Metformin Selectively Attenuates Mitochondrial H2O2 Emission without Affecting Respiratory Capacity in Skeletal Muscle of Obese Rats (original) (raw)
. Author manuscript; available in PMC: 2011 Sep 15.
Abstract
Metformin is a widely prescribed drug for treatment of type 2 diabetes, although no cellular mechanism of action has been established. To determine whether in vivo metformin treatment alters mitochondrial function in skeletal muscle, respiratory O2 flux and H2O2 emission were measured in saponin-permeabilized myofibers from lean and obese (fa/fa) Zucker rats treated for 4 wks with metformin. Succinate- and palmitoyl-carnitine- supported respiration generated >2-fold higher rates of H2O2 emission in myofibers from untreated obese versus lean rats, indicative of an obesity-associated increased mitochondrial oxidant emitting potential. In conjunction with improved glycemic control, metformin treatment reduced H2O2 emission in muscle from obese rats to rates near or below those observed in lean rats during both succinate- and palmitoyl-carnitine- supported respiration. Surprisingly, metformin treatment did not affect basal or maximal rates of O2 consumption in muscle from obese or lean rats. Ex vivo dose-response experiments revealed that metformin inhibits complex I-linked H2O2 emission at a concentration ∼2 orders of magnitude lower than that required to inhibit respiratory O2 flux. These findings suggest that therapeutic concentrations of metformin normalize mitochondrial H2O2 emission by blocking reverse electron flow without affecting forward electron flow or respiratory O2 flux in skeletal muscle.
Keywords: metformin, reactive oxygen species, mitochondria, skeletal muscle, respiration, diabetes
Dimethylbiguanide, popularly known as metformin, is among the most widely prescribed drugs for the treatment of type 2 diabetes. Improved glycemic control has been attributed to two effects: decreased hepatic glucose production [1-3] and increased glucose disposal in skeletal muscle [4-7]. However, despite the routine prescription of metformin, its mechanism of action remains unclear. Two potential cellular targets of metformin have been identified: adenosine monophosphate-activated protein kinase (AMPK) [8], a major regulator of cellular glucose and lipid metabolism, and complex I of the mitochondrial respiratory chain [9, 10]. Both in vitro and in vivo exposure to metformin increases AMPK activity [6, 8, 11] and inhibits mitochondrial respiration [9, 10, 12, 13] in skeletal muscle and liver. Cell free assays indicate that metformin does not activate AMPK or its upstream kinases directly [14], suggesting that activation of AMPK is due to an increase in [AMP]/[ATP] ratio. However, the degree of inhibition of complex I by metformin is mild and does not appear to affect overall cellular energy charge [14]. Thus, although there is considerable circumstantial evidence, a direct link between AMPK and the insulin sensitizing action of metformin has not been established [15].
In addition to increasing insulin-stimulated glucose disposal [4-6], metformin treatment has also been reported to reduce oxidative stress in target tissues [16-20], raising the alternative possibility that its mechanism of action may be related to reactive oxygen species (ROS) generation and/or neutralization. Batandier et al. [21] recently reported that metformin decreases complex I-linked H2O2 production during succinate-supported respiration in mitochondria isolated from rat liver. Succinate, a complex II substrate, induces high rates of ROS production by generating a backflow of electrons through complex I which dramatically accelerates electron leak and superoxide formation at complex I [22, 23]. While respiration supported exclusively by succinate is unphysiological, the findings suggest that substrate/respiration conditions in vivo that result in a greater proportion of reducing equivalents feeding into the electron transport system beyond complex I may lead to elevated ROS production; e.g., during basal (resting) respiration supported by fatty acids [24, 25]. In support of this contention, we have recently found that elevated mitochondrial-derived H2O2 emission is a primary factor in the etiology of dietary fat-induced muscle insulin resistance [26]. In the present study, we determined whether treatment with metformin daily for 4 weeks alters mitochondrial H2O2 emission and/or O2 respiration in skeletal muscle of obese Zucker (fa/fa) rats, an animal model of obesity-induced insulin resistance. Our findings reveal that, in addition to improved whole-body glycemic control, metformin treatment reduces the potential for succinate- and fatty acid- supported mitochondrial H2O2 emission in skeletal muscle without affecting O2 respiratory control. Moreover, dose-response experiments conducted ex vivo on control myofibers demonstrate that complex I-linked mitochondrial H2O2 emission is far more sensitive than complex I-linked respiration to inhibition by metformin.
Materials and methods
Animals
All animal studies were approved by the East Carolina University Institutional Animal Care and Use Committee. Sixteen age-matched male Zucker rats (8 lean, 8 fa/fa obese rats; Harlan Laboratories, Inc.) were housed in single cages in a temperature (22°C) and light-controlled (12:12 hour light-dark cycle) room with ad libitum access to standard chow and water for the duration of the study. Sprague-Dawley rats (Charles River Laboratories, Inc.) were used in control dose-response experiments and were housed as described above.
