Coxiella burnetii Phase I and II Variants Replicate with Similar Kinetics in Degradative Phagolysosome-Like Compartments of Human Macrophages (original) (raw)
Abstract
Coxiella burnetii infects mononuclear phagocytes, where it directs biogenesis of a vacuolar niche termed the parasitophorous vacuole (PV). Owing to its lumenal pH (∼5) and fusion with endolysosomal vesicles, the PV is considered phagolysosome-like. However, the degradative properties of the mature PV are unknown, and there are conflicting reports on the maturation state and growth permissiveness of PV harboring virulent phase I or avirulent phase II C. burnetii variants in human mononuclear phagocytes. Here, we employed infection of primary human monocyte-derived macrophages (HMDMs) and THP-1 cells as host cells to directly compare the PV maturation kinetics and pathogen growth in cells infected with the Nine Mile phase I variant (NMI) or phase II variant (NMII) of C. burnetii. In both cell types, phase variants replicated with similar kinetics, achieving roughly 2 to 3 log units of growth before they reached stationary phase. HMDMs infected by either phase variant secreted similar amounts of the proinflammatory cytokines interleukin-6 and tumor necrosis factor alpha. In infected THP-1 cells, equal percentages of NMI and NMII PVs decorate with the early endosomal marker Rab5, the late endosomal/lysosomal markers Rab7 and CD63, and the lysosomal marker cathepsin D at early (8 h) and late (72 h) time points postinfection (p.i.). Mature PVs (2 to 4 days p.i.) harboring NMI or NMII contained proteolytically active cathepsins and quickly degraded Escherichia coli. These data suggest that C. burnetii does not actively inhibit phagolysosome function as a survival mechanism. Instead, NMI and NMII resist degradation to replicate in indistinguishable digestive PVs that fully mature through the endolysosomal pathway.
Coxiella burnetii is a wide-ranging facultative intracellular bacterium (37) that causes the zoonosis Q fever, a disease that generally manifests as an acute, debilitating flu-like illness (34). A small developmental form of the pathogen confers pronounced environmental stability (21), a characteristic that facilitates aerosol transmission of the organism. Human infection primarily occurs via inhalation of contaminated material generated by domestic livestock, the primary animal reservoirs of C. burnetii. The organism is highly infectious, with the infective dose approaching one bacterium (35). The main target cells of C. burnetii during natural infection are mononuclear phagocytes, such as alveolar macrophages (27, 48). Consequently, infection of cultured primary or immortalized human monocytes/macrophages is considered the most physiologically relevant in vitro model of _C. burnetii_-host cell interactions (52). In human mononuclear phagocytes and other cell types, C. burnetii replicates within a membrane-bound compartment termed the parasitophorous vacuole (PV) (52).
The genetic intractability of C. burnetii has limited the availability of knowledge of the pathogen's virulence mechanisms and host-pathogen interactions. Currently, lipopolysaccharide (LPS) is the only confirmed virulence factor of the organism (35). Full-length LPS is produced by virulent phase I organisms isolated from natural sources and infections, typified by the Nine Mile phase I variant (NMI) reference strain (strain RSA493). Serial passage of phase I C. burnetii in embryonated eggs or tissue culture selects for phase II bacteria, which produce a severely truncated LPS that lacks the O antigen and some core sugars (20, 35). A cloned phase II variant originating from NMI, termed Nine Mile phase II variant (NMII; strain RSA439, clone 4), has an ∼26-kb chromosomal deletion that eliminates multiple genes involved in LPS biosynthesis (24, 35) and is avirulent for immunocompetent mice and guinea pigs (4, 35). NMII is a biosafety level 2 organism, while biosafety level 3 is required for all other C. burnetii strains.
A conundrum in C. burnetii biology is whether the virulence properties of NMI and NMII are associated with the ultimate maturation state of their respective PVs in resting primary human monocytes and/or macrophages (17, 52). PVs of both phase variants decorate with the late endosomal/lysosomal markers lysosome-associated membrane protein 1 (LAMP-1), CD63 (LAMP-3), and the vacuolar type H+ ATPase and are moderately acidic (pH ∼5) (17). However, on the basis of the minimal recruitment of cathepsin D and the small GTPase Rab7, it has been suggested that maturation of PVs containing NMI stalls at a late endosomal stage (17). This trafficking behavior correlates with pathogen survival but in most cases little to no replication (8, 17, 18, 23). Conversely, PVs sheltering NMII are proposed to fully mature into a bactericidal phagolysosomal compartment that contains active lysosomal hydrolases (17, 18, 23).
In conflict with the phase-specific trafficking model in human mononuclear phagocytes is the observation that NMI and NMII both grow robustly in CD63-positive PVs of human monocyte-derived dendritic cells (DCs) (47). Moreover, phase variants productively infect THP-1 cells and primary nonhuman primate alveolar macrophages, where they induce similar host cell prosurvival responses (53, 54). In animal cell lines, NMI and NMII replicate equally in vacuoles that fully mature to contain lysosomal markers (5). For example, PVs harboring replicating NMI in murine L-929 fibroblasts and J774 macrophages clearly fuse with lysosomes, as evidenced by the presence of active acid phosphatase and 5′-nucleotidase (2, 11, 25). NMII has also recently been demonstrated to replicate in a cathepsin D-positive vacuole in human HeLa epithelial cells (1).
Because multiple laboratories have recently employed avirulent NMII to investigate C. burnetii infection of host cells (1, 30, 38, 50, 54), it is important to ascertain the degree to which in vitro infection by NMII recapitulates infection by virulent NMI, particularly with respect to PV maturation in human mononuclear phagocytes. To this end, we directly compared the growth kinetics and PV maturation of NMI and NMII in human monocyte-derived macrophages (HMDMs) and phorbol 12-myristate 13-acetate (PMA)-differentiated THP-1 cells, which accurately mimic the properties of human primary macrophages (29). Additionally, the cytokine responses of infected HMDMs were examined, as were the degradative properties and cathepsin activities of PVs. We conclude that human macrophages respond similarly to NMI and NMII C. burnetii by delivering organisms to phenotypically indistinguishable, degradative, phagolysosome-like compartments.
MATERIALS AND METHODS
C. burnetii and mammalian cell culture.
C. burnetii NMI (strain RSA493) and NMII (strain RSA439, clone 4) were propagated in African green monkey kidney (Vero) cells (CCL-81; ATCC, Manassas, VA). Chlamydia trachomatis LGV-434, serotype L2, was cultivated in HeLa 229 cells (CCL-2; ATCC). Bacteria were isolated from infected cells by Renografin density gradient centrifugation, as described previously (13, 45), and stored at −80°C. The full-length and truncated LPSs of NMI and NMII, respectively, were validated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining, as described previously (7). Recent work in our laboratory has also confirmed the virulence and avirulence of NMI and NMII, respectively, for C57BL/6 mice (44; J. G. Shannon and R. A. Heinzen, unpublished data).
