Forced Abstinence Model of Relapse to Study Pharmacological Treatments of Substance Use Disorder (original) (raw)
. Author manuscript; available in PMC: 2010 Aug 4.
Published in final edited form as: Curr Drug Abuse Rev. 2009 May;2(2):184–194. doi: 10.2174/1874473710902020184
Abstract
Understanding and preventing relapse to drug use is one of the most difficult challenges faced by clinicians and practitioners in the struggle to help people remain abstinent. In this paper, we review basic preclinical research on forced abstinence periods that identify the neural substrates involved and neural adaptations that occur after a drug-free period. Our attention focuses on forced abstinence after self-administration because of its promise for translational research in the development of candidate medications to reduce relapse. This model requires subjects (often rats) to initially acquire drug self-administration. However, rather than extinguishing behavior with daily drug-free sessions as in the reinstatement model of drug seeking, subjects are removed from the self-administration situation and do not receive any exposure to the drug. Notably, the integrity of the drug-taking behavior and the drug-associated cues in the drug-taking environment are preserved because they are not experienced in the absence of the drug. Research shows time dependent increases in drug-seeking following forced abstinence periods. More so, neural substrates and adaptations within the mesocorticolimbic system and the nigrostriatal system have been identified that contribute to increased drug seeking following abstinence. From a translational perspective, behavioral and pharmacological treatment of substance use disorder often starts during this initial abstinence period (either forced or voluntary). The forced abstinence model simulates some of the features of this treatment situation and thus allows for the study of potential treatments that alter relapse of drug-seeking behaviors along with the accompanying neurobiological changes.
Keywords: cocaine, drug addiction, medication development, methamphetamine, reinstatement, self-administration, drug-seeking, reward
BACKGROUND
Drug addiction is defined as a “chronic relapsing brain disease that is characterized by compulsive drug seeking and use, despite harmful consequences” [1]. Understanding and preventing relapse to drug use is among the most difficult problems faced by clinicians and practitioners in the struggle to help people remain abstinent [2, 3]. Indeed, the National Institute on Drug Abuse (NIDA) reports that 40 to 60% of drug-addicted patients relapse [1, 4]. Given the high rate of relapse, there is still much room for improving treatment approaches to substance use disorder. Such improvements that increase rates and length of abstinence will come from theoretical and empirical advances in our understanding of the behavioral, neurobiological, and genetic mechanisms mediating relapse to drug use. One way such advances will be made is through the development and refining of preclinical animal models that capture key aspects of addiction, abstinence, and relapse. With this in mind, the present paper will focus on the “forced abstinence” model of relapse to self-administration behavior in rodents. As described in the following section, this underutilized model is of much interest because it simulates several key features of an individual with substance use disorder attempting to quit and/or receiving treatment. After a detailed description of the forced abstinence model, we will provide an overview of what is already understood about the behavioral and neural adaptations that occur following forced abstinence. We will then discuss the model within a translational approach to medication development research for the treatment of substance use disorder. Finally, throughout this review we will suggest some possible directions for future behavioral and neurobiological research using this model of relapse. Before discussing the forced abstinence model of relapse, however, it will be instructive to describe the widely employed reinstatement model.
TWO MODELS OF RELAPSE
Reinstatement
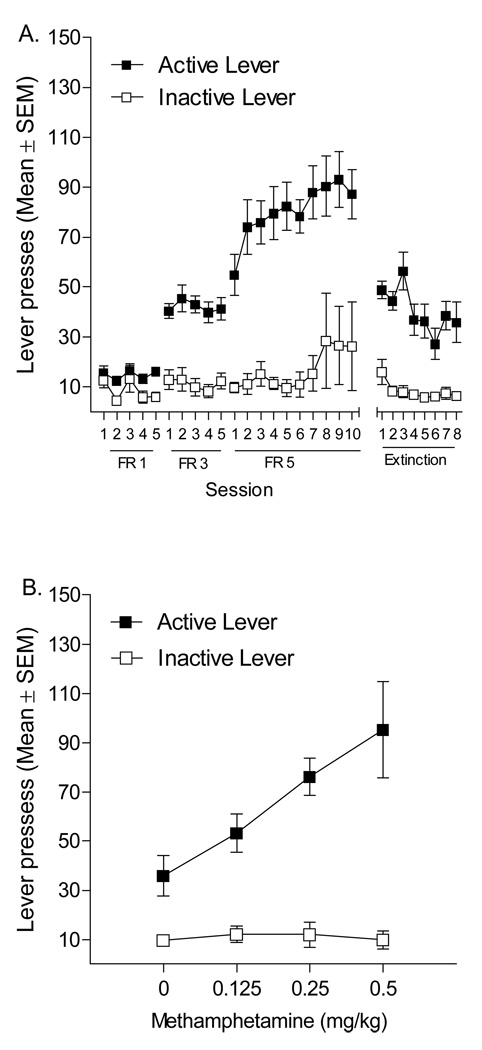
In the typical reinstatement study there are 3 phases: self-administration, extinction, and reinstatement (relapse). In the self-administration phase, subjects (often rats) prepared with indwelling jugular catheters are trained to press a lever resulting in an intravenous (IV) infusions of drug. As an exemplar, the left-most panel of Figure 1A shows data from our laboratory demonstrating acquisition and maintenance of methamphetamine self-administration. Briefly, in 1-h sessions pressing the active (drug) lever produced a 1-sec, 35.74-µl infusion of 0.05 mg/kg methamphetamine and a simultaneous 1-sec presentation of a cue light above the active lever; pressing a second inactive lever had no programmed consequence. To prevent accidental overdose both levers were retracted for 1 min immediately upon earning an infusion. After 5 days of this fixed ratio (FR) 1 schedule of reinforcement, the response requirement was increased to 3 presses per infusion on the active lever (FR3) for 5 days and then to an FR5 for 10 days. As can be seen in the graph, rats readily discriminated the active from the inactive lever and active lever presses increased to match the increases in response requirement. The extinction phase of a reinstatement experiment often commences once self-administration behavior has stabilized. In the extinction phase, the drug-taking behavior (lever pressing in our case) and some aspects of the drug-taking situation are presented (i.e., response contingent presentation of a cue light, retraction of the levers, activation of the infusion pump), but the drug is no longer available. The left panel of Figure 1A shows the extinction of active lever presses with all experimental procedures held constant (cue-light illumination, activation of the infusion pump, and retraction of levers) except that no methamphetamine was delivered. Responding on the active lever fell to below 50% of that during methamphetamine self-administration phase.
Figure 1.
This figure shows active and inactive lever presses (Mean ± SEM) for rats (n= 14) trained to self-administer 0.05 mg/kg/infusion methamphetamine. Panel A depicts the acquisition of the lever press response on FR 1, 3, and 5 schedules of reinforcement and the extinction of such a response. Panel B illustrates drug-primed reinstatement tested with 0, 0.125, 0.25, and 0.5 mg/kg methamphetamine IP.
Sometime following the last extinction session (e.g., 24 h) is the primary phase of interest—reinstatement. In the reinstatement phase, the rat is often exposed to a drug-associated stimulus (termed cue-induced reinstatement), a challenge injection of the drug that was self-administered (drug-induced reinstatement), or a stressor such as foot-shock (stress-induced reinstatement). These three triggers for reinstatement are mediated by different neuronal events and cue- and stress-induced reinstatement are sensitive to length of withdrawal periods [5]. In all models of reinstatement, the degree to which the extinguished drug-taking behavior re-emerges in the absence of any drug is taken as a measure of drug-seeking. Figure 1B shows the reinstatement of active lever pressing using a within-subject drug-primed reinstatement design. That is, rats received intraperitoneal (IP) injections of methamphetamine (0.125, 0.25, 0.5 mg/kg) or saline 5 min before a 1-h extinction test session. Doses were given in an ascending order on consecutive days. As can be seen, responding on the active lever, considered a measure of relapse to drug seeking, increased in dose-dependent manner. In fact, the 0.5 mg/kg methamphetamine dose reinstated responding to a pre-extinction level. This reinstatement model of drug-taking behavior has been widely used to study the behavioral and neurobiological substrates of drug relapse. There have been many excellent reviews of this literature [5–10], as well as published discussions of the pros and cons of this model [e.g., 11, 12]. Recapitulating these reviews and the discussion are beyond the scope and purpose of this article. Accordingly, we refer to the reader to the cited papers for a more detailed analysis of the reinstatement model of relapse.