Metformin treatment and oral glucose tolerance testing
At 9-10 weeks of age, obese and lean Zucker rats were randomly assigned to receive either control (water) or metformin (320 mg/kg/day) by gavage for four weeks (n = 4/group). At the end of the fourth week, rats were fasted 10 h and an oral glucose tolerance test (OGTT; 2 g/kg BW by oral gavage of dextrose) was performed. Blood glucose (glucose oxidase method, One Touch Ultra glucose analyzer; Lifescan, Milpitas, CA) and insulin levels (ELISA, Linco Research, St. Charles, MO) were determined in the fasting condition and at time 30, 60, and 120 min after dextrose administration. After an additional three days of treatment, rats were anesthetized (ketamine:xylazine, 10 mg/0.1 kg of 9:1 mixture, i.p.) ∼4 h after their final metformin dose. The gastrocnemius muscle was removed and red portions of the muscle dissected and separated for preparation of fiber bundles (mitochondrial function studies) or quick-frozen in liquid N2. All rats were fasted 10 h prior to sacrifice.
Preparation of permeabilized myofibers
This technique is partially adapted from previous methods [27, 28] and has been described in detail previously [22, 24, 26]. Briefly, after dissection, connective tissue was removed and fiber bundles were separated with fine forceps under a binocular dissecting microscope in ice cold buffer X, containing (in mM): 60 K-MES, 35 KCl, 7.23 K2EGTA, 2.77 CaK2EGTA, 20 Imidazole, 0.5 DTT, 20 Taurine, 5.7 ATP, 15 PCr, 6.56 MgCl2-6H2O (pH 7.4, 295 mOsm). After separation, myofiber bundles were placed in buffer X containing 50 μg/ml saponin for 30 minutes and then were washed in ice-cold Buffer Z containing (in mM) 110 K-MES, 35 KCl, 1 EGTA, 10 K2HPO4, 3 MgCl2-6H2O, 5 mg/ml BSA, 0.1 glutamate and 0.05 malate (pH 7.4, 295 mOsm) until analysis (<1 hour). Fibers used in the H2O2 emission experiments were briefly washed in cold buffer Z containing 10 mM pyrophosphate prior to analysis to prevent Ca+2-independent contraction. Due to their positive charge, biguanides bio-accumulate by ∼100-fold in mitochondria [29] and are unlikely to be affected by cell permeabilization given that mitochondrial membrane potential is maintained.
Mitochondrial respiration and H2O2 emission measurements in permeabilized myofibers from lean and obese Zucker rats
O2 consumption rate was measured by high resolution respirometry (Oroboros Oxygraph-2k, Innsbruck, AT) at 30°C in Buffer Z + 20 mM creatine hydrate and 50 μM _N_-benzyl-_p_-toluene sulphonamide (BTS, a myosin II ATPase inhibitor that blocks contraction) under the following two protocols: Respirometric protocol A - 2 mM glutamate + 1 mM malate (complex I substrates) followed by sequential additions of 2 mM ADP, 3 mM succinate (complex II substrate), 10 μg/ml oligomycin (inhibitor of mitochondrial ATP synthase), and finally 2 μM carbonylcyanide-p-trifluoromethoxyphenylhydrazone (FCCP, a protonophoric uncoupler); Respirometric protocol B - 25 μM palmitoyl-carnitine + 1 mM malate followed by sequential additions of 2 mM glutamate and 3 mM succinate. Mitochondrial H2O2 emission was measured at 30°C in Buffer Z during state 4 respiration by continuously monitoring oxidation of Amplex Red using a Spex Fluoromax 3 (Jobin Yvon, Ltd.) spectrofluorometer under the following two protocols: Fluorometric protocol A - 25 μM palmitoyl-carnitine + 1 mM malate followed by sequential additions of 2 mM glutamate and 3 mM succinate; Fluorometric protocol B -succinate titration (in mM), 0.1, 0.25, 0.5, 0.75, 1.5 and 3.0. At the conclusion of each experiment, permeabilized fiber bundles were washed in distilled H2O to remove salts and freeze-dried in a lyophilizer (LabConco). Mitochondrial respiration rates are expressed as pmol·s-1·mg-1 dry weight and H2O2 emission rates as pmol·min-1·mg-1 dry weight.
Whole muscle protein extraction, citrate synthase activity, GSH and GSSG concentrations
Frozen red gastrocnemius muscle samples (50-80 mg) were homogenized in ice-cold lysis buffer [50 mM HEPES, 50 mM Na+ pyrophosphate, 100 mM Na+ fluoride, 10 mM EDTA, 10 mM Na+ orthovanadate, 1% Triton X-100, and protease and phosphatase (1 and 2) inhibitor cocktails (Sigma, St. Louis, MO)]. Homogenates were sonicated for 10 sec then rotated for 2 h at 4°C. After centrifugation for 25 min at 15,000 × g, supernatants were extracted and protein concentration was determined (BCA protein assay, Pierce, Rockford, IL) and individual homogenate volumes were separated into 50 μg of protein aliquots, frozen in liquid nitrogen and stored at -80°C. Citrate synthase activity was determined using the methods of Srere [30]. Separate muscle extracts were prepared for determination of oxidized glutathione (GSSG) and total glutathione (GSH) content from frozen muscle as previously described [26].
