α2 nicotine receptors function as a molecular switch to continuously excite a subset of interneurons in rat hippocampal circuits (original) (raw)
. Author manuscript; available in PMC: 2010 Aug 4.
Abstract
Rapid activation of nicotinic acetylcholine receptors (nAChRs) at various anatomical and cellular locations in the hippocampus differentially modulates the operation of hippocampal circuits. However, it is largely unknown how the continued presence of nicotine affects the normal operation of hippocampal circuits. Here, we used single and dual whole-cell recordings to address this question. We found that horizontally oriented interneurons in the stratum oriens/alveus continuously discharged action potentials in the presence of nicotine. In these interneurons, bath application of nicotine produced slow inward currents that were well maintained and inhibited by the non-α7 antagonist dihydro-β-erythroidine. Single-cell reverse transcription-polymerase chain reaction analysis showed that nicotine-responding interneurons were consistently positive for the α2 subunit mRNA. These observations suggest that in the presence of nicotine, a subset of interneurons in the stratum oriens/alveus are continuously excited due to the sustained activation of α2* nAChRs. These interneurons were synaptically connected to pyramidal cells, and nicotine increased inhibitory baseline currents at the synapses and suppressed phasic inhibition at the same synapses. Nicotine-induced inhibitory activity increased background noise and masked small phasic inhibition in pyramidal cells, originating from other interneurons in the stratum radiatum. Thus, the continued presence of nicotine alters the normal operation of hippocampal circuits by gating inhibitory circuits through activating a non-desensitizing α2 nAChR subtype on a distinct population of interneurons.
Keywords: nicotinic acetylcholine receptors, α2 subunit, stratum oriens/alveus, dual whole-cell recordings, inhibitory postsynaptic currents
Introduction
γ-aminobutyric acid (GABA)ergic interneurons, which are a target of cholinergic inputs to the hippocampus, have a central role in the control of synaptic plasticity and hippocampus-dependent learning. Discrete subtypes of these interneurons in turn provide selective innervation of specific postsynaptic membrane domains (the somata, axon initial segment, proximal dendrites, or distal dendrites) of excitatory principal cells (pyramidal cells and granule cells) with each subtype likely having a unique operational role in the hippocampal circuitry (Freund & Buzaki, 1996; Maccaferri et al., 2000; Maccaferri, 2005). Many of these interneurons express nicotinic acetylcholine receptors (nAChRs) (Alkondon & Albuquerque, 2001; Alkondon et al., 2000; McQuiston & Madison, 1999; Sudweeks & Yakel, 2000; Ji et al., 2001; Buhler & Dunwiddie, 2001, 2002; Frazier et al., 1998a, 1998b, 2003). By differentially activating discrete interneuron subtypes through nAChRs, cholinergic afferents may switch synaptic inhibition between distinct postsynaptic membrane domains, thereby altering the operation of hippocampal circuits.
Nicotine enhances several types of hippocampus-dependent learning (Abdulla et al., 1996; Levin & Simon, 1998; Marti Barros et al., 2004; Gould, 2006; Davis et al., 2007) and modulates the induction of long-term potentiation (LTP), which is considered to be a cellular substrate of learning and memory, in the hippocampus (Sawada et al., 1994; Hamid et al., 1997; Fujii et al., 1999; Matsuyama et al., 2000; Mann & Greenfield, 2003; Welsby et al., 2006; Nakauchi et al., 2007a). These behavioral and cellular effects of nicotine are mediated by its interaction with nAChRs that are normally activated by the neurotransmitter acetylcholine (ACh). However, it is largely unknown which nAChR subtypes mediate these effects of nicotine. Interneurons in different anatomical layers of the hippocampal CA1 region contain non-α7 nAChR (α3β4*, α4β2*) and α7 nAChR subtypes (Alkondon & Albuquerque, 2001; Alkondon et al., 2000; McQuiston & Madison, 1999; Sudweeks & Yakel, 2000; Ji et al., 2001; Buhler & Dunwiddie, 2001, 2002; Frazier et al., 1998a, 1998b, 2003). In addition, α2 mRNAs are expressed by interneurons in the stratum oriens/alveus (Wada et al., 1989; Sudweeks & Yakel, 2000; Ishii et al., 2005), suggesting the presence of a functional α2* nAChR subtype. Rapid application of ACh or nicotine onto interneurons in hippocampal slices activates various nAChR subtypes (Frazier et al., 1998a; McQuiston & Madison, 1999; Alkondon & Albuquerque, 2001; Alkondon et al., 2000, 2003; Sudweeks & Yakel, 2000). However, bath application of nicotine, which might mimic slow delivery of nicotine into the brain via cigarette smoking or systemic nicotine injection, fails to elicit nAChR-mediated responses in interneurons due to desensitization (Yamazaki et al., 2006). Furthermore, both synaptically elicited- and exogenous ACh mediated-nicotinic responses in interneurons are blocked in the presence of nicotine at the concentration achieved by heavy smokers (Frazier et al., 1998a, 1998b; McQuiston & Madison, 1999; Alkondon et al., 2000; Alkondon & Albuquerque, 2005; Yamazaki et al., 2005, 2006). These results suggest that in the presence of nicotine, nicotinic cholinergic control of GABAergic interneurons is blocked via nAChR desensitization. Interestingly, however, bath application of nicotine in hippocampal slices increases the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) in pyramidal cells in the presence of glutamatergic inhibitors, which is blocked by the Na+ channel blocker tetrodotoxin (TTX) (Yamazaki et al., 2005). The implication of this observation is that nicotine continuously excites GABAergic interneurons via a non-desensitizing nAChR subtype. This nAChR subtype becomes a critical component in hippocampal circuitry in the presence of nicotine, and potentially serves as a molecular switch for gating information flow and synaptic plasticity by altering local GABAergic inhibition. However, the location of a non-desensitizing nAChR subtype, which is responsible for the increase in the frequency of sIPSCs in pyramidal cells, remains to be determined.
A single pressure pulse application of ACh onto interneurons in the stratum oriens/alveus elicits slow non-α7 nAChR-mediated responses, which are confined primarily to horizontally oriented oriens lacunosum-moleculare cells (McQuiston & Madison, 1999; Sudweeks & Yakel, 2000). These responses are reduced in the presence of nicotine, due to nicotine-induced desensitization. It was not tested whether bath application of nicotine elicited non-α7 nAChR-mediated responses in these interneurons. In the present study, we searched for a non-desensitizing nAChR subtype on interneurons of hippocampal CA1 region, and found a non-desensitizing α2 nAChR subtype on horizontally oriented interneurons in the stratum oriens/alveus.
Materials and Methods
All animal procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and with protocols approved by the Institutional Animal Care and Use Committee of the University of California at Irvine. Efforts were made to minimize animal suffering and numbers of rats used.
Slice preparation
Sprague-Dawley rats (18- to 54-day-old; Harlan, Indianapolis, IN, USA) were anesthetized with urethane (1.25 g/kg) and killed by decapitation. Transverse hippocampal slices (375 μm) were prepared and maintained at 30-32°C in artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl 124, KCl 5, NaH2PO4 1.25, MgSO4 2, CaCl2 2.5, NaHCO3 22, and glucose 10 and oxygenated with 95% O2/5% CO2, for at least 1 hour before recordings.
Electrophysiological recordings
Current- and voltage-clamp recordings were made from the somatic region of pyramidal cells and interneurons as described previously (Yamazaki et al., 2005, 2006). Slices were placed in a recording chamber, submerged, and continuously perfused at 2-3 ml/min with oxygenated ACSF at 30°C. Neurons were visualized using a 40x water-immersion objective and infrared differential interference contrast (IR-DIC) system under infrared light (Axioskop, Zeiss, Germany). Patch electrodes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL, USA) using a micropipette puller (P-97, Sutter Instrument, Novato, CA, USA). The pipettes had a resistance of 3-8 MΩ after being filled with pipette solution. For current-clamp recording, the patch pipettes were filled with solution 1 (120 mM K-gluconate, 10 mM KCl, 2 mM ATP, 0.2 mM Na-GTP, 2 mM MgCl2, 10 mM HEPES, 10 mM EGTA). For voltage-clamp recordings, unless otherwise mentioned, the patch pipettes were filled with solution 2 (130 mM Cs-methanesulfonate, 10 mM TEA, 5 mM BAPTA, 10 mM HEPES, 4 mM ATP, 5 mM QX-314). Responses of currents and potentials were recorded using Axopatch-200B or Axoclamp-2B (Axon Instruments, Union City, CA, USA), and were filtered (1-2 kHz), digitized at 1-5 kHz, and stored on a computer. All drugs were applied by introduction into the bath perfusion system.
Single-cell reverse transcription (RT)-multiplex polymerase chain reaction (PCR)
To monitor the expression of different subtypes of nAChR subunits (α2-α6) in recorded cells, single-cell RT-multiplex PCR was performed by the procedure previously described (Porter et al., 1999). After recordings, the cytoplasm of individual neurons was harvested by applying suction on a patch pipette filled with sterile intracellular pipette solution. The contents of the pipette were expelled into a PCR tube by the application of positive pressure, quickly frozen on dry ice, stored at −80°C overnight, and subjected to RT-multiplex PCR using the Titan One Tube RT-PCR System (Roche Molecular Biochemicals, Indianapolis, IN, USA) and different pairs of PCR primers for simultaneous amplification of different subtypes of nAChR subunits (α2-α6). This was followed by second rounds of PCR using 1 μl of the first PCR product as a template. In this second round, each target cDNA was amplified individually using its specific primer pair. Each individual PCR reaction was run on an agarose gel stained with ethidium bromide for identification of target cDNAs.