Forced abstinence
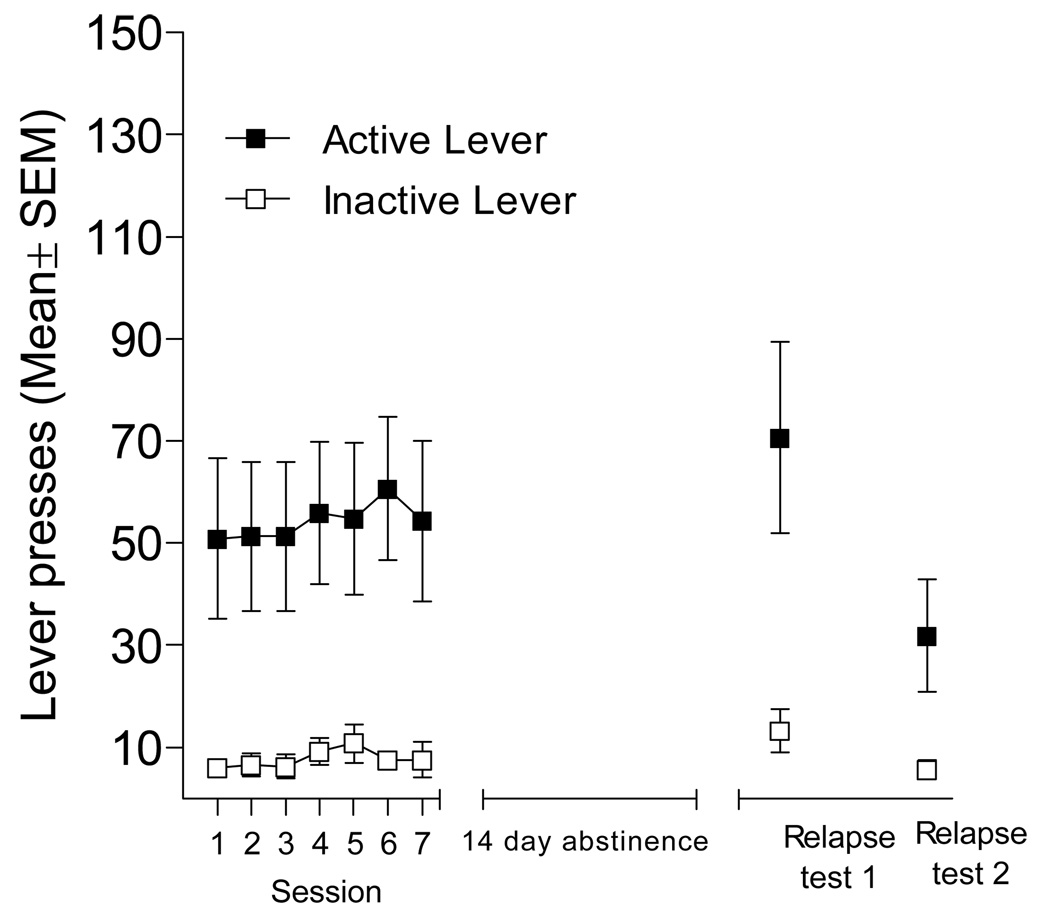
For the reinstatement model of relapse just described, drug abstinence (withdrawal) begins with the extinction phase. In the forced abstinence model, the drug abstinence period does not involve extinction of drug-taking behaviors or the situational stimuli in which drug taking occurs. Similar to other authors, we will use the term “forced abstinence” to describe this scenario [cf. 13–16]. Other terms used to describe this experimental situation in the literature include “withdrawal” or “no extinction” or “deprivation effect” [7, 17–19]. In this model of relapse, subjects acquire drug self-administration (i.e., lever pressing reinforced by IV infusion of drug) as described previously. However, rather than extinguishing the self-administration behavior with daily drug-free sessions, subjects are removed from the self-administration situation for a prescribed amount of time. Thus, at the time of relapse testing the drug-reinforced associations including lever press (i.e., drug-taking) behavior and environmental stimuli in the self-administration situation are fully intact. As an exemplar, Figure 2 shows the results of a recent forced abstinence experiment conducted in our laboratory. Rats acquired methamphetamine (0.05 mg/kg/infusion) self-administration and responding was stable for the last 7 days of training on an FR5 schedule of reinforcement (left-most graph). Rats were then kept in their home cage in the colony room for 14 days. In this abstinence period they were handled daily and received standard care (e.g., cage cleaning, water change, etc.). The two 1-h relapse tests were conducted under extinction conditions (24 h apart); identical to the earlier FR5 training except methamphetamine was not available. After 14 days of abstinence, active lever pressing (drug seeking) on the first extinction relapse test was comparable to the end of training (right-most panel of Figure 2). Albeit lower, active lever pressing still persisted above that of the inactive lever on the second relapse test.
Figure 2.
This figure shows active and inactive lever presses (Mean ± SEM) for rats (n=10) trained to self-administer 0.05 mg/kg/infusion methamphetamine. Following stable methamphetamine self-administration (last 7 days of drug access are shown), rats were given 14 days of forced abstinence followed by 2 consecutive relapse tests occurring 24 hr apart. These tests were conducted with contingent presentations of the stimulus complex on an FR5 schedule of reinforcement.
IMPORTANT FEATURES OF FORCED ABSTINENCE MODEL
Of interest from a translational research and treatment development perspective is that this forced abstinence model simulates some key, yet understudied, aspects of drug relapse in humans. Whether mandated (e.g., drug court), forced (e.g., hospitalization, incarceration), or voluntarily (e.g., check self into treatment clinic), a chronic drug user will be abstinent for some time period before a relapse opportunity presents itself. Further, in these example situations there is little to no opportunity for drug-taking behaviors or their associated stimuli to be extinguished. In fact, even if someone is entering treatment, typical manualized treatment and treatment-as-usual protocol for substance use disorder do not include forced extinction of drug-taking behaviors or the stimuli associated with drug taking [see 20, for a review]. As such, when these individuals are faced with a scenario that might precipitate relapse such as running into a fellow user or coming across drug paraphernalia, the past conditioning history with the stimuli associated with the drug and the drug-taking behaviors are fully intact. This is a critical point considering that drug users consistently report that exposure to drug-related stimuli (e.g., drug paraphernalia, videos of purchasing drugs, and imagery of drug use) evoke feelings of drug craving during abstinence from cocaine, morphine, ethanol, and nicotine [21–26]. For instance, human cocaine addicts shown cocaine-associated stimuli reported increased subjective feelings of craving in comparison to conditions when non-drug neutral or arousing stimuli were viewed [27]. More so, exposure to cocaine cues increased brain activity in sensory, motor, and cognitive-emotional processing areas and these physiological responses were better predictors of relapse than self-reported craving [28].
These changes in behavior, subjective states, and brain processes are thought to arise, at least in part, from Pavlovian conditioning processes. That is, situational stimuli and interoceptive states that occur in a contiguous manner with the nervous system effects of a drug will enter into an association such that the stimuli acquire the ability to evoke drug-related responses. According to this perspective, such widely used constructs as urges, cravings, withdrawal, and seeking are conceptualized as conditioned responses evoked by these stimuli. These conditioning processes have been given prominent roles in many theories of addiction and are thought to be critical for acquisition and maintenance of drug-taking behavior, as well as contributing to relapse [5, 29–36]. Preclinical models that provide a better understanding of the role of such stimuli and the behavioral, neurobiological, and genetic mechanisms mediating their influence on relapse will be important to study because such associative processes are active in abstaining individuals well after physiological withdrawal from the drug. We believe that the forced abstinence model is one such tool.