Acute metformin incubations
To directly compare the metformin dose-response effect on maximal mitochondrial respiration versus maximal H2O2 production, skeletal muscle was obtained from adult male Sprague-Dawley rats and processed as described above with the exception that fiber bundles were washed (Buffer Z) without or with metformin (0.1, 1, 5 or 10 mM) for 20 min. Maximal respiration rate was determined in the presence of 5 mM glutamate, 2 mM malate, 5 mM pyruvate and 8 mM ADP. Maximal H2O2 emission was determined during state 4 respiration supported by 3 mM succinate. Control experiments verified that metformin, which was maintained at the designated concentrations in both the respiration and H2O2 emission buffers, did not affect background O2 flux or oxidation of Amplex Red.
Statistical analyses
Data are presented as mean ± SEM. Statistical analyses were performed with GraphPad Prism (GraphPad Software, Inc.) using a two-way ANOVA with Bonferroni post-hoc analysis of significance in experiments involving Zucker rats; one-way ANOVA with Tukey's post-hoc analysis for experiments involving Sprague-Dawley rats; and a curve-fitting model for kinetic analyses of the succinate titrations. The alpha level for significance was established a priori at P ≤ 0.05.
Results
Metformin improves glycemic control in obese rats
Fasting blood glucose and plasma insulin were significantly (P<0.05) higher in obese (124.0 ±4.9 mg/dl and 17.1 ±3.2 ng/ml, respectively) compared with lean (96.5 ±1.2 mg/dl and 0.61 ±0.11 ng/ml, respectively) Zucker rats. Metformin treatment reduced both fasting blood glucose (105.0 ±0.71 mg/dl) and plasma insulin (9.9 ±2.1 ng/ml) in obese rats only. To further examine whole body glycemic control, oral glucose tolerance tests were performed in lean and obese Zucker rats. As expected, obese Zucker rats were characterized by greater (main effect, P < 0.001) area under the curve (AUC) for blood glucose (Supplemental Figure 1C) and plasma insulin (Supplemental Figure 1D) compared with lean Zucker rats, consistent with peripheral insulin resistance in the obese rats. Also as expected, metformin treatment significantly (P < 0.05) reduced both glucose and insulin AUC in the obese rats, demonstrating improved glycemic control with the drug. No difference in glucose or insulin AUC was observed in lean rats treated with metformin. Metformin treatment did not affect body weight (supplementary Figure 2) suggesting improvements in glucose tolerance in the obese rats occurred independent of body mass. Fasting serum NEFA concentration was greater in obese compared with lean rats (1.26 ±0.20 and 0.90 ±0.09 mM, respectively) and, interestingly, was slightly higher in obese rats treated with metformin (1.84 ±0.15 mM, P < 0.05)). Serum lactate concentrations were similar in lean and obese with or without metformin treatment (not shown).
Metformin treatment does not affect mitochondrial respiration in permeabilized skeletal myofibers
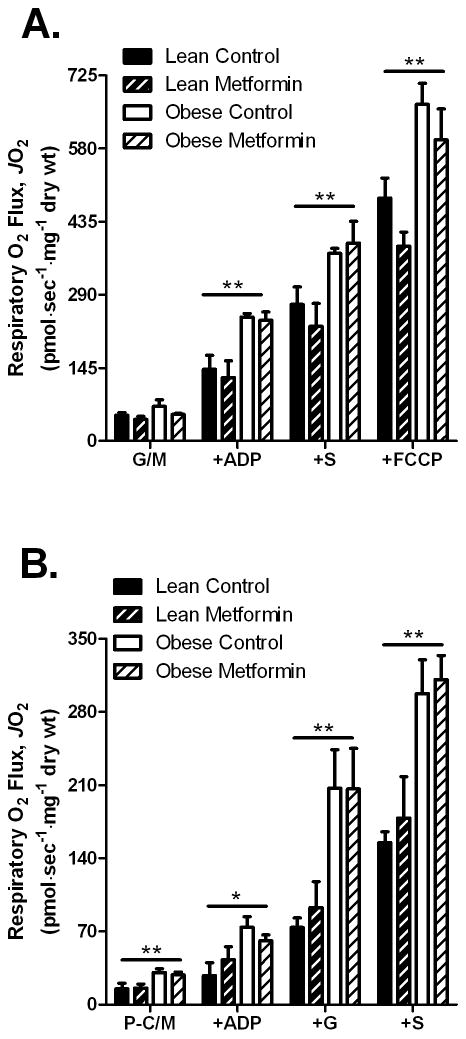
Previous reports indicate that metformin causes a mild to moderate decrease in oxygen consumption in isolated mitochondria or intact cells [9, 10, 12], although the effect appears to require high concentrations (i.e., millimolar) and/or long exposure time. To determine if four weeks of oral metformin treatment leads to inhibition of mitochondrial respiration in skeletal muscle of lean and obese Zucker rats, we measured respiratory O2 flux in permeabilized fibers from the red gastrocnemius muscle. Metformin treatment did not affect complex I-linked (glutamate/malate) basal (state 4) or ADP-stimulated (state 3) respiration, either alone or in combination with the complex II substrate succinate, nor maximal uncoupled FCCP-stimulated respiration in either lean or obese animals (Figure 1A). Metformin also had no effect on palmitoyl-carnitine supported respiration under any of the conditions tested (Figure 1B). Taken together, these results indicate that oral metformin treatment does not inhibit complex I-linked respiration (i.e., forward electron flux) in skeletal muscle of lean or obese Zucker rats under the conditions employed in the current study.
