LP-211 is a brain penetrant selective agonist for the serotonin 5-HT7 receptor (original) (raw)
. Author manuscript; available in PMC: 2011 Aug 30.
Abstract
We have determined the pharmacological profile of the new serotonin 5-HT7 receptor agonist _N_-(4-cyanophenylmethyl)-4-(2-diphenyl)-1-piperazinehexanamide (LP-211). Radioligand binding assays were performed on a panel of 5-HT receptor subtypes. The compound was also evaluated in vivo by examining its effect on body temperature regulation in mice lacking the 5-HT7 receptor (5-HT7−/−) and their 5-HT7+/+ sibling controls. Disposition studies were performed in mice of both genotypes. It was found that LP-211 was brain penetrant and underwent metabolic degradation to 1-(2-diphenyl)piperazine (RA-7). In vitro binding assays revealed that RA-7 possessed higher 5-HT7 receptor affinity than LP-211 and a better selectivity profile over a panel of 5-HT receptor subtypes. In vivo it was demonstrated that LP-211, and to a lesser degree RA-7, induced hypothermia in 5-HT7+/+ but not in 5-HT7−/− mice. Our results suggest that LP-211 can be used as a 5-HT7 receptor agonist in vivo.
Keywords: serotonin 5-HT7 receptor, agonist, LP-211, RA-7, brain distribution, body temperature
The 5-HT7 receptor has been cloned from mouse, rat, guinea-pig, pig and human and the binding profile is consistent across species and between cloned and native receptors [1–3]. 5-HT7 receptors are defined pharmacologically by their high affinity for 5-CT (5-carboxamidotryptamine), 5-HT (5-hydroxytryptamine), 5-MeOT (5-methoxytryptamine) and methiothepin, moderate affinity for 8-OH-DPAT (8-hydroxy-N,_N_-dipropylaminotetraline) and ritanserin and low affinity for pindolol, sumatriptan and buspirone [4].
In brain tissue, 5-HT7 receptor mRNA is localized within the thalamus, hypothalamus, limbic and cortical regions [4–6]. Neurochemical lesioning indicate that, at least in the hypothalamus, they are located post-synaptically [7]. Autoradiography has shown a pattern of 5-HT7 binding sites in the brain [4,8,9] which matches, to a large extent, the mRNA distribution.
Studies using selective antagonists such as SB-258719 ((R)-3,_N_-dimethyl-N_-[1-methyl-3-(4-methylpiperidin-1-yl)propyl]benzene sulfonamide) and SB-269970 ((2_R)-1-[(3-hydroxyphenyl)sulfonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl]pyrrolidine), as well as mice lacking the receptor, have suggested that targeting 5-HT7 receptors may have therapeutic applications in depression [3] and may play a role in cognitive processes [10,11].
In recent years considerable efforts have been focused on the development of selective 5-HT7 receptor agonists and antagonists [12,13]. The first selective antagonist, SB-258719, was identified in 1997 by GlaxoSmithKline, who subsequently identified SB-269970 that is considered to date the standard selective 5-HT7 receptor antagonist [14]. However, further studies with SB-269970 have been hampered by its rapid clearance in vivo. The antagonist SB-656104 ((6-((R)-2-[2-[4-(4-chloro-phenoxy)-piperidin-1-yl]-ethyl]-pyrrolidine-1-sulphonyl)-1H-indole) showed an improved pharmacokinetic profile, but displayed modest selectivity over 5-HT2A, 5-HT2B, and 5-HT1D receptors [15]. On the agonists side, the mixed 5-HT1A/5-HT7 ligand 8-OH-DPAT has been used to activate 5-HT7 receptors in the presence of the 5-HT1A receptor antagonist WAY 100635 (_N_-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N_-(2-pyridyl)cyclohexanecarboxamide) [10]. Recently characterization of the selective 5-HT7 agonists AS-19 ((2_S)-(+)-5-(1,3,5-trimethylpyrazol-4-yl)-2-(dimethylamino)tetralin) and E-55888 (dimethyl-{2-[3-(1,3,5-trimethyl-1H-pyrazol-4-yl)-phenyl]-ethyl}-amine dihydrochloride) has been reported [16]. We have recently developed a series of arylpiperazine derivatives as potential 5-HT7 receptor agonists [17]. Among these _N_-(4-cyanophenylmethyl)-4-(2-diphenyl)-1-piperazinehexanamide (LP-211, identified as compound 25 in [17]) displayed potent 5-HT7 receptor agonist activity while maintaining relatively low affinity for 5-HT1A and dopamine D2 receptors. In the present study we report on the in vitro and in vivo activity profile of LP-211 by studying radioligand binding, body temperature regulation in mice lacking the 5-HT7 receptor (5-HT7−/−) and their 5-HT7+/+ sibling controls, and pharmacokinetic disposition in these mice as well as in rats. Also, a similar pharmacological characterization was carried out on 1-(2-diphenyl)piperazine (RA-7), the main metabolite of LP-211.