BEHAVIORAL FACTORS AND FORCED ABSTINENCE
In treatment studies, a key variable used to determine success is duration of abstinence once the intervention has started. Basic research with the forced abstinence model supports its use as measure of treatment success. That is, the extent and degree of relapse varies as a function of time since last drug experience. Consider the seminal work by Tran-Nguyen and colleagues [37] demonstrating that forced abstinence periods increased cocaine seeking. In this study, rats were trained to self-administer 0.75 mg/kg per 0.1-ml infusion of cocaine; each infusion earned on a variable ratio (VR) 5 schedule of reinforcement was briefly preceded by a stimulus complex consisting of a light and tone. Rats subsequently underwent exposure to one of three differing abstinence periods: 1 day, 1 week, or 1 month. Testing occurred after completion of the abstinence period in a 6-h test session and consisted of extinction, cue-induced and drug-primed reinstatement tests using a within-subject and within-session design. That is, upon return to the self-administration environment, the initial 2 h was an extinction session in which responding on the active lever was not reinforced. The following 2 h was cue-induced reinstatement in which the light plus tone compound stimulus previously paired with cocaine was presented independent of the rat’s behavior (non-contingent). The final 2 h of the test assessed drug-primed reinstatement with IP administration of 15 mg/kg cocaine. Cocaine seeking indexed as a difference between the responses on active and inactive levers was moderately elevated after 1 month of abstinence relative to 1 day during the extinction test and the drug-primed reinstatement test [37].
In a subsequent study, Grimm et al. [38] found that this enhanced relapse of cocaine seeking was time dependent. In that report, rats were re-exposed to the drug-taking environment following abstinence (1, 2, 4, 7, 15, 29, or 60 days) from cocaine using two different testing procedures. First, rats were tested in extinction (in the manner described above). That is, rats were able to press a cocaine-associated lever without access to drug or the stimuli previously paired with the drug. Second, they evaluated cue-induced reinstatement of cocaine-seeking using contingent (response dependent) presentations of the stimulus complex [cf. 38]. Both methods of testing produced a time-dependent enhancement of cocaine-seeking behavior. Specifically, 7 to 60 days of abstinence resulted in significantly greater responding on the active lever in comparison to 1, 2, or 4 days of abstinence. The increase in drug seeking following abstinence (or withdrawal) can be considered as an index of craving, defined by “a motivational state elicited by exposure to drug-associated cues that often precedes and accompanies drug-seeking” (p. 215) [17]. Because enhancement requires some time post drug self-administration to develop, this phenomenon has been termed “incubation” of drug-associated cues [38].
Related, there is a substantial literature showing that intake of alcohol increases following a period of forced abstinence. This alcohol deprivation effect has been suggested to model relapse to alcohol [39]. Typically, subjects are first given chronic access to alcohol via two (or more)-bottle choice or operant self-administration procedures [for a review see 40]. Forced abstinence follows in which alcohol is not available for a prescribed duration. Subsequent testing allows the animal to again have access to alcohol. Intake of alcohol increases relative to basal drinking levels. This effect has been widely replicated and observed in rats, mice, monkeys, and humans [for a review see 40]. In contrast to the incubation research described earlier, this model of relapse usually measures drug [alcohol] consumption rather than persistence of a non-reinforced drug-taking behavior. Thus, the alcohol deprivation effect is based on a measure of drug taking rather than drug seeking. However, testing on a progressive ratio schedule of reinforcement indicates that during periods of deprivation from alcohol rats show an increased motivation to work for alcohol [39]. When considered together, this research supports using forced abstinence models in the study of relapse to drug seeking.
Incubation has been observed with heroin [41], methamphetamine [42], and alcohol (i.e., alcohol deprivation effect, [6, 39,40]. Incubation does not seem to be permanent. Rather, it appears to follow an inverted U-shaped function. For example, heroin withdrawal impacted drug seeking during extinction testing depending upon the length of withdrawal; specifically, higher responding occurred on days 6, 12, or 25 but not after 1 or 66 days of withdrawal [41]. Cocaine withdrawal results in a progressive increase in drug seeking to cocaine-associated stimuli that peaks at 1 to 3 months but declines 6 months after withdrawal [38, 43]. Notably, the decline in cocaine seeking was only observed when cocaine was not present; drug-primed reinstatement occurred at all time periods [43]. For methamphetamine, time-dependent increases in extinction responding were observed at 21 and 51 days of withdrawal [42]; the descending limb of the incubation effect for methamphetamine has not been determined.
Importantly, increased cocaine seeking following forced abstinence relies on the integrity of the drug-paired cues being maintained during the abstinence period. A learning history that includes extinction of drug-associated stimuli prevented increased responding for cocaine-associated cues after a 21 day forced abstinence period [44], perhaps due to the ability of extinction training to regulate neuroadaptations occurring during withdrawal [45]. Indeed, direct comparisons between rats that undergo extinction training vs. forced abstinence confirm that extinction training abolishes, whereas abstinence promotes cocaine-seeking behavior [18, 19]. In these studies, rats were first trained to press a lever on a VR5 schedule of cocaine reinforcement (0.5 or 0.75 mg/kg per 0.1 ml, IV). Once cocaine intake stabilized, rats were divided into extinction or forced abstinence (i.e., no extinction) groups. For the extinction groups, extinction training began the day after self-administration training was completed and consisted of non-contingent [18] or contingent [19] presentations of the stimulus complex previously paired with cocaine. This training continued for 21 consecutive daily 2-h sessions. The forced abstinence groups, on the other hand, were transported to a different testing room and placed in an alternative environment than that in which the self-administration training occurred. On test, both methods of extinction training (contingent and non-contingent) eliminated cocaine-seeking behavior; forced abstinence, however, produced a robust increase in cocaine seeking [18, 19].
Notably, incubation is not specific to drug reinforcers. Responding reinforced by sucrose also follows time-dependent patterns with peak responding occurring at a much shorter time interval than that of cocaine [15, 17]. In brief, extinction tests allowed rats to press a sucrose-associated lever without access to sucrose or the stimuli previously paired with the sucrose. Under these conditions, incubation of sucrose seeking peaked after 7 days of withdrawal then declined at 1 month to levels seen after 1 day without sucrose [17]. When re-exposed to the stimulus complex associated with sucrose via a contingent response, sucrose seeking was increased only at 1 month [17]. Follow-up studies designed to characterized incubation of sucrose craving determined that shortened session lengths (i.e., 6 h reduced to 2 h) have no impact on incubation [15]. Given that motivation engendered by sucrose or other types of food reinforcers are subject to satiation, further tests determined whether satiation impacts incubation. In that study, rats were allowed unlimited access to sucrose solution in their home cages for 17 h before being placed back into the operant chambers. This sucrose pre-loading manipulation reduced sucrose seeking on day 1 and 30 during extinction testing but not during a test where responding produced presentation of the sucrose-paired cue. In fact, when an active lever press resulted in a contingent presentation of the sucrose-associated cue there were no differences in responding between rats that had 2 or 6 h session lengths or sucrose pre-loading — all groups increased responding for the cue at 7 and 30 days post abstinence [15]. This distinction is important because the overlap in drug and non-drug reinforced responding for associated cues may indicate common neural networks involved in incubation of reward seeking or craving. In fact, Grimm et al. [15] suggests “incubation of craving may be an exaggerated expression of an adaptive behavior–exaggerated due to neuroplastic changes mediated by the effects of high-density reward on brain reward circuitry” (p. 78).