Statistical analysis
Data were analyzed off-line using Mini analysis program (Synaptosoft Inc., Decatur, GA, USA), Origin (OriginLab, Northampton, MA, USA), and pCLAMP 7 (Axon Instruments). Data were expressed as means ± SEM. Sample size n refers to the number of neurons analyzed in electrophysiological recordings from hippocampal slices. Significant changes in frequency of sIPSCs, membrane depolarization, and action potential firing frequency (before vs. after drug application, or between two drugs) were assessed using a paired or an unpaired, two-tailed Student's _t_-test where appropriate. The statistical tests for the data presented in Fig. 2E were carried out on the percentage data. For all recordings, 5 min of baseline data were acquired. Average pre-drug sIPSC frequency for a 2 min period immediately prior to drug application and average sIPSC frequency during 2 min drug application were normalized to 5 min of baseline data. These normalized data were then compared using a paired _t_-test. Additional statistical tests for the data presented in Fig. 2E were carried out on the percentage data. In these tests, we compared the effects of two drug groups using an unpaired _t_-test and the results were presented in the text. The statistical tests for the data presented in Fig. 3E were carried out on the unnormalized data. Average pre-drug frequency for a 2 min period immediately prior to drug application and average frequency during 2 min drug application were compared using a paired _t_-test. Additional statistical tests for the data presented in Fig. 3E were carried out on the unnormalized data. In these tests, we compared the effects of two drug groups using an unpaired _t_-test and the results were presented in the text. The Kolmogorov-Smirnov test was applied to compare distributions. A comparison was considered statistically significant if P < 0.05.
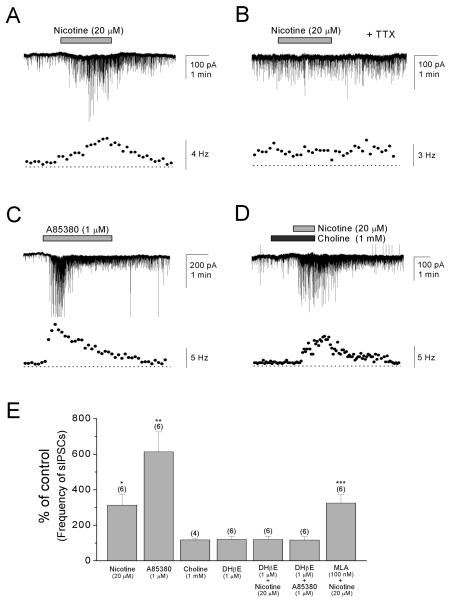
Figure 2. Nicotine increases the frequency of sIPSCs in pyramidal cells via activation of non-α7 nAChRs.
(A) Bath application of nicotine (20 μM) in the presence of DNQX (20 μM) and AP5 (40 μM) reversibly increased the frequency of sIPSCs in pyramidal cells voltage-clamped at −70 mV. (B) Preapplication of TTX (0.2-0.5 μM) blocked the effect of nicotine. (C) The non-α7 nAChR agonist A85380 (1 μM) mimicked the effect of nicotine. (D) The α7 nAChR agonist choline (1 mM) had no significant effect on the frequency of sIPSCs. (E) Summary plot of the effects of different nicotinic agonists and antagonists on the frequency of sIPSCs as a percentage of control. *P < 0.05, **P < 0.01, ***P < 0.001.
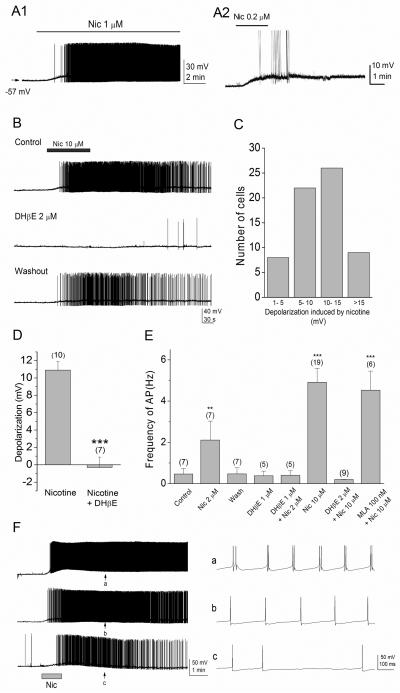
Figure 3. Nicotine depolarizes horizontally oriented interneurons in the stratum oriens/alveus and increases interneuronal spiking rate.
(A1, A2) Bath application of low concentrations of nicotine in the presence of DNQX (20 μM) and AP5 (40 μM) caused a depolarization and increased action potential firing in current-clamped interneurons. (A1) The interneurons remained depolarized during 10-min application of 1 μM nicotine. (A2) Nicotine at a low concentration found in cigarette smokers excites interneurons. (B) Bath application of 10 μM nicotine reversibly induced a depolarization of interneurons and increased the rate of action potential firing (top). These effects were blocked by 2 μM DHβE (middle). The blocking effect of DHβE was reversible after washout of the drug (bottom). (C) The magnitude of nicotine-induced depolarization varied among interneurons. (D) Summary graph showing the magnitude of depolarization observed in the presence of nicotine (10 μM) and nicotine (10 μM) + DHβE (2 μM). Note that nicotine depolarizes interneurons and the effect of nicotine was blocked by DHβE. (E) Summary graph showing the frequency of action potential observed in the absence (control) and presence of nicotine, DHβE, nicotine + DHβE, and nicotine + MLA, and after washout of nicotine (wash). Note that nicotine reversibly increased the rate of action potential firing in a dose-dependent manner, and the effect was blocked by DHβE, but not MLA. (F) Nicotine-responding interneurons exhibited different firing patterns. On the right, representative traces from three different interneurons exhibiting clustered (top), regular (middle), and irregular (bottom) firing patterns at arrows (on the left, a, b, c) are shown on an expanded time scale. **P < 0.01, ***P < 0.001.
Results
Bath application of nicotine increases tonic inhibition in pyramidal cells via activation of non-α7 nAChRs
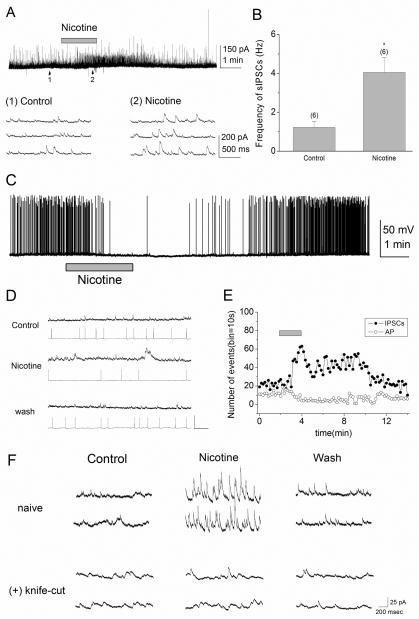
Whole-cell voltage-clamp recordings were obtained from pyramidal cells in the presence of the glutamate receptor antagonists 6, 7-dinitro-quinoxaline-2, 3-dione (DNQX; 20 μM) and 2-amino-5-phosphopentanoate (AP5; 40 μM) to eliminate excitatory synaptic activity. We confirmed the previous finding (Yamazaki et al., 2005) that bath application of nicotine (10 μM) caused a significant increase in the frequency of sIPSCs in pyramidal cells (Fig. 1A, B). On average, after nicotine application the frequency of sIPSCs increased from 1.2 ± 0.3 to 4.0 ± 0.8 Hz (n = 6, _t_5 = −3.40, P = 0.019). We then examined whether the nicotine-induced increases in the frequency of sIPSCs in turn influenced action potential discharges in pyramidal cells. For this purpose, current-clamp responses to a current pulse (~80 pA) were recorded in pyramidal cells and the effect of nicotine on the action potential firings was monitored. We found that bath application of nicotine (10 μM) effectively prevented action potential discharges (Fig. 1C) in the recorded cells. To further examine these effects of nicotine, we simultaneously recorded from two pyramidal cells, one voltage-clamped and the other current-clamped. In all cases (n=6), bath application of nicotine (10 μM) caused an increase in the frequency of sIPSCs in the voltage-clamped cell, whereas it resulted in a decrease in the frequency of action potentials in the current-clamped cell (Fig. 1D, E). These effects of nicotine were most likely related (Fig. 1E) and were reversed upon washout of nicotine (Fig. 1D). These observations indicate that nicotine causes the action potential discharge of interneurons, leading to an increase of tonic inhibition in pyramidal cells.
Figure 1. Nicotine increases tonic inhibition in pyramidal cells via activation of interneurons in the stratum oriens/alveus.