Fig. 1.
Effects of oral metformin treatment on skeletal muscle respiratory O2 flux measured in permeabilized myofibers from lean and obese Zucker rats. Respiratory O2 flux in permeabilized fiber bundles from red gastrocnemius muscle from lean and obese rats +/- metformin treatment during respiration supported by (A) complex I-linked substrates glutamate/malate (G/M, 2/1 mM) followed by additions of ADP (2 mM), succinate (+S, 3 mM), and the chemical uncoupler FCCP (2 μM + 10 μg/ml oligomycin) or (B) during respiration supported by activated fatty acid palmitoyl-carnitine and malate (P-C/M, 25 μM/1 mM) followed by additions of ADP (2 mM), glutamate (+G, 2 mM), and succinate (+S, 3 mM). The data represent means ± SEM (n = 4/group). Main effect for obesity *P < 0.05; **P < 0.01. No effect of metformin treatment on respiratory O2 flux was observed in A or B.
Maximal O2 consumption was significantly greater in skeletal muscle of obese verses lean rats under nearly all respiratory conditions (Figure 1). Citrate synthase activity, a marker of mitochondrial content, tended to be higher (P = 0.061) in the muscle from obese rats (Supplementary Figure 3). Citrate synthase activity was not different between metformin treated and control Zucker rats, indicating the effects of metformin treatment were not due to a change in mitochondrial content.
Metformin treatment attenuates elevated mitochondrial H2O2 emission associated with obesity
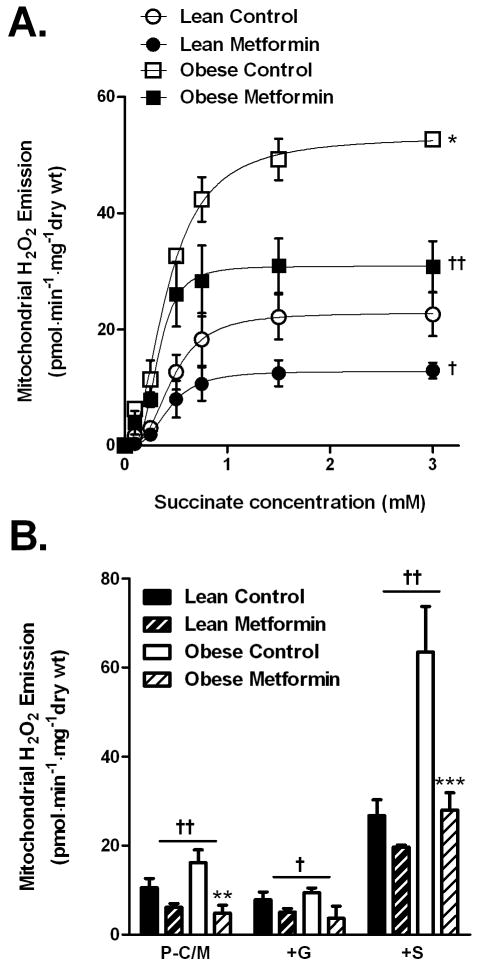
Metformin has been shown to reduce ROS production at complex I in isolated mitochondria obtained from rat liver pre-perfused with 10 mM metformin [31]. To determine if metformin attenuates reverse electron flux-mediated ROS production at complex I in skeletal muscle, mitochondrial H2O2 emission was measured in permeabilized fibers from red gastrocnemius muscle of lean and obese Zucker rats treated for four weeks with metformin. Titration of succinate to induce reverse electron flux revealed a more than 2-fold greater (P < 0.05) potential for complex I-linked H2O2 emission in skeletal muscle from untreated obese versus lean rats (53.2 ± 2.3 vs. 22.8 ± 2.3 pmol H2O2·min-1·mg-1dry wt, respectively, Figure 2A). Metformin treatment significantly (P < 0.05) reduced maximal succinate-induced H2O2 emission in the obese Zucker rats (30.9 ± 2.5 pmol H2O2·min-1·mg-1dry wt) to levels near that of untreated leans. In parallel experiments, when respiration was supported by palmitoyl-carnitine, mitochondrial H2O2 emission was markedly elevated in muscle from obese rats and whereas in muscle from obese rats treated with metformin, H2O2 emission rates were similar to levels obtained from lean rats, particularly upon addition of succinate (Figure 2B). These findings demonstrate that: 1) obesity in the Zucker rat model is associated with a marked increase in the potential for skeletal muscle mitochondrial H2O2 emission and 2) oral metformin treatment significantly attenuates mitochondrial H2O2 emission associated with reverse electron flux at complex I.
Fig. 2.