The ability of an abstinence period to increase drug-seeking behavior has not been systematically characterized in regards to drug doses or reinforcement schedules. Doses ranging from 0.2 to 0.33 mg/dose and 0.35 to 1 mg/kg per infusion of cocaine have been used to study forced abstinence from self-administered cocaine with the most common schedule of reinforcement being an FR1. Table 1 provides a list of experimental parameters (e.g., cocaine dose, reinforcement schedule) from abstinence studies that have shown enhanced cocaine seeking after a forced abstinence period. The main focus of these papers has been on discovering the molecular mechanisms and/or neuroanatomical substrates that contribute to increased cocaine seeking in the presence of drug-associated cues following forced abstinence (see next section). As such, the preponderance of this research has focused on the duration of withdrawal to define parameters in which incubation occurs; the importance that other behavioral factors (i.e., drug dose, reinforcement schedule, experiences during abstinence, etc.) that might contribute to the extent of relapse post-abstinence have been left largely unexplored.
Table 1.
Overview of experimental parameters in forced abstinence studies with cocaine
| Reference | Cocaine dose | Reinforcementschedule | Abstinenceperiod | Behavioral test(s) following forced abstinence |
|---|---|---|---|---|
| Fuchs et al., 2006 [13] | 0.2 mg/dose | FR1 | 14 days | Extinction – responding on active lever had no scheduled consequence |
| Berglind et al., 2007 [72] | 0.2 mg/dose | FR1 | 22 h & 6 days | Extinction – contingent presentation of stimulus complex |
| Gal & Gyertyan, 2007 [74] | 0.25 mg/dose | FR1 | 3 weeks | Extinction – contingent presentation of stimulus complex |
| Hollander & Carelli, 2005 [66] | 0.33 mg/dose | FR1 | 1 day & 1 month | Extinction – responding on active lever had no scheduledDrug-primed reinstatement |
| Hollander & Carelli, 2007 [67] | 0.33 mg/dose | FR1 | 1 day & 1 month | Extinction – contingent presentations of stimulus complex |
| Ghitza et al., 2003 [65] | 0.35 mg/kg/inf | FR1 | 3–4 weeks | Extinction – presentation of a tone discriminitive stimulus |
| Neisewander et al., 2000 [18] | 0.5 mg/kg/inf | VR5 | 21 days | Extinction – non-contingent presentation of stimulus complex |
| Grimm et al., 2001[38] | 0.5 mg/kg/inf | FR1 | 1, 2, 4, 7, 15, 29,& 60 days | Extinction – responding on active lever had no scheduled consequenceExtinction – contingent presentation of stimulus complex |
| Graham et al., 2007 [14] | 0.5 mg/kg/inf | FR5 | 10 days | Extinction – responding on active lever had no scheduled consequence |
| Hearing et al., 2008 [70] | 0.6 mg/kg/inf | FR1 | 22 h & 15 days | Extinction – responding on active lever had no scheduled consequence |
| See et al., 2007 [16] | 0.6 mg/kg/inf | FR1 | 14 days | Extinction – responding on active lever had no scheduled consequence |
| Zavala et al., 2007 [19] | 0.75 mg/kg/inf | VR5 | 22 days | Extinction – contingent presentation of stimulus complex |
| Tran-Nguyen et al., 1989 [37] | 0.75 mg/kg/inf | VR5 | 1 day, 1 week, &1 month | Extinction – responding on active lever had no scheduled consequenceCue-induced reinstatement – non-contingent presentation of stimulus complexDrug-primed reinstatement |
| Lu et al., 2007 [75] | 0.75 mg/kg/inf | FR1 | 3 & 21 days | Extinction – contingent presentation of stimulus complex |
| Lu et al., 2004 [43] | 1.0 mg/kg/inf | FR1 | 24 h, 1, 3, & 6 months | Extinction – contingent presentation of stimulus complexDrug-primed reinstatement |
| Grimm et al., 2003 [63] | 1.0 mg/kg/inf | FR1 | 1, 30, & 90 days | Extinction – responding on active lever had no scheduled consequenceCue-induced reinstatement – contingent presentation of stimulus complex |
NEURAL FACTORS AND FORCED ABSTINENCE
The neurobiology of relapse has been reviewed and commented upon in detail [9, 10, 17, 46, 47]. These reviews include coverage of the neural substrates involved in relapse [9, 10, 46, 47], general molecular neuroadaptations [46, 48, 49], or more focused reviews of the glutamate [50] or dopamine systems [51], incubation of cocaine craving [17], or extinction training vs. forced abstinence periods [45]. Given the number and extent of these thoughtful and comprehensive reviews, this section will not duplicate these efforts. Instead, we will focus on research using forced abstinence and highlight the importance of the mesocorticolimbic circuitry and the neuroadaptations within that circuitry. We will not discuss research that provides extinction training during the abstinence period because it produces a different behavioral profile [18, 19] and regulates neuroadaptations related to relapse [45, 52, 53].
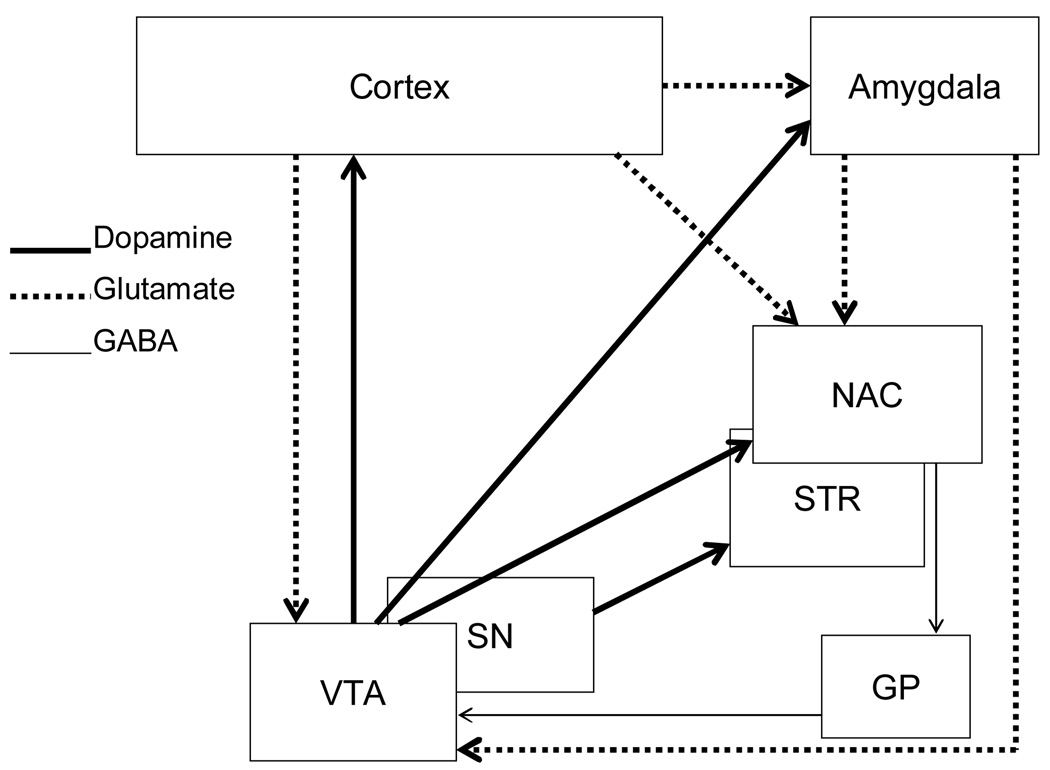
There are two main dopamine pathways by which various drugs of abuse exert their affective, incentive motivational, rewarding, and locomotor activating effects: the mesocorticolimbic and the nigrostriatal systems. The latter dopamine system consists of dopamine cell bodies located in the substantia nigra and project to the striatum (caudate/putamen) [54, 55]. The mesocorticolimbic system consists of the ventral tegmental area, nucleus accumbens, amygdala, and prefrontal cortex. These areas interact via dopaminergic and glutamatergic connections (see Figure 3). The connections include dopaminergic cell bodies located in the ventral tegmental area and project to the nucleus accumbens, prefrontal cortex, and amygdala [54–56]. Glutamatergic cortical output neurons project from the frontal cortex to the nucleus accumbens, ventral tegmental area, and amygdala [see 57 for a review]. The amygdala also sends glutamatergic input to the accumbens [see 58 and 50 for reviews]. Accumbal output neurons project from the nucleus accumbens to the ventral pallidum, and then from the ventral pallidum to the ventral tegmental area [see 59]. Many of these brain regions have been implicated in drug seeking (especially cocaine) and there is clear evidence of neuroadaptations in these regions when rats are re-exposed to drug-related stimuli after a forced abstinence period.