(A) Bath application of nicotine (10 μM) in the presence of DNQX (20 μM) and AP5 (40 μM) reversibly increased the frequency of sIPSCs in pyramidal cells voltage-clamped at 0 mV. The traces below show sIPSCs from the regions indicated by the arrows 1 and 2 on an expanded time scale. (B) Summary plot of the effect of nicotine on the frequency of sIPSCs (mean ± SEM). Numbers in parentheses in this and the following figures indicate the numbers of experiments. (C) Bath application of nicotine (10 μM) in the presence of DNQX (20 μM) and AP5 (40 μM) reversibly reduced the frequency of action potentials in current-clamped pyramidal cells. To induce the repetitive firing of action potentials, depolarizing current (~80 pA) was injected. (D, E) Simultaneous recordings from two pyramidal cells, one voltage-clamped and the other current-clamped. (D) sIPSCs (top) and action potentials (bottom) recorded in the absence (Control) and presence of nicotine (10 μM), and after washout of nicotine are shown. (E) Nicotine reversibly increased the frequency of sIPSCs, while decreasing the rate of action potentials triggered by depolarizing current injection. The horizontal bar indicates bath application of nicotine (10 μM). (F) A knife cut in the stratum oriens, parallel to stratum pyramidale, prevented the nicotine-induced increase in the frequency of sIPSCs in voltage-clamped pyramidal cells (Vh = 0 mV). *P < 0.05
Interneurons in the CA1 region contain somatodendritic nAChRs (McQuiston & Madison, 1999; Alkondon et al., 2000; Sudweeks & Yakel, 2000). However, it is unknown whether bath application of nicotine continuously activates a distinct nAChR subtype, thereby exciting discrete interneurons in a particular anatomical layer. In our effort to identify the regional location of nicotine-responding interneurons, we made a knife cut along the pyramidal layer in the stratum oriens to sever the axons of interneurons projecting to the somata and apical dendrites of pyramidal cells. In such slices, the effect of nicotine on the frequency of sIPSCs was not observed (Fig. 1F), suggesting that interneurons in the stratum oriens/alveus are selectively excited in the presence of nicotine and are synaptically connected to pyramidal cells.
Subsequently, we carried out the pharmacology of the nicotine response using agonists and antagonists. In these experiments, pyramidal cells were voltage-clamped at −70 mV and sIPSCs were recorded with CsCl-filled pipettes. A85380 has been used as a non-α7-selective agonist with higher affinity for β2* nAChRs than β4* nAChRs (Whiteaker et al., 2002; Perry et al., 2002). Choline has been used as an α7-selective agonist (Papke et al., 1996; Alkondon et al., 1997), but it also weakly activates α3β4 receptors (Alkondon et al., 1997). Bath application of nicotine (20 μM) significantly increased the frequency of sIPSCs in pyramidal cells (Fig. 2A, E; 313 ± 60% change of pre-drug value, n = 6, _t_5 = −3.56, P = 0.016). This effect of nicotine depended on action potential discharges as the increase was completely prevented by preapplication of TTX (0.2-0.5 μM, n=6; Fig. 2B). Bath application of A85380 (1 μM) mimicked the effect of nicotine (Fig. 2C, E), causing an increase in the frequency of sIPSCs in pyramidal cells (614 ± 112% change of pre-drug value, n = 6, _t_5 = −4.59, P = 0.0059). Bath application of choline (1 mM) had no significant effect on the frequency of sIPSCs (Fig. 2D, E; 98 ± 16% change of pre-drug value, n = 4, _t_3 = 0.16, P = 0.89). In some recordings, however, we noticed that the frequency of sIPSCs briefly increased immediately after choline application, perhaps due to very brief activation and subsequent desensitization of α7 nAChRs. The non-α7 antagonist dihydro-β-erythroidine (DHβE; 1 μM) alone did not significantly alter the baseline frequency of sIPSCs (Fig. 2E; 121 ± 16% change of pre-drug value, n = 6, _t_5 = −1.31, P = 0.25), but completely blocked the nicotine-induced increases (Fig. 2E; nicotine, 313 ± 60% change of pre-drug value, n =6, vs. DHβE + nicotine 120 ± 18% change of pre-drug value, n = 6, _t_10 = −3.10, P = 0.011) and the A85380-induced increases (Fig. 2E; A85380, 614 ± 112% change of pre-drug value, n = 6, vs. DHβE + A85380 117 ± 18% change of pre-drug value, n = 6, _t_10 = −4.39, P = 0.0014). In contrast, the α7 nAChR antagonist methyllycaconitine (MLA; 100 nM) had no significant effect on the nicotine-induced increases (Fig. 2E; MLA + nicotine, 346 ± 12% change of pre-drug value, n = 6, _t_5 = −21.21, P < 0.001; nicotine vs. MLA + nicotine, _t_10 = 0.53, P = 0.61). These findings suggest that the effect of nicotine is mediated via the activation of non-α7 nAChRs.
Nicotine continuously excites interneurons in the stratum oriens/alveus
To directly demonstrate that bath application of nicotine induces sustained action potential discharges in interneurons of the stratum oriens/alveus, we performed current-clamp recordings. We found that a subset of interneurons located at the stratum oriens/alveus border generated action potentials in response to bath application of 0.2-1 μM nicotine (Fig. 3A1, A2), a concentration that can be achieved in the plasma during cigarette smoking (Benowitz et al., 1989; Henningfield et al., 1993). The effect persisted throughout the application of nicotine (Fig. 3A1, A2, B) and, thus, nicotine-responding interneurons were continuously excited during 10 min bath application of nicotine, the longest application tested (Fig. 3A1). On average, the application of nicotine (10 μM) induced a depolarization in these interneurons ranging from 5.1 to 14.9 mV (Fig. 3C) in a manner sensitive to DHβE (Fig. 3B, D; nicotine 10.9 ± 1.0 mV, n = 10, vs. nicotine + DHβE −0.3 ± 1.2 mV, n = 7, _t_15 = 7.21, P < 0.001), suggesting that they expressed varying numbers of non-α7 nAChRs. We then compared the frequency of action potential before and after application of different nicotinic drugs. On average, pre-drug values were very similar in these experiments (data not shown) and were around 0.5 Hz (Fig. 3E, control). The frequency of action potentials increased in the presence of nicotine in a concentration dependent manner (Fig. 3E; 2 μM nicotine 2.1 ± 0.9 Hz, _n_ = 7, _t_6 = −5.00, _P_ = 0.0015; 10 μM nicotine 4.9 ± 0.7 Hz, _n_= 19, _t_18 = 3.95, _P_ < 0.001). This effect of nicotine was reversed upon washout (Fig. 3B, E; **0.5 ± 0.3 Hz, _n_ = 7**) and was blocked by DHβE (Fig. 3B, E; 1 μM DHβE alone 0.4 ± 0.2 Hz, _n_ = 5; 2 μM nicotine + 1 μM DHβE 0.4 ± 0.2 Hz, _n_ = 5; 10 μM nicotine + 2 μM DHβE 0.2 ± 0.1 Hz, _n_ = 9; 2 μM nicotine vs. 2 μM nicotine + 1 μM DHβE, _t_10 = −4.05, _P_ = 0.0023; 10 μM nicotine vs. 10 μM nicotine + 2 μM DHβE, _t_26 = −4.79, _P_ < 0.001), but not MLA (Fig. 3E; MLA + nicotine, 4.5 ± 0.9 Hz, _n_ = 6, _t_5 = 4.79, _P_ <0.001; 10 μM nicotine vs. MLA + nicotine, _t_23 = −0.28, _P_ = 0.78). These results suggest that non-α7 nAChRs mediate the effect of nicotine. Under IR-DIC optics, most, if not all, of these interneurons appeared to be horizontally oriented cells, but not all horizontally oriented cells responded to bath application of 10 μM nicotine (>3 mV depolarization in approximately 80% of recorded cells). Perhaps the number of nicotine-responding cells is somewhat over-represented, because late in the study we were often, but not always, able to identify them before recording, on the basis of the presence of perineuronal glial cells alongside the soma.
GABAergic interneurons exhibit at least three different firing patterns (regular, irregular, and clustered) that occur after maintained depolarizing current injections (Parra et al., 1998; Minneci et al., 2007). Thus, we next examined whether nicotine-responding interneurons exhibit particular firing characteristics. Bath application of nicotine (10 μM) triggered different patterns of action potential discharges in these interneurons. At least three distinct patterns of inhibitory cell discharge were evident (Fig. 3F), which were similar to those previously reported (Parra et al., 1998; Minneci et al., 2007). There were interneurons exhibiting clusters of two or more regularly occurring spikes (~3%; Fig. 3F, a) and regularly firing interneurons with constant interspike intervals (~16%; Fig. 3F, b). However, the majority of cells (~81%) exhibited the irregular firing pattern characterized by variable interspike intervals (Fig. 3F, c). These findings suggest that nicotine is able to generate at least three distinct temporal sequences of inhibitory postsynaptic potentials (IPSPs) in pyramidal cells.
Nicotine-responding interneurons at the stratum oriens/alveus border contain non-desensitizing non-α7 nAChRs
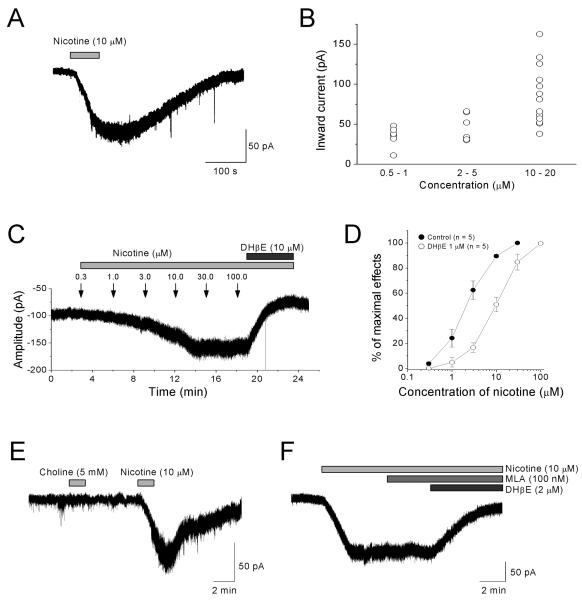
Because nicotine-responding interneurons were continuously excited, we predicted the presence of non-desensitizing nAChRs in horizontally oriented interneurons. Previous studies found that a single pressure pulse application of ACh onto interneurons in the stratum oriens/alveus elicits fast α7 nAChR-mediated and slow non-α7 nAChR-mediated responses, which are reduced in the presence of nicotine, due to nicotine-induced desensitization (McQuiston & Madison, 1999; Sudweeks & Yakel, 2000). Thus, our effort was focused on whether horizontally oriented interneurons in the stratum oriens/alveus express non-desensitizing nAChRs. To record nAChR-mediated responses, whole-cell voltage-clamp recordings (holding potential = −70 mV) were carried out in the presence of DNQX (20 μM), AP5 (40 μM), and the GABAergic antagonist bicuculline (10 μM). Bath application of nicotine (0.5-100 μM) produced long-lasting inward currents (Fig. 4A-C) that were inhibited by DHβE (1 or 10 μM; Fig. 4C, D, F), but not MLA (100 nM, n=4; Fig. 4F). Bath application of choline (5 mM, n=4) had no effect (Fig. 4E). These results suggest that non-α7 nAChRs mediate the effect of nicotine. The amplitudes of peak inward currents varied among the interneurons (Fig. 4B), but increased in a concentration-dependent manner with an EC50 of approximately 2.4 μM (Fig. 4C, D). DHβE (1 μM) shifted the concentration-response curve to the right in a parallel manner (Fig. 4D), which is consistent with a competitive block of nAChRs. Furthermore, nicotine-induced currents continued even when synaptic transmission was blocked by TTX (0.2-0.5 μM; data not shown). These results suggest that the responses were due to direct activation of non-α7 nAChRs on these interneurons.
