryocytes with atypical nuclear features and prominent emperipolesis (Figure 1I). CD41+ cells were increased in Jak2+/VF BM (p=0.05), platelet counts were not increased and no differences were observed in megakaryocyte ploidy between Jak2+/VF and Jak2+/+ mice (Figures S1C, S1D, S1E). WBC counts were increased in Jak2+/VF mice (p<0.0001) although we did not observe an increase in Mac1+Gr1+ or Mac1+cells relative to total Jak2+/VF BM cells (Figures S1F, S1G, S1H). Reticulin fibrosis was absent in both Jak2+/VF BM and spleen, even in mice that were 6 months old (Figure 1J) and the development of acute leukemia was not observed in any animals. In aggregate, these findings demonstrate that Jak2+/VF knock-in mice develop a MPN reminiscent of human PV with a short disease latency and reduced survival.
Figure 1. Physiological Jak2V617F expression causes a lethal MPN resembling PV.
(A) Targeting vector schema for the Jak2V617F conditional KI mouse.
(B) PCR analysis of tail DNA from Jak2+/+, Jak2+/Fl and Jak2+/VF mice for genotyping (Geno), detection of floxed band (Flox) and detection of excised band (Exc);(Fl = Floxed).
(C) Sequencing analysis of unfractionated BM cells from Jak2+/+ and Jak2+/VF mice.
(D) Kaplan-Meier analysis of a cohort of Jak2+/VF mice (n=17) and littermate controls (n=13).
(E) Composite data from age-matched littermates of Jak2+/+ and Jak2+/VF mice (unpaired two-tailed t test; mean +/− SEM; n=4 in each group) demonstrating increased in CD71+, Ter119+ erythroid precursors in Jak2+/VF spleens.
(F) Hematocrits of age-matched littermate Jak2+/+ and Jak2+/VF mice aged 4–14 weeks (mean +/− SEM; n=16 in each group).
(G) Photograph of spleens from Jak2+/+ (upper; weight = 0.08G) and Jak2+/VF mice (lower; weight = 3.1G) demonstrating marked splenomegaly in Jak2+/VF mouse.
(H) Representative flow-cytometric analysis of single cell suspensions of spleen from age-matched littermate Jak2+/+ and Jak2+/VF mice, indicating expansion of CD71+ Ter119+ cells (subpopulations I and II) in Jak2+/VF mice.
(I) Histopathologic H & E sections of bone marrow (BM) and spleen (SPL) from representative Jak2+/+ and Jak2+/VF mice demonstrating megakaryocyte hyperplasia with clustering and emperipolesis in Jak2+/VF BM and marked erythroid hyperplasia with complete effacement of white pulp in Jak2+/VF SPL when compared with unremarkable BM and SPL from Jak2+/+ controls (BM: first column [scale bars 250µM], second column [scale bars 50µM]; SPL: third column [scale bars 500µM], fourth column [scale bars 125µM].
(J) Histopathologic reticulin sections of bone marrow (BM) and spleen (SPL) from representative Jak2+/+ and Jak2+/VF mice demonstrating absence of reticulin fibrosis (BM: first and second panels [scale bars 250µM]; SPL: third and fourth panels [scale bars 250µM].
See also Figure S1.
Erythroid skewing in the myeloid progenitor compartment of Jak2V617F mice
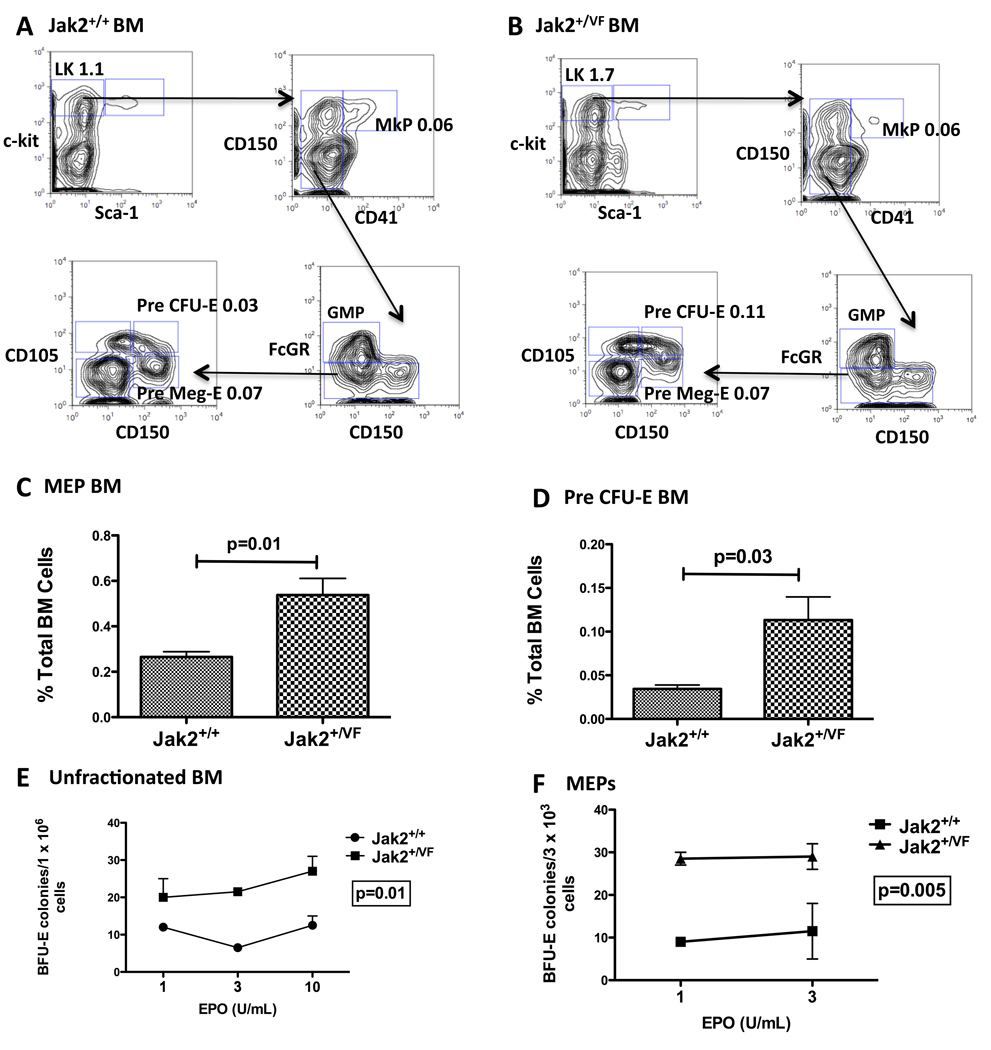
Having demonstrated that Jak2+/VF mice developed elevated HCT and expanded erythroid precursor cells, we undertook a quantitative evaluation of the BM myeloid progenitor compartment of Jak2+/VF or Jak2+/+ mice. We found that immunophenotypically-defined myeloid progenitor cells (LineagelowSca1−cKithigh) were increased in Jak2+/VF mice (Figure S2A, p=0.002) mainly as a result of expansion of the megakaryocytic/erythroid progenitor (MEP) population within this compartment (Figure 2C; Figure S2B, S2C). The ratio of common myeloid progenitor (CMP) and granulocyte/macrophage progenitors (GMP) cells to total BM cells was unchanged when comparing Jak2+/VF and Jak2+/+ mice (Figures S2D, S2E). We then performed a more detailed examination of megakaryocytic and erythroid progenitor populations using the additional markers CD150, CD41, and CD105 (Pronk et al., 2007). These studies showed an increase in lineagelowcKithighCD150+CD41−CD105+ Pre-CFU-E cells (that have only erythroid lineage potential), relative to lineagelowcKithighCD150+CD41−CD105− Pre-MegE cells (that have mixed erythroid/megakaryocytic potential) and lineagelowcKithighCD41+ MkP cells (that are lineage restricted to a mature megakaryocytic fate) (Figures 2A, 2B, 2D), in Jak2+/VF mice compared with Jak2+/+ mice. The Jak2+/VF expanded CD71+ Ter119+ proerythroblast population is contained within the CD150−, CD105+ compartment. These results demonstrate that Jak2V617F causes marked erythroid skewing of progenitor populations: disproportionately increasing MEP cells over other myeloid progenitors and increasing Pre-CFU-E cells relative to megakaryocyte progenitors.