_K_i determinations were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program under the direction of Bryan L. Roth and Jamie Driscoll (Contract # NO1MH32004, NIMH PDSP).
Competition binding assays were performed using transfected or stably expressing cell membrane preparations as previously described [18]. Radioligand identity, radioligand concentration, and _K_i values of reference compounds are listed in Table 1. Incubation buffer was 50 mM Tris HCl, 10 mM MgCl2, 0.1 mM EDTA, pH 7.4 in all cases, except SERT where 50 mM Tris HCl, 150 mM NaCl, 5 mM KCl, pH 7.4 was used. In all cases the incubation time was 1.5 h. Additional information is available online (http://pdsp. med.unc.edu/UNC-CH%20Protocol%20Book.pdf).
Table 1.
_K_i estimates for LP-211 and RA-7 at a panel of human 5-HT receptors and at human SERT
| Target | _K_i (nM) | Radioligand | Reference Compound (_K_i, nM) | |
|---|---|---|---|---|
| LP-211 | RA-7 | |||
| h5-HT7 | 15 ± 1 | 1.4 ± 0.2 | 1.1 nM [3H]LSD | Chlorpromazine (64) |
| h5-HT1A | 379 ± 118 | 99 ± 22 | 0.5 nM [3H]8-OH-DPAT | Methysergide (38) |
| h5-HT1B | 215 ± 10 | 1021 ± 121 | 0.29 nM [3H]GR125743 | Ergotamine (2.7) |
| h5-HT1D | 394 ± 74 | 302 ± 39 | 1.4 nM [3H]5-CT | Ergotamine (4.7) |
| h5-ht1E | >10,000 | >10,000 | 3 nM [3H]5-HT | 5-HT (7.5) |
| h5-HT2A | 626 ± 141 | 1190 ± 288 | 0.5 nM [3H]ketanserin | Chlorpromazine (9.6) |
| h5-HT2B | 67 ± 10 | 178 ± 19 | 1.0 nM [3H]LSD | Methysergide (2.4) |
| r5-HT2C | 91 ± 9 | 171 ± 14 | 0.5 nM [3H]mesulergine | Chlorpromazine (22) |
| h5-HT3 | >10,000 | 228 ± 34 | 0.29 nM [3H]LY278584 | LY278584 (1.7) |
| h5-ht5A | 178 ± 26 | 76 ± 14 | 0.99 nM [3H]LSD | Ergotamine (7) |
| h5-HT6 | 1571 ± 193 | 596 ± 70 | 0.97 nM [3H]LSD | Chlorpromazine (32) |
| hSERT | 812 ± 156 | 439 ± 41 | 0.79 nM [3H]citalopram | Amytriptyline (9.3) |
The effect of LP-211 and RA-7 on body temperature was studied using ten-to-twelve week old male 5-HT7−/− mice with their male 5-HT7+/+ siblings used as controls. The generation of the 5-HT7−/− mouse strain has been described previously [19]. The experiments were performed at The Scripps Research Institute (TSRI) where the mice were housed in a 12-hour light/dark cycle (lights on at 06.00 and off at 18.00) and had free access to water and food pellets. The experiments were done in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the US National Institutes of Health, and were approved by the Animal Care and Use Committee at TSRI. Every effort was made to reduce the number of animals used and to minimize potential suffering.