Human monocyte-like (THP-1) cells (TIB-202; ATCC) were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (HyClone, Logan, UT) at 37°C in 5% CO2. Prior to infection, THP-1 cells were differentiated into adherent, macrophage-like cells by treating freshly plated cells with PMA (200 nM; EMD Biosciences, San Diego, CA) for 24 h. Human monocytes and HMDMs were derived from human peripheral blood mononuclear cells (PBMCs) from whole blood of human donors. Buffy coats enriched in PBMCs were isolated by centrifugation of whole blood through a Ficoll-Paque Plus (Amersham Pharmacia Biotech, Uppsala, Sweden) density gradient. PBMCs were enriched for monocytes (CD14+ cells) using a RossetteSep monocyte enrichment kit (Stem Cell Technologies, Vancouver, BC, Canada). To differentiate monocytes into macrophages, monocytes were resuspended at 1 × 106 cells per ml in macrophage medium (RPMI plus Glutamax [Invitrogen], 10% fetal bovine serum [FBS] containing recombinant human macrophage colony-stimulating factor [M-CSF] at 50 ng/ml [Peprotech, Rocky Hill, NJ]) and cultured for 6 days with addition of fresh cytokines on day 3. On day 6, the culture medium was removed and the cells were washed with phosphate-buffered saline (PBS; 1 mM KH2PO4, 155 mM NaCl, 3 mM Na2HPO4, pH 7.4). Adherent HMDMs were detached by incubation on ice in PBS, followed by gentle scraping. The cells were then plated in 24-well plates at a density of 1 × 105 cells per well in macrophage medium.
One-step growth curves.
The growth kinetics of C. burnetii NMI and NMII were established by using HMDMs or THP-1 cells (1 × 105) cultivated in 24-well tissue culture plates (Corning Inc., Charlotte, NC). Here and elsewhere, cells were infected with NMI and NMII at multiplicities of infection (MOIs) of 100 and 10, respectively, by addition of organisms to cell culture medium. This time was considered 0 h postinfection (p.i.). A lower MOI was used for NMII because this variant is roughly 10-fold more infectious for cultured cells than NMI (52). The MOI was based on C. burnetii genome equivalents, as described previously (14). Unless otherwise noted, the inoculum was left on the cells for 24 h, and then the cultures were washed and replenished with fresh medium.
C. burnetii replication was determined using quantitative PCR (qPCR) of genome equivalents. Samples were harvested from triplicate wells for each time point, and DNA from total infected cell lysates was isolated using an UltraClean microbial DNA isolation kit (MoBio Laboratories, Carlsbad, CA). C. burnetii genomes were quantified using a primer/probe set specific for C. burnetii dotA, as described previously (14). A standard curve was generated using purified plasmid containing C. burnetii dotA as a template. qPCR was performed using TaqMan universal PCR master mix and a Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA).
Cytokine measurement.
HMDMs were infected with NMI or NMII for 48 h without removal of the inoculum. The levels of tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) present in the culture supernatants were determined using the BioPlex multiplex cytokine assay (Bio-Rad Laboratories, Hercules, CA), according to the manufacturer's instructions. As a control, Escherichia coli O111:B4 LPS was added to uninfected cell cultures at a final concentration of 0.5 μg/ml and was left in the medium throughout the 48 h of incubation.
NMI and NMII coinfections.
To assess the trafficking of NMI and NMII in coinfected cells, HMDMs and THP-1 cells (1 × 105 cells) cultivated on 12-mm glass coverslips in 24-well plates were infected with both phase variants. At the indicated times p.i., the cells were fixed and permeabilized with 100% cold methanol and then blocked for 1 h in PBS containing 5% bovine serum albumin (BSA). NMII was specifically stained with undiluted monoclonal A6 hybridoma culture supernatant directed against NMII LPS (6) and anti-mouse Alexa Fluor-647 immunoglobulin G (IgG). NMI was specifically stained with diluted (1:2,500) rabbit polyclonal serum directed against NMI (6) and anti-rabbit Alexa Fluor-594. The secondary antibodies used here and elsewhere were acquired from Invitrogen. The PV membrane was immunostained with CD63 (47) using a monoclonal antibody (clone H5C6) conjugated to fluorescein isothiocyanate (BD Biosciences, San Jose, CA).
Cells were viewed by confocal fluorescence microscopy using a modified Perkin-Elmer UltraView spinning-disch confocal system connected to a Nikon Eclipse Ti-E inverted microscope. Confocal images (0.2-μm sections) were acquired with a ×60 oil immersion objective (numerical aperture, 1.4) and a Photometrics Cascade II:512 digital camera (Princeton Instruments, Trenton, NJ) using Metamorph software (Molecular Devices, Inc., Downingtown, PA). All images were processed similarly using ImageJ software (written by W. S. Rasband at the U.S. National Institutes of Health, Bethesda, MD, and available from http://rsb.info.nih.gov/ij/) and Adobe Photoshop (Adobe Systems, San Jose, CA). ImageJ was also used to quantify the fluorescence intensity.
Trafficking of endolysosomal markers.
PV recruitment of the endolysosomal markers Rab5, Rab7, CD63, and cathepsin D was investigated using THP-1 cells cultivated on 12-mm glass coverslips in 24-well plates. Cells were infected with NMI or NMII for 2 h and then washed, and the medium was replenished. To assess the trafficking of Rab5 and Rab7, infected cells were transfected with pEGFP-Rab5 (36) or pEGFP-Rab7 (10) using the Polyplus jetPEI macrophage transfection reagent (Genesee Scientific, San Diego, CA) at 2 or 66 h p.i. Transfected cells were fixed for 20 min in 4% paraformaldehyde plus PBS, followed by permeabilization for 5 min in 0.1% Triton X-100 in PBS. To evaluate the trafficking of CD63 and cathepsin D, infected cells were fixed and permeabilized by treatment with 100% cold methanol for 10 min. Following fixation, the cells were blocked for 1 h in PBS containing 5% BSA. C. burnetii was labeled with guinea pig polyclonal serum directed against formalin-fixed NMII and anti-guinea pig Alexa Fluor-594 IgG. CD63 was labeled with a mouse monoclonal antibody (clone H5C6; BD Biosciences) and anti-mouse Alexa Fluor-488 IgG. Cathepsin D was labeled with rabbit polyclonal serum directed against the human enzyme (Upstate Biotechnology, Lake Placid, NY) and anti-rabbit Alexa Fluor-484 IgG. Host and C. burnetii DNAs were labeled with DRAQ5 (Biostatus Limited, Leicestershire, United Kingdom). Cells were viewed by confocal fluorescence microscopy as described above. Endolysosomal markers were considered colocalized with the PV membrane if the average membrane fluorescence intensity was 20% or greater than the average total cell fluorescence.
PV degradative activity.
The general degradative activity of NMI and NMII PVs was assessed by superinfecting _C. burnetii_-infected THP-1 cells with E. coli expressing mCherry red fluorescent protein. Degradation of E. coli within PVs was examined using static and time-lapse imaging of live cells and by transmission electron microscopy (TEM) of fixed cells. For static live-cell imaging, THP-1 cells were plated in 35-mm glass-bottomed petri dishes (1.5 × 106 cells). At 48 h p.i., NMI- or NMII-infected cells were superinfected with E. coli suspended in RPMI medium with 10% FBS at an MOI of 50. Noninternalized E. coli cells were washed from the monolayer after 1 h, and the cells were incubated for an additional 2 h to allow fusion of E. coli phagosomes with PV. Degradation of E. coli was assessed by phase-contrast and epifluorescence microscopy using a Nikon TE-2000 microscope equipped with a CoolSNAP HQ digital camera (Roper Scientific, Tuscon, AZ). Images were acquired using Metamorph software and processed using ImageJ and Adobe Photoshop. For time-lapse video microscopy of live cells, THP-1 cells (2 × 105) were cultured in 24-well glass-bottomed SensoPlates (Greiner Bio-One North America, Inc., Monroe, NC). E. coli was added to wells containing THP-1 cells infected with NMII for 72 h. The culture plate was placed into a LiveCell stage top incubation system (Pathology Devices, Inc., Westminster, MD) and time-lapse video microscopy was conducted using a spinning-disk confocal fluorescence microscope as described above.