Figure 2. Erythroid skewing in the myeloid progenitor compartment of Jak2V617F mice.
(A – B) Multiparameter flow cytometry of Jak2+/+ and Jak2+/VF BM. Representative contour plots indicating LK subsets (MkPs, GMPs, Pre Meg-Es and Pre CFU-Es) are shown. All values are mean frequency of total BM cells. Representative data from one of 2 independent experiments (n=4 in each group).
(C) Composite data from age-matched littermates of Jak2+/+ and Jak2+/VF mice demonstrating significantly increased numbers of MEP cells in Jak2+/VF BM over Jak2+/+ BM (unpaired two-tailed t test; mean +/−SEM; n=4 in each group).
(D) Composite data from age-matched littermates of Jak2+/+ and Jak2+/VF mice demonstrating significantly increased numbers of Pre CFU-E cells in Jak2+/VF BM over Jak2+/+ BM (unpaired two-tailed t test; mean +/−SEM; n=4 in each group).
(E – F) Unfractionated BM and MEP cells from Jak2+/+ and Jak2+/VF mice were plated and scored for BFU-E colony formation 7–10 days later. Results are the average of two independent experiments performed in duplicate (unpaired two-tailed t test; mean +/−SEM).
See also Figure S2.
One of the pathognomonic features of PV is hypersensitivity of erythroid progenitors to erythropoietin (EPO), and growth with a low plating efficiency even in the absence of EPO. We plated unfractionated BM cells or highly purified MEP cells derived from Jak2+/VF or Jak2+/+ mice in media containing growth factors supplemented with decreasing concentrations of EPO. We observed EPO hypersensitivity in both Jak2+/VF unfractionated BM and Jak2+/VF MEPs, although we did not observe endogenous erythroid colony formation (Figures 2E, 2F). Consistent with this, we also found that Jak2+/VF MEP cells demonstrated increased phospho-Stat5 signaling in response to stimulation with EPO and interleukin-3 (IL3) as compared with Jak2+/+ MEP cells (Figure S2F).
The Jak2V617F MPN-initiating population is contained within the HSC-enriched LSK population
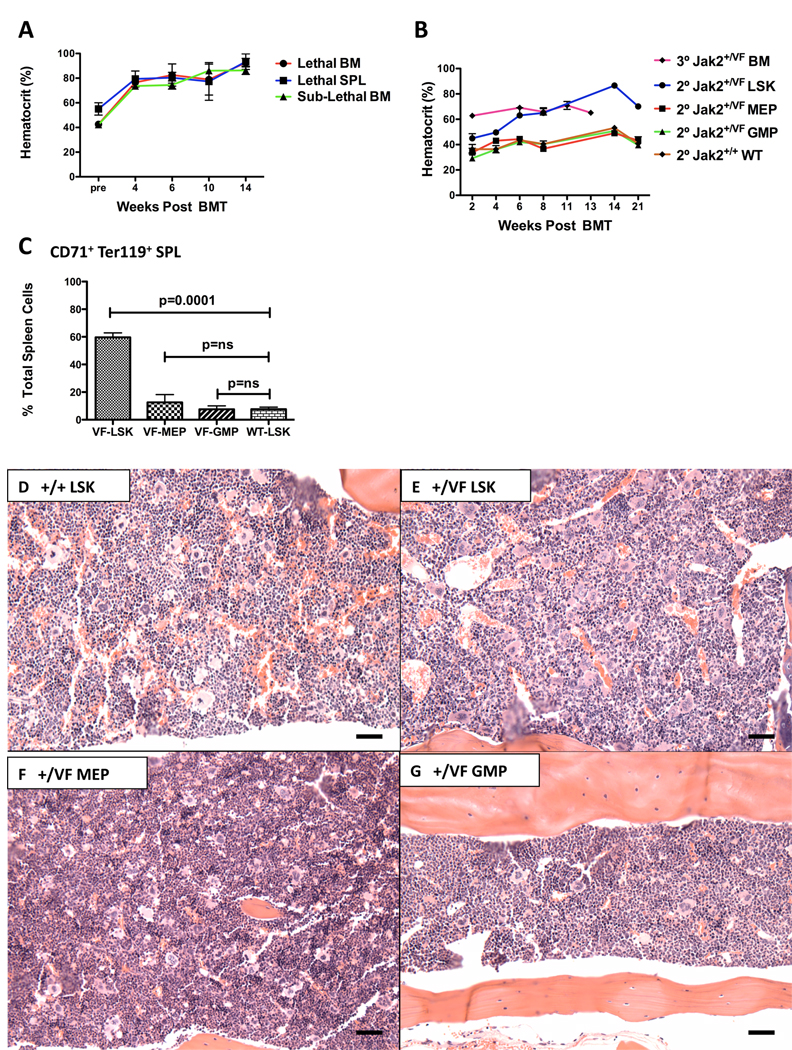
Existing murine MPN models have generally demonstrated poor transplantability of the primary disease (Chan et al., 2004), (Braun et al., 2004), (Lee et al., 2007), (Chan et al., 2009), limiting the evaluation of the disease-initiating population in these models. To assess the transplantability of Jak2V617F evoked MPN, we first transplanted unfractionated BM cells from diseased Jak2+/VF mice into lethally or sub-lethally irradiated littermate recipients; and unfractionated spleen cells from diseased Jak2+/VF mice into lethally irradiated littermate recipients. In all cases a MPN developed in secondary recipients, characterized by elevated HCT as early as 4 weeks post transplantation (Figure 3A) and a median survival of 342 days in this mixed group of transplants.
Figure 3. The Jak2V617F MPN-initiating population is contained within the LSK population.
(A) HCT of lethally (Lethal) or sub-lethally (Sublethal) irradiated littermate secondary recipients of unfractionated BM or SPL cells from diseased Jak2+/VF mice measured 4–14 weeks post transplantation (mean +/−SEM; n = 3 in each group).
(B) HCT of lethally irradiated secondary (2°) recipients of purified Jak2+/VF BM sub-populations (LSKs, MEPs and GMPs) and corresponding Jak2+/+ BM sub-populations; and of lethally irradiated tertiary (3°) recipients of unfractionated BM, measured 2–21 weeks post transplantation (mean +/−SEM; n = 5 in each group).
(C) Composite data from secondary recipients of purified Jak2+/VF BM sub-populations (LSKs, MEPs and GMPs), demonstrating increased CD71+, Ter119+ erythroid precursors in spleens of secondary recipients of purified Jak2+/VF LSK cells (unpaired two-tailed t test; mean +/− SEM; n=3 in each group).
(D – G) Histopathologic H & E sections of BM from representative secondary recipients of purified Jak2+/+ and Jak2+/VF LSK cells, Jak2+/VF MEP cells and Jak2+/VF GMP cells, demonstrating erythroid hyperplasia, increased megakaryocytes in clusters with large forms and emperipoliesis in Jak2+/VF LSK; slight increase in megakaryocytes in Jak2+/VF MEP and mild myeloid hyperplasia in Jak2+/VF GMP as compared with unremarkable BM from Jak2+/+ LSK (BM: [scale bars 250µM]).
See also Figure S3.