Figure 4. Nicotine persistently activates non-α7 nAChRs on interneurons in the stratum oriens/alveus.
(A, C, E, F) Bath application of nicotine in the presence of DNQX (20 μM), AP5 (40 μM), and bicuculline (10 μM) induced inward currents in interneurons voltage-clamped at −70 mV. (A) The response elicited by bath application of 10 μM nicotine is shown. (B) Low concentrations of nicotine found in cigarette smokers induced inward currents and the amplitude of nicotine-induced currents varied among interneurons. (C) Successive bath application of nicotine with increasing concentrations increased the amplitude of inward currents rather than desensitizing the non-α7 nAChRs. (C, D) Nicotine induced inward currents in a dose-dependent manner and DHβE blocked the nicotine-induced inward currents. (E) Bath application of choline (5 mM) onto nicotine-responding interneurons had no effect. (F) MLA (100 nM) had no significant effect on nicotine-induced inward currents.
Nicotine-sensitive interneurons in the stratum oriens/alveus contain α2* nAChRs
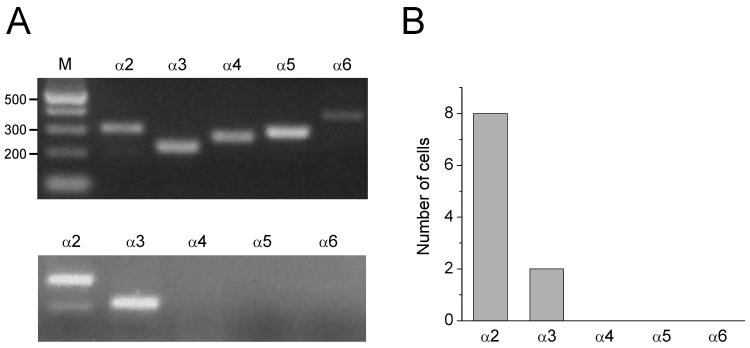
Bath application of low concentrations of nicotine desensitizes α4β2* nAChRs and α7 nAChRs in CA1 interneurons (Frazier et al., 1998a, 1998b; McQuiston & Madison, 1999; Alkondon et al., 2000; Yamazaki et al., 2005, 2006). However, a nAChR subtype expressed on the interneurons was apparently not desensitized in the presence of a wide range of nicotine concentrations and was continuously activated (Fig. 4C). Given the restricted localization of α2 mRNA in the stratum oriens/alveus (Wada et al., 1989; Ishii et al., 2005), these interneurons may contain non-desensitizing α2* nAChRs. To examine the expression of different α subunit subtypes (α2-α6) in nicotine-sensitive interneurons, we synthesized specific sets of PCR primers that were successfully used for single-cell RT-PCR by others (Porter et al., 1999). The efficiency and specificity of the RT-multiplex PCR using the mixture of 5 primer sets were tested on total RNA isolated from rat hippocampi (Fig. 5A, top). The 5 specific DNA fragments were detected, each corresponding to the size predicted by its mRNA sequence (Porter et al., 1999). When we combined whole-cell recordings and single-cell RT-multiplex PCR to monitor nicotinic responses and expression of different α subunit mRNAs (α2-α6), nicotine-responding interneurons were consistently positive for the α2 subunit mRNA (in 8 of 8 interneurons) (Fig. 5A, bottom, B). All cells tested were negative for the α4 subunit mRNA (Fig. 5A, bottom, B), but two cells were positive for the α3 subunit mRNA (Fig. 5A, bottom, B). These results suggest that nicotine-sensitive interneurons in the stratum oriens/alveus contain α2* nAChRs.
Figure 5. Nicotine-sensitive interneurons in the stratum oriens/alveus contain the α2 subunit mRNA.
(A) RT-multiplex PCR amplification of different nAChR subunit sequences. (A, top) Hippocampal RNA (500 pg) was subjected to the RT-multiplex PCR protocol to detect the expression of different nAChR subunits (α2-α6). (A, bottom) RT-multiplex PCR applied on a single nicotine-responding interneuron after electrophysiological recording to detect expression of (α2-α6). An example of single-cell RT-multiplex PCR, showing the presence of α2 and α3 subunits in a recorded cell. RT-PCR products were separated by agarose gel electrophoresis and visualized with ethidium bromide. The amplified fragments had the sizes (in bp) predicted by the mRNA sequences: 300 (α2), 221 (α3), 265 (α4), 291 (α5), 367 (α6). A 100 bp DNA ladder was used as a molecular weight marker (A, top, M). (B) Summary data of single-cell RT-multiplex PCR.
Nicotine-sensitive interneurons are synaptically connected to pyramidal cells
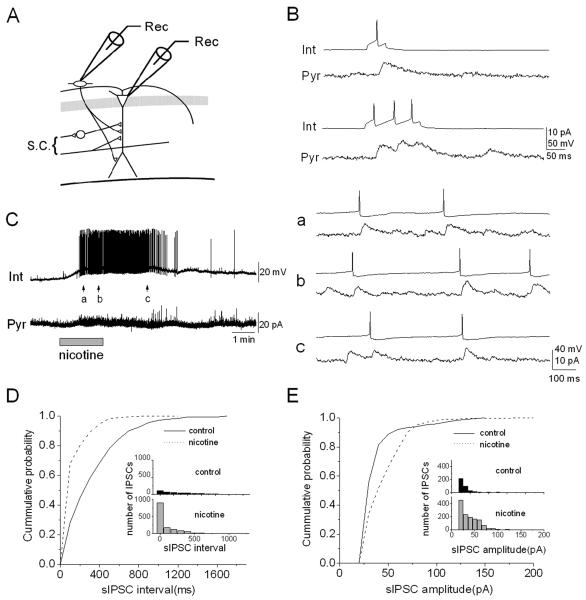
To directly examine whether nicotine-induced excitation of interneurons in the stratum oriens/alveus produces sIPSCs in pyramidal cells, we performed dual whole-cell recordings (Fig. 6A). Interneurons were current-clamped and pyramidal cells were voltage-clamped at a holding potential of 0 mV in the presence of DNQX (20 μM) and AP5 (40 μM). In a pair, interneuronal spikes, triggered by depolarizing current injection (~18 pA) with different durations (50-150 ms) successfully evoked unitary inhibitory postsynaptic currents (uIPSCs) that were superimposed on random background sIPSC activity in the pyramidal cell (Fig. 6B, C), confirming that they are synaptically connected. Bath application of nicotine induced a depolarization of the interneuron and interneuronal action potentials (Fig. 6C, left), confirming the presence of non-desensitizing nAChRs on the interneuron. During the discharges in the interneuron, the frequency of sIPSCs in the pyramidal cell increased (Fig. 6C, left), and the interneuronal discharges were temporally correlated with sIPSCs in the pyramidal cell (Fig. 6C, right, a, b, c). The sIPSCs, which were temporally uncorrelated with the interneuronal discharges, were also observed (Fig. 6C, right, a, b, c). These sIPSCs might originate from other nicotine-sensitive oriens/alveus interneurons, spontaneously active interneurons, or both. In different cell pairs, the effects of nicotine on the frequency and amplitude distributions of sIPSCs were examined. Nicotine produced a significant leftward shift in the cumulative probability distribution of sIPSC intervals (Fig. 6D; Kolmogorov-Smirnov test, P < 0.001), indicating an increase in frequency. The cumulative probability distribution of the peak amplitude of sIPSCs was significantly shifted toward larger values in the presence of nicotine as compared to controls (Fig. 6E; Kolmogorov-Smirnov test, P < 0.001). Such significant shifts were observed in 4 of the 6 pairs (67%) tested. These data suggest that nicotine-induced increases in the frequency of sIPSCs in the pyramidal cell are indeed due to nicotine-induced depolarization of horizontally oriented interneurons via non-desensitizing α2* nAChRs.
Figure 6. Non-α7 nAChR-expressing interneurons are synaptically connected to pyramidal cells.