LP-211 (as tartrate salt), RA-7 (as hydrochloride salt) (both synthesized at the Dipartimento Farmaco-Chimico, Università degli Studi di Bari), WAY 100135 ((S)-N-tert-butyl-3-(4-(2-methoxyphenyl)-piperazin-1-yl)-2-phenylpropanamide), or SB-269970 were given as single intraperitoneal injections in a volume of 0.2 ml. WAY 100135 and SB-269970 were dissolved in 0.9% saline and administered 30 min prior to LP-211. LP-211 and RA-7 were dissolved in 100% ethanol to a 10× concentration and then diluted in saline immediately prior to injection, and 10% ethanol in saline was used as control. The doses used in this study were chosen based on published data [20,19,21].
Core body temperature was measured using a rectal thermometer probe as previously described [19,21]. A basal value was measured immediately before injections and then measurements were made 15 minutes after the injection and subsequently every 30 minutes for two hours. All experiments were started at 09.00 h to control for circadian variability in body temperature.
In disposition experiments different groups of 5-HT7+/+ and 5-HT7−/− mice were given LP-211 as above. Thirty minutes after injection, the mice were anaesthetized with an isoflurane overdose. Trunk blood was collected and allowed to coagulate. The brain was removed and stored at −80 °C until analysis. The blood was centrifuged and the serum was stored at −80 °C until analysis.
Compounds LP-211 and RA-7 were extracted from plasma and brain homogenate (distilled water, 1 g ·10 mL−1) and quantified by reversed-phase high-performance liquid chromatography with UV detection (230 nm), with slight modifications to the procedures previously described [17]. Analytes were identified on the basis of their retention time, 8.6 min for LP-211, 6.4 min for RA-7 and 10.9 min for the internal standard. Coefficient of variation (C.V.) for the precision and reproducibility of the assay at the lower limit of quantification (50 ng · mL−1 or g−1) was 15–20%. Higher concentrations gave C.V. generally less than 10%.
Values reported in Tables 1 and 2 and Figure 1 are expressed as means ± standard errors of mean (S.E.M.). Possible differences between genotypes and/or drug treatments were analyzed using two-way analysis of variance (ANOVA) with genotype as one variable and drug treatment as the other variable. The ANOVA was followed by an appropriate Bonferroni posttest. Differences were considered significant at P < 0.05.
Table 2.
Effects of LP-211 and its main metabolite RA-7 on body temperature in 5-HT7+/+ and 5-HT7−/− mice.
| Treatment | 5-HT7+/+ | 5-HT7−/− | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Basal value | Changes | n | Basal value | Changes | |||
| (°C) | Peak (%) | Overall (arbitrary units) | (°C) | Peak (%) | Overall (arbitrary units) | |||
| Vehicle | 14 | 34.91 ± 0.09 | −1.44 ± 0.33 | −21.83 ± 9.92 | 14 | 35.11 ± 0.07 | −1.00 ± 0.23 | −22.53 ± 6.73 |
| LP-211 (3 mg/kg) | 6 | 35.01 ± 0.04 | −0.76 ± 0.21 | −17.48 ± 5.14 | 6 | 34.98 ± 0.12 | −0.48 ± 0.30 | 0.12 ± 11.43 |