Figure 3.
This figure is a schematic representation of the mesocorticolimbic and nigrostriatal systems that are important in relapse to cocaine seeking following abstinence. Abbreviations: NAC, nucleus accumbens; STR, striatum (caudate and putamen); GP, globus pallidus; SN, substantia nigra; VTA, ventral tegmental area.
Evidence exists from inactivation studies to implicate the involvement of both dopamine pathways in cocaine seeking after forced abstinence. Inactivation of the dorsal lateral caudate putamen (dlCPu) with a baclofen and muscimol (GABAA and GABAB agonists, respectively) combination decreased cocaine seeking following 2 weeks of abstinence [13, 16]. In these experiments, rats trained to self-administer cocaine underwent 14 days of forced abstinence. Inactivation occurred immediately before testing in a relapse condition (i.e., the first day back in the self-administration environment). Additional extinction sessions were conducted for 4 days. During these sessions no further differences were apparent between inactivated and non-inactivated groups [16]. Thus, inactivation of the dlCPu only impacted cocaine seeking on the initial relapse test. Similarly, inactivation of the cell body regions (i.e., substantia nigra and ventral tegmental area) decreased active lever responding on a relapse test [16]. In contrast, similar inactivation of the nucleus accumbens core or shell had no impact on the initial relapse test but increased cocaine seeking on ensuing abstinence tests (i.e., extinction sessions) [16]. The differences observed between inactivation of the dlCPu and nucleus accumbens suggest that these substrates have distinct roles in cocaine seeking following relapse. Because the striatum is more commonly associated with habit formation, authors suggest that dlCPu may have a prominent role in the habitual components of relapse [13, 60, 61]. The role of the nucleus accumbens may be to process critical information about the relapse event because differences only emerged after the initial relapse event occurred [16].
Changes in cell firing and multiple neuroadaptations also occur in the nucleus accumbens. These adaptations include increased Fos expression [18, 19], protein kinase A activity [62], brain-derived neurotrophic factor [BDNF, 63], dopamine uptake [64], and expression of glutamate receptor subunits (GluR1, GluR2, and NMDAR1) depending upon the duration of abstinence period [62] (see Table 2). Time-dependent increases in the firing patterns of accumbal neurons also occur after forced abstinence from cocaine [65–67]. Electrophysiology studies have identified 4 specific patterns of short duration accumbal cell firing occurring within seconds of an operant response for cocaine that encode specific aspects of cocaine self-administration [68, 69]. One set of neurons increases firing rate immediately before the reinforced response, whereas others increase or decrease firing rates immediately after the completion of the response. Yet another set exhibits increased firing immediately before and after the response with a period of inhibition in between. In regards to abstinence, it appears that prolonged periods without the drug increases the percentage of all 4 populations of neurons in a manner that is regionally specific to the core of the nucleus accumbens [67]. Combined, these studies demonstrate the importance of the nucleus accumbens in relapse after abstinence from cocaine.
Table 2.
Overview of abstinence related brain changes from forced abstinence studies with cocaine
| Reference | Cocaine dose | Abstinence period | Abstinence related brain changes |
|---|---|---|---|
| Cortex | |||
| Hearing et al., 2008 [71] | 0.6 mg/kg/inf | 22 h & 15 days | Anterior cingulate – increased zif/268 and c-fos at 22 h and 15 days, increased Arc mRNA at 22 h |
| Hearing et al., 2008 [70] | 0.6 mg/kg/inf | 22 h & 15 days | Dorsomedial PFC – increased c-fos, zif/268, arc, and BDNF (BDNF only increased when drugassociated lever was available) at 22 h, all were increased at 15 days of abstinenceOrbital frontal – increased c-fos, zif/268, arc, and BDNF at 22 h and 15 days |
| Neisewander et al., 2000[18] | 0.5 mg/kg/inf | 21 days | Increased Fos in anterior cingulate |
| Zavala et al., 2007 [19] | 0.75 mg/kg/inf | 22 days | Increased Fos in prelimbic, infralimbic, orbital frontal, and anterior cigulate; increased percentage ofFos cells colabeled with GluR1 in anterior cigulate; and co-labled GluR5 in infralimbic cortex |
| Amygdala | |||
| Tran-Nguyen et al., 1989[37] | 0.75 mg/kg/inf | 1 day, 1 week, &1 month | Increased basal and extracellular dopamine concentrations following 1 month abstinence |
| Grimm et al., 2003 [63] | 1.0 mg/kg/inf | 1, 30, & 90 days | Increased BDNF but not NGFat 30 and 90 days |
| Neisewander et al., 2000[18] | 0.5 mg/kg/inf | 21 days | Increased Fos in basolateral amygdala |
| Nucleus Accumbens | |||
| Samuvel et al., 2008 [64] | 0.6 mg/kg/inf | 21 days | Increased dopamine uptake |
| Hearing et al., 2008 [71] | 0.6 mg/kg/inf | 22 h & 14 days | Increased zif/268 and Arc mRNA at 22 h and 15 days abstinence |
| Lu et al., 2003 [62] | 1 mg/kg/inf | 1, 30, or 90 days | Increases GluR1 and NMDAR1 on days 1 and 90, increased GluR2 levels on days 1 and 30 |
| Grimm et al., 2003 [63] | 1.0 mg/kg/inf | 1, 30, & 90 days | Increased BDNF but not NGFat 30 and 90 days |
| Neisewander et al., 2000[18] | 0.5 mg/kg/inf | 21 days | Increased Fos in core and shell |
| Zavala et al., 2007 [19] | 0.75 mg/kg/inf | 22 days | Increased Fos in core and shell; increased percentage ofFos co-labled with GluR1 in core |
| Caudate Putamen | |||
| Samuvel et al., 2008 [64] | 0.6 mg/kg/inf | 21 days | Increased dopamine uptake, increased surface expression of dopamine transporter, decreasedintracellular levels |
| Hearing et al., 2008 [71] | 0.6 mg/kg/inf | 22 h & 14 days | Increased zif/268 and Arc mRNA at 22 h and 15 days abstinence |
| Zavala et al., 2007 [19] | 0.75 mg/kg/inf | 22 days | Increased Fos in dorsal lateral caudate putamen |
| Ventral Tegmental Area | |||
| Lu et al., 2003 [62] | 1 mg/kg/inf | 1, 30, or 90 days | Increases NMDAR1 on days 1, 30 and 90, increased GluR2, TH, Cdk5 levels on day 1 |
| Grimm et al., 2003 [63] | 1.0 mg/kg/inf | 1, 30, & 90 days | Increased BDNF but not NGFat 30 and 90 days |
Labeling studies show Fos protein immunoreactivity is increased in cortical and subcortical areas involved in drug reward [18,19]. Fos is the protein product of the early response gene c-fos and is used as an index for neuronal activity because expression occurs when neurons fire. Rats that underwent forced abstinence from cocaine for 21 consecutive days had elevated Fos expression in the nucleus accumbens core and shell, basolateral amygdala, dentate gyrus, hippocampal CA1, and central gray relative to extinction and control (rats that had not received cocaine) groups [18]. A similar study replicated the observed increase in Fos in these areas and extends this result to cortical (i.e., prelimbic, infralimbic, orbital frontal, and anterior cingulated cortices) and limbic (i.e., dorsal caudate putamen, lateral and central amygdala, hippocampal CA3, and dorsal hippocampus) brain regions [19]. More so, rats that have undergone forced abstinence have an increased number Fos cells co-labeled with AMPA glutamate receptors (i.e, GluR1, GluR2/3, and GluR4) relative to extinction and control rats. Specifically, a greater percentage of Fos cells co-labeled with GluR1 receptors were identified in the anterior cingulate and nucleus accumbens shell and co-labeled with GluR5 in the infralimbic cortex. Combined these studies show the importance of regional and subtype specificity of AMPA glutamate receptors and Fos activation in cocaine-seeking following abstinence [19].