(A) Schematic of recording electrode positions in the hippocampal CA1 region. (B) An example of dual whole-cell recordings from a synaptically coupled interneuron (Int) and pyramidal cell (Pyr). Unitary IPSCs in the pyramidal cell evoked by short (50 msec, upper traces) and long (150 msec, lower traces) depolarization of the interneuron. (C) Nicotine (10 μM) caused the interneuron to depolarize and fire repetitively and simultaneously increased the frequency of sIPSCs in the pyramidal cell (left). The records at arrows are shown on an expanded time scale on the right. Note that each action potential in the interneuron is precisely correlated with a sIPSC in the pyramidal cell. Other sIPSCs, which did not correlate with action potentials, might originate from other interneurons activated by nicotine. (D), (E) The effects of nicotine on the interval and amplitude distributions of sIPSCs were examined. (D) Cumulative distributions of sIPSC intervals. (E) Cumulative distributions of sIPSC amplitudes. The Kolmogorov-Smirnov test indicates that the interval (D) and amplitude (E) distributions of sIPSCs were significantly altered (P <0.001) in the presence of nicotine.
Nicotine increases inhibitory baseline currents and suppresses phasic inhibition originating from nicotine-sensitive interneurons in pyramidal cells
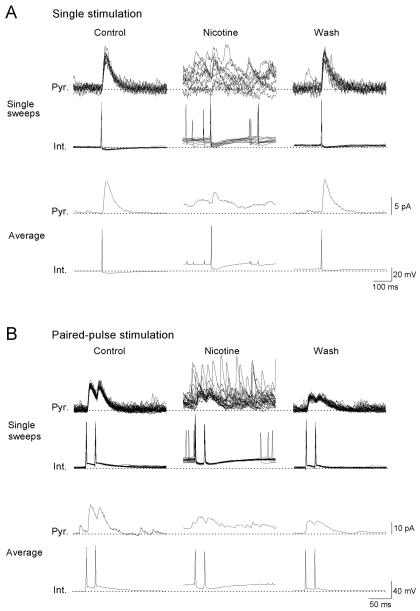
Our results above showed that in the presence of nicotine, a subset of interneurons in the stratum oriens/alveus persistently generated action potentials, which in turn dramatically increased the frequency of sIPSCs in postsynaptic cells. To examine the consequence of this effect of nicotine for evoked IPSCs at the same synapses, dual recordings were performed from synaptically coupled pairs of presynaptic nicotine-sensitive interneurons and postsynaptic pyramidal cells. Delivery of single depolarizing stimuli to the patched interneurons induced interneuronal spikes (Fig. 7A, Int.) and evoked uIPSCs in the pyramidal cell without failures (Fig. 7A, Pyr.). The mean peak amplitude of uIPSCs was 26 ± 10 pA (n=7). Paired-pulse stimulation was also delivered at a 20-ms or 100-ms (data not shown) interval to an interneuron, which triggered paired spikes (Fig. 7B, Int.) and evoked uIPSCs in the postsynaptic cell (Fig. 7B, Pyr.). Under these conditions, there were no failures in the absence or presence of nicotine (10 μM), and no significant differences were observed between the amplitude of the first and second averaged uIPSCs in the absence or presence of nicotine (Fig. 7B, Pyr.). These observations suggest that single action potentials reliably evoke GABA release at the synapse during nicotine exposure despite the nicotine-induced increases in interneuronal spiking and sIPSC frequency. Bath application of nicotine (10 μM) caused depolarization and action potential discharge in the presynaptic interneuron (Fig. 7A, B, Int., Nicotine, Single sweeps) and increased the frequency of sIPSCs in the postsynaptic pyramidal cell (Fig. 7A, B, Pyr., Nicotine, Single sweeps), which was most likely due to the activation of non-desensitizing nAChRs on the patched interneuron. Some sIPSCs might originate from other nicotine-sensitive oriens/alveus interneurons connected to the patched pyramidal cell. Nicotine also caused a shift of the baseline current (Fig. 7A, B, Pyr., Nicotine), perhaps due to the summation of nicotine-induced sIPSCs. Evoked IPSCs were depressed during nicotine exposure (Fig. 7A, B, Pyr., Nicotine), although delivery of depolarizing current pulses still reliably induced presynaptic action potentials (Fig. 7A, B, Int., Nicotine). The nicotine-induced shift of the inhibitory baseline current was reversed upon washout of nicotine (Fig. 7A, B, Pyr., Wash), whereas the nicotine-induced depression of evoked IPSCs often persisted for over 10 min after washout of nicotine (Fig. 7A, B, Pyr. Wash). Similar effects of nicotine were also observed in other pairs (n=3). These results suggest that nicotine increases tonic inhibition and decreases phasic inhibition between nicotine-sensitive horizontally oriented interneurons and pyramidal cells.
Figure 7. Nicotine causes tonic and phasic inhibitions at synapses between interneurons in the stratum oriens/alveus and pyramidal cells.
A dual whole-cell recording from a current-clamped interneuron and a voltage-clamped pyramidal cell at 0 mV was carried out. Trains of single (A) or paired spikes at the 20 ms interval (B) in the interneuron (Int.) evoked uIPSCs in the pyramidal cell (Pyr.). Ten superimposed traces and averaged traces recorded in the absence (Control) and presence of nicotine (Nicotine) and 10 min after washout of nicotine (Wash) are shown. Bath application of nicotine (10 μM) increased the frequency of sIPSCs and simultaneously depressed evoked uIPSCs. (A, B, Nicotine). Note that nicotine also caused a large shift of the baseline current during the nicotine-induced increase in the frequency of sIPSCs, perhaps due to the summation of nicotine-induced sIPSCs. The broken lines indicate the baseline potential and current in the control condition.
Nicotine-induced inhibitory activity increases background noise and masks small phasic inhibition originating from stratum radiatum interneurons in pyramidal cells
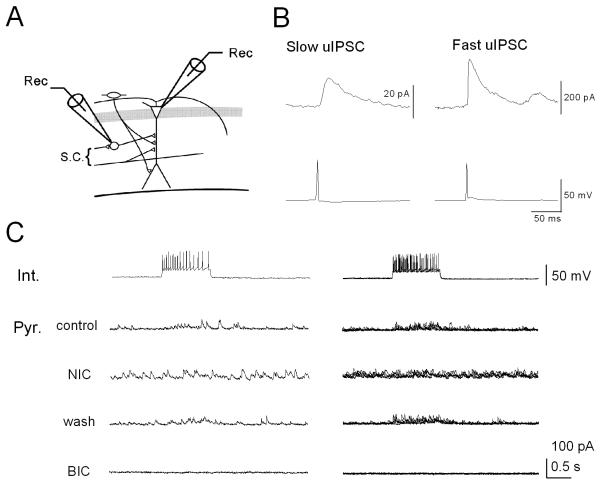
Our results demonstrate that nicotine produces robust firing of interneurons in the stratum oriens/alveus, resulting in increases in GABA release onto postsynaptic pyramidal cells. During the period of intense synaptic activity, GABA escaping the synaptic cleft can potentially reach pre- and post-synaptic GABA receptors in neighboring synapses, thereby affecting synaptic transmission. Thus, we next investigated whether evoked IPSCs, originating from feedforward interneurons in the stratum radiatum, in pyramidal cells were affected during the sustained excitation of interneurons in the stratum oriens/alveus using dual whole-cell recording (Fig. 8A). Synaptically coupled pairs of interneurons and pyramidal cells were found, and uIPSCs were recorded in the pyramidal cells by eliciting interneuronal action potentials through injection of a suprathreshhold current pulse. A few pairs showed occasional synaptic failure (data not shown) and there were at least two kinetically distinct uIPSCs, slow and fast (Fig. 8B), as previously described by others (Pearce, 1993; Banks et al., 1998; Vida et al., 1998; Jiang et al., 2000). The amplitude of fast uIPSCs was always larger than that of slow uIPSCs (Fig. 8B). We found that slow, but not fast, uIPSCs were depressed in the presence of 10 μM nicotine (4 of 7 pairs). In a pair, a trains of interneuronal spikes (Fig. 8C, Int.; a single trace on the left, superimposed single traces on the right) successfully evoked uIPSCs (Fig. 8C, Pyr. control; a single trace on the left, superimposed single traces on the right), which were blocked by 10 μM bicuculline (Fig. 8C, Pyr. BIC), confirming that they were GABAergic events. Upon bath application of nicotine (10 μM), interneuronal action potentials were still reliably induced (Fig. 8C, Int.). The frequency of sIPSCs was increased and evoked IPSCs in the pyramidal cell were suppressed simultaneously (Fig. 8C, Pyr. NIC). These effects were reversed upon washout of nicotine (Fig. 8C, Pyr. wash). Because bath application of nicotine did not generate spontaneous action potentials in the patched interneuron (Fig. 8C, Int.), an increase in the frequency of sIPSCs in the pyramidal cell is most likely due to the activation of non-desensitizing α2* nAChRs on interneurons in the stratum oriens/alveus, projecting to the patched pyramidal cell. Although the mechanisms underlying the effect of nicotine remain to be determined, these observations provide evidence that relatively small phasic inhibition arriving at specific membrane domains of pyramidal cells are masked by ongoing nicotine-induced inhibitory synaptic activity.
Figure 8. Nicotine-induced sIPSCs mask evoked uIPSCs originating from feedforward interneurons in the stratum radiatum in pyramidal cells.
Dual whole-cell recordings from current-clamped interneurons and voltage-clamped pyramidal cells at 0 mV were performed. (A) Schematic of recording electrode positions in the hippocampal CA1 region. (B) Slow and fast uIPSCs recorded by eliciting a spike in the interneuron by injection of a suprathreshold square current pulse. (C) On the left, a train of interneuronal spikes generated by 0.1 nA current injection evoked uIPSCs in pyramidal cells in the absence (Control) and presence of 10 μM nicotine (NIC) and after washout of nicotine (wash). All responses recorded in the pyramidal cell were blocked in the presence of bicuculline (BIC, 10 μM). On the right, five consecutive current or potential traces recorded from a pyramidal cell-interneuron pair were superimposed. Note that bath application of nicotine increased background noise and simultaneously masked evoked uIPSCs in the pyramidal cell.