| LP-211 (10 mg/kg) | 8 | 35.03 ± 0.22 | −2.82 ± 1.03*** | −73.58 ± 27.99* | 8 | 35.03 ± 0.15 | −1.63 ± 0.84† | −31.08 ± 15.84 |
| LP-211 (30 mg/kg) | 8 | 34.99 ± 0.04 | −5.10 ± 0.80*** | −124.22 ± 33.58*** | 8 | 34.83 ± 0.12 | −1.85 ± 0.66*†† | −18.04 ± 14.86††† |
| WAY-100135 (10 mg/kg) | 5 | 35.02 ± 0.10 | −0.19 ± 0.34 | N/D | 5 | 34.96 ± 0.04 | −0.31 ± 0.32 | N/D |
| LP-211 (30 mg/kg) + WAY-100135(10 mg/kg) | 5 | 34.95 ± 0.06 | −6.45 ± 0.65*** | −211.62 ± 23.02*** | 5 | 34.85 ± 0.08 | −4.54 ± 0.62*** | −104.76 ± 21.54**†† |
| SB-269970 (10 mg/kg) | 5 | 35.04 ± 0.04 | −0.10 ± 0.15 | N/D | 5 | 35.00 ± 0.05 | 0.06 ± 0.21 | N/D |
| LP-211 (30 mg/kg) + SB-269970 (10 mg/kg) | 5 | 35.00 ± 0.02 | −2.21 ± 0.17 | −61.5 ± 7.92 | 5 | 35.02 ± 0.03 | −1.84 ± 0.18 | −62.57 ± 7.87 |
| RA-7 (3 mg/kg) | 6 | 34.97 ± 0.08 | 1.12 ± 0.16*** | 2.72 ± 8.41 | 6 | 34.99 ± 0.09 | 0.64 ± 0.13* | −9.28 ± 8.28 |
| RA-7 (10 mg/kg) | 11 | 35.26 ± 0.15 | −2.13 ± 0.49 | −65.88 ± 16.31*** | 10 | 35.24 ± 0.10 | −0.87 ± 0.54 | −23.79 ± 12.17 |
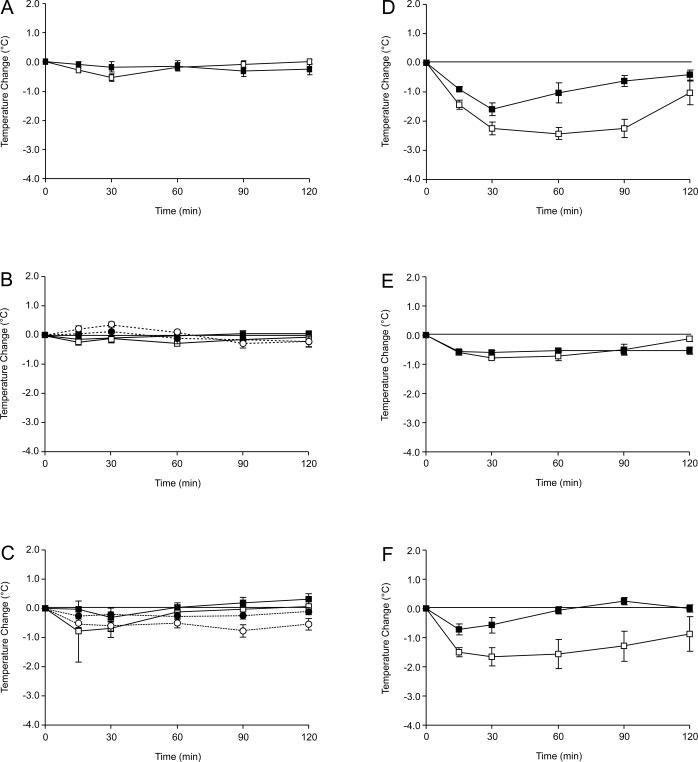
Fig. 1.
Effects of LP-211 (square symbols) and RA-7 (round symbols) on body temperature in 5-HT7+/+ (open symbols) and 5-HT7−/− mice (filled symbols). (A) vehicle (10% ethanol in saline); (B) LP-211 and RA-7, 3 mg · kg−1 i.p.; (C) LP-211 and RA-7, 10 mg · kg−1 i.p.; (D) LP-211, 30 mg · kg−1 i.p.; (E) LP-211 30 mg · kg−1 + SB-269970 10 mg · kg−1 i.p.; (F) LP-211 30 mg · kg−1 + WAY 100135 10 mg · kg−1 i.p. For basal values and statistical evaluation see Table 2.
The radioligand binding screening confirmed that LP-211 bound to 5-HT7 receptors with high affinity (_K_i = 15 nM). LP-211 was also at least 5-fold selective over a range of 5-HT receptor subtypes, and 54-fold selective over SERT. RA-7 also displayed high affinity for the 5-HT7 receptor (_K_i = 1.4 nM) and was > 54-fold selective over a range of 5-HT receptor subtypes and SERT (Table 1).
There was no difference in basal body temperature between 5-HT7+/+ and 5-HT7−/− mice (Table 2), and vehicle-treated animals maintained a stable body temperature which fluctuated by no more than 0.5 °C over the 2h recording period, regardless of genetic status (Fig. 1). LP-211 (3, 10, and 30 mg · kg−1, i.p.) dose-dependently reduced body temperature in wild-type mice with a peak effect at 15–30 min after the injection (Fig. 1). For the doses tested body temperature returned to base level within the registration period. Statistically, the effects of LP-211 and RA-7 were evaluated as peak change and as overall change (Table 2). The lowest dose of LP-211 (3 mg · kg−1) tested did not alter body temperature in either 5 HT7+/+ or 5 HT7−/− mice. At 10 mg · kg−1 LP-211 had a peak effect in 5-HT7+/+ mice, but not in 5-HT7−/− mice. There was also an overall hypothermic effect in the 5 HT7+/+ mice. At the highest dose tested (30 mg · kg−1), LP-211 had a significant effect in 5-HT7+/+ mice both when analyzing peak change and overall change. At this dose (30 mg · kg−1), LP-211 also had a significant, but less pronounced, effect on peak change in 5-HT7−/− mice.