For TEM, THP-1 cells (5 × 105 cells) in 24-well plates containing Thermanox coverslips (VWR, West Chester, PA) were infected and superinfected with C. burnetii and E. coli, respectively, as described above for static live-cell imaging. Monolayers were washed in cold PBS and fixed overnight at 4°C in 4% glutaraldehyde-4% paraformaldehyde. Following primary fixation, the samples were processed using a model 3451 laboratory microwave system (Ted Pella, Inc., Redding, CA) at ambient temperature, as follows. Samples were rinsed in 0.1 M sodium phosphate buffer, pH 7.4, at 80 W for 45 s and then postfixed in a mixture of 1% osmium tetroxide and 0.8% potassium ferrocyanide in phosphate buffer at 80 W with two cycles of 2 min on, 2 min off, and 2 min on. Following one wash in phosphate buffer and two washes in water for 45 s each at 80 W, the samples were stained en bloc with 1% aqueous uranyl acetate, as described above for postfixation. The samples were then rinsed in water for 45 s at 80 W. Samples were dehydrated in a series of 70%, 100%, and 100% ethanol for 45 s each at 250 W and then infiltrated with Spurr's resin in a series of 50%, 75%, 100%, 100%, and 100% for 3 min each at 250 W. The resin blocks were polymerized overnight at 65°C. Coverslips were removed from the blocks after exposure to liquid nitrogen for 5 s. The embedded cells were sectioned with a diamond knife, poststained with 1% uranyl acetate and 1% lead citrate, and examined with a model H7500 electron microscope (Hitachi High-Technologies USA, Pleasanton, CA) at 80 kV. Digital images were collected with an XR100 charge-coupled-device camera (Advanced Microscopy Techniques, Danvers, MA).
Evaluation of PV cathepsin activities.
To quantitatively assess PV cathepsin D activity, THP-1 cells (1.5 × 106) in 35-mm glass-bottomed petri dishes (MatTek, Ashland, MA) were infected with NMII for 32 h and then incubated for 16 h in medium containing Alexa Fluor-594 dextran (150 mg/ml; _M_r, 10,000; Invitrogen) and DQ Green BSA (500 mg/ml; Invitrogen). The cells were then washed three times with tissue culture medium and incubated for 2 h at 37°C with fresh medium alone or medium containing DQ Green BSA with or without the cathepsin D inhibitor pepstatin A (100 μM; Sigma Aldrich, St. Louis, MO). The cells were washed once with cold PBS, and confocal fluorescence microscopy was performed as described above to quantify the fluorescence generated by the proteolysis of DQ Green BSA. Images for ratiometric calculations were acquired at wavelengths of 515 nm (DQ Green BSA) and 594 nm (Alexa Fluor-594 dextran). Data are expressed as the ratio of cleaved DQ Green BSA/Alexa Fluor-594 fluorescence signal intensities that were obtained from centrally located 0.2-μm sections of individual PVs in a _Z_-series stack.
To qualitatively examine PV cathepsin D activity, THP-1 cells (2 × 105) in 24-well glass-bottomed SensoPlates were infected for 48 h with NMI or NMII and then incubated for 2 h with DQ Red BSA (500 mg/ml; Invitrogen) with or without pepstatin A. Cells were visualized by phase-contrast and epifluorescence microscopy.
Cathepsin B, K, and L activities were detected using the Magic Red (MR) fluorogenic substrates MR-(RR)2, MR-(LR)2, and MR-(FR)2, respectively (Immunochemistry Technologies, LLC, Bloomington, MN), and the methods recommended by the supplier. Briefly, THP-1 cells (2 × 105) in individual wells of a 24-well glass-bottomed SensoPlate were infected with NMI or NMII for 72 h or C. trachomatis (MOI = 10) for 24 h. The monolayers were washed twice with medium, and then 300 μl of medium containing MR substrate was added to the culture dishes. The cells were incubated for 30 min at 37°C and then visualized live by phase-contrast and epifluorescence microscopy. Time-lapse video confocal fluorescence microscopy of MR-(RR)2 cleavage in NMII-infected THP-1 cells (72 h p.i.) was conducted as described above.
RESULTS
NMI and NMII replicate with similar kinetics in HMDM and THP-1 cells.
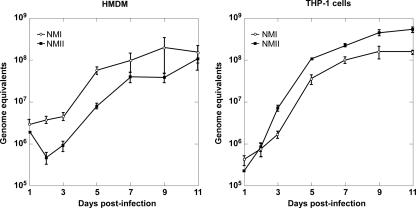
There are conflicting data on the growth permissiveness of human mononuclear phagocytes for C. burnetii phase variants (16, 17, 47, 54). Therefore, one-step growth curves were generated for NMI and NMII in both HMDMs and THP-1 cells using qPCR to quantify genome equivalents (14). The growth kinetics of NMI and NMII were similar in both cell types (Fig. 1). Over an 11-day incubation, approximately 1.7- and 2.4-log-unit increases in genome equivalents were observed in HMDMs and THP-1 cells, respectively. Generation times during exponential phase (3 to 5 days p.i.) for NMI and NMII in HMDMs were 13.2 and 15.4 h, respectively. Faster growth was observed in THP-1 cells, with generation times being 11.0 and 12.6 h for NMI and NMII, respectively. NMI and NMII also grew similarly in undifferentiated primary human monocytes, with approximately 2 log units of growth being observed at 6 days p.i. (data not shown).
FIG. 1.
NMI and NMII grow at similar rates in HMDMs and THP-1 cells. Cell monolayers were infected with C. burnetii, and genome equivalent assays were conducted to quantify pathogen replication, as described in Materials and Methods. The results are expressed as the means of three biological replicates from one experiment, with the error bars representing the standard deviations.
HMDMs infected with NMI and NMII secrete comparable levels of proinflammatory cytokines.
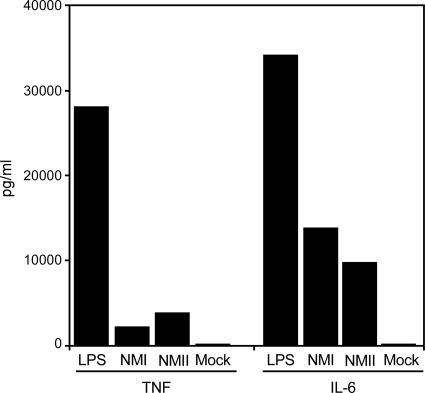
We have previously shown that innate immune recognition of NMI by human DCs is attenuated (47). Evidence suggests that this effect is mediated by the shielding of NMI surface toll-like receptor (TLR) ligands by full-length LPS (47). Conversely, interactions between NMII and human DCs result in the significant maturation and release of proinflammatory cytokines (47). To examine whether this behavior extends to interactions between C. burnetii phase variants and HMDMs, cells were infected for 48 h with NMI or NMII, and then the cell culture medium concentrations of TNF-α and IL-6 were determined. Similar amounts of each proinflammatory cytokine were secreted by cells infected with either phase variant (Fig. 2).