We next sought to identify the hematopoietic developmental stage that contains the disease-initiating cell. Highly purified BM LSK, MEP or GMP cell populations from Jak2+/VF and Jak2+/+ animals, respectively, were transplanted into lethally irradiated congenic secondary recipients and full donor engraftment was confirmed in all recipients. Recipient mice that received Jak2+/VF LSK cells developed a MPN that was similar to that which developed in primary mice, characterized by elevated HCT (Figure 3B), increased WBC and platelet counts (Figures S3A, S3B), prominent extramedullary erythropoiesis (Figure 3C), increased MEP cells (Figure S3C) and erythroid and megakaryocytic hyperplasia in the BM (Figures 3D, 3E). In contrast, animals that received either the Jak2+/VF MEP or Jak2+/VF GMP committed progenitor populations did not develop MPN with > 6 months of followup (Figures 3B, 3F, 3G). Furthermore, lethally irradiated tertiary recipients of unfractionated BM from mice that were transplanted with Jak2+/VF LSK cells 6 months earlier also developed elevated HCT (Figure 3B) and upon sacrifice histopathologic evidence of MPN was evident, demonstrating that the disease is serially transplantable (Figures S3D, S3E). These experiments demonstrate that _Jak2V617F_-expressing LSK cells, but not committed myeloid progenitors, are able to initiate and maintain the MPN in vivo.
Jak2V617F directs differentiation within the LSK compartment but has otherwise modest effects on LSK cells
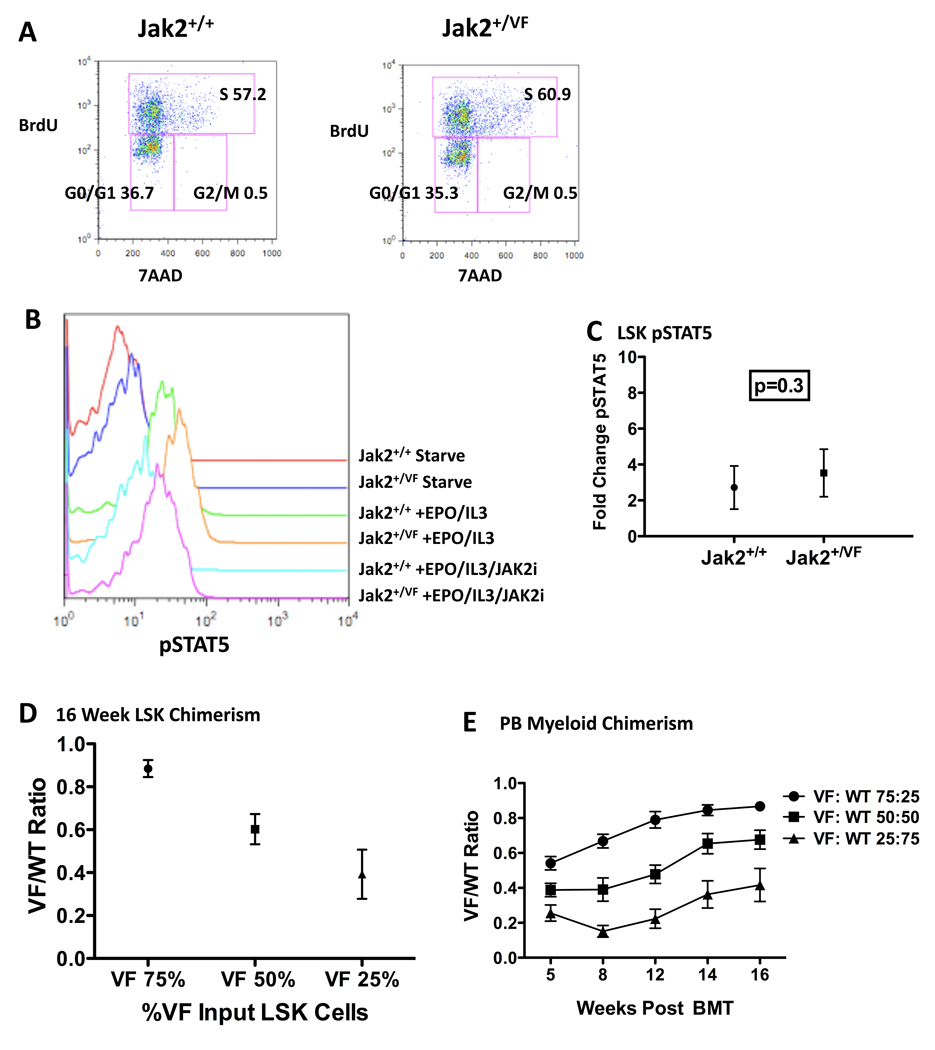
We observe that the MPN-initiating population is contained within the LSK compartment, and the Jak2V617F mutation has been identified in HSCs from human MPN patients (Jamieson et al., 2006). Based on these insights, we further assessed the LSK compartment that contains long-term (LT) and short-term (ST) HSCs (Spangrude et al., 1988), as well as multipotent progenitor cells (MPPs). We found no significant quantitative differences between Jak2+/VF and Jak2+/+ LSK cells (Figure S4A). Similarly, we did not observe quantitative differences in immunophenotyically defined LT-HSCs (CD150+CD48-LSKs) (Kiel et al., 2005) when comparing Jak2+/VF and Jak2+/+ BM (Figure S4B). In an assessment of the functional effects of the Jak2V617F mutation in LSK cells, we did not observe any significant differences between Jak2+/+ and Jak2+/VF LSK cells with regard to cell cycle status (Figure 4A).
Figure 4. Jak2V617F has modest effects in the LSK compartment and does not confer a significant competitive advantage to LSK cells.
(A) Representative multiparameter flow cytometry of LSK populations in Jak2+/+ and Jak2+/VF mice demonstrating no significant differences in cell numbers in different phases of cell cycle. All values are mean frequency of total LSK cells. Representative data from one of two independent experiments (n=4 in each group).
(B) Representative flow cytometric analysis of levels of phospho-STAT5 (p-STAT5) in LSK cells from Jak2+/+ and Jak2+/VF littermate controls, following serum starvation (starve) or stimulation with EPO (3U/mL) and IL3 (10ng/mL) for 10 mins, in the absence (EPO/IL3) or presence of the JAK2 inhibitor, TG101348 (EPO/IL3/JAK2i). Representative data from one of three independent experiments.
(C) Composite data from Jak2+/+ and Jak2+/VF LSK cells purified from primary mice (unpaired two-tailed t test; mean +/− SEM), demonstrating no significant difference in phosphoSTAT5 activation after stimulation with EPO/IL3 (n=4 in each group).
(D) Jak2+/VF(VF) to Jak2+/+(WT) chimerism ratios assessed in BM LSK compartment from lethally irradiated secondary recipients of Jak2+/VF and Jak2+/+ LSK cells in 75:25, 50:50 and 25:75 ratios respectively, measured 16 weeks post transplantation (mean +/− SEM; n=4 in each group).
(E) Jak2+/VF(VF) to Jak2+/+(WT) myeloid chimerism ratios assessed in peripheral blood (PB) from lethally irradiated secondary recipients of Jak2+/VF and Jak2+/+ LSK cells in 75:25, 50:50 and 25:75 ratios respectively, measured 5–16 weeks post transplantation (mean +/− SEM; n=6 in each group).
See also Figure S4 and Table S1.
These observations suggested that JAK-STAT signal transduction might not be differentially activated between Jak2+/VF and Jak2+/+ LSK cells. To assess this possibility, we used flow cytometry to assess phospho-Stat5 levels within Jak2+/VF and Jak2+/+ LSK cells using a phospho-specific Stat5 antibody (Figure 4B). Consistent with our colony-forming cell (CFC) data (described above), we observed no difference in basal Stat5 phosphorylation signaling after serum starvation between Jak2+/VF and Jak2+/+ LSK cells. Similarly, there was no statistically significant difference in Stat5 activation between these populations in response to stimulation with EPO plus IL3 (Figure 4C). We also assessed Stat5 activation in Jak2+/VF or Jak2+/+ LSK cells in response to low dose EPO and IL3 stimulation and obtained similar results to those obtained at the higher doses (Figure S4C). As expected, Stat5 activation after cytokine stimulation was inhibited by in vitro treatment with the JAK2 inhibitor TG101348 (300nM), although phospho-Stat5 did not return to basal levels under these experimental conditions (Figure 4B).