Effects of oral metformin treatment on skeletal muscle mitochondrial H2O2 emission in permeabilized myofibers from lean and obese Zucker rats. (A) H2O2 emission rates in response to titration of succinate during basal (i.e., state 4) respiration in permeabilized fiber bundles from red gastrocnemius muscle from lean and obese rats +/- metformin treatment. Data represent means ± SEM (n = 4/group). *P < 0.05 for Vmax, obese versus lean. †P <0.05, ††P < 0.01 for Vmax, metformin treated vs untreated. (B) H2O2 emission rates during basal (i.e., state 4) respiration supported by activated fatty acid palmitoyl-carnitine and malate (P-C/M, 25 μM/1 mM) followed by additions of glutamate (+G, 2 mM), and succinate (+S, 3 mM). Data represent means ± SEM (n = 4/group). †P < 0.05, ††P < 0.01 for main effect of metformin treatment. **P < 0.01, ***P < 0.001 within group effect of metformin treatment.
Metformin does not affect muscle glutathione redox buffering system in Zucker rats
To determine whether the changes in mitochondrial H2O2 emitting potential are associated with alterations in the redox buffering system, GSSG concentration, GSH/GSSG ratio and total glutathione concentration were determined in frozen muscle. Neither obesity nor metformin treatment affected any of the measured parameters (Supplemental Fig 4).
H2O2 emission much more sensitive than O2 respiration to inhibition by metformin
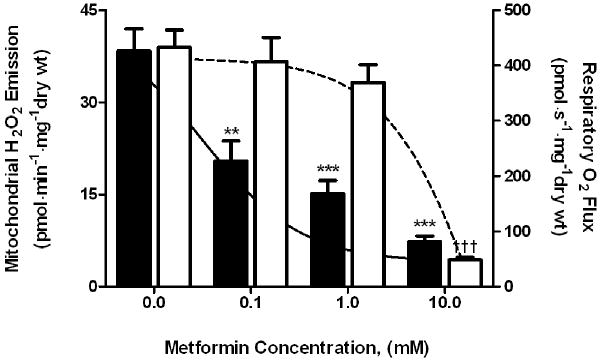
To examine the direct effects of metformin on forward (respiration) and reverse (H2O2 emission) electron flux at mitochondrial complex I in skeletal muscle, fiber bundles from the red portion of the gastrocnemius muscle of Sprague-Dawley rats, after permeabilization, were pre-incubated (∼20 min) in increasing concentrations of metformin (0-10 mM). Maximal succinate-generated H2O2 emission was reduced (P < 0.01) by ∼50% at the lowest concentration of metformin tested (100 μM) and was further inhibited (P < 0.001) to <25% of the maximal rate at 10 mM metformin (Figure 3). In stark contrast, maximal ADP-stimulated O2 consumption was maintained at the lower concentrations of metformin (0.1 and 1.0 mM) and inhibited (P < 0.001) only at a metformin concentration of 10 mM (Figure 3). These findings demonstrate that metformin inhibits reverse electron flux-mediated H2O2 emission at complex I of the mitochondrial electron transport chain in skeletal muscle at concentrations approximately two orders of magnitude lower than that required to inhibit electron flux in the forward direction.
Fig. 3.
Dose-dependent effects of meformin on respiratory O2 flux and mitochondrial H2O2 emission. Parallel control permeabilized red gastrocnemius myofibers were pre-incubated in metformin (0-10 mM) for 20 min. Maximal mitochondrial H2O2 emission rates (black bars, left y-axis) during succinate (3 mM) supported respiration are plotted with maximal ADP (8 mM) stimulated respiratory O2 flux (white bars, right y-axis) during complex I (glutamate/pyruvate/malate, 5/5/2 mM) supported respiration at each concentration of metformin. **P < 0.01, ***P < 0.001 versus no metformin for H2O2 emission. †††P < 0.001 versus no metformin for O2 flux.
Discussion
The results of the present study demonstrate a marked reduction in the potential for mitochondrial H2O2 emission with metformin treatment in skeletal muscle of the obese Zucker fa/fa rat, concurrent with improved whole-body glycemic control. Although previous reports have provided evidence that metformin mildly inhibits complex I-supported respiration [9, 10, 12, 13], we found no difference in ADP-stimulated submaximal or maximal O2 consumption rates in red gastrocnemius myofibers from lean or obese Zucker rats treated with metformin. Interestingly however, ex vivo experiments using increasing concentrations of metformin revealed that metformin is indeed capable of inhibiting ADP-stimulated respiration, but at concentrations two orders of magnitude greater than that required to inhibit complex I-linked H2O2 production. Thus, in view of recent data linking mitochondrial H2O2 emission in skeletal muscle to the development of high fat diet-induced insulin resistance [26], the findings of the present study raise the possibility that the insulin-sensitizing actions of metformin in muscle [32] may be mediated by the drug's ability to inhibit complex I-linked H2O2 emission while minimally affecting complex I-linked respiration.