FIG. 2.
Human monocyte-derived macrophages infected with NMI or NMII secrete similar amounts of the proinflammatory cytokines TNF-α and IL-6. Cell monolayers were mock infected or infected with C. burnetii. Uninfected cell cultures were treated with E. coli LPS as a control. Cell culture supernatants were assayed for TNF-α and IL-6 concentrations at 48 h p.i. or after LPS addition. The results shown are from one experiment and are representative of those from three independent experiments.
NMI and NMII replicate within the same PVs in HMDMs and THP-1 cells.
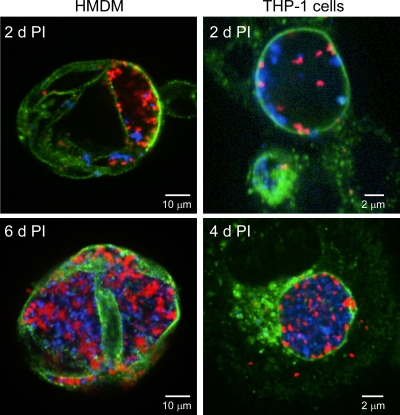
As an initial examination of the trafficking properties of NMI and NMII in human macrophages, HMDM and THP-1 cells were coinfected with both phase variants, and the localization of the organisms to common or distinct CD63-positive PVs was assessed by confocal fluorescence microscopy. Roughly equal numbers of NMI and NMII were found in a common large and spacious PV at 2 days p.i. in both cell types (Fig. 3). A similar observation was made at 4 and 6 days p.i. in THP-1 cells and HMDMs, respectively, with the PVs being nearly filled with replicating organisms at these time points. These results show that a single PV can support growth of both phase variants.
FIG. 3.
NMI and NMII replicate within the same PV of coinfected HMDMs and THP-1 cells. Cell monolayers were infected with C. burnetii as described in Materials and Methods. Infected cells were fixed with methanol at the indicated days p.i.; and NMI (red), NMII (blue), and CD63 (green) was stained by indirect immunofluorescence. Confocal fluorescence micrographs show similar numbers NMI and NMII in cohabited PVs.
PVs harboring NMI or NMII PV decorate similarly with endolysosomal markers.
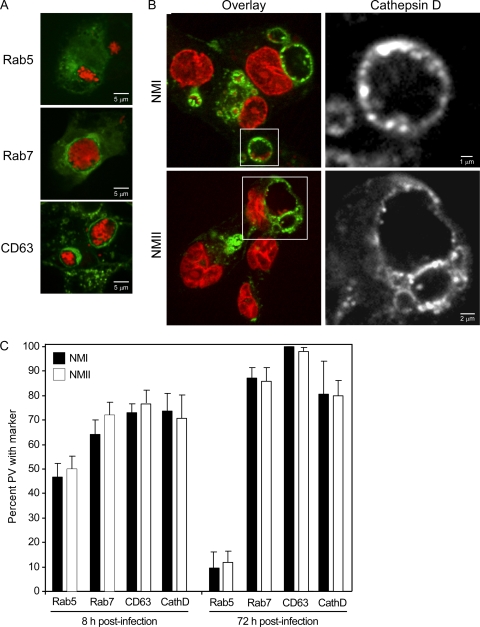
The cohabitation of both phase variants in a common PV within coinfected human macrophages suggested that PVs harboring just NMI or NMII mature to a similar stage in the endolysosomal cascade. To examine PV maturation, trafficking of the early endosome marker Rab5, the late endosome marker Rab7, the late endosome/lysosome marker CD63 (Fig. 4 A), and the lysosome marker cathepsin D (Fig. 4B) (43) was examined in infected THP-1 cells. The percentages of early (8 h p.i.) or late (72 h p.i.) NMI or NMII PVs that decorated with each marker were statistically the same (Fig. 4C). The percentages of PVs positive for late endosome/lysosome markers increased from 8 to 72 h p.i. For example, the proportion of NMII PVs positive for CD63 increased from 77.0% ± 5.1% to 98.0% ± 1.7%. Moreover, at 72 h p.i., both NMI and NMII PVs showed high percentages of labeling for the lysosomal aspartate protease cathepsin D (81.0% ± 13.3% and 80.0% ± 6.1%, respectively). Acquisition of late endosome/lysosome markers correlated with decreased labeling for Rab5. For example, the percentage of NMII positive for Rab5 decreased from 50.0% ± 5.3% to 12.0% ± 4.6% between 8 and 72 h p.i. Collectively, these data suggest that PVs harboring NMI or NMII mature similarly through the endolysosomal pathway to ultimately acquire characteristics of a phagolysosome.
FIG. 4.
PVs harboring NMI or NMII decorate similarly with endolysosomal markers. THP-1 cells were infected with C. burnetii, as described in Materials and Methods. (A) Cells were transfected with pEGFP-Rab5 (green) and pEGFP-Rab7 (green) to assess trafficking of Rab5 and Rab7, respectively. CD63 (green) and C. burnetii (red) were labeled by indirect immunofluorescence. Representative images of NMI-infected macrophages at 72 h p.i. show negative PV membrane decoration by Rab5 and positive decoration by Rab7 and CD63. (B) Representative images showing decoration of the NMI and NMII PV membrane by cathepsin D (72 h p.i.). Cathepsin D (green) was labeled by indirect immunofluorescence, and C. burnetii (red) and host cell nuclei (red) were labeled by DRAQ5. (C) Quantification of colocalization of Rab5, Rab7, CD63, and cathepsin D to early (8 h p.i.) and late (72 h p.i.) PVs containing NMI or NMII. The percentage of PVs colocalizing with a marker is expressed as the mean ± standard deviation of three independent experiments, where at least 30 PVs were evaluated in each experiment. CD63 labeled all NMI PVs at 72 h p.i.
NMI and NMII PVs are degradative, proteolytic compartments that contain active cathepsins.
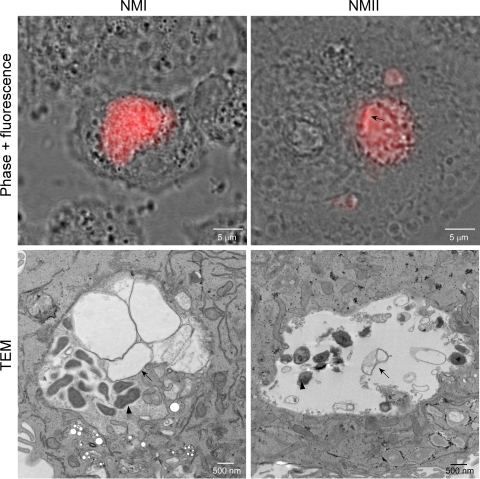
Typical phagolysosomes are degradative compartments due to a diverse array of acid-activated lysosomal hydrolases (31). The degradative capacity of the C. burnetii PV is unknown. Thus, as an initial probe to determine whether C. burnetii recruitment of lysosomal markers correlates with degradative function, THP-1 cells infected with NMI or NMII for 48 h were superinfected for 3 h with E. coli expressing mCherry red fluorescent protein. Live cells were then visualized by phase-contrast and fluorescence microscopy. E. coli cells that had trafficked to either NMI or NMII PVs were quickly degraded, indicated by the presence of disrupted organisms and released mCherry protein in the PV lumen (Fig. 5). Indeed, time-lapse video microscopy shows the leakage of mCherry protein by E. coli within 5 min of entry into PVs, with some organisms being completely destroyed within 15 min of entry (see Video S1 in the supplemental material). Severely disrupted E. coli cells that appeared to be under osmotic stress were also evident by TEM (Fig. 5).