The 5-HT7 receptor antagonist SB-269970 (10 mg · kg−1, i.p.) blocked the effect of LP-211 (30 mg · kg−1) on body temperature (Fig. 1E, Table 2). SB-269970 itself had no effect on body temperature. The 5-HT1A receptor antagonist WAY 100135 (10 mg · kg−1, i.p.) failed to counteract the hypothermic effect of LP-211 (Fig. 1F); it rather appeared to unmask other effects on body temperature, the cause of which remains to be determined.
The effect of RA-7 on body temperature was tested for 3 and 10 mg · kg−1 (Fig. 1B, C; Table 2). Interestingly, at 3 mg · kg−1 RA-7 had a slight but significant hyperthermic effect. At 10 mg · kg−1 RA-7 induced hypothermia to small degree that reached significance for overall change in the 5 HT7+/+ mice.
It should be noted that, apart from the hypothermia, LP-211 or RA-7 did not induce any visible adverse effects in the mice.
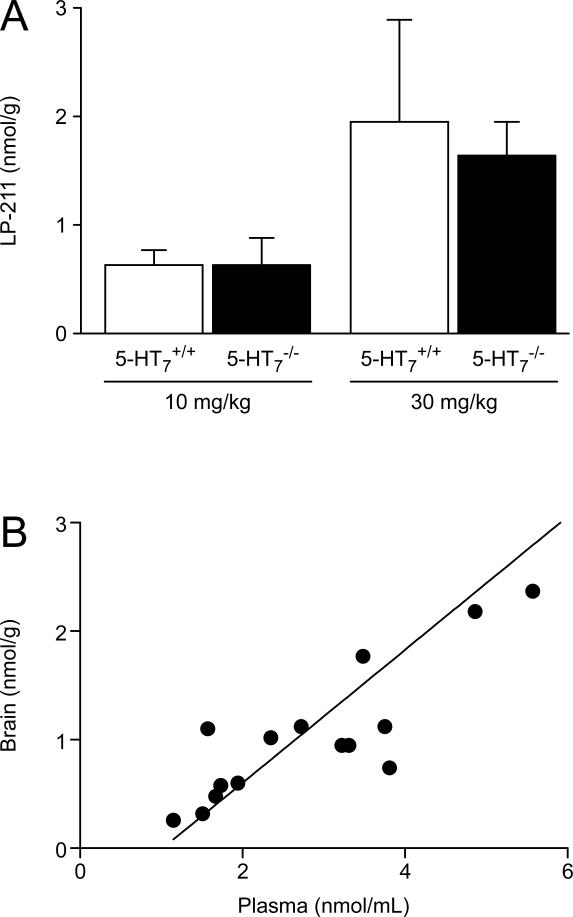
Other groups of 5-HT7−/− and 5-HT7+/+ mice were given 10 and 30 mg · kg−1 LP-211 i.p. and were killed 30 min thereafter to obtain basic information on the parent compound and metabolite concentrations achieved in plasma and brain. Plasma concentrations of LP-211 at approximately the time of peak effect on body temperature were essentially dose-related, ranging from 2.1 ± 0.3 and 1.8 ± 0.3 nmol · mL−1 at 10 mg · kg−1 to 4.5 ± 0.7 and 4.1 ± 0.5 at 30 mg · kg−1 in 5-HT7−/− and 5-HT7+/+ mice, respectively. Brain concentrations (Fig. 2A) reflected plasma concentrations with mean brain-to-plasma distribution ratios around 0.3–0.4, regardless of genotype. As shown in Fig. 2B there was a linear relationship between plasma and brain concentrations 30 min after i.p. doses of 10–30 mg · kg−1 in these mice (brain = −291 + 0.61 plasma; r2 = 0.78), indicating that LP-211 passes through the blood-brain barrier by passive diffusion and that plasma concentrations accurately predict whole brain concentrations.
Fig. 2.