To further explore the functional consequences of Jak2V617F expression in the LSK compartment, and in view of the recently described role of JAK2 as an epigenetic regulator through phosphorylation of Tyr 41 (Y41) on histone H3 (Dawson et al., 2009), we analyzed Jak2+/VF or Jak2+/+ LSK cells by gene expression profiling. In a supervised analysis of Jak2+/VF versus Jak2+/+ LSK cells, no individual genes were significantly differentially expressed between the two states (FDR <0.05). Given that we observed expansion and erythroid skewing of the myeloid progenitor compartment of Jak2+/VF mice and that recipients of Jak2+/VF BM developed elevated HCT as soon as 3 weeks post transplantation (Figures 3A, 3B), we used gene set enrichment analysis (GSEA) (Subramanian et al., 2005) to compare murine myeloid progenitor signatures in Jak2+/VF or LSK Jak2+/+ cells. We observed that MEP, CMP and GMP differentiation signatures (Krivtsov et al., 2006) were significantly enriched in Jak2+/VF LSK cells (Table S1, Figure S4D). We also found significant enrichment of erythroid and megakaryocytic progenitor differentiation signatures (Pronk et al., 2007) in Jak2+/VF LSK cells (Table S1). These findings indicate that although the Jak2V617F mutation does not expand LSK cell numbers it directs hematopoietic differentiation within the LSK compartment.
Jak2V617F does not confer a significant competitive advantage to LSK cells
Our observations on LSK cell number and gene expression; cell cycle and Stat5 signaling indicate that in aggregate, physiologic expression of the Jak2V617F allele has quite modest effects on the LSK compartment.
To further assess LSK function and to determine whether the Jak2V617F allele conferred a selective advantage to Jak2+/VF LSK cells when compared with Jak2+/+ LSK cells, we performed competitive transplantation experiments. We transplanted LSK cells into lethally irradiated congenic recipients using the following Jak2+/VF to Jak2+/+ ratios: 75:25, 50:50 or 25:75, respectively, in combination with 250,000 WT supportive BM cells. All recipient groups developed HCT > 55% that was sustained over 16 weeks, all developed the same degree of expansion of CD71+ Ter119+ cells in the spleen and all demonstrated the same degree of splenomegaly (Figures S4E – S4G). LSK cell chimerism in the BM at 16 weeks showed that the ratios of Jak2+/VF to Jak2+/+ LSK cells were mildly increased from the input Jak2+/VF to Jak2+/+ ratios in all groups (Figure 4D). Peripheral blood myeloid chimerism demonstrated similar results to LSK chimerism (Figure 4E) and there was no significant difference in the percentage of peripheral blood Mac1+ Gr1+ myeloid cells between the groups (Figure S4H). These data demonstrate that Jak2V617F confers at most, a small selective competitive advantage on the HSC-enriched LSK population and that the presence of even a minority of Jak2+/VF LSK cells in the BM is sufficient to cause a marked expansion of erythroid precursors in the spleen and the development of a PV phenotype.
Inhibiting JAK2 kinase does not eradicate the MPN-initiating population
Together, these data indicate that Jak2V617F has nominal effects on the size or function of the LSK compartment. A clinically relevant prediction of these observations is that inhibition of Jak2V617F might be expected to have minimal effects on this compartment. If this hypothesis were correct, it could have implications for this population as a resistant reservoir of MPN-initiating cells – and for the efficacy of JAK2 inhibitors as curative rather than remitting therapy.
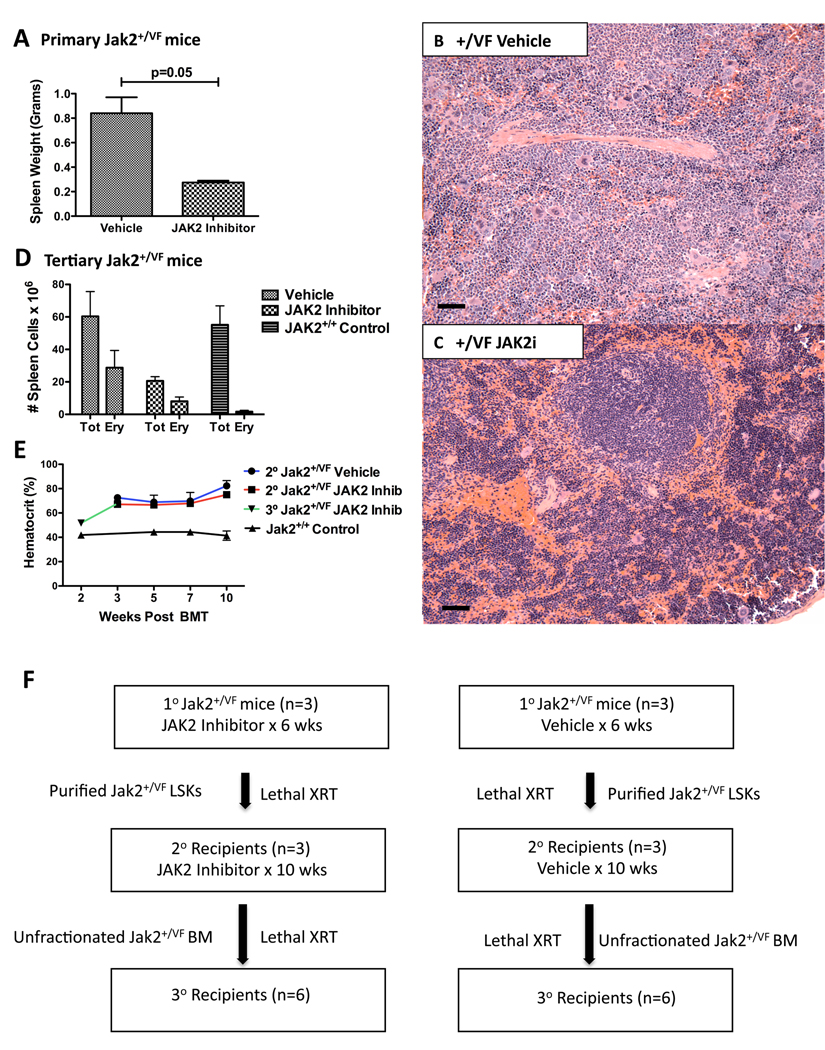
To first evaluate if Jak2+/VF mice respond to treatment with a JAK2 inhibitor, we treated primary mice with the JAK2 kinase inhibitor, TG101348 (Wernig et al., 2008) or vehicle for 6 weeks by oral gavage (60mg/kg twice daily). Primary Jak2+/VF mice responded to treatment with a statistically significant reduction in spleen size (Figure 5A) and histopathological improvement of the erythroid hyperplasia in the BM and spleen (Figures 5B, 5C), compared with mice treated with vehicle. HCT remained elevated in treated mice, possibly a reflection of the long life span of red blood cells. We also treated lethally irradiated congenic tertiary recipients of unfractionated Jak2+/VF BM with TG101348 for 6 weeks by oral gavage (60mg/kg twice daily). Tertiary recipients also responded to treatment and in addition to a statistically significant reduction in spleen size (Figure S4I), decreased erythroid precursors were observed in the spleens of TG101348 treated mice, compared with tertiary recipient mice treated with vehicle (Figure 5D).
Figure 5. JAK2 kinase inhibition does not eradicate the MPN-initiating population.