FIG. 5.
PVs harboring NMI or NMII are degradative compartments. THP-1 cells were infected with C. burnetii, as described in Materials and Methods. At 48 h p.i., cells were incubated with E. coli expressing mCherry red fluorescent protein for 3 h and then viewed live by phase-contrast and fluorescence microscopy (upper panels) or fixed and viewed by TEM (lower panels). NMI and NMII PVs contain mCherry released from E. coli organisms that have trafficked to the vacuoles. A rod-shaped E. coli cell (arrow) in the process of degrading is still evident in the NMII PV. By TEM, an E. coli cell (arrows) apparently undergoing osmotic lysis is next to an intact C. burnetii cell (arrowheads). Representative images are shown.
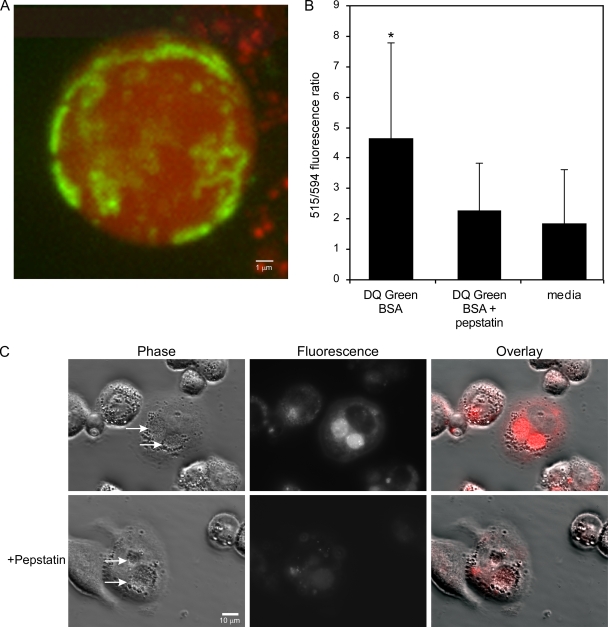
Lysosomal proteases in C. burnetii PVs could contribute to degradation of E. coli. To determine if the vacuole is proteolytically active, THP-1 cells were infected with NMII for 32 h and then incubated with Alexa Fluor-594 dextran and DQ Green BSA for 16 h to allow fluid-phase uptake and trafficking to the PVs (22). Alexa Fluor-594 dextran was employed to label the PVs with a nondigestible fluorescent probe. DQ Green BSA was used to qualitatively assess the proteolytic properties of the PVs. The molecule is a self-quenched 4,4-difluoro-4-bora-3a,4a-diaza-S-indacene (BODIPY) dye conjugate of BSA where quenching is relieved upon proteolysis of the protein to single dye-labeled peptides (41). A typical merged _Z_-series of confocal fluorescence micrographs showed a uniform distribution of red fluorescent dextran in the PV lumen. Conversely, green fluorescent cleaved DQ Green BSA showed a ring-like association with the PV membrane and a mottled distribution in the PV lumen (Fig. 6 A). Membrane-associated fluorescence was also clearly evident in 0.2-μm confocal slices (data not shown).
FIG. 6.
Proteolytically active cathepsin D contributes to PV degradative activity. (A) THP-1 cells in 35-mm glass-bottomed petri dishes were infected with NMII, as described in Materials and Methods. At 32 h p.i., cells were incubated for 16 h with Alexa Fluor-594 dextran and DQ Green BSA to allow delivery of probes to PVs by fluid-phase endocytosis. The cells were then washed and incubated for 2 h with fresh medium alone or medium containing DQ Green BSA with or without pepstatin. (A) Representative confocal fluorescence micrograph showing a merged _Z_-series (0.2-μm sections) of a PV with uniform red dextran and mottled cleaved DQ Green BSA fluorescence. (B) Ratio of cleaved DQ Green BSA fluorescence (515 nm) to Alexa Flour-594 fluorescence (594 nm). The 515-nm/594-nm fluorescence ratio is expressed as the mean ± standard deviation of three independent experiments, where at least 10 PVs from each condition were evaluated in each experiment. As determined by the Student t test, a significantly higher ratio (P < 0.01) was observed with PVs secondarily loaded with DQ Green BSA than with PVs secondarily loaded with DQ Green BSA plus pepstatin A or medium alone, indicating the presence of active cathepsin D. (C) DQ Red BSA is degraded by the cathepsin D present in NMI PVs of THP-1 cells. Cells infected for 48 h in a 24-well glass-bottomed tissue culture plate were incubated for 2 h with DQ Red BSA with or without pepstatin. By phase-contrast and epifluorescence microscopy, NMI PVs showed substantial red fluorescence, indicating proteolysis of the DQ Red BSA substrate. Fluorescence was considerably reduced in cells treated with pepstatin A.
The distribution of fluorescent DQ Green BSA suggested that proteolytic activity is associated with the PV membrane. As shown in Fig. 4B, cathepsin D localizes to the PV membrane; however, the protease has inactive proenzyme and active mature forms that are not differentiated by the polyclonal serum used in this study (55). Therefore, we determined whether DQ Green BSA proteolysis and, potentially, presentation correlated with active cathepsin D. The assay was repeated with NMII-infected THP-1 cells that were incubated for 2 h prior to microscopy with medium alone or medium containing DQ Green BSA with or without pepstatin A, an inhibitor of cathepsin D. DQ Green BSA/Alexa Fluor-594 dextran fluorescence ratios were then determined, as described in Materials and Methods. A significantly higher ratio was observed with PVs secondarily loaded with DQ Green BSA than with PVs secondarily loaded with DQ Green BSA plus pepstatin A or medium alone (Fig. 6B), indicating that active cathepsin D contributes to DQ Green BSA degradation.
The lack of a confocal microscope in our biosafety level 3 laboratory precluded a similar quantitative analysis of NMI PV cathepsin D activity. However, a qualitative evaluation of activity was conducted by loading THP-1 cells infected for 48 h with DQ Red BSA and visualizing the PVs by phase-contrast and epifluorescence microscopy. NMI PVs showed substantial red fluorescence, indicating proteolysis of the substrate. The fluorescence was considerably reduced in cells treated with pepstatin A (Fig. 6C). A similar result was observed for NMII PV (data not shown).