Mild to moderate inhibition of the respiratory system by metformin was first reported nearly a decade ago and thus raised the prospect that the anti-diabetic actions of metformin may be related to inhibition of mitochondrial respiration [9, 10]. However, the effect requires relatively high concentrations (>10 mM) of metformin in isolated mitochondria [9, 12, 13] or extended exposure time in intact cells [10]. This time-dependency of metformin action is attributed to the slow membrane potential-driven rate at which metformin accumulates in the mitochondrial matrix [29]. Complex I has been identified as a potential site of metformin action based on the finding that respiration is inhibited by metformin only when supported by complex I but not complex II substrates [9, 12, 13]. However, even at high concentrations, the degree of inhibition of respiration by metformin is only a fraction of that observed with the classical complex I inhibitor rotenone [21]. Nevertheless, it has been proposed in liver that even mild inhibition of mitochondrial respiration, which has a high flux-control coefficient over gluconeogenesis, may represent a mechanism by which metformin reduces hepatic glucose output [10]. In skeletal muscle, decreased cellular energy charge and increased AMPK activity have been reported with metformin treatment [6, 8], but a definitive link has not been established. Moreover, treatment with oral metformin in rats requires at least 5 hours before an increase in AMPK phosphorylation in skeletal muscle is observed [33]. Thus, the mechanism by which metformin activates AMPK, and whether AMPK-induced signaling constitutes the mechanism responsible for the insulin-sensitizing actions of metformin remains unknown.
An alternative mechanism of action for metformin recently emerged from studies of the mitochondrial permeability transition pore (PTP). The PTP is a large conductance channel within the inner mitochondrial membrane that opens in response to a number of physiological factors and various forms of cellular stress (e.g., large increases in intracellular [Ca+2]), triggering collapse of the protonmotive force and release of pro-apoptotic factors [34]. Although the molecular composition of the PTP is unknown, complex I has been demonstrated to play a role in PTP opening and is even suggested to comprise part of the pore complex [35]. Leverve and co-workers [17, 18] recently found that metformin inhibits PTP opening in permeabilized cells in response to Ca2+ overload and in intact cells in response to high glucose concentrations or the oxidizing agent t-butyl hydroperoxide. In fact, metformin was found to be nearly as effective as cyclosporin A in preventing PTP opening in intact and permeabilized cells [17, 18]. Inclusion of the antioxidant N-acetyl-L-cysteine prevents hyperglycemia-induced opening of the PTP [17], suggesting that oxidative stress may represent the underlying link between high glucose, PTP opening and the therapeutic effects of metformin. Indeed, metformin has been reported to reduce oxidative stress in many [16-21] though not all [13, 36] studies. Notably, Batandier et al. [21] found that ROS production induced by reverse electron flux at complex I in isolated liver mitochondria is inhibited by metformin in a manner similar to rotenone, implying that complex I is the source of free radical production associated with hyperglycemia. The results of the present study demonstrate that in skeletal muscle, H2O2 emission associated with reverse electron flux at complex I is reduced by metformin treatment, potentially contributing to metformin's therapeutic action in vivo.
The present study also demonstrates that the maximal rate of mitochondrial H2O2 emission induced by succinate-driven reverse electron flux at complex I is more than 2-fold greater in skeletal muscle from obese vs. lean Zucker rats (Figure 2). This difference is nearly identical to the increase in H2O2 emitting potential previously observed in skeletal muscle of both rats and mice fed a high fat diet or humans after consuming a high fat meal [26]. Moreover, in rats injected with a mitochondrial-targeted antioxidant, or in mice genetically-engineered to express catalase in their muscle mitochondria, mitochondrial H2O2 emitting potential was restored to normal and rodents were protected against high fat diet-induced insulin resistance [26]. In the present study, metformin had nearly an identical effect, decreasing complex I-derived H2O2 emitting potential in the muscle of obese rats to a rate nearly equal to that observed in lean rats in conjunction with improving glucose tolerance. Suprisingly however, the elevated H2O2 emitting potential evident in muscle of obese rats was not associated with a reduced GSH/GSSG ratio nor was the reduced H2O2 emitting potential induced by metformin treatment associated with an increased GSH/GSSG ratio. These findings contrast with recent data from Sprague-Dawley rats and humans in which muscle GSH/GSSG was found to be acutely and chronically reduced by high fat feeding [26]. Interestingly, total muscle glutathione concentration in muscle from Zucker rats is nearly double that in Sprague-Dawley rats, raising the possibility that the Zucker strain is uniquely adapted. Clearly, further work will be required to determine whether reduced mitochondrial H2O2 emission accounts for the insulin sensitizing actions of metformin.