(A) Composite data from primary Jak2+/VF age-matched littermates treated with vehicle or the JAK2 inhibitor, TG101348 for 6 weeks, demonstrating reduced spleen weight in mice treated with TG101348 as compared with those treated with vehicle (unpaired two-tailed t test; mean +/− SEM; n=3 in each group).
(B – C) Histopathologic H & E sections of spleen from representative primary Jak2+/VF mice treated with vehicle or JAK2 inhibitor, TG101348 for 6 weeks, demonstrating complete effacement of normal splenic architecture in vehicle treated mice and improvement towards normal in TG101348 treated mice (SPL: [scale bars 250µM]).
(D) Composite data from tertiary Jak2+/VF recipients treated with vehicle or TG101348 for 6 weeks and from untreated Jak2+/+ controls, demonstrating reduced splenic cellularity and reduced number of CD71+ Ter119+ erythroid precursor cells in mice treated with TG101348 (mean +/−SEM; n=4 in each group; Tot = Total; Ery = Erythroid).
(E) HCTs of lethally irradiated secondary recipients of purified Jak2+/VF LSKs from mice treated with vehicle or JAK2 inhibitor, TG101348 for 6 weeks pre-transplant and resumed 3 weeks post transplantation. HCTs of lethally irradiated secondary recipients of purified Jak2+/+ control LSKs, measured 3–10 weeks post transplantation also shown. HCTs of tertiary recipients of unfractionated Jak2+/VF BM from treated secondary recipients measured 3 weeks post transplantation are also shown (mean +/−SEM; n=3 in each group for secondary recipients; n=6 for tertiary recipients).
(F) Schema outlining schedule for primary, secondary and tertiary Jak2+/VF mice with treated with TG101348 or vehicle.
To assess the effect of Jak2 inhibition on the MPN-initiating cell population, we performed serial transplantation experiments (see Figure 5F for schema). We first purified LSK cells from primary Jak2+/VF mice that had been treated with TG101348 or vehicle for 6 weeks by oral gavage (60mg/kg twice daily), and transplanted equal numbers of cells into lethally irradiated congenic secondary recipients. Three weeks later, blood counts demonstrated full hematopoietic reconstitution, with elevated HCT in all secondary recipient mice (Figure 5E), demonstrating that the MPN-initiating population was not eliminated in primary treated mice. To investigate if treatment for a longer duration could eradicate the disease-initiating population, TG101348 treatment was initiated in secondary recipients of LSK cells from a TG101348 treated primary mouse and vehicle treatment was initiated in secondary recipients of LSK cells from a vehicle treated primary mouse. Both the treatment and vehicle groups showed sustained elevation in HCT (Figure 5E). After 10 weeks of treatment with TG101348, unfractionated BM was then transplanted from treated secondary recipients into lethally irradiated congenic tertiary recipients and at 3 weeks post transplantation these tertiary recipients also demonstrated elevated HCT (Figure 5E), indicating that the MPN-initiating population was not eliminated in secondary treated mice.
In aggregate, these data indicate that although the JAK2 kinase inhibitor, TG101348 demonstrated therapeutic efficacy against serially transplanted Jak2V617F evoked MPN with regard to reduction in spleen size and in erythroid precursor cell number, treatment with the drug did not eliminate the MPN-initiating population in vivo.
DISCUSSION
Eradicating the disease-initiating cells within a tumor is the definitive goal of curative cancer therapy. In murine models of leukemia, the cell populations that possess the capacity to initiate disease vary depending on the oncogene driving the leukemia (Cleary, 2009). In general, murine MPN models have been limited in their ability to address questions pertaining to the MPN-initiating cell population due to poor transplantability of the primary disease (Chan et al., 2004), (Braun et al., 2004), (Lee et al., 2007), (Chan et al., 2009). We report a murine knock-in model in which Jak2V617F is expressed from its endogenous promoter, and that the MPN that arises is serially transplantable. We demonstrate the presence of at least two distinct cellular subfractions that are essential for MPN pathogenesis. The Jak2V617F LSK population is enriched for disease-initiating activity but has few phenotypic attributes that distinguish it from a wild-type LSK population. In contrast, Jak2V617F MEP cells are expanded in number and drive the disease phenotype in vivo, but do not transplant the MPN. Concordantly, treatment with a JAK2 inhibitor attenuates the disease phenotype, but the MPN-initiating population retains its ability to re-constitute disease.
The Jak2V617F knock-in murine model we report was engineered to faithfully reproduce the human situation, and the murine phenotype closely recapitulates many of the features of human PV. Jak2+/VF MEPs exhibit hypersensitivity to EPO stimulation as manifested by enhanced growth of erythroid colonies in methylcellulose media supplemented with low dose EPO but do not demonstrate endogenous erythroid colony formation. These results are consistent with in vitro studies of primary human PV patient samples (Jamieson et al., 2006), (Dupont et al., 2007). The absence of reticulin fibrosis in this model is compatible with the existence of strain-specific and micro-environmental modifiers of fibrosis. This phenotype is more prominent in Balb/c mice in the retroviral JAK2V617F model (Wernig et al., 2006) and rapidly progressive BM fibrosis is seen in the TEL-JAK2 NOD/SCID model, in which JAK2 is activated as a result of a chromosomal translocation (Kennedy et al., 2006). Our transplantation experiments demonstrate that the development of _Jak2V617F_–evoked MPN is cell autonomous, consistent with the finding that the JAK2V617F mutation is not detectable in the stroma of patients with JAK2V617F positive MPN (Pieri et al., 2008), (Mercier et al., 2009).
Our preliminary observations suggest that lethality of the MPN in this model may have been related to thrombotic events. Animals died suddenly, precluding detailed histopathologic analysis, but among a small cohort of animals who had limited necropsy, gangrenous bowel was observed that would be consistent with thrombosis. We also note that thrombotic events were not observed in retroviral transduction or transgenic JAK2V617F models (Wernig et al., 2006), (Lacout et al., 2006), (Zaleskas et al., 2006), (Tiedt et al., 2008), (Xing et al., 2008), (Shide et al., 2008), and that cardiac thrombosis was indicated as a cause of death in homozygous Jak2V617F expressing animals in the recently published conditional knock-in model of Akada et al (Akada et al.). Collectively, these data suggest the possibility that physiologic expression of the Jak2V617F allele from the endogenous Jak2 promoter may enable preclinical studies of thrombosis in MPN.
JAK2 was recently identified to have a previously unrecognized nuclear role, directly phosphorylating Tyr 41 (Y41) on histone H3 and preventing binding of the transcriptional repressor, heterochromatin protein 1∝ (HP1∝) to H3 (Dawson et al., 2009). We did not find any significantly differentially expressed genes within the LSK compartment of Jak2+/VF mice, indicating that endogenous heterozygous Jak2V617F expression has minimal effects on global HSPC gene expression. Microarray profiling of Jak2+/VF LSKs demonstrated a robust enrichment of myeloid progenitor differentiation signatures including those derived from MEP (Krivtsov et al., 2006) and Pre-CFU-E cells (Pronk et al., 2007) respectively, indicating that Jak2V617F instructs HSC differentiation, consistent with what has been observed in vitro in HSCs from PV patients (Jamieson et al., 2006). Our demonstration that Jak2+/VF LSK cells have little selective advantage over Jak2+/+ LSK cells in competitive transplant experiments are in keeping with NOD/SCID models of JAK2V617F PV, in which the majority of SCID repopulating cells (SRCs) are wild type cells and transformed SRCs have no proliferative advantage over normal SRC (James et al., 2008). Moreover, human patients with ET, who are heterozygous for the JAK2V617F mutation, can maintain a stable JAK2V617F clone over years (Gale et al., 2007).