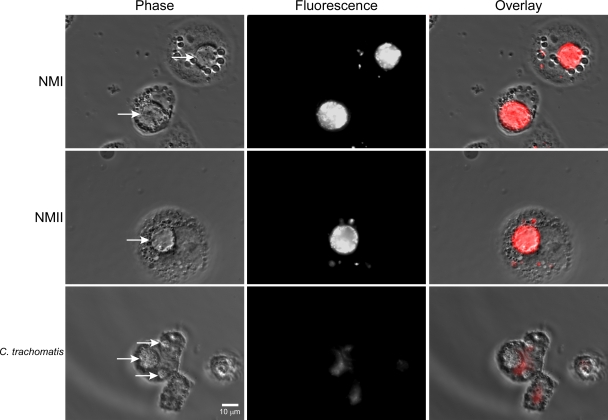
To determine whether active cysteine proteases are also present in C. burnetii PVs, THP-1 cells infected for 72 h were stained with MR-(RR)2, a membrane-permeant cresyl violet-conjugated peptide that is a specific substrate of cathepsin B (15). As a control, cells containing C. trachomatis PVs (24 h p.i.), which have negligible interactions with the endocytic pathway (22), were also stained. Intact MR-(RR)2 is nonfluorescent, while the enzymatically cleaved substrate generates red fluorescence when it is excited at 500 to 590 nm. By phase-contrast and epifluorescence microscopy, PVs containing NMI or NMII showed intense red fluorescence 30 min after substrate addition (Fig. 7). Time-lapse video microscopy shows the appearance of red fluorescence in PVs as early as 18 s after substrate addition (see Video S2 in the supplemental material). As expected, C. trachomatis PVs showed no fluorescence (Fig. 7). Similar results were obtained using MR substrates specific for cathepsins K and L (data not shown). Collectively, these data indicate that multiple proteolytically active cathepsins are present in both NMI and NMII PVs.
FIG. 7.
Active cathepsin B is present in PVs. THP-1 cells in 24-well glass-bottomed tissue culture plates were infected with NMI or NMII, as described in Materials and Methods. At 72 h p.i., cells were incubated for 30 min with the fluorogenic cathepsin B substrate MR-(RR)2. By phase-contrast and epifluorescence microscopy, PVs containing NMI or NMII showed intense red fluorescence, indicating the presence of active cathepsin B. In contrast, C. trachomatis PVs showed no fluorescence. Arrows, pathogen PVs.
DISCUSSION
Here, we show that virulent NMI and avirulent NMII traffic similarly in HMDMs and THP-1 cells to reside in a degradative, phagolysosome-like compartment that is permissive for growth. In each cell type they replicate with comparable kinetics; however, on the basis of the net increase in the numbers of C. burnetii genome equivalents between lag and stationary phases, THP-1 cells appear to be moderately more permissive for growth, showing a 2.5-log-unit increase, which is similar to that observed in nonphagocytic Vero cells (14). The occurrence of replicating NMI and NMII within the same PVs of coinfected human macrophages is consistent with the results of a previous study (6) and further supports the idea that phase variants do not direct maturation of biologically distinct PV.
Although we did not specifically examine vacuolar pH, studies using ratiometric, pH-sensitive probes and different cell types have consistently shown that NMI and NMII PVs have a similar phagolysosome-like pH (∼5) (2, 19, 32, 33). This degree of acidification, along with the presence of active lysosomal hydrolases, is a reliable indicator of lysosome fusion (41). Akporiaye et al. (2) determined that the overall activity of multiple lysosomal enzymes in cellular extracts of NMI-infected J774 murine macrophage-like cells is unaltered and, along with Howe and Mallavia (25), demonstrated that the lumen of individual PVs contains active acid phosphatase. This study demonstrates that PVs harboring NMI or NMII are proteolytically active and that both cysteine and aspartate cathepsins contribute to proteolysis. Cathepsin D has also been localized to NMII PVs in CHO and HeLa cells, where the vacuoles degrade DQ Green BSA (1, 40). Notably, NMII shows no growth defect in either cell line (1, 40). Interestingly, a recent report demonstrates that pretreatment of HMDMs with apoptotic lymphocytes enhances NMI replication, with a corresponding increase in the percentage of early PVs that decorate with cathepsin D (8). Thus, fuller phagosome maturation in this context does not correlate with increased C. burnetii killing.
How C. burnetii resists degradation by the lysosomal constituents of its PV is a puzzle. Resistance does not appear to require pathogen metabolism, as chloramphenicol-treated organisms remain viable for several days in lysosome-like vacuoles of Vero cells (26). Furthermore, our results showing similar growth of NMI and NMII in human macrophages indicate that full-length LPS is not required for protection. One possible resistance mechanism is the production by C. burnetii of peptidoglycan-associated proteins that are protease resistant (3).
Avirulence in intracellular bacteria is often associated with defects in phagosome modification. For example, mutants of Mycobacterium tuberculosis deficient in phagosome arrest are quickly killed by macrophages (39). NMII avirulence is unrelated to phagosome arrest; instead, it appears to be strictly related to production of truncated LPS (24). Indeed, using resequencing microarrays, we have recently found that, in addition to the 25,992-bp deletion of LPS biosynthesis genes, NMII has 13 single nucleotide polymorphisms relative to the sequence of NMI, but none are predicted to disrupt the proteins required for intracellular growth and virulence (P. A. Beare and R. A. Heinzen, unpublished data). Full-length C. burnetii LPS acts as a virulence factor by shielding the outer membrane, thereby conferring resistance to complement-mediated killing (51) and masking surface TLR ligands from innate immune recognition by human DCs (46, 47). Exposure of NMII TLR surface ligands is thought to stimulate the potent activation, maturation, and release of proinflammatory cytokines (i.e., IL-12 and TNF-α) observed during in vitro infection of DCs (47). Despite the differential activation of human DCs, NMI and NMII grow at equal rates in these cells (47). However, in vivo, this behavior is predicted to result in potentiated innate and adaptive immune responses to NMII relative to the response to NMI (47). Unlike DCs, HMDMs infected by NMI or NMII produce similar amounts of proinflammatory cytokines (i.e., TNF-α and IL-6). Phase variants also induce similar levels of TNF-α early after infection of murine P388D1 macrophage-like cells (49) and replicate similarly in these cells (5).
In addition to primary human macrophages and DCs, NMI and NMII show similar growth characteristics in primary guinea pig macrophages (28) and all continuous cell lines examined, including murine macrophage-like cells (5, 52). However, NMII does have severe growth defects relative to the growth of NMI in primary mouse macrophages (9, 42, 56, 57). NMII activation of the primary mouse macrophage pathogen recognition system by exposed TLR ligands may induce production of a cellular effector that limits replication.
NMI and NMII appear to engage different macrophage/monocyte receptors (12). However, in our hands, this does not result in different phagosome maturation states or pathogen growth. We conclude that infection of primary human macrophages and human macrophage/monocyte-like cell lines by avirulent NMII represents a physiologically accurate system to model _C. burnetii_-host cell interactions.
Supplementary Material
[Supplemental material]
Acknowledgments
We thank Stacey Gilk, Jean Celli, and Shelly Robertson for critical review of the manuscript and Anita Mora for graphic illustrations. The pEGFP-Rab5 and pEGFP-Rab7 constructs were kindly provided by Marino Zerial and Cecilia Bucci, respectively.
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases.
Footnotes
▿
Published ahead of print on 1 June 2010.