Other types of gene expression are changed in cortical areas following abstinence that have demonstrated the importance of cortical involvement in regards to relapse. For example, rats exposed to 22-h and 15-day abstinence from cocaine displayed an increase in activity-related gene expression (e.g., c-fos, zif/268, arc, and BDNF) in the medial prefrontal, orbital frontal cortices, and anterior cingulated cortex when re-introduced to the self-administration environment [70, 71]. More so, changes in gene expression (increased zif/268 and arc) in the caudate/putamen and nucleus accumbens support the involvement of the cortical-striatal and cortical-accumbens pathways in cocaine seeking after abstinence [71]. It is interesting to note that medial prefrontal cortical inactivation did not reduce cocaine-seeking behavior following abstinence [13], which would lead to speculation that cortical involvement may not have a primary role in cocaine seeking following abstinence. However, inactivation of cortical areas before testing would not circumvent molecular adaptations that occur early in the abstinence period. That is, changes in gene expression occur as early as 22 h into the withdrawal period, yet inactivation just before testing takes place about 2 weeks after those neuroadaptations occur [13, 70, 71]. Consider the case of cortical BDNF infusions. BDNF is a neurotrophic factor that helps to support the survival of neurons and encourages the growth and differentiation of new neurons and synapses. When cortical BDNF infusions occurred before a 6 day abstinence period, cocaine seeking in response to cocaine-paired cues decreased; however, when BDNF was infused after 6 days of abstinence, cocaine seeking was not affected [72]. Combined, these studies demonstrate involvement of cortical pathways in relapse following abstinence periods and even more specifically suggest that BDNF may be important for diminishing cocaine-seeking behavior early in the abstinence period.
In fact, BDNF increases in a time-dependent manner in brain areas that receive glutamatergic output neurons from the prefrontal cortex. That is, BDNF protein levels progressively increased in the nucleus accumbens, ventral tegmental area, and amygdala after 30 to 90 days abstinence from cocaine self-administration [63]–this time course parallels that of incubation of cocaine seeking [17, 38]. Given this relation between increased BDNF and incubation, follow-up studies assessed whether infusions of BDNF into these brain areas would increase cocaine seeking. A single infusion of BDNF into the ventral tegmental area after the last cocaine training session potentiated cocaine seeking after 10 and 30 days of abstinence relative to control animals [71]. Importantly, this increase was specific to the ventral tegmental area because BDNF infusions into the substantia nigra did not impact cocaine seeking on extinction tests. Repeated BDNF infusions into the nucleus accumbens shell also increased cocaine seeking after forced abstinence. Specifically, Graham and colleagues [14] injected BDNF into the nucleus accumbens shell immediately following each cocaine self-administration session for 5 consecutive days. Rats then underwent a 10-day forced abstinence period before being returned to the self-administration environment for 5 days of extinction testing. BDNF treated rats had an increase in cocaine lever pressing on the initial relapse test (i.e., the first day of extinction) that returned to control levels on the ensuing extinction sessions. Similar infusions into the caudate/putamen were without an effect. Combined, these findings indicate that BDNF in the mesocorticolimbic pathway has an important role in relapse following forced abstinence, but the precise role and the differences in cortical vs. subcortical BDNF need to be further elucidated.
The vast majority of the research on forced abstinence has focused on cocaine. This brief review of the neural processes clearly indicates a role for the dopaminergic and glutamatergic pathways of the mesocorticolimbic system for cocaine seeking following forced abstinence. Whether these findings extend to other abused substances still remains to be determined. Such research will be critical for a better understanding of relapse, as well as for the development of pharmacological interventions that aid in the prevention of relapse once a user is abstinent.
USING THE FORCED ABSTINENCE MODEL TO DEVELOP PHARMACOTHERAPIES
The research just described demonstrates that brain changes occurring during a forced abstinence period have a profound impact on cocaine-seeking behaviors. As such, some researchers have incorporated forced abstinence models to study mechanisms underlying relapse [42, 74, 75]. In these examples, rats initially undergo drug-self administration training, followed by a time period away from the drug-taking environment (i.e., without extinction sessions) before being returned to the self-administration situation. The mechanisms mediating relapse are tested by administration of a treatment drug just before the relapse test. For instance, Gal and Gyertyan [74] investigated the importance of dopamine D2 and D3 receptor subtypes in relapse following a 21-day abstinence period. Initially rats were allowed to self-administer cocaine (0.25 mg/kg per infusion) for 20 days. Following the 21 days of abstinence, separate groups of rats were injected with saline, SB-277011 (D3 antagonist), BR-897 (D3 partial agonist/D2 antagonist), or haloperidol (D2 antagonist) before placement into the self-administration situation. All drugs reduced cocaine-seeking (i.e., number of active lever presses) in comparison to controls, indicating a role of both D2 and D3 receptor subtypes in relapse to cocaine seeking following abstinence.
This approach has initiated our interest in expanding the forced abstinence model for use as preclinical screen for candidate medications for abused drugs. This approach would be accomplished by administering a candidate medication during the forced abstinence period. Treatment during abstinence simulates an important and common aspect of the treatment situation that has received very little empirical attention. Whether forced or voluntarily, chronic drug users often enter treatment to either gain or maintain abstinence. Often the behavioral and cognitive interventions, along with any medication, start early in abstinence. As such, assessment of potential medications (or behavioral interventions) within this time frame may provide a valuable tool to assess efficacy of treatment compounds for addiction. This strategy has been implemented in alcohol studies to access treatment medications during the alcohol deprivation effect [76, 77]. Such a maneuver has face validity with the clinical treatment situation and may serve to enhance the translational relevance of preclinical animal tests for medications to treat substance use disorders [see References 12, 17, and 39 for a critical discussion of the validity of this approach].
Very few studies exist (outside of the alcohol deprivation effect) that have administered a treatment medication during a forced abstinence period. Those studies available provide insight into the utility of such an approach. One recent Society for Neuroscience abstract assessed the impact of drug treatment during a methamphetamine abstinence period [78]. In that abstract, the maintenance self-administration schedule was an FR5 using 0.1 mg/kg/infusion of methamphetamine. Rats were then given forced abstinence for 12 days in which some rats were treated with 5 mg/kg of the antidepressant mirtazapine for the first 10 days of this abstinence period. This treatment regimen decreased active lever presses when rats were reintroduced to the self-administration situation. This finding demonstrates the potential efficacy of mirtazapine for treating methamphetamine seeking and provides encouraging data to further study this model within the context of medication development.
Notably, treatment efficacy may not be expressed upon return to the self-administration situation but may occur during ensuing extinction or reinstatement periods. Such an outcome was reported in a recent methamphetamine self-administration study. Davidson et al. [79] trained rats to self-administer 0.1 mg/kg/infusion methamphetamine on an FR5. When responding stabilized, rats remained in their home cages for 11 days. Treatment began on day 3 and lasted 5 days. Rats were injected subcutaneously with either vehicle or a pergolide/ondansetron combination. When returned to the self-administration situation groups did not differ during the first extinction test (i.e., a measure of relapse) or on the ensuing extinction sessions that measure the persistence of relapse-related behaviors. Following these extinction sessions, drug-primed reinstatement was tested by systemic injections of 0.1, 0.2, 0.3, and 0.4 mg/kg IP methamphetamine before placement into the self administration chamber. Reinstatement tests occurred in an ascending order on consecutive days. Drug-primed reinstatement differed between the pergolide/ondansetron and vehicle control groups with 0.3 and 0.4 mg/kg methamphetamine reinstating drug seeking only in the control group. Thus, pergolide/ondansetron treatment during abstinence blocked later drug-primed reinstatement, but did not alter initial relapse when the drug-associated stimuli and the response had yet to be experienced in the absence of methamphetamine.