As we could not generate homozygote Jak2V617F expressing mice in this model we were unable to address the impact of allelic dosage on disease phenotype. However, given that the phenotype of heterozygote Jak2V617F expressing mice is more consistent with PV than ET it may be that additional somatic mutations and/or host modifiers (Pardanani et al., 2008) contribute to MPN phenotypic pleiotropy. In support of this hypothesis, another conditional Jak2V617F knock-in model was published during the course of the preparation of this manuscript, in which both heterozygote and homozygote mice demonstrated a similar PV phenotype, with more severe disease observed in the homozygote animals (Akada et al.). We observe minor phenotypic differences between the models, including the development of thrombocytosis, mild splenic reticulin fibrosis and expansion of the LSK population that we did not see in heterozygote Jak2V617F expressing mice in our study. These differences may be explained in part by the use of Mx1Cre recombinase by Akada et al., requiring the administration of polyinosinic-polycytidylic acid (pIpC) for induction, since it has recently been demonstrated that interferon-∝ can cause proliferation of HSCs in vivo (Essers et al., 2009), (Sato et al., 2009).
JAK2 small molecule inhibitors are currently in early phase clinical trials in patients with advanced myelofibrosis (Pardanani et al., 2009) (Verstovsek et al., 2009b). Although treated patients have generally been noted to experience rapid, marked decreases in spleen size and hematological responses, these findings have not been consistently associated with a reduction in JAK2V617F allelic burden (Verstovsek et al., 2008). Furthermore, patients without JAK2 mutations who were enrolled in these trials have also demonstrated clinical responses, indicating that clonal JAK2V617F positive disease is not being selectively targetted by these compounds (Verstovsek, 2009). The clinical trial results are consistent with our study, where we observed a statistically significant reduction in spleen weight and reduced erythroid precursor cell numbers in Jak2+/VF mice treated with TG101348 but this was not associated with elimination of the MPN-initiating population. While we cannot rule out that treatment with a higher dose of TG101348 or for a longer duration could potentially diminish or possibly even eliminate the MPN initiating population, we observed clear evidence of differential sensitivity to JAK2 kinase inhibition in vivo between different Jak2+/VF cellular compartments, with JAK2 selective effects on Jak2+/VF progenitors as compared with Jak2+/VF LSKs. Furthermore, our demonstration that Jak2V617F has modest effects on LSK phenotype suggest that JAK2 inhibitors regardless of efficacy, dose or treatment duration are unlikely to have a curative therapeutic index in JAK2V617F mediated MPN.
The therapeutic eradication of MPN-initiating cells will require insights into the differential molecular circuitry of normal and JAK2V617F mutant stem cells. Maintenance of JAK2V617F disease-initiating cells may require normal self-renewal pathways in a manner analogous to the dependence of CML stem cells on the Wnt-β-catenin (Zhao et al., 2007) and Hedgehog signaling pathways (Dierks et al., 2008), (Zhao et al., 2009). However unlike chronic phase CML, which appears to be a mono-allelic neoplasm driven by BCR-ABL, there is accumulating evidence that additional genetic lesions occur within the HSC compartment of BCR-ABL negative MPN. At least 50% patients with a JAK2V617F positive MPN transform to a JAK2V617F negative acute leukemia, suggesting that JAK2V617F may not be the disease-initiating mutation in many cases of MPN (Campbell et al., 2006), (Theocharides et al., 2007). Recently, inactivating acquired mutations in TET2 (Delhommeau et al., 2009), ASXL1 (Gelsi-Boyer et al., 2009) and IDH1/2 (Abdel-Wahab et al.), (Green and Beer) have been identified in multipotent MPN progenitors. CD34+ HSCs with mutations in TET2 in addition to the JAK2V617F mutation show an increased capacity over JAK2V617F single-mutant CD34+ HSCs to repopulate NOD-SCID mice (Delhommeau et al., 2009) and both TET2 and IDH1/2 mutations have been associated with leukemic transformation in MPN (Abdel-Wahab et al.), (Green and Beer), suggesting that these loss of function mutations may enhance HSC self-renewal. Little is known about the function of TET2, but it was recently shown that TET1 has an enzymatic function in epigenetic regulation (Tahiliani et al., 2009). Identifying strategies to target TET2 therapeutically in the HSC compartment will require the development of pre-clinical genetic murine models that integrate TET2 loss into the Jak2V617F context. Such models will enable the interrogation of the therapeutic susceptibilities of MPN alleles within different hematopoietic compartments.
In conclusion, we report a Jak2V617F heterozygote knock-in mouse model that develops a MPN resembling human PV. We found that the LSK compartment contained the MPN-initiating cell population but that Jak2V617F had modest effects on the size of the LSK compartment or it’s subpopulations; and on cell cycle, signal transduction or gene expression of LSK cells. Furthermore, in functional studies employing competitive repopulation assays Jak2V617F did not confer a significant competitive advantage to the LSK compartment. Concordant with this, the disease-initiating population was not eradicated with therapeutic dosing of a JAK2 kinase inhibitor. This model will be valuable in evaluating the curative potential of MPN therapies. Effective elimination of MPN disease-initiating cells will require insights into the differential molecular dependencies of normal and JAK2V617F mutant HSCs.
EXPERIMENTAL PROCEDURES
Generation of Jak2V617F knock-in mice
The Jak2V617F knock-in construct was generated using the recombineering method (Liu et al., 2003), (Copeland et al., 2001) (see Supplemental Information for full details). Exposure to Cre recombinase results in inversion of the mutant exon followed by an excision reaction that removes the WT exon, one LoxP site, and one LoxP511 site, fixing the inversion in place (Fig 1A). All results described pertain to Jak2V617F germline expressing heterozygous mice. We did not obtain any viable germline homozygous Jak2V617F expressing animals and as a result we believe germline homozygosity for Jak2V617F to be embryonically lethal. We were unable to generate viable homozygous floxed animals (Jak2Fl/Fl) as the targeting construct appeared to interfere with WT Jak2 expression such that Jak2Fl/Fl functioned as a Jak2 null which is known to be embryonically lethal (Parganas et al., 1998), (Neubauer et al., 1998). Heterozygous floxed mice (Jak2+/Fl) had no phenotype. All experiments were conducted using an IUCAC-approved animal protocol at our institution.
Survival analysis
Jak2+/VF or age matched Jak2+/+ control mice were monitored biweekly and were sacrificed when visibly ill (hunched posture, weight loss). Statistical analysis was performed using Prism 5 software (GraphPad, San Diego, CA) using the Kaplan-Meier method.
Histopathology
Tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin or with reticulin, to assess for fibrosis. Images of histological slides were obtained on a Nikon Eclipse E400 microscope (Nikon, Tokyo, Japan) equipped with a SPOT RT color digital camera model 2.1.1 (Diagnostic Instruments, Sterling Heights, MI).
Flow Cytometry
BM cells were isolated from age-matched littermates between 1–6 months of age backcrossed to C57BL/6 and were flushed from hind leg bones with PBS (GIBCO) + 2% FBS + Penicillin/Streptomycin (Cambrex, Biowhittaker), lysed on ice with red blood cell (RBC) lysis solution (Puregene), and washed in PBS (GIBCO) + 2% FBS. Single-cell suspensions of spleen were prepared by pressing tissue through a cell strainer and lysed and washed in the same manner as BM. Cells were stained with monoclonal antibodies in 2%FBS/PBS for 30 min on ice.
HIGHLIGHTS
- Pre-clinical murine model of human PV in which MPN is serially transplantable
- Distinct cell populations responsible for disease initiation and MPN phenotype
- No significant selective competitive advantage for _Jak2V617F_–expressing HSCs
- Treatment with a JAK2 inhibitor did not eradicate MPN-initiating population
Supplementary Material
01
ACKNOWLEDGEMENTS
We gratefully acknowledge transgenic core facilities supported by NIH P30 DK49216, NIDDK Centers of Excellence in Hematology for generating chimeric mice and TargeGen Inc. (San Diego, CA, USA) for supplying TG101348. We warmly thank Drs. Ross Levine and Demetrios Kalaitzidis for critically reviewing the manuscript and all members of the Gilliland and Ebert laboratories for their insights and collegiality.