REFERENCES
- 1.Aguilera, M., R. Salinas, E. Rosales, S. Carminati, M. I. Colombo, and W. Beron. 2009. Actin dynamics and Rho GTPases regulate the size and formation of parasitophorous vacuoles containing Coxiella burnetii. Infect. Immun. 77**:**4609-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akporiaye, E. T., J. D. Rowatt, A. A. Aragon, and O. G. Baca. 1983. Lysosomal response of a murine macrophage-like cell line persistently infected with Coxiella burnetii. Infect. Immun. 40**:**1155-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amano, K., J. C. Williams, T. F. McCaul, and M. G. Peacock. 1984. Biochemical and immunological properties of Coxiella burnetii cell wall and peptidoglycan-protein complex fractions. J. Bacteriol. 160**:**982-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andoh, M., G. Zhang, K. E. Russell-Lodrigue, H. R. Shive, B. R. Weeks, and J. E. Samuel. 2007. T cells are essential for bacterial clearance, and gamma interferon, tumor necrosis factor alpha, and B cells are crucial for disease development in Coxiella burnetii infection in mice. Infect. Immun. 75**:**3245-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baca, O. G., E. T. Akporiaye, A. S. Aragon, I. L. Martinez, M. V. Robles, and N. L. Warner. 1981. Fate of phase I and phase II Coxiella burnetii in several macrophage-like tumor cell lines. Infect. Immun. 33**:**258-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beare, P. A., D. Howe, D. C. Cockrell, and R. A. Heinzen. 2007. Efficient method of cloning the obligate intracellular bacterium Coxiella burnetii. Appl. Environ. Microbiol. 73**:**4048-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beare, P. A., J. E. Samuel, D. Howe, K. Virtaneva, S. F. Porcella, and R. A. Heinzen. 2006. Genetic diversity of the Q fever agent, Coxiella burnetii, assessed by microarray-based whole-genome comparisons. J. Bacteriol. 188**:**2309-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benoit, M., E. Ghigo, C. Capo, D. Raoult, and J. L. Mege. 2008. The uptake of apoptotic cells drives Coxiella burnetii replication and macrophage polarization: a model for Q fever endocarditis. PLoS Pathog. 4**:**e1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan, R. E., K. Russell, G. Zhang, and J. E. Samuel. 2004. Both inducible nitric oxide synthase and NADPH oxidase contribute to the control of virulent phase I Coxiella burnetii infections. Infect. Immun. 72**:**6666-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucci, C., P. Thomsen, P. Nicoziani, J. McCarthy, and B. van Deurs. 2000. Rab7: a key to lysosome biogenesis. Mol. Biol. Cell 11**:**467-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton, P. R., N. Kordova, and D. Paretsky. 1971. Electron microscopic studies of the rickettsia Coxiella burnetii: entry, lysosomal response, and fate of rickettsial DNA in L-cells. Can. J. Microbiol. 17**:**143-150. [DOI] [PubMed] [Google Scholar]
- 12.Capo, C., A. Moynault, Y. Collette, D. Olive, E. J. Brown, D. Raoult, and J. L. Mege. 2003. Coxiella burnetii avoids macrophage phagocytosis by interfering with spatial distribution of complement receptor 3. J. Immunol. 170**:**4217-4225. [DOI] [PubMed] [Google Scholar]
- 13.Cockrell, D. C., P. A. Beare, E. R. Fischer, D. Howe, and R. A. Heinzen. 2008. A method for purifying obligate intracellular Coxiella burnetii that employs digitonin lysis of host cells. J. Microbiol. Methods 72**:**321-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman, S. A., E. R. Fischer, D. Howe, D. J. Mead, and R. A. Heinzen. 2004. Temporal analysis of Coxiella burnetii morphological differentiation. J. Bacteriol. 186**:**7344-7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Creasy, B. M., C. B. Hartmann, F. K. White, and K. L. McCoy. 2007. New assay using fluorogenic substrates and immunofluorescence staining to measure cysteine cathepsin activity in live cell subpopulations. Cytometry 71(Pt A)**:**114-123. [DOI] [PubMed] [Google Scholar]
- 16.Ghigo, E., C. Capo, D. Raoult, and J. L. Mege. 2001. Interleukin-10 stimulates Coxiella burnetii replication in human monocytes through tumor necrosis factor down-modulation: role in microbicidal defect of Q fever. Infect. Immun. 69**:**2345-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghigo, E., C. Capo, C. H. Tung, D. Raoult, J. P. Gorvel, and J. L. Mege. 2002. Coxiella burnetii survival in THP-1 monocytes involves the impairment of phagosome maturation: IFN-gamma mediates its restoration and bacterial killing. J. Immunol. 169**:**4488-4495. [DOI] [PubMed] [Google Scholar]
- 18.Ghigo, E., A. Honstettre, C. Capo, J. P. Gorvel, D. Raoult, and J. L. Mege. 2004. Link between impaired maturation of phagosomes and defective Coxiella burnetii killing in patients with chronic Q fever. J. Infect. Dis. 190**:**1767-1772. [DOI] [PubMed] [Google Scholar]
- 19.Grieshaber, S., J. A. Swanson, and T. Hackstadt. 2002. Determination of the physical environment within the Chlamydia trachomatis inclusion using ion-selective ratiometric probes. Cell. Microbiol. 4**:**273-283. [DOI] [PubMed] [Google Scholar]
- 20.Hackstadt, T., M. G. Peacock, P. J. Hitchcock, and R. L. Cole. 1985. Lipopolysaccharide variation in Coxiella burnetii: intrastrain heterogeneity in structure and antigenicity. Infect. Immun. 48**:**359-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinzen, R. A., T. Hackstadt, and J. E. Samuel. 1999. Developmental biology of Coxiella burnetii. Trends Microbiol. 7**:**149-154. [DOI] [PubMed] [Google Scholar]
- 22.Heinzen, R. A., M. A. Scidmore, D. D. Rockey, and T. Hackstadt. 1996. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect. Immun. 64**:**796-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honstettre, A., E. Ghigo, A. Moynault, C. Capo, R. Toman, S. Akira, O. Takeuchi, H. Lepidi, D. Raoult, and J. L. Mege. 2004. Lipopolysaccharide from Coxiella burnetii is involved in bacterial phagocytosis, filamentous actin reorganization, and inflammatory responses through Toll-like receptor 4. J. Immunol. 172**:**3695-3703. [DOI] [PubMed] [Google Scholar]
- 24.Hoover, T. A., D. W. Culp, M. H. Vodkin, J. C. Williams, and H. A. Thompson. 2002. Chromosomal DNA deletions explain phenotypic characteristics of two antigenic variants, phase II and RSA 514 (crazy), of the Coxiella burnetii Nine Mile strain. Infect. Immun. 70**:**6726-6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howe, D., and L. P. Mallavia. 2000. Coxiella burnetii exhibits morphological change and delays phagolysosomal fusion after internalization by J774A.1 cells. Infect. Immun. 68**:**3815-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howe, D., J. Melnicakova, I. Barak, and R. A. Heinzen. 2003. Maturation of the Coxiella burnetii parasitophorous vacuole requires bacterial protein synthesis but not replication. Cell. Microbiol. 5**:**469-480. [DOI] [PubMed] [Google Scholar]