Discussion
The present study shows that nicotine continuously excites distinct GABAergic interneurons in the stratum oriens/alveus via activating a non-desensitizing α2 nAChR subtype. This, in turn, causes the sustained release of GABA onto specific postsynaptic membrane domains of pyramidal cells, leading to an increase in inhibitory baseline currents at the synapses and a decrease in phasic inhibition at the same synapse as well as other synapses. These results suggest that in the presence of nicotine, the local balance between excitation and inhibition is altered, setting up a unique operation of hippocampal circuits. This effect of nicotine may underlie the nicotine-induced modulation of LTP induction and, thus, hippocampal-dependent learning.
The α7 nAChR is the most abundant subtype in the hippocampus. The fast α7 nAChR-mediated responses were previously found in all layers of the CA1 region, whereas the slow non-α7 nAChR-mediated responses elicited by rapid ACh application were confined primarily to horizontally oriented cells in the stratum oriens/alveus (Alkondon & Albuquerque, 2001; Alkondon et al., 2000; McQuiston & Madison, 1999; Sudweeks & Yakel, 2000; Ji et al., 2001; Buhler & Dunwiddie, 2001, 2002; Frazier et al., 1998a, 1998b, 2003). Given the presence of α4β2 nAChRs, which have the highest affinity for nicotine and is the primary candidate for mediating nicotine's central effects, in some GABAergic interneurons in the stratum oriens/alveus (Rogers et al., 1998), it is possible that these slow responses are mediated by α4β2 nAChRs. However, these responses were less sensitive than α4β2-mediated responses to DHβE (McQuiston & Madison, 1999), suggesting the involvement of a nAChR subtype that may or may not contain α4 and β2 subunits. Are these non-α7 nAChRs the same as those identified in our current study? The slow non-α7 nAChR-mediated responses in horizontally oriented cells described previously desensitize in the presence of nicotine (McQuiston & Madison, 1999). In contrast, non-α7 nAChRs identified in the current study are continuously activated by bath application of nicotine, suggesting the presence of different subtypes of non-α7 nAChRs in stratum oriens/alveus interneurons. The α2* nAChR is the most sparsely expressed nAChR subtype in the brain, but a subset of interneurons in the stratum oriens/alveus do contain α2 subunit mRNAs (Wada et al., 1989; Sudweeks & Yakel, 2000; Ishii et al., 2005). Some of these interneurons also contain α4 subunit mRNAs (Sudweeks & Yakel, 2000). In the current study, interneurons that are continuously excited in the presence of nicotine contained α2 subunit mRNAs, but not α4 subunit mRNAs. These observations suggest that there is a distinct population of horizontally oriented interneurons in the stratum oriens/alveus, expressing a non-desensitizing α2 nAChR subtype. However, in our RT-PCR study, the absence of α4 subunit mRNAs can easily be due to lack of the sensitivity needed to detect less-abundant nAChR subunit mRNAs. Thus, we are unable to exclude the possibility that a subset of interneurons in the stratum oriens/alveus contain both α2* nAChRs and α4β2* nAChRs.
The slow non-α7 nAChR-mediated responses in horizontally oriented interneurons in the stratum oriens/alveus were not affected by the α3β2-selective antagonist α-Conotoxin ImI (McQuiston & Madison, 1999). Our data demonstrate that choline, which can weakly activate α3β4 receptors (Alkondon et al., 1997), has no effect on α2* nAChR-expressing horizontally oriented interneurons in the stratum oriens/alveus. These observations suggest that the involvement of α3β2 or α3β4 in the responses is unlikely. However, in our RT-PCR study, 2 of 8 α2 subunit mRNA-containing cells express α3 subunit mRNAs. Although we are unable to rule out the possibility that an α3-containing nAChR subtype is expressed in these interneurons in addition to an α2-containing nAChR subtype, a possible explanation for this is that there are at least two different α2* nAChR types, one lacking the α3 subunit and the other containing the α3 subunit, which are differentially expressed in distinct subsets of interneurons. Defining the subunit composition of α2* nAChRs, which mediates non-desensitizing nicotine responses, remains a major challenge.
Are non-desensitizing α2* nAChRs an important component in hippocampal circuitry? Hippocampal CA1 pyramidal cells, which provide the major output of the hippocampus, receive two major excitatory synaptic inputs, the Schaffer collateral (SC) path and the temporoammonic (TA) path. One set of inputs comes through the hippocampal trisynaptic circuit [entorhinal cortex-dentate gyrus-CA3-CA1] and terminates on the proximal dendritic regions (the SC path). The other set of inputs represents a direct connection from the entorhinal cortex and terminates on the very distal dendritic regions (the TA path). At the SC path, nicotine promotes the induction of LTP (Fujii et al., 1999; Mann & Greenfield, 2003; Nakauchi et al., 2007a). In contrast, at the TA path, nicotine suppresses LTP induction (Nakauchi et al., 2007a). These opposing effects of nicotine are absent or greatly reduced in α2 knockout mice (Nakauchi et al., 2007a), suggesting not only that activation of α2* nAChRs mediates these effects, but also that this subtype is an important component in hippocampal circuitry. However, the locations of α2* nAChRs involved in these effects of nicotine and the underlying mechanisms remain unknown. Because there are relatively few neurons expressing α2 subunit mRNAs in the brain (Wada et al., 1989; Ishii et al., 2005), a non-desensitizing α2 nAChR subtype expressed in stratum oriens/alveus interneurons that we identified in the current study may be involved in these effects.
How can activation of α2* nAChRs in stratum oriens/alveus interneurons differentially modulate LTP induction at the SC and TA paths? Our previous findings showed that bath application of nicotine reversibly suppressed field excitatory postsynaptic potentials (EPSPs) at the TA path (Nakauchi et al., 2007a,b). This suppressive effect of nicotine at the TA path is mediated by the activation of non-α7 nAChRs and depends on the presence of GABAergic inhibition (Nakauchi et al., 2007b). Furthermore, a knife cut in the stratum oriens (parallel to stratum pyramidale), which severs the axons of interneurons projecting to the apical dendrites of pyramidal cells, prevents the suppressive effect of nicotine (Nakauchi et al., 2007b). These observations suggest that excitation of GABAergic interneurons in the stratum oriens is involved in nicotine's effects. Is this effect of nicotine mediated through the same interneurons described here? In the literature (Maccaferri et al., 2000; Maccaferri, 2005), there are at least three different subtypes of horizontally oriented interneurons (oriens-lacunosum moleculare cells, basket cells, and oriens-bistratified cells) that innervate pyramidal cells. In the current study, a few interneurons used for the recordings were passively filled with biocytin through the recording pipette for subsequent morphological identification. They showed morphological characteristics of oriens-lacunosum moleculare cells (unpublished results), which project to the most distal dendrites of pyramidal cells (Freund & Buzsaki, 1996; Maccaferri et al., 2000; Maccaferri, 2005). Although we need to expand these preliminary findings to determine whether nicotine selectively activates oriens-lacunosum moleculare cells, our observations suggest that nicotine excites oriens-lacunosum moleculare cells to cause sustained GABA release at the TA path termination zones. Because the induction of LTP at the TA path requires sufficient depolarization of pyramidal cells to activate both _N_-methyl-d-aspartate receptor receptors (NMDARs) and voltage-gated Ca2+ channels (Remondes & Schuman, 2003; Nakauchi et al., 2007b), increased GABA release at the TA path would be a straightforward mechanism for the suppression of field EPSPs (Yanovsky et al., 1997; Nakauchi et al., 2007b) and LTP induction (Nakauchi et al., 2007a). However, the facilitation of LTP induction at the SC path via the activation of oriens-lacunosum moleculare cell would need novel mechanisms such that increasing inhibition at the TA path facilitates LTP induction at the SC path (Remondes & Schuman, 2002). Because changes in neural activity at the TA path affect synaptic plasticity at the SC path (Remondes & Schuman, 2002), the possibility that nicotine-induced GABA release at the TA path modulates LTP induction at the SC path does in fact exist. Oriens-lacunosum moleculare cells make synaptic contacts with interneurons in the stratum lacunosum moleculare in addition to their more frequent contacts onto the distal dendrites of pyramidal cells (Katona et al., 1999; Elfant et al., 2008). The involvement of these interneurons, at least some of which express non-α7 nAChRs (McQuiston & Madison, 1999; Alkondon et al., 2000), in the nicotinic modulation of LTP induction remains possible.
Nicotine application also has a lamina-selective effect in the CA3 regions, causing changes in field EPSPs only in the stratum lacunosum moleculare (Giocomo & Hasselmo, 2005). The effect of nicotine in CA3 stratum lacunosum moleculare was characterized by a transient suppression of field EPSPs followed by an enhancement of field EPSPs (Giocomo & Hasselmo, 2005), which is different from the effect observed in CA1 stratum lacunosum moleculare (TA path; Nakauchi et al., 2007a,b). The different effects are most likely due to different nAChR subtypes expressed in the inhibitory circuits involved (Giocomo & Hasselmo, 2005; Nakauchi et al., 2007a,b).