Mean brain concentrations of LP-211 (A) and the relationship (individual animals) between brain and plasma concentrations (B) after intraperitoneal doses in 5-HT7−/− and 5-HT7+/+ mice. Values are mean ± S.D. for 5 mice per treatment group.
The metabolite RA-7 was undetectable in plasma and brain of 5-HT7−/− and 5-HT7+/+ mice 30 min after the lowest dose used in the LP-211-induced hypothermia test. It became detectable in the samples of some mice (n = 2–3 per group) at 30 mg · kg−1. At this time mean metabolite plasma concentrations approached only about 3–4% of LP-211 plasma concentrations, on a molar basis. Mean brain concentrations (about 0.3 nmol ·g−1 in both groups) were higher than plasma concentrations, in agreement with previous reports of concentrations of this [17] and other arylpiperazine derivatives in rodent brain [22]. Therefore, the metabolite-to-parent drug ratio was slightly higher in brain (about 0.5, on a molar basis) than in plasma, although varied largely among animals of both groups.
The compound LP-211 has recently been identified as a 5-HT7 receptor agonist [17]. It showed a _K_i value of 0.58 nM at rat cloned 5-HT7 receptors, and good selectivity (>300-fold) over the 5-HT1A receptor. LP-211 was characterized as a competitive agonist at the 5-HT7 receptor by measuring relaxation of substance P induced contraction in an isolated guinea pig ileum assay [17]. LP-211 behaved as a full agonist (82% maximal activity compared to 5-CT) with an EC50 value of 0.60 μM which is close to that of 5-CT (EC50 = 0.63 μM). The observed effect was reversed by the selective 5-HT7 receptor antagonist SB-269970. Disposition studies in mouse demonstrated that LP-211 rapidly entered the brain achieving whole-brain concentrations comparable to those in plasma. Like arylpiperazine derivatives in general [22], metabolism of LP-211 included _N_-dealkylation of the aliphatic chain attached to the piperazine nitrogen, resulting in the formation of 1-(2-diphenyl)piperazine (RA-7). This potentially active metabolite concentrated in mouse brain, yielding a mean metabolite-to-parent compound ratio of about 2 on a molar basis. Other 1-arylpiperazine's metabolites have similar pharmacokinetic behavior in rodents, concentrating in the brain more than their parent drug(s) and possibly contributing to or accounting for some of their central effects [22]. All these data prompted us to investigate further the in vitro binding profile of LP-211 and also its activity profile in a simple test (body temperature regulation) in mice lacking the 5-HT7 receptor (5-HT7−/−) and their 5-HT7+/+ sibling controls. The same evaluations were also performed on RA-7 to assess its possible contribution to the in vivo activity of LP-211.
As illustrated in Table 1, LP-211 showed a _K_i value of 15 nM at human cloned 5-HT7 receptors, which is 25-fold greater than the _K_i value previously found at rat cloned 5-HT7 receptor (_K_i = 0.58 nM) [17]. The difference could be due to differences between receptors cloned from different species or to different binding protocols. Moreover, the affinity profile of LP-211 seems preferable compared to that of 8-OH-DPAT which has been reported to have _K_i = 467 nM at the human cloned 5-HT7 receptor and _K_i = 3.8 nM at human cloned 5-HT1A [23]. The selectivity of LP-211 against 5-HT1B, 5-HT2B, 5-HT2C and 5-HT5A was modest (5-14-fold), whereas significantly higher selectivities were observed for the subtypes 5-HT1A (25-fold), 5-HT1D (26-fold), 5-HT2A (40-fold) and 5-HT6 (105-fold). The selectivity observed at 5-HT1A receptor might, at least in most cases, be sufficient to avoid interference from biological response mediated by this receptor in vivo. Finally, LP-211 did not significantly bind at 5-HT3 and 5-HT1E receptors, or at SERT. Interestingly, RA-7 appeared to be a potent ligand for the human 5-HT7 receptor with a _K_i (1.4 nM), approximately 10-fold lower than the corresponding value for LP-221. Overall, RA-7 was more specific than LP-211 in vitro, mainly because RA-7 showed higher affinity for the 5-HT7 receptor than LP-211. Moreover, RA-7 was less than 100-fold selective only over 5-HT5A and 5-HT1A receptors (54- and 71-fold).