This work was supported with funding from the Myeloproliferative Disorders Foundation (Chicago, IL); R01 HL082950 (NIH/NHLBI) and P01 CA 66996-11 (NIH/NCI). A.M. has received T32HL0763 funding (NIH/NHLBI); support from the Jeanne D. Housman Fund for Research on Myeloproliferative Disorders and is a 2010 ASH Scholar recipient.
Authorship Contributions: A.M., S.W.L., D.G.G., and B.L.E. designed experiments. A.M., S.W.L., B.B., C.M., R.O., and F.S. performed experiments and analyzed data. A.M. wrote the manuscript with assistance from S.W.L. All authors provided critical review of the manuscript.
B.L.E. has received research support from GlaxoSmithKline. D.G.G. is now an employee of Merck.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Accession Number
Gene expression data is available in GEO database with the accession number GSE21842.
See supplementary information for: Flow Cytometry; Megakaryocyte Ploidy Analysis; Cytokine Stimulation and Intracellular Phosphoprotein Analysis; Cell Cycle; Colony-Forming Assays; Gene Expression Profiling; Bone Marrow Transplantation; JAK2 Inhibitor Studies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
REFERENCES
- Abdel-Wahab O, Manshouri T, Patel J, Harris K, Yao J, Hedvat C, Heguy A, Bueso-Ramos C, Kantarjian H, Levine RL, Verstovsek S. Genetic analysis of transforming events that convert chronic myeloproliferative neoplasms to leukemias. Cancer Res. 70:447–452. doi: 10.1158/0008-5472.CAN-09-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akada H, Yan D, Zou H, Fiering S, Hutchison RE, Mohi MG. Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood. doi: 10.1182/blood-2009-04-215848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- Braun BS, Tuveson DA, Kong N, Le DT, Kogan SC, Rozmus J, Le Beau MM, Jacks TE, Shannon KM. Somatic activation of oncogenic Kras in hematopoietic cells initiates a rapidly fatal myeloproliferative disorder. Proc Natl Acad Sci U S A. 2004;101:597–602. doi: 10.1073/pnas.0307203101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell PJ, Baxter EJ, Beer PA, Scott LM, Bench AJ, Huntly BJ, Erber WN, Kusec R, Larsen TS, Giraudier S, et al. Mutation of JAK2 in the myeloproliferative disorders: timing, clonality studies, cytogenetic associations, and role in leukemic transformation. Blood. 2006;108:3548–3555. doi: 10.1182/blood-2005-12-013748. [DOI] [PubMed] [Google Scholar]
- Chan G, Kalaitzidis D, Usenko T, Kutok JL, Yang W, Mohi MG, Neel BG. Leukemogenic Ptpn11 causes fatal myeloproliferative disorder via cell-autonomous effects on multiple stages of hematopoiesis. Blood. 2009;113:4414–4424. doi: 10.1182/blood-2008-10-182626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan IT, Kutok JL, Williams IR, Cohen S, Kelly L, Shigematsu H, Johnson L, Akashi K, Tuveson DA, Jacks T, Gilliland DG. Conditional expression of oncogenic K-ras from its endogenous promoter induces a myeloproliferative disease. J Clin Invest. 2004;113:528–538. doi: 10.1172/JCI20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary ML. Regulating the leukaemia stem cell. Best Pract Res Clin Haematol. 2009;22:483–487. doi: 10.1016/j.beha.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland NG, Jenkins NA, Court DL. Recombineering: a powerful new tool for mouse functional genomics. Nat Rev Genet. 2001;2:769–779. doi: 10.1038/35093556. [DOI] [PubMed] [Google Scholar]
- Dawson MA, Bannister AJ, Gottgens B, Foster SD, Bartke T, Green AR, Kouzarides T. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009;461:819–822. doi: 10.1038/nature08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Masse A, Kosmider O, Le Couedic JP, Robert F, Alberdi A, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360:2289–2301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- Delhommeau F, Dupont S, Tonetti C, Masse A, Godin I, Le Couedic JP, Debili N, Saulnier P, Casadevall N, Vainchenker W, Giraudier S. Evidence that the JAK2 G1849T (V617F) mutation occurs in a lymphomyeloid progenitor in polycythemia vera and idiopathic myelofibrosis. Blood. 2007;109:71–77. doi: 10.1182/blood-2006-03-007146. [DOI] [PubMed] [Google Scholar]
- Dierks C, Beigi R, Guo GR, Zirlik K, Stegert MR, Manley P, Trussell C, Schmitt-Graeff A, Landwerlin K, Veelken H, Warmuth M. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14:238–249. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Dupont S, Masse A, James C, Teyssandier I, Lecluse Y, Larbret F, Ugo V, Saulnier P, Koscielny S, Le Couedic JP, et al. The JAK2 617V>F mutation triggers erythropoietin hypersensitivity and terminal erythroid amplification in primary cells from patients with polycythemia vera. Blood. 2007;110:1013–1021. doi: 10.1182/blood-2006-10-054940. [DOI] [PubMed] [Google Scholar]
- Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- Gale RE, Allen AJ, Nash MJ, Linch DC. Long-term serial analysis of X-chromosome inactivation patterns and JAK2 V617F mutant levels in patients with essential thrombocythemia show that minor mutant-positive clones can remain stable for many years. Blood. 2007;109:1241–1243. doi: 10.1182/blood-2006-06-029769. [DOI] [PubMed] [Google Scholar]
- Gelsi-Boyer V, Trouplin V, Adelaide J, Bonansea J, Cervera N, Carbuccia N, Lagarde A, Prebet T, Nezri M, Sainty D, et al. Mutations of polycomb-associated gene ASXL1 in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br J Haematol. 2009;145:788–800. doi: 10.1111/j.1365-2141.2009.07697.x. [DOI] [PubMed] [Google Scholar]
- Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- Green A, Beer P. Somatic mutations of IDH1 and IDH2 in the leukemic transformation of myeloproliferative neoplasms. N Engl J Med. 362:369–370. doi: 10.1056/NEJMc0910063. [DOI] [PubMed] [Google Scholar]
- Holyoake T, Jiang X, Eaves C, Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–2064. [PubMed] [Google Scholar]
- Ishii T, Bruno E, Hoffman R, Xu M. Involvement of various hematopoietic-cell lineages by the JAK2V617F mutation in polycythemia vera. Blood. 2006;108:3128–3134. doi: 10.1182/blood-2006-04-017392. [DOI] [PubMed] [Google Scholar]
- James C, Mazurier F, Dupont S, Chaligne R, Lamrissi-Garcia I, Tulliez M, Lippert E, Mahon FX, Pasquet JM, Etienne G, et al. The hematopoietic stem cell compartment of JAK2V617F–positive myeloproliferative disorders is a reflection of disease heterogeneity. Blood. 2008;112:2429–2438. doi: 10.1182/blood-2008-02-137877. [DOI] [PubMed] [Google Scholar]
- James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- Jamieson CH, Gotlib J, Durocher JA, Chao MP, Mariappan MR, Lay M, Jones C, Zehnder JL, Lilleberg SL, Weissman IL. The JAK2 V617F mutation occurs in hematopoietic stem cells in polycythemia vera and predisposes toward erythroid differentiation. Proc Natl Acad Sci U S A. 2006;103:6224–6229. doi: 10.1073/pnas.0601462103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy JA, Barabe F, Patterson BJ, Bayani J, Squire JA, Barber DL, Dick JE. Expression of TEL-JAK2 in primary human hematopoietic cells drives erythropoietin-independent erythropoiesis and induces myelofibrosis in vivo. Proc Natl Acad Sci U S A. 2006;103:16930–16935. doi: 10.1073/pnas.0604902103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- Krivtsov AV, Twomey D, Feng Z, Stubbs MC, Wang Y, Faber J, Levine JE, Wang J, Hahn WC, Gilliland DG, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 2006;442:818–822. doi: 10.1038/nature04980. [DOI] [PubMed] [Google Scholar]