In the present study, horizontally oriented interneurons in the stratum oriens/alveus responded to bath application of nicotine with at least three distinct firing patterns, generating the different temporal sequences of IPSPs in pyramidal cells. These firing patterns are similar to those observed with enhanced green fluorescent protein (EGFP)-positive, somatostatin-containing interneurons in the stratum oriens/alveus of transgenic mice (Minneci et al., 2007). Because at least some of these EGFP-positive interneurons are electrically coupled via gap junctions (Minneci et al., 2007), bath application of nicotine may excite a large population of interneurons in the stratum oriens/alveus. These interneurons may be connected to different postsynaptic membrane domains of pyramidal cells and other interneurons, thereby affecting the normal operation of local circuits.
Our current study shows that slow uIPSCs originating from feedforward interneurons at the SC path are depressed in the presence of nicotine-induced increases in the frequency of sIPSCs. uIPSCs from dendritic synapses rise more slowly than uIPSCs from perisomatic synapses due to dendritic filtering (Major et al., 1994; Jiang et al., 2000; Maccaferri et al., 2000) and molecular heterogeneity of postsynaptic GABAA receptors (Banks et al., 1998; Pearce, 1993), raising the possibility that nicotine selectively inhibits uIPSCs originating from dendritic synapses. There are multiple loci where nicotine may act to influence uIPSCs. It is possible that sustained activation of presynaptic non-α7 nAChRs suppresses evoked GABA release (Yamazaki et al., 2005) and/or activation of postsynaptic nAChRs depresses GABAA receptor-mediated responses via stimulating a Ca2+ signaling pathway (Wanaverbecq et al., 2007; Zhang & Berg, 2007). Alternatively, the activation of oriens-bistratified cells, whose axons primarily terminate in the stratum radiatum and overlap the dendritic trees of pyramidal cells (Maccaferri et al., 2000), might increase GABA release at the SC path. This might cause GABA spillover and lead to desensitization of postsynaptic GABAA receptors at neighboring inhibitory synapses, which receive synaptic input from feedforward interneurons. Activation of presynaptic GABAB autoreceptors (Davies et al., 1990) might also contribute to the nicotine-induced depression of uIPSCs originating from feedforward interneurons at the SC path. Reduced excitability of feedforward interneurons and/or suppression of GABAergic synaptic transmission onto pyramidal cells are potential mechanisms for enhancing NMDAR-mediated responses, which would promote LTP induction at the SC path (Bliss & Collingridge, 1993). Because activation of α2* nAChRs facilitates the induction of LTP (Nakauchi et al., 2007a), the observed suppressive effect of nicotine on slow uIPSCs may be involved in the nicotine-induced facilitation of LTP induction at the SC path. However, it remains to be determined whether activation of non-desensitizing α2* nAChRs in stratum oriens/alveus interneurons mediates the facilitative effect of nicotine on LTP induction.
Nicotine increases inhibitory baseline currents and suppresses phasic inhibition between nicotine-responding horizontally oriented interneurons and pyramidal cells. These effects of nicotine have similarities to the effects of kainate on oriens-lacunosum moleculare cells and other interneurons described previously, in which bath application of kainate increases the frequency of sIPSCs in CA1 pyramidal cells through an increase in interneuronal spiking while simultaneously suppressing evoked IPSCs (Cossart et al., 1998; Frerking et al., 1998, 1999). Kainate-induced suppression of evoked IPSCs appears to be mediated by increasing spontaneous GABA release and subsequently activating presynaptic GABAB autoreceptors, which depresses GABA release, and postsynaptic GABAA receptors, which increases shunt effects (Frerking et al., 1999). It is also interesting to note that activation of muscarinic receptors on interneurons increases the excitability of the interneurons, causing an increase in the frequency of sIPSCs and a decrease in evoked IPSCs in pyramidal cells (Pitler & Alger, 1992; Behrends & ten Bruggencate, 1993; Patil & Hasselmo, 1999). This suppression of evoked IPSCs involves the activation of presynaptic muscarinic receptors at GABAergic terminals (Behrends & ten Bruggencate, 1993). Based on the observed similarities between the effects of nicotine, kainate, and a muscarinic agonist, it is possible that nicotine-induced depression of phasic inhibition at synapses between nicotine-sensitive interneurons and pyramidal cells is mediated by the same mechanisms involving presynaptic GABAB autoreceptors, postsynaptic GABAA receptors, and presynaptic nAChRs. The functional effects of combined depolarization of interneurons (increasing background inhibition) and suppression of GABAergic synaptic transmission (decreasing evoked inhibition) are analyzed in computational modeling (Patil & Hasselmo, 1999).
In conclusion, the present data indicate that during nicotine exposure, local circuit activity is altered due to the sustained activation of a α2 nAChR subtype. This subtype is an important component in hippocampal circuitry and potentially serves as a molecular switch for gating information flow and synaptic plasticity by altering local GABAergic inhibition.
Acknowledgements
This research was supported by the National Institute on Drug Abuse (DA14542).
Abbreviations
ACh
acetylcholine
ACSF
artificial cerebrospinal fluid
AP5
2-amino-5-phosphopentanoate
DHβE
dihydro-β-erythroidine
DNQX
6, 7-dinitroquinoxaline-2, 3-dione
EGFP
enhanced green fluorescent protein
EPSPs
excitatory postsynaptic potentials
GABA
γ-aminobutyric acid
IPSPs
inhibitory postsynaptic potentials
IR-DIC
infrared-differential interference contrast
LTP
long-term potentiation
MLA
methyllycaconitine
nAChRs
nicotinic acetylcholine receptors
NMDARs
_N_-methyl-d-aspartate receptors
RT
reverse transcription
PCR
polymerase chain reaction
SC
Schaffer collateral
sIPSCs
spontaneous inhibitory postsynaptic currents
TA
temporoammonic
TTX
tetrodotoxin
uIPSCs
unitary inhibitory postsynaptic currents
References
- Abdulla FA, Bradbury E, Calaminici MR, Lippiello PM, Wonnacott S, Gray JA, Sinden JD. Relationship between up-regulation of nicotine binding sites in rat brain and delayed cognitive enhancement observed after chronic or acute nicotinic receptor stimulation. Psychopharmacology. 1996;124:323–331. doi: 10.1007/BF02247437. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur. J. Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Almeida LE, Randall WR, Albuquerque EX. Nicotine at concentrations found in cigarette smokers activates and desensitizes nicotinic acetylcholine receptors in CA1 interneurons of rat hippocampus. Neuropharmacology. 2000;39:2726–39. doi: 10.1016/s0028-3908(00)00156-8. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J. Neurophysiol. 2001;86:3043–55. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Albuquerque EX. NMDA and AMPA receptors contribute to the nicotinic cholinergic excitation of CA1 interneurons in the rat hippocampus. J. Neurophysiol. 2003;90:1613–1625. doi: 10.1152/jn.00214.2003. [DOI] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic receptor subtypes in rat hippocampal slices are differentially sensitive to desensitization and early in vivo functional up-regulation by nicotine and to block by bupropion. J. Pharmacol. Exp. Ther. 2005;313:740–750. doi: 10.1124/jpet.104.081232. [DOI] [PubMed] [Google Scholar]
- Banks MI, Li TB, Pearce RA. The synaptic basis of GABAA,slow. J. Neurosci. 1998;18:1305–1317. doi: 10.1523/JNEUROSCI.18-04-01305.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrends JC, ten Bruggencate G. Cholinergic modulation of synaptic inhibition in the guinea pig hippocampus in vitro: excitation of GABAergic interneurons and inhibition of GABA-release. J. Neurophysiol. 1993;69:626–629. doi: 10.1152/jn.1993.69.2.626. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Jacob P. Nicotine dependence and tolerance in man: pharmacokinetic and pharmacodynamic investigations. Prog. Brain Res. 1989;79:279–287. doi: 10.1016/s0079-6123(08)62487-5. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Buhler AV, Dunwiddie TV. Regulation of the activity of hippocampal stratum oriens interneurons by alpha7 nicotinic acetylcholine receptors. Neuroscience. 2001;106:55–67. doi: 10.1016/s0306-4522(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Buhler AV, Dunwiddie TV. alpha7 nicotinic acetylcholine receptors on GABAergic interneurons evoke dendritic and somatic inhibition of hippocampal neurons. J. Neurophysiol. 2002;87:548–557. doi: 10.1152/jn.00316.2001. [DOI] [PubMed] [Google Scholar]
- Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat. Neurosci. 1998;1:470–478. doi: 10.1038/2185. [DOI] [PubMed] [Google Scholar]
- Davies CH, Davies SN, Collingridge GL. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J. Physiol., (Lond.) 1990;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. J. Neurosci. 2007;27:10870–10877. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfant D, Pál BZ, Emptage N, Capogna M. Specific inhibitory synapses shift the balance from feedforward to feedback inhibition of hippocampal CA1 pyramidal cells. Eur. J. Neurosci. 2008;27:104–113. doi: 10.1111/j.1460-9568.2007.06001.x. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an α-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J. Neurosci. 1998a;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Buhler AV, Weiner JL, Dunwiddie TV. Synaptic potentials mediated via α-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J. Neurosci. 1998b;18:8228–8235. doi: 10.1523/JNEUROSCI.18-20-08228.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Strowbridge BW, Papke RL. Nicotinic receptors on local circuit neurons in dentate gyrus: a potential role in regulation of granule cell excitability. J. Neurophysiol. 2003;89:3018–3028. doi: 10.1152/jn.01036.2002. [DOI] [PubMed] [Google Scholar]