Compound LP-211 seemed to dose-dependently reduce body temperature in 5-HT7+/+ mice but not in 5-HT7−/− mice when analyzing both peak and overall effect. At the higher dose tested, body temperature was reduced also in 5-HT7−/− mice when analyzing peak effect, but not when analyzing overall effect. The peak effect in 5-HT7−/− mice can most likely be attributed to an action at 5-HT1A receptors [21]. The 5-HT1A receptor antagonist WAY 100135 failed to block the hypothermic effect of LP-211. WAY 100135 rather appeared to unmask other effects on body temperature, the cause of which remains to be determined. It should however be noted that WAY 100135 is a partial agonist at 5-HT1A receptors which will also lower body temperature when activated [21,24]. The 5-HT7 receptor antagonist SB-269970 blocked the effect of LP-211 on body temperature as the effects seen when combining LP-211 and SB-269970 were not different from vehicle. Taking into account the pharmacokinetic results showing that plasma and brain concentrations of LP-211 and its active metabolite RA-7 at approximately the time of peak effect on body temperature were essentially similar in either wild-type and knockout mice, it can be concluded that the hypothermic effect of LP-211, at least primarily, is mediated by the 5-HT7 receptor. In support it should be noted that the ability of LP-211 to induce hypothermia was similar to that seen for the unselective agonists 5-HT, 5-CT, and 8-OH-DPAT, but possibly with an enhanced ability for SB-269970 to inhibit the response [19,21].
Interestingly, the metabolite RA-7 also affected body temperature, but for most parts in a way that was not different between 5-HT7+/+ and 5-HT7−/− mice. This observation stands in contrast to the apparent 5-HT7 receptor selectivity seen for RA-7 in the binding profile. As mentioned, RA-7 rapidly entered the brain achieving higher concentrations than in plasma.
Based on the affinity profiles, it cannot be excluded that at higher doses, LP-211 and RA-7 act also on 5-HT1A receptors to induce hypothermia [21]. This is consistent with the finding that WAY 100135, a partial agonist, failed to counteract the effect of LP-211. Further studies are needed to fully determine the mechanisms at play.
In conclusion, our quest to produce new ligands for the serotonin 5-HT7 receptor has resulted in the development of the 5-HT7 receptor agonist LP-211, which shows suitable pharmacodynamic and pharmacokinetic properties for in vivo studies aimed to characterize the functional role of this receptor. Continued work comparing LP-211 with other recently described 5-HT7 receptor agonists such as AS-19 and E-55888 is needed to fully establish their actual in vivo usability.
Acknowledgments
This work was supported by NIMH MH073923 (P.B.H.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Thomas DR, Hagan JJ. 5-HT7 receptors. Curr. Drug Target CNS Neurol. Disord. 2004;3:81–90. doi: 10.2174/1568007043482633. [DOI] [PubMed] [Google Scholar]
- [2].Hedlund PB, Sutcliffe JG. Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol. Sci. 2004;25:481–486. doi: 10.1016/j.tips.2004.07.002. [DOI] [PubMed] [Google Scholar]
- [3].Hedlund PB. The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology (Berl.) 2009;206:345–354. doi: 10.1007/s00213-009-1626-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].To ZP, Bonhaus DW, Eglen RM, Jakeman LB. Characterization and distribution of putative 5-ht7 receptors in guinea- pig brain. Br. J. Pharmacol. 1995;115:107–116. doi: 10.1111/j.1476-5381.1995.tb16327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J. Biol. Chem. 1993;268:23422–23426. [PubMed] [Google Scholar]