- Lacout C, Pisani DF, Tulliez M, Gachelin FM, Vainchenker W, Villeval JL. JAK2V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006;108:1652–1660. doi: 10.1182/blood-2006-02-002030. [DOI] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci U S A. 1996;93:5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Tothova Z, Levine RL, Anderson K, Buza-Vidas N, Cullen DE, McDowell EP, Adelsperger J, Frohling S, Huntly BJ, et al. FLT3 mutations confer enhanced proliferation and survival properties to multipotent progenitors in a murine model of chronic myelomonocytic leukemia. Cancer Cell. 2007;12:367–380. doi: 10.1016/j.ccr.2007.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Loriaux M, Huntly BJ, Loh ML, Beran M, Stoffregen E, Berger R, Clark JJ, Willis SG, Nguyen KT, et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood. 2005a;106:3377–3379. doi: 10.1182/blood-2005-05-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ, Boggon TJ, Wlodarska I, Clark JJ, Moore S, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005b;7:387–397. doi: 10.1016/j.ccr.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–484. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Levine R, Tong W, Wernig G, Pikman Y, Zarnegar S, Gilliland DG, Lodish H. Expression of a homodimeric type I cytokine receptor is required for JAK2V617F–mediated transformation. Proc Natl Acad Sci U S A. 2005;102:18962–18967. doi: 10.1073/pnas.0509714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Monczak Y, Francois M, Prchal J, Galipeau J. Bone marrow mesenchymal stromal cells of patients with myeloproliferative disorders do not carry the JAK2-V617F mutation. Exp Hematol. 2009;37:416–420. doi: 10.1016/j.exphem.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Michor F, Hughes TP, Iwasa Y, Branford S, Shah NP, Sawyers CL, Nowak MA. Dynamics of chronic myeloid leukaemia. Nature. 2005;435:1267–1270. doi: 10.1038/nature03669. [DOI] [PubMed] [Google Scholar]
- Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- Pardanani A, Fridley BL, Lasho TL, Gilliland DG, Tefferi A. Host genetic variation contributes to phenotypic diversity in myeloproliferative disorders. Blood. 2008;111:2785–2789. doi: 10.1182/blood-2007-06-095703. [DOI] [PubMed] [Google Scholar]
- Pardanani A, Gotlib J, Jamieson CH, Cortes JMT, Stone R, Silverman M, Shorr J, Gilliland DG, Tefferi A. A Phase I Evaluation of TG101348, a Selective JAK2 Inhibitor, in Myelofibrosis: Clinical Response Is Accompanied by Significant Reduction in JAK2V617F Allele Burden. Blood. 2009;114 Abstract 755. [Google Scholar]
- Parganas E, Wang D, Stravopodis D, Topham DJ, Marine JC, Teglund S, Vanin EF, Bodner S, Colamonici OR, van Deursen JM, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- Pieri L, Guglielmelli P, Bogani C, Bosi A, Vannucchi AM. Mesenchymal stem cells from JAK2(V617F) mutant patients with primary myelofibrosis do not harbor JAK2 mutant allele. Leuk Res. 2008;32:516–517. doi: 10.1016/j.leukres.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Pronk CJ, Rossi DJ, Mansson R, Attema JL, Norddahl GL, Chan CK, Sigvardsson M, Weissman IL, Bryder D. Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell. 2007;1:428–442. doi: 10.1016/j.stem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Ren R. Modeling the dosage effect of oncogenes in leukemogenesis. Curr Opin Hematol. 2004;11:25–34. doi: 10.1097/00062752-200401000-00005. [DOI] [PubMed] [Google Scholar]
- Sato T, Onai N, Yoshihara H, Arai F, Suda T, Ohteki T. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat Med. 2009;15:696–700. doi: 10.1038/nm.1973. [DOI] [PubMed] [Google Scholar]
- Shide K, Shimoda HK, Kumano T, Karube K, Kameda T, Takenaka K, Oku S, Abe H, Katayose KS, Kubuki Y, et al. Development of ET, primary myelofibrosis and PV in mice expressing JAK2 V617F. Leukemia. 2008;22:87–95. doi: 10.1038/sj.leu.2405043. [DOI] [PubMed] [Google Scholar]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- Steensma DP, Dewald GW, Lasho TL, Powell HL, McClure RF, Levine RL, Gilliland DG, Tefferi A. The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both "atypical" myeloproliferative disorders and myelodysplastic syndromes. Blood. 2005;106:1207–1209. doi: 10.1182/blood-2005-03-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theocharides A, Boissinot M, Girodon F, Garand R, Teo SS, Lippert E, Talmant P, Tichelli A, Hermouet S, Skoda RC. Leukemic blasts in transformed JAK2-V617F–positive myeloproliferative disorders are frequently negative for the JAK2-V617F mutation. Blood. 2007;110:375–379. doi: 10.1182/blood-2006-12-062125. [DOI] [PubMed] [Google Scholar]
- Tiedt R, Hao-Shen H, Sobas MA, Looser R, Dirnhofer S, Schwaller J, Skoda RC. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood. 2008;111:3931–3940. doi: 10.1182/blood-2007-08-107748. [DOI] [PubMed] [Google Scholar]
- Verstovsek S. Therapeutic potential of JAK2 inhibitors. Hematology Am Soc Hematol Educ Program. 2009:636–642. doi: 10.1182/asheducation-2009.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstovsek S, Kantarjian H, Mesa R, Cortes J, Pardanani A, Thomas D, Estrov Z, Bradley E, Erickson-Viitanen S, Vaddi K, et al. Long-Term Follow up and Optimized Dosing Regimen of INCB018424 in Patients with Myelofibrosis: Durable Clinical, Functional and Symptomatic Responses with Improved Hematological Safety. Blood. 2009b;114 [Google Scholar]
- Verstovsek S, Kantarjian H, Pardanani A, Burn T, Vaddi K, Redman J, Bradley E, Levy R, Friedman S, Hollis G, Tefferi A. Characterization of JAK2 V617F Allele Burden in Advanced Myelofibrosis (MF) Patients: No Change in V617F:WT JAK2 Ratio in Patients with High Allele Burdens despite Profound Clinical Improvement Following Treatment with the JAK Inhibitor, INCB018424. Blood. 2008;112 Abstract 2802. [Google Scholar]
- Wernig G, Kharas MG, Okabe R, Moore SA, Leeman DS, Cullen DE, Gozo M, McDowell EP, Levine RL, Doukas J, et al. Efficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F–induced polycythemia vera. Cancer Cell. 2008;13:311–320. doi: 10.1016/j.ccr.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Wernig G, Mercher T, Okabe R, Levine RL, Lee BH, Gilliland DG. Expression of Jak2V617F causes a polycythemia vera-like disease with associated myelofibrosis in a murine bone marrow transplant model. Blood. 2006;107:4274–4281. doi: 10.1182/blood-2005-12-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing S, Wanting TH, Zhao W, Ma J, Wang S, Xu X, Li Q, Fu X, Xu M, Zhao ZJ. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood. 2008;111:5109–5117. doi: 10.1182/blood-2007-05-091579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaleskas VM, Krause DS, Lazarides K, Patel N, Hu Y, Li S, Van Etten RA. Molecular pathogenesis and therapy of polycythemia induced in mice by JAK2 V617F. PLoS One. 2006;1:e18. doi: 10.1371/journal.pone.0000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, Lagoo A, Reya T. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Chen A, Jamieson CH, Fereshteh M, Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01