Treatment during forced abstinence has also been shown to enhance relapse behavior (i.e., increased active lever responding). This outcome was recently reported in a cocaine self-administration study [80]. In that study, rats were trained to self-administer cocaine (0.25 mg/infusion). Once responding stabilized on an FR1 schedule a 5 day forced abstinence period was started. Rats remained in the colony and were treated with WIN 55,212-2, a cannabinoid agonist, during the abstinence period. Twenty-four to 48 h after stopping abstinence treatment, rats were reintroduced to the self-administration situation. Abstinence treatment with the low dose of WIN 55,212-2 (0.3 mg/kg) increased active lever responses in this relapse test; inactive lever presses were not altered. Higher doses of the cannabinoid agonist had no effect on relapse of cocaine-seeking behavior. This type of outcome is an important observation that suggests additional studies defining the empirical limits of such an effect. Although this study was not focused on medication development, such a finding with a potential medication would provide a cautionary note when considering the drug for the treatment of relapse to substance use. Indeed, the importance of determining the empirical limits of such and effect was demonstrated in an alcohol deprivation experiment. Chronic naloxone was administered via osmotic mini pumps (0, 0.5, or 1 mg/kg per hour) during a 14 day forced abstinence period [77]. Drug treatment occurred on days 10–14. This chronic dosing regimen increased rather than decreased alcohol consumption. In contrast, intermittent injections of naltrexone (2×5 mg/kg per day SC) reduced alcohol consumption.
CLOSING COMMENTS
Given the research indicating the pernicious nature of chronic drug use there is a need for the development of better treatment approaches including the use of medications to aid with the chronic relapsing nature of drug use. The overarching goal of this review was to focus on the behavioral and neurobiological mechanisms specific to forced abstinence (i.e., withdrawal) periods. Beyond this goal we wanted to emphasize studies that administer potential medications during these forced abstinence periods. This feature simulates an important aspect of a typical treatment situation where an individual often shows up at a treatment facility abstinent either by force (i.e., hospitalization or incarceration) or by choice. Findings that emerge from the basic research identifying neural substrates involved and neural adaptations that occur during forced abstinence will inform drug development of potential targets for treating substance abuse disorder.
Key Learning Objectives
Drug use is marked by chronic relapse despite negative consequences resulting from the resumption of drug use. This review presents an overview of behavioral models of relapse focusing on forced abstinence following drug self-administration. Further, suggestions are made to expand the current model to have a more translational perspective for medication development.
Future Research Questions
Do treatments administered during a forced abstinence period impact drug-seeking behavior upon re-exposure to the drug taking situation? Also, what mechanisms should be targeted for candidate medications administered during forced abstinence?
ACKNOWLEDGMENTS
We thank Jennifer Murray for help in collecting the data and for comments on an earlier version of the manuscript. R. A. Bevins was supported in part by a United States Public Health Service Grant DA018114 while writing this report. C. M. Reichel was supported by DA023283 while preparing this manuscript for publication. This research was partially funded by Promunne Inc and monies from Nebraska Tobacco Settlement Biomedical Research Development Funds.
References
- 1.Drugs, Brains, and Behavior: The Science of Addiction. National Institute of Health Publication Number 07-5605 [Google Scholar]
- 2.Mendelson JH, Mello NK. Management of cocaine abuse and dependence. The New England Journal of Medicine. 1996;334:965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien CP. A range of research-based pharmacotherapies for addiction. Science. 1997;278:66–70. doi: 10.1126/science.278.5335.66. [DOI] [PubMed] [Google Scholar]
- 4.McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implication for treatment, insurance, and outcomes evaluation. JAMA. 284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 5.Shaham Y, Shalev U, Lu L, de Wit H, Stewart J. The reinstatement model of relapse: histroy, methodology and major findings. Psychopharmacol. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 6.Panlilio LV, Goldberg SR. Self-administration of drugs in animals and humans as a model and an investigative tool. Addiction. 2007;102:1863–1870. doi: 10.1111/j.1360-0443.2007.02011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addiction Biology. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 8.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. Journal of Psychiatry & Neuroscience. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart J. Pathways to relapse: Reinitiation of drug seeking after abstinence. In: Bevins RA, Bardo MT, editors. Nebraska Symposium on Motivation: Vol. 50. Motivational factors in the etiology of drug abuse; University of Nebraska Press; Lincoln, NE. 2004. pp. 197–234. [PubMed] [Google Scholar]
- 11.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: An assessment of the validity of the reinstatement procedure. Psychopharmacol. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacol. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: A critical role for the dorsolateral caudate-putamen. J of Neuroscience. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neuroscience. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- 15.Grimm JW, Fyall AM, Osincup DP. Incubation of sucrose craving: Effects of reduced training and sucrose pre-loading. Physiol Behav. 2005;84:73–79. doi: 10.1016/j.physbeh.2004.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacol. 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- 17.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacol. 2004;47:214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Neisewander JL, Baker DA, Fuchs RA, et al. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J of Neuroscience. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zavala AR, Biswas S, Harlan RE, Neisewander JL. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145:438–452. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling W, Rawson R, Shoptaw S, Ling W. Management of methamphetamine abuse and dependence. Current Psychiatry Reports. 2006;8:345–354. doi: 10.1007/s11920-006-0035-x. [DOI] [PubMed] [Google Scholar]
- 21.Ehrman R, Robbins S, Childress A, O'Brien C. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacol. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- 22.Franklin TR, Wang Z, Wang J, et al. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: a perfusion fMRI study. Neuropsychopharmacol. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- 23.Kilts CD, Schweitzer JB, Quinn CK, et al. Neural activity related to drug craving in cocaine addiction. Archives of General Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 24.Robbins SJ, Ehrman RN, Childress AR, O'Brien CP. Relationships among physiological and self-report responses produced by cocaine-related cues. Addictive Behaviors. 1997;22:157–167. doi: 10.1016/s0306-4603(96)00007-x. [DOI] [PubMed] [Google Scholar]
- 25.Sell LA, Morris JS, Bearn J, Frackowiak RSJ, Friston KJ, Dolan RJ. Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug and Alcohol Dependence. 2000;60:207–216. doi: 10.1016/s0376-8716(99)00158-1. [DOI] [PubMed] [Google Scholar]
- 26.Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 27.Johnson BA, Chen YR, Schmitz J, Bordnick P, Shafer A. Cue reactivity in cocaine-dependent subjects: effects of cue type and cue modality. Addict Behav. 1998;23:7–15. doi: 10.1016/s0306-4603(97)00014-2. [DOI] [PubMed] [Google Scholar]
- 28.Kosten TR, Scanley BE, Tucker KA, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacol. 2006;31:644–650. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- 29.Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behavioral Neuroscience. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- 30.Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behavioral and Cognitive Neuroscience Reviews. 2004;3:143–158. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- 31.Di Chiara G. Drug addiction as dopamine-dependent associative learning disorder. E J Pharmacol. 1999;375:13–30. doi: 10.1016/s0014-2999(99)00372-6. [DOI] [PubMed] [Google Scholar]
- 32.Koob GF. Allostatic view of motivation: Implications for psychopathology. In: Bevins RA, Bardo MT, editors. Nebraska Symposium on Motivation, 50, Motivational Factors in the Etiology of Drug Abuse; University of Nebraska Press; Lincoln. 2004. pp. 1–18. [PubMed] [Google Scholar]