- 27.Khavkin, T., and S. S. Tabibzadeh. 1988. Histologic, immunofluorescence, and electron microscopic study of infectious process in mouse lung after intranasal challenge with Coxiella burnetii. Infect. Immun. 56**:**1792-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kishimoto, R. A., and J. S. Walker. 1976. Interaction between Coxiella burnetii and guinea pig peritoneal macrophages. Infect. Immun. 14**:**416-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loeuillet, C., F. Martinon, C. Perez, M. Munoz, M. Thome, and P. R. Meylan. 2006. Mycobacterium tuberculosis subverts innate immunity to evade specific effectors. J. Immunol. 177**:**6245-6255. [DOI] [PubMed] [Google Scholar]
- 30.Lubick, K., M. Radke, and M. Jutila. 2007. Securinine, a GABAA receptor antagonist, enhances macrophage clearance of phase II C. burnetii: comparison with TLR agonists. J. Leukoc. Biol. 82**:**1062-1069. [DOI] [PubMed] [Google Scholar]
- 31.Luzio, J. P., P. R. Pryor, and N. A. Bright. 2007. Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 8**:**622-632. [DOI] [PubMed] [Google Scholar]
- 32.Maurin, M., A. M. Benoliel, P. Bongrand, and D. Raoult. 1992. Phagolysosomal alkalinization and the bactericidal effect of antibiotics: the Coxiella burnetii paradigm. J. Infect. Dis. 166**:**1097-1102. [DOI] [PubMed] [Google Scholar]
- 33.Maurin, M., A. M. Benoliel, P. Bongrand, and D. Raoult. 1992. Phagolysosomes of _Coxiella burnetii_-infected cell lines maintain an acidic pH during persistent infection. Infect. Immun. 60**:**5013-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maurin, M., and D. Raoult. 1999. Q fever. Clin. Microbiol. Rev. 12**:**518-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moos, A., and T. Hackstadt. 1987. Comparative virulence of intra- and interstrain lipopolysaccharide variants of Coxiella burnetii in the guinea pig model. Infect. Immun. 55**:**1144-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nielsen, E., F. Severin, J. M. Backer, A. A. Hyman, and M. Zerial. 1999. Rab5 regulates motility of early endosomes on microtubules. Nat. Cell Biol. 1**:**376-382. [DOI] [PubMed] [Google Scholar]
- 37.Omsland, A., D. C. Cockrell, D. Howe, E. R. Fischer, K. Virtaneva, D. E. Sturdevant, S. F. Porcella, and R. A. Heinzen. 2009. Host cell-free growth of the Q fever bacterium Coxiella burnetii. Proc. Natl. Acad. Sci. U. S. A. 106**:**4430-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan, X., A. Luhrmann, A. Satoh, M. A. Laskowski-Arce, and C. R. Roy. 2008. Ankyrin repeat proteins comprise a diverse family of bacterial type IV effectors. Science 320**:**1651-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pethe, K., D. L. Swenson, S. Alonso, J. Anderson, C. Wang, and D. G. Russell. 2004. Isolation of Mycobacterium tuberculosis mutants defective in the arrest of phagosome maturation. Proc. Natl. Acad. Sci. U. S. A. 101**:**13642-13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Romano, P. S., M. G. Gutierrez, W. Beron, M. Rabinovitch, and M. I. Colombo. 2007. The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell. Microbiol. 9**:**891-909. [DOI] [PubMed] [Google Scholar]
- 41.Russell, D. G., B. C. Vanderven, S. Glennie, H. Mwandumba, and R. S. Heyderman. 2009. The macrophage marches on its phagosome: dynamic assays of phagosome function. Nat. Rev. Immunol. 9**:**594-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sauer, J. D., J. G. Shannon, D. Howe, S. F. Hayes, M. S. Swanson, and R. A. Heinzen. 2005. Specificity of Legionella pneumophila and Coxiella burnetii vacuoles and versatility of Legionella pneumophila revealed by coinfection. Infect. Immun. 73**:**4494-4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scott, C. C., R. J. Botelho, and S. Grinstein. 2003. Phagosome maturation: a few bugs in the system. J. Membr. Biol. 193**:**137-152. [DOI] [PubMed] [Google Scholar]
- 44.Shannon, J. G., D. C. Cockrell, K. Takahashi, G. L. Stahl, and R. A. Heinzen. 2009. Antibody-mediated immunity to the obligate intracellular bacterial pathogen Coxiella burnetii is Fc receptor- and complement-independent. BMC Immunol. 10**:**26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shannon, J. G., and R. A. Heinzen. 2007. Infection of human monocyte-derived macrophages with Coxiella burnetii. Methods Mol. Biol. 431**:**189-200. [DOI] [PubMed] [Google Scholar]
- 46.Shannon, J. G., D. Howe, and R. A. Heinzen. 2005. Lack of dendritic cell maturation following infection by Coxiella burnetii synthesizing different lipopolysaccharide chemotypes. Ann. N. Y. Acad. Sci. 1063**:**154-160. [DOI] [PubMed] [Google Scholar]
- 47.Shannon, J. G., D. Howe, and R. A. Heinzen. 2005. Virulent Coxiella burnetii does not activate human dendritic cells: role of lipopolysaccharide as a shielding molecule. Proc. Natl. Acad. Sci. U. S. A. 102**:**8722-8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stein, A., C. Louveau, H. Lepidi, F. Ricci, P. Baylac, B. Davoust, and D. Raoult. 2005. Q fever pneumonia: virulence of Coxiella burnetii pathovars in a murine model of aerosol infection. Infect. Immun. 73**:**2469-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tujulin, E., B. Lilliehook, A. Macellaro, A. Sjostedt, and L. Norlander. 1999. Early cytokine induction in mouse P388D1 macrophages infected by Coxiella burnetii. Vet. Immunol. Immunopathol. 68**:**159-168. [DOI] [PubMed] [Google Scholar]
- 50.Vazquez, C. L., and M. I. Colombo. 2010. Coxiella burnetii modulates Beclin 1 and Bcl-2, preventing host cell apoptosis to generate a persistent bacterial infection. Cell Death Differ. 17**:**421-438. [DOI] [PubMed] [Google Scholar]
- 51.Vishwanath, S., and T. Hackstadt. 1988. Lipopolysaccharide phase variation determines the complement-mediated serum susceptibility of Coxiella burnetii. Infect. Immun. 56**:**40-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voth, D. E., and R. A. Heinzen. 2007. Lounging in a lysosome: the intracellular lifestyle of Coxiella burnetii. Cell. Microbiol. 9**:**829-840. [DOI] [PubMed] [Google Scholar]
- 53.Voth, D. E., and R. A. Heinzen. 2009. Sustained activation of Akt and Erk1/2 is required for Coxiella burnetii antiapoptotic activity. Infect. Immun. 77**:**205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voth, D. E., D. Howe, and R. A. Heinzen. 2007. Coxiella burnetii inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages. Infect. Immun. 75**:**4263-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zaidi, N., A. Maurer, S. Nieke, and H. Kalbacher. 2008. Cathepsin D: a cellular roadmap. Biochem. Biophys. Res. Commun. 376**:**5-9. [DOI] [PubMed] [Google Scholar]
- 56.Zamboni, D. S. 2004. Genetic control of natural resistance of mouse macrophages to Coxiella burnetii infection in vitro: macrophages from restrictive strains control parasitophorous vacuole maturation. Infect. Immun. 72**:**2395-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zamboni, D. S., R. A. Mortara, E. Freymuller, and M. Rabinovitch. 2002. Mouse resident peritoneal macrophages partially control in vitro infection with Coxiella burnetii phase II. Microbes Infect. 4**:**591-598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental material]