- Frerking M, Malenka RC, Nicoll RA. Synaptic activation of kainate receptors on hippocampal interneurons. Nat. Neurosci. 1998;1:479–486. doi: 10.1038/2194. [DOI] [PubMed] [Google Scholar]
- Frerking M, Petersen CC, Nicoll RA. Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus. Proc. Natl. Acad. Sci. USA. 1999;96:12917–12922. doi: 10.1073/pnas.96.22.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Fujii S, Ji Z, Morita N, Sumikawa K. Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Res. 1999;846:137–143. doi: 10.1016/s0006-8993(99)01982-4. [DOI] [PubMed] [Google Scholar]
- Giocomo LM, Hasselmo ME. Nicotinic modulation of glutamatergic synaptic transmission in region CA3 of the hippocampus. Eur. J. Neurosci. 2005;22:1349–1356. doi: 10.1111/j.1460-9568.2005.04316.x. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning: implications for addiction. Mol. Neurobiol. 2006;34:93–107. doi: 10.1385/MN:34:2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid S, Dawe GS, Gray JA, Stephenson JD. Nicotine induces long-lasting potentiation in the dentate gyrus of nicotine-primed rats. Neurosci. Res. 1997;29:81–85. doi: 10.1016/s0168-0102(97)00074-6. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend. 1993;33:23–29. doi: 10.1016/0376-8716(93)90030-t. [DOI] [PubMed] [Google Scholar]
- Ishii K, Wong JK, Sumikawa K. A comparison of α2 nicotinic acetylcholine receptor subunit mRNA expression in the central nervous system of rats and mice. J. Comp. Neurol. 2005;493:241–260. doi: 10.1002/cne.20762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Jiang L, Sun S, Nedergaard M, Kang J. Paired-pulse modulation at individual GABAergic synapses in rat hippocampus. J. Physiol. (Lond.) 2000;523:425–439. doi: 10.1111/j.1469-7793.2000.t01-1-00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Acsády L, Freund TF. Postsynaptic targets of somatostatin-immunoreactive interneurons in the rat hippocampus. Neuroscience. 1999;88:37–55. doi: 10.1016/s0306-4522(98)00302-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology. 1998;138:217–230. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Roberts JDB, Szucs P, Cottingham CA, Somogyi P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurons in rat hippocampus in vitro. J. Physiol. (Lond.) 2000;524:91–116. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri G. Stratum oriens horizontal interneurone diversity and hippocampal network dynamics. J. Physiol. (Lond.) 2005;562:73–80. doi: 10.1113/jphysiol.2004.077081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major G, Larkman AU, Jonas P, Sakmann B, Jack JJ. Detailed passive cable models of whole-cell recorded CA3 pyramidal neurons in rat hippocampal slices. J. Neurosci. 1994;14:4613–4638. doi: 10.1523/JNEUROSCI.14-08-04613.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EO, Greenfield SA. Novel modulatory mechanisms revealed by the sustained application of nicotine in the guinea-pig hippocampus in vitro. J. Physiol., (Lond.) 2003;551:539–550. doi: 10.1113/jphysiol.2003.045492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti Barros D, Ramirez MR, Dos Reis EA, Izquierdo I. Participation of hippocampal nicotinic receptors in acquisition, consolidation and retrieval of memory for one trial inhibitory avoidance in rats. Neuroscience. 2004;126:651–656. doi: 10.1016/j.neuroscience.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Matsumoto A, Enomoto T, Nishizaki T. Activation of nicotinic acetylcholine receptors induces long-term potentiation in vivo in the intact mouse dentate gyrus. Eur. J. Neurosci. 2000;12:3741–3747. doi: 10.1046/j.1460-9568.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J. Neurosci. 1999;19:2887–2896. doi: 10.1523/JNEUROSCI.19-08-02887.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minneci F, Janahmadi M, Migliore M, Dragicevic N, Avossa D, Cherubini E. Signaling properties of stratum oriens interneurons in the hippocampus of transgenic mice expressing EGFP in a subset of somatostatin-containing cells. Hippocampus. 2007;17:538–553. doi: 10.1002/hipo.20291. [DOI] [PubMed] [Google Scholar]
- Nakauchi S, Brennan RJ, Boulter J, Sumikawa K. Nicotine gates long-term potentiation in the hippocampal CA1 region via the activation of alpha2* nicotinic ACh receptors. Eur. J. Neurosci. 2007a;25:2666–2681. doi: 10.1111/j.1460-9568.2007.05513.x. [DOI] [PubMed] [Google Scholar]
- Nakauchi S, Yamazaki Y, Sumikawa K. Chronic nicotine exposure affects the normal operation of hippocampal circuits. NeuroReport. 2007b;18:87–91. doi: 10.1097/WNR.0b013e328011b883. [DOI] [PubMed] [Google Scholar]
- Papke RL, Bencherif M, Lippiello P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the alpha 7 subtype. Neurosci. Lett. 1996;213:201–204. doi: 10.1016/0304-3940(96)12889-5. [DOI] [PubMed] [Google Scholar]
- Parra P, Gulyás AI, Miles R. How many subtypes of inhibitory cells in the hippocampus? Neuron. 1998;20:983–993. doi: 10.1016/s0896-6273(00)80479-1. [DOI] [PubMed] [Google Scholar]
- Patil MM, Hasselmo ME. Modulation of inhibitory synaptic potentials in the piriform cortex. J. Neurophysiol. 1999;81:2103–2118. doi: 10.1152/jn.1999.81.5.2103. [DOI] [PubMed] [Google Scholar]
- Pearce RA. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993;10:189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- Perry DC, Xiao Y, Nguyen HN, Musachio JL, Dávila-García MI, Kellar KJ. Measuring nicotinic receptors with characteristics of alpha4beta2, alpha3beta2 and alpha3beta4 subtypes in rat tissues by autoradiography. J. Neurochem. 2002;82:468–481. doi: 10.1046/j.1471-4159.2002.00951.x. [DOI] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Cholinergic excitation of GABAergic interneurons in the rat hippocampal slice. J. Physiol., (Lond.) 1992;450:127–142. doi: 10.1113/jphysiol.1992.sp019119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, Cauli B, Tsuzuki K, Lambolez B, Rossier J, Audinat E. Selective excitation of subtypes of neocortical interneurons by nicotinic receptors. J. Neurosci. 1999;19:5228–5235. doi: 10.1523/JNEUROSCI.19-13-05228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Molecular mechanisms contributing to long-lasting synaptic plasticity at the temporoammonic-CA1 synapse. Learn. Mem. 2003;10:247–252. doi: 10.1101/lm.59103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Direct cortical input modulates plasticity and spiking in CA1 pyramidal neurons. Nature. 2002;416:736–740. doi: 10.1038/416736a. [DOI] [PubMed] [Google Scholar]
- Rogers SW, Gahring LC, Collins AC, Marks M. Age-related changes in neuronal nicotinic acetylcholine receptor subunit alpha4 expression are modified by long-term nicotine administration. J. Neurosci. 1998;18:4825–32. doi: 10.1523/JNEUROSCI.18-13-04825.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada S, Yamamoto C, Ohno-Shosaku T. Long-term potentiation and depression in the dentate gyrus, and effects of nicotine. Neurosci. Res. 1994;20:323–329. doi: 10.1016/0168-0102(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Sudweeks SN, Yakel JL. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat CA1 hippocampal neurons. J. Physiol. (Lond.) 2000;527:515–528. doi: 10.1111/j.1469-7793.2000.00515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida I, Halasy K, Szinyei C, Somogyi P, Buhl EH. Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J. Physiol., (Lond.) 1998;506:755–773. doi: 10.1111/j.1469-7793.1998.755bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J. Comp. Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wanaverbecq N, Semyanov A, Pavlov I, Walker MC, Kullmann DM. Cholinergic axons modulate GABAergic signaling among hippocampal interneurons via postsynaptic alpha 7 nicotinic receptors. J. Neurosci. 2007;27:5683–5693. doi: 10.1523/JNEUROSCI.1732-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsby P, Rowan M, Anwyl R. Nicotinic receptor-mediated enhancement of long-term potentiation involves activation of metabotropic glutamate receptors and ryanodine-sensitive calcium stores in the dentate gyrus. Eur. J. Neurosci. 2006;24:3109–3118. doi: 10.1111/j.1460-9568.2006.05187.x. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Peterson CG, Xu W, McIntosh JM, Paylor R, Beaudet AL, Collins AC, Marks MJ. Involvement of the alpha3 subunit in central nicotinic binding populations. J. Neurosci. 2002;22:2522–2529. doi: 10.1523/JNEUROSCI.22-07-02522.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Jia Y, Hamaue N, Sumikawa K. Nicotine-induced switch in the nicotinic cholinergic mechanisms of facilitation of long-term potentiation. Eur. J. Neurosci. 2005;22:845–860. doi: 10.1111/j.1460-9568.2005.04259.x. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Fujii S, Jia Y, Sumikawa K. Nicotine withdrawal suppresses nicotinic modulation of long-term potentiation induction in the hippocampal CA1 region. Eur. J. Neurosci. 2006b;24:2903–2916. doi: 10.1111/j.1460-9568.2006.05160.x. [DOI] [PubMed] [Google Scholar]
- Yanovsky Y, Sergeeva OA, Freund TF, Haas HL. Activation of interneurons at the stratum oriens/alveus border suppresses excitatory transmission to apical dendrites in the CA1 area of the mouse hippocampus. Neuroscience. 1997;77:87–96. doi: 10.1016/s0306-4522(96)00461-7. [DOI] [PubMed] [Google Scholar]
- Zhang J, Berg DK. Reversible inhibition of GABAA receptors by alpha7-containing nicotinic receptors on the vertebrate postsynaptic neurons. J. Physiol., (Lond.) 2007;579:753–763. doi: 10.1113/jphysiol.2006.124578. [DOI] [PMC free article] [PubMed] [Google Scholar]