- 33.O’Brien CP, Childress AR, Mclellan TA, Ehrman R. Classical conditioning in drug dependent humans. Ann. N. Y. Acad. Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 34.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 35.Siegel S, Ramos BM. Applying laboratory research: drug anticipation and the treatment of drug addiction. Exper and Clin Psychopharmacol. 2002;10:162–183. doi: 10.1037//1064-1297.10.3.162. [DOI] [PubMed] [Google Scholar]
- 36.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 37.Tran-Nguyen LTL, Fuchs RA, Coffey GP, et al. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdale during cocaine withdrawal. Neuropsychopharmacol. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- 38.Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spanagel R, Holter SM. Pharmacological validation of a new animal model of alcoholism. J Neural Trans. 2000;107:669–680. doi: 10.1007/s007020070068. [DOI] [PubMed] [Google Scholar]
- 40.Le AD, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol. Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 41.Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacol. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- 42.Shepard JD, Bossert JM, Liu SY, Shaham The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 43.Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods: Different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacol. 2004;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- 44.Di Ciano P, Everitt BJ. Reinstatement and spontaneous recovery of cocaine-seeking following extinction and different durations of withdrawal. Behav Pharmacol. 2002;13:397–405. doi: 10.1097/00008877-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Self DW, Choi KH, Simmons D, Walker JR, Smajula CS. Extinction training regulates neuroadaptive responses to withdrawal from chronic cocaine self-administration. Learning Memory. 2004;11:648–657. doi: 10.1101/lm.81404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rebec GV, Sun WL. Neuronal substrates of relapse to cocaine-seeking behavior: role of prefrontal cortex. J Exp Analysis Behav. 2005;84:653–666. doi: 10.1901/jeab.2005.105-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.See RE. Neural substrates of cocaine-cue associations that trigger relapse. Europ J Pharmacol. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 48.Shaham Y, Hope BT. The role of neuroadaptations in relapse to drug seeking. Nat Neuroscience. 2005;8:1437–1439. doi: 10.1038/nn1105-1437. [DOI] [PubMed] [Google Scholar]
- 49.Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. British J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalivas PW. Glutamate systems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacol. 2004;47:242–255. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt EF, Sutton MA, Schad CA, et al. Extinction training regulates tyrosine hydroxylase during withdrawal from cocaine self administration. J Neuroscience. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-07-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sutton MA, Schmidt EF, Choi KH, et al. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- 54.Fallon JH. Topographic organization of ascending dopaminergic projections. Ann NY Acad Sci. 1988;537:216–227. doi: 10.1111/j.1749-6632.1988.tb42093.x. [DOI] [PubMed] [Google Scholar]
- 55.Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiologica Scandinavica. 1971;367:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- 56.Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- 57.Tzschentke TM. Pharmacology and behavioral pharmacology of the mesocortical dopamine system. Prog Nerurobiol. 2001;63:241–320. doi: 10.1016/s0301-0082(00)00033-2. [DOI] [PubMed] [Google Scholar]
- 58.Baker DA, Cornish JL, Kalivas PW. Glutamate and dopamine interactions in the motive circuit. In: Herman BH, editor. Glutamate and Addiction. Totowa NJ: Humana Press; 2003. pp. 143–156. [Google Scholar]
- 59.Kalivas PW, Churchill L, Romanides A. Involvement of the pallidal-thalamocortical circuit in adaptive behavior. Ann NY Acad Sci. 1999;877:64–70. doi: 10.1111/j.1749-6632.1999.tb09261.x. [DOI] [PubMed] [Google Scholar]
- 60.Faure A, Haberland U, Conde F, El Massioui N. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neuroscience. 2005:2771–2780. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jog MS, Kubota Y, Connolly CI, Hillegaart V, Graybiel AM. Building neural representations of habits. Science. 1999;286:1745–1749. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- 62.Lu L, Grimm JW, Shaham Y, Hope BT. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochemistry. 2003;86:1604–1613. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- 63.Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: Implications for incubation of cocaine craving. J Neuroscience. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Samuvel DJ, Jayanthi LD, Manohar S, Kaliyaperumal K, See RE, Ramamoorthy S. Dysregulation of dopamine transporter trafficking and function after abstinence from cocaine self-administration in rats: Evidence for differential regulation in caudate putamen and nucleus accumbens. J Pharmacol Exper Therap. 2008;325:293–301. doi: 10.1124/jpet.107.130534. [DOI] [PubMed] [Google Scholar]
- 65.Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neuroscience. 2003;23:7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hollander JA, Carelli RM. Abstinence from cocaine self-administration heightens neural encoding of goal-directed behaviors in the accumbens. Neuropsychopharmacol. 2005;30:1464–1474. doi: 10.1038/sj.npp.1300748. [DOI] [PubMed] [Google Scholar]
- 67.Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neuroscience. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carelli RM, Deadwyler SA. A comparison of nucleus accumbens neuronal firing patterns during cocaine self-administration and water reinforcement in rats. J Neuroscience. 1994;14:7735–7746. doi: 10.1523/JNEUROSCI.14-12-07735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carelli RM, Deadwyler SA. Cellular mechanisms underlying reinforcement-related processing in the nucleus accumbens: electrophysiological studies in behaving animals. Pharmacology, Biochemistry, and Behavior. 1997;57:495–504. doi: 10.1016/s0091-3057(96)00442-x. [DOI] [PubMed] [Google Scholar]
- 70.Hearing MC, Miller SW, See RE, McGinty Relapse to cocaine seeking increases activity-regulated gene expression differentially in the prefrontal cortex of abstinent rats. Psychopharmacol. 2008;198:77–91. doi: 10.1007/s00213-008-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hearing MC, See RE, McGinty JF. Relapse to cocaine-seeking increases activity-regulated gene expression differentially in the striatum and cerebral cortex of rats following short or long periods of abstinence. Brain Struct Funct. 2008 May 17; doi: 10.1007/s00429-008-0182-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berglind WJ, See RE, Fuchs RA, et al. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Europ J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- 73.Lu L, Dempsey J, Liu SY, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasing potentiation of cocaine seeking after withdrawal. J Neurosci. 2004;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gal K, Gyertyan I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug Alcoh Depend. 2006;81:63–70. doi: 10.1016/j.drugalcdep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 75.Lu L, Uejina JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR2/3 agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 76.Heyser CJ, Moc K, Koob GF. Effects of naltrexone alone and in combination with acamprosate on the alcohol deprivation effect in rats. Neuropsychopharmacol. 2003;28:1463–1471. doi: 10.1038/sj.npp.1300175. [DOI] [PubMed] [Google Scholar]
- 77.Holter SM, Spanagel R. Effects of opiate antagonist treatment on the alcohol deprivation effect in long-term ethanol-experienced rats. Psychopharmacol. 1999;145:360–369. doi: 10.1007/s002130051069. [DOI] [PubMed] [Google Scholar]
- 78.Fox RG, Dhonnchadha NIC, Cunningham KA, Specio SE, Napier T. Program No. 813.14. 2007 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2007. Self-administration of methamphetamine: reduction in drug-seeking during forced abstinence following repeated mirtazapine treatment. Online. [Google Scholar]
- 79.Davidson CD, Gopalan R, Ahn C, et al. Reduction in methamphetamine induced sensitization and reinstatement after combined pergolide plus ondansetron treatment during withdrawal. Europ J Neuroscience. 2007;565:113–118. doi: 10.1016/j.ejphar.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 80.González-Cuevas G, Aujla H, Martin-Fardon R, López-Moreno JA, Navarro M, Weiss F. Subchronic cannabinoid agonist (WIN 55,212-2) treatment during cocaine abstinence alters subsequent cocaine seeking behavior. Neuropsychopharmacol. 2007;32:2260–2266. doi: 10.1038/sj.npp.1301365. [DOI] [PubMed] [Google Scholar]