Metformin did not affect the rate of O2 consumption during basal (state 4) respiration supported by either glutamate/malate or palmitoyl-carnitine/malate, indicating that the decrease in H2O2 emission induced by metformin was not due to an uncoupling effect. Surprisingly, in contrast to the fairly well-established mild inhibitory action of metformin on ADP-stimulated respiration [9, 10, 12, 13], we found no evidence of impaired ADP-stimulated, complex I-supported respiration in muscles from metformin treated lean or obese rats (Figure 1). To further explore this discrepancy, we tested the concentration-dependent effects of metformin on mitochondrial H2O2 emission and O2 consumption in parallel. Interestingly, metformin was found to be capable of suppressing mitochondrial H2O2 emission at concentrations two orders of magnitude lower than that needed to inhibit respiration (Figure 3). In the context of metformin's action in vivo, these findings are significant as they suggest that at therapeutic doses in humans (i.e., ∼1000 mg/dose, ∼0.5-1 mg/L or ∼2.5-5 μM peak plasma bio-accumulating to ∼100-500 μM in mitochondria) [37, 38], metformin is capable of suppressing muscle mitochondrial H2O2 emission without affecting respiratory control or lactate production unless concentrations reach the mM range in mitochondria.
In summary, the current study demonstrates that complex I-linked mitochondrial H2O2 emitting potential is ∼2-fold higher in skeletal muscle of obese rats but is reduced by 4 weeks of metformin treatment to rates observed in lean rats. At the cellular level, pre-treatment of permeabilized skeletal muscle fiber bundles with metformin limits reverse electron flux-associated H2O2 production at complex I at concentrations at least two orders of magnitude less than that required to inhibit forward electron flux (i.e., respiration). Further research will be necessary to determine whether the intrinsic mehanisms by which metformin exerts its therapeutic effects are mediated by its ability to limit mitochondrial oxidant emission in target tissues such as skeletal muscle and liver.
Supplementary Material
01
Acknowledgments
This study was supported by U.S. National Institute of Health grants R01 [DK061314] & [DK075880] (RNC) and [DK073488] (PDN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, Shulman GI. Efficacy and Metabolic Effects of Metformin and Troglitazone in Type II Diabetes Mellitus. N Engl J Med. 1998;338:867–873. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]
- 2.Johnson AB, Webster JM, Sum CF, Heseltine L, Argyraki M, Cooper BG, Taylor R. The impact of metformin therapy on hepatic glucose production and skeletal muscle glycogen synthase activity in overweight type II diabetic patients. Metabolism. 1993;42:1217–1222. doi: 10.1016/0026-0495(93)90284-u. [DOI] [PubMed] [Google Scholar]
- 3.Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic Effects of Metformin in Non-Insulin-Dependent Diabetes Mellitus. N Engl J Med. 1995;333:550–554. doi: 10.1056/NEJM199508313330903. [DOI] [PubMed] [Google Scholar]
- 4.Hother-Nielsen O, Schmitz O, Andersen PH, Beck-Nielsen H, Pedersen O. Metformin improves peripheral but not hepatic insulin action in obese patients with type II diabetes. Acta Endocrinol (Copenh) 1989;120:257–265. doi: 10.1530/acta.0.1200257. [DOI] [PubMed] [Google Scholar]
- 5.Kim YB, Ciaraldi TP, Kong A, Kim D, Chu N, Mohideen P, Mudaliar S, Henry RR, Kahn BB. Troglitazone but not Metformin Restores Insulin-Stimulated Phosphoinositide 3-Kinase Activity and Increases p110Î2 Protein Levels in Skeletal Muscle of Type 2 Diabetic Subjects. Diabetes. 2002;51:443–448. doi: 10.2337/diabetes.51.2.443. [DOI] [PubMed] [Google Scholar]
- 6.Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 7.Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, Inzucchi SE, Schumann WC, Petersen KF, Landau BR, Shulman GI. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Mir MY, Nogueira V, Fontaine E, Averet N, Rigoulet M, Leverve X. Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J Biol Chem. 2000;275:223–228. doi: 10.1074/jbc.275.1.223. [DOI] [PubMed] [Google Scholar]
- 10.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348(Pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunmair B, Staniek K, Gras F, Scharf N, Althaym A, Clara R, Roden M, Gnaiger E, Nohl H, Waldhausl W, Furnsinn C. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes. 2004;53:1052–1059. doi: 10.2337/diabetes.53.4.1052. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho C, Correia S, Santos MS, Seica R, Oliveira CR, Moreira PI. Metformin promotes isolated rat liver mitochondria impairment. Mol Cell Biochem. 2008;308:75–83. doi: 10.1007/s11010-007-9614-3. [DOI] [PubMed] [Google Scholar]
- 14.Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420–2425. doi: 10.2337/diabetes.51.8.2420. [DOI] [PubMed] [Google Scholar]
- 15.Hardie DG. Neither LKB1 nor AMPK are the direct targets of metformin. Gastroenterology. 2006;131:973. doi: 10.1053/j.gastro.2006.07.032. author reply 974-975. [DOI] [PubMed] [Google Scholar]