- [6].Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, Danielson PE, Sutcliffe JG, Erlander MG. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- [7].Clemett DA, Kendall DA, Cockett MI, Marsden CA, Fone KCF. Pindolol-insensitive [3H]-5-hydroxytryptamine binding in the rat hypothalamus; identity with 5-hydroxytryptamine7 receptors. Br. J. Pharmacol. 1999;127:236–242. doi: 10.1038/sj.bjp.0702503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gustafson EL, Durkin MM, Bard JA, Zgombick J, Branchek TA. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br. J. Pharmacol. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bonaventure P, Nepomuceno D, Hein L, Sutcliffe JG, Lovenberg T, Hedlund PB. Radioligand binding analysis of knockout mice reveals 5-hydroxytryptamine7 receptor distribution and uncovers 8-hydroxy-2-(di-npropylamino)tetralin interaction with α2 adrenergic receptors. Neuroscience. 2004;124:901–911. doi: 10.1016/j.neuroscience.2004.01.014. [DOI] [PubMed] [Google Scholar]
- [10].Eriksson TM, Golkar A, Ekström JC, Svenningsson P, Ögren SO. 5-HT7 receptor stimulation by 8-OH-DPAT counteracts the impairing effect of 5-HT1A receptor stimulation on contextual learning in mice. Eur. J. Pharmacol. 2008;596:107–110. doi: 10.1016/j.ejphar.2008.08.026. [DOI] [PubMed] [Google Scholar]
- [11].Sarkisyan G, Hedlund PB. The 5-HT7 receptor is involved in allocentric spatial memory information processing. Behav. Brain Res. 2009;202:26–31. doi: 10.1016/j.bbr.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Leopoldo M. Serotonin7 receptors (5-HT7Rs) and their ligands. Curr. Med. Chem. 2004;11:629–661. doi: 10.2174/0929867043455828. [DOI] [PubMed] [Google Scholar]
- [13].Pittalà V, Salerno L, Modica M, Siracusa MA, Romeo G. 5-HT7 receptor ligands: recent developments and potential therapeutic applications. Mini Rev. Med. Chem. 2007;7:945–960. doi: 10.2174/138955707781662663. [DOI] [PubMed] [Google Scholar]
- [14].Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, Smith MI, Upton N, Medhurst AD, Middlemiss DN, Riley GJ, Lovell PJ, Bromidge SM, Thomas DR. Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br. J. Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Forbes IT, Douglas S, Gribble AD, Ife RJ, Lightfoot AP, Garner AE, Riley GJ, Jeffrey P, Stevens AJ, Stean TO, Thomas DR. SB-656104-A: A novel 5-HT(7) receptor antagonist with improved in vivo properties. Bioorg. Med. Chem. Lett. 2002;12:3341–3344. doi: 10.1016/s0960-894x(02)00690-x. [DOI] [PubMed] [Google Scholar]
- [16].Brenchat A, Romero L, García M, Pujol M, Burgueño J, Torrens A, Hamon M, Baeyens JM, Buschmann H, Zamanillo D, Vela JM. 5-HT7 receptor activation inhibits mechanical hypersensitivity secondary to capsaicin sensitization in mice. Pain. 2009;141:239–247. doi: 10.1016/j.pain.2008.11.009. [DOI] [PubMed] [Google Scholar]
- [17].Leopoldo M, Lacivita E, De Giorgio P, Fracasso C, Guzzetti S, Caccia S, Contino M, Colabufo NA, Berardi F, Perrone R. Structural modifications of N-(1,2,3,4-tetrahydronaphthalen-1-yl)-4-aryl-1-piperazinehexanamides: Influence on lipophilicity and 5-HT7 receptor activity. Part III. J. Med. Chem. 2008;51:5813–5822. doi: 10.1021/jm800615e. [DOI] [PubMed] [Google Scholar]
- [18].Strachan RT, Ferrara G, Roth BL. Screening the receptorome: an efficient approach for drug discovery and target validation. Drug Discov. Today. 2006;11:708–716. doi: 10.1016/j.drudis.2006.06.012. [DOI] [PubMed] [Google Scholar]
- [19].Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc. Natl. Acad. Sci. USA. 2003;100:1375–1380. doi: 10.1073/pnas.0337340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol. Psychiatry. 2005;58:831–837. doi: 10.1016/j.biopsych.2005.05.012. [DOI] [PubMed] [Google Scholar]
- [21].Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur. J. Pharmacol. 2004;487:125–132. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- [22].Caccia S. N-dealkylation of arylpiperazine derivatives: disposition and metabolism of the 1-aryl-piperazines formed. Curr. Drug Metab. 2007;8:612–622. doi: 10.2174/138920007781368908. [DOI] [PubMed] [Google Scholar]
- [23].Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- [24].Assie MB, Koek W. Effects of 5-HT1A receptor antagonists on hippocampal 5-hydroxytryptamine levels: (S)-WAY100135, but not WAY100635, has partial agonist properties. Eur. J. Pharmacol. 1996;304:15–21. doi: 10.1016/0014-2999(96)00086-6. [DOI] [PubMed] [Google Scholar]