- 16.Bonnefont-Rousselot D, Raji B, Walrand S, Gardes-Albert M, Jore D, Legrand A, Peynet J, Vasson MP. An intracellular modulation of free radical production could contribute to the beneficial effects of metformin towards oxidative stress. Metabolism. 2003;52:586–589. doi: 10.1053/meta.2003.50093. [DOI] [PubMed] [Google Scholar]
- 17.Detaille D, Guigas B, Chauvin C, Batandier C, Fontaine E, Wiernsperger N, Leverve X. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54:2179–2187. doi: 10.2337/diabetes.54.7.2179. [DOI] [PubMed] [Google Scholar]
- 18.Guigas B, Detaille D, Chauvin C, Batandier C, De Oliveira F, Fontaine E, Leverve X. Metformin inhibits mitochondrial permeability transition and cell death: a pharmacological in vitro study. Biochem J. 2004;382:877–884. doi: 10.1042/BJ20040885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouslimani N, Peynet J, Bonnefont-Rousselot D, Therond P, Legrand A, Beaudeux JL. Metformin decreases intracellular production of reactive oxygen species in aortic endothelial cells. Metabolism. 2005;54:829–834. doi: 10.1016/j.metabol.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Rosen P, Wiernsperger NF. Metformin delays the manifestation of diabetes and vascular dysfunction in Goto-Kakizaki rats by reduction of mitochondrial oxidative stress. Diabetes Metab Res Rev. 2006;22:323–330. doi: 10.1002/dmrr.623. [DOI] [PubMed] [Google Scholar]
- 21.Batandier C, Guigas B, Detaille D, El-Mir MY, Fontaine E, Rigoulet M, Leverve XM. The ROS production induced by a reverse-electron flux at respiratory-chain complex 1 is hampered by metformin. J Bioenerg Biomembr. 2006;38:33–42. doi: 10.1007/s10863-006-9003-8. [DOI] [PubMed] [Google Scholar]
- 22.Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H(2)O(2) generation. Am J Physiol Cell Physiol. 2006;290:C844–851. doi: 10.1152/ajpcell.00402.2005. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- 24.Anderson EJ, Yamazaki H, Neufer PD. Induction of endogenous uncoupling protein 3 suppresses mitochondrial oxidant emission during fatty acid-supported respiration. J Biol Chem. 2007;282:31257–31266. doi: 10.1074/jbc.M706129200. [DOI] [PubMed] [Google Scholar]
- 25.St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 26.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuznetsov AV, Tiivel T, Sikk P, Kaambre T, Kay L, Daneshrad Z, Rossi A, Kadaja L, Peet N, Seppet E, Saks VA. Striking differences between the kinetics of regulation of respiration by ADP in slow-twitch and fast-twitch muscles in vivo. Eur J Biochem. 1996;241:909–915. doi: 10.1111/j.1432-1033.1996.00909.x. [DOI] [PubMed] [Google Scholar]
- 28.Tonkonogi M, Henriksson J, Cotgreave IA. Human skeletal muscle interstitial glutathione levels are elevated in comparison to adipose tissue and blood plasma. Arch Biochem Biophys. 2003;413:147–149. doi: 10.1016/s0003-9861(03)00121-8. [DOI] [PubMed] [Google Scholar]
- 29.Dykens JA, Jamieson J, Marroquin L, Nadanaciva S, Billis PA, Will Y. Biguanide-induced mitochondrial dysfunction yields increased lactate production and cytotoxicity of aerobically-poised HepG2 cells and human hepatocytes in vitro. Toxicology and applied pharmacology. 2008;233:203–210. doi: 10.1016/j.taap.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Srere PA. Citrate synthase. Methods Enzymol. 1969;13:3–5. [Google Scholar]
- 31.Batandier C, Leverve X, Fontaine E. Opening of the Mitochondrial Permeability Transition Pore Induces Reactive Oxygen Species Production at the Level of the Respiratory Chain Complex I. J Biol Chem. 2004;279:17197–17204. doi: 10.1074/jbc.M310329200. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Wan Q, Guan Q, Gao L, Zhao J. High-fat diet feeding impairs both the expression and activity of AMPKa in rats' skeletal muscle. Biochem Biophys Res Commun. 2006;339:701–707. doi: 10.1016/j.bbrc.2005.11.068. [DOI] [PubMed] [Google Scholar]
- 33.Suwa M, Egashira T, Nakano H, Sasaki H, Kumagai S. Metformin increases the PGC-1alpha protein and oxidative enzyme activities possibly via AMPK phosphorylation in skeletal muscle in vivo. J Appl Physiol. 2006;101:1685–1692. doi: 10.1152/japplphysiol.00255.2006. [DOI] [PubMed] [Google Scholar]
- 34.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Fontaine E, Eriksson O, Ichas F, Bernardi P. Regulation of the Permeability Transition Pore in Skeletal Muscle Mitochondria. Modulation by Electron Flow Through the Respiratory Chain Complex I. J Biol Chem. 1998;273:12662–12668. doi: 10.1074/jbc.273.20.12662. [DOI] [PubMed] [Google Scholar]
- 36.Anedda A, Rial E, Gonzalez-Barroso MM. Metformin induces oxidative stress in white adipocytes and raises uncoupling protein 2 levels. J Endocrinol. 2008;199:33–40. doi: 10.1677/JOE-08-0278. [DOI] [PubMed] [Google Scholar]
- 37.Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 1996;30:359–371. doi: 10.2165/00003088-199630050-00003. [DOI] [PubMed] [Google Scholar]
- 38.Bailey CJ. Biguanides and NIDDM. Diabetes Care. 1992;15:755–772. doi: 10.2337/diacare.15.6.755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01