Evidence that fold-change, and not absolute level, of β-catenin dictates Wnt signaling (original) (raw)
. Author manuscript; available in PMC: 2010 Dec 11.
SUMMARY
In the canonical Wnt pathway, binding of the Wnt ligand to its transmembrane receptors leads to an inhibition of the degradation of β-catenin; as a result, β-catenin accumulates to a point where it activates target genes. Using mathematical modeling and experiments in mammalian cells, we examined the robustness of the β-catenin response to Wnt stimulation. We found that the final (post-Wnt) level of β-catenin is very sensitive to all perturbations in the Wnt signaling pathway, such that mild genetic or environmental variation would be expected to change the final level of β-catenin, and alter the output of the pathway. By contrast, one unusual parameter was robust: the fold-change in β-catenin (post- Wnt level / pre-Wnt level). Furthermore, in Xenopus embryos, dorsal-anterior development and the corresponding target gene expression are robust to the same perturbations that alter the final level but leave the fold-change intact. These results suggest: First, despite noise and variation, within a range the cell maintains a constant fold-change in β-catenin for a given Wnt stimulation. Second, the transcriptional machinery downstream of the Wnt pathway is constructed to read the robust fold-change and not simply the final level of β-catenin. In analogy to Weber’s law in sensory physiology, some gene transcription networks may be tuned to respond to fold-changes, rather than absolute levels of signals, as a way to reduce the consequences of stochastic, genetic and environmental variation.
INTRODUCTION
The canonical Wnt signaling pathway is highly conserved and broadly employed in multicellular animals (for example, in patterning embryonic body plans (Riggleman et al., 1990) and maintaining stem cell homeostasis (Korinek et al., 1998)). Mutations in the Wnt pathway are implicated in disease, notably colorectal cancer (Rubinfeld et al., 1993; Su et al., 1993) and hepatocellular carcinoma (Satoh et al., 2000). The intermediary signal in the Wnt pathway is a protein called β-catenin. In the absence of a Wnt signal, β-catenin is maintained at low levels by rapid degradation (van Leeuwen et al., 1994) (Figure 1A). Degradation is mediated by a protein complex, whose components include multiple kinases (GSK3βand CKIα) and scaffolds (APC and Axin1) (Hart et al., 1998; Ikeda et al., 1998; Itoh et al., 1998; Liu et al., 2002; Peifer et al., 1994b; Rubinfeld et al., 1993; Salic et al., 2000; Su et al., 1993; Zeng et al., 1997). The destruction complex phosphorylates β-catenin and targets it to a ubiquitin ligase and ultimately to the proteosome (Aberle et al., 1997; Liu et al., 2002; Orford et al., 1997; Peifer et al., 1994a; van Noort et al., 2002; Yost et al., 1996). Binding of the Wnt ligand to the extracellular domains of two transmembrane receptors leads to the inhibition of the activity of the destruction complex in the cytosol (Bhanot et al., 1996; Pinson et al., 2000; Tamai et al., 2000; Wehrli et al., 2000). As a result, β-catenin accumulates to a high level (Figure 1A).
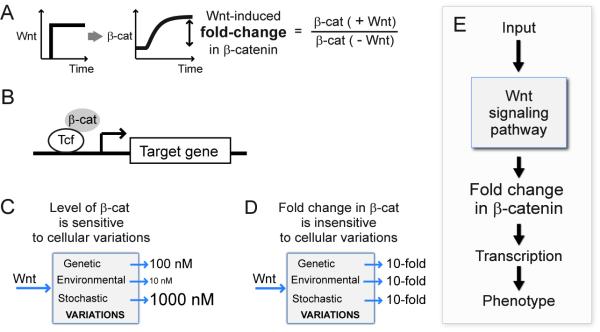
Figure 1. What is the cellular reporter of Wnt stimulation?
(A) Wnt stimulation induces accumulation of β-catenin. (B) Together with the Tcf/Lef transcription factor, β-catenin activates transcription of specific target genes. (C) We found that the absolute level of β-catenin is sensitive to non-specific variation that may naturally occur in cells. (D) Interestingly, the fold-change in β-catenin induced by Wnt stimulation is buffered against variation in parameters. (E) Together with functional data from Xenopus embryos, we propose that fold-changes in β-catenin, and not absolute levels of β-catenin, is the output of Wnt signaling.
The increased amount of β-catenin, in conjunction with Tcf/Lef transcription factors, activates transcription of specific target genes (Figure 1B) (Behrens et al., 1996; Molenaar et al., 1996; Peifer et al., 1994b; van Leeuwen et al., 1994). In this way it is thought that phenotypic responses to β-catenin, whether normal or pathological, are consequences of the elevated level of the protein. While it was observed that a high level of β-catenin in cancer cells correlates with hyper-active Wnt responses (Klaus and Birchmeier, 2008), the quantitative features of this correlation have not been fully explored. What is not known, for instance, is how precisely the cell controls the level of β-catenin in the absence of the Wnt ligand, and how precisely it controls the level of β-catenin in response to the Wnt ligand, and if these levels are robustly maintained, and by what means they are controlled.
Given the importance of Wnt signaling, we were somewhat surprised to find, first through theory and then through experiments, that response of this pathway, the level of β-catenin, was very sensitive to relatively small perturbations in Wnt pathway parameters (Figure 1C). Parameters in the Wnt pathway include levels of scaffolds, kinases, kinetic rate constants, binding equilibrium and so on. This result holds for the level of β-catenin either before or, more significantly, after Wnt stimulation. This means that natural variation, either genetic, environmental or stochastic, could significantly change the output of Wnt signaling. This is a real concern as there is increasing evidence that the level of a protein, for example, might vary by 4-fold across a population of cells due to stochasticity alone (Alon, 2007; Bar-Even et al., 2006; Blake et al., 2006; Blake et al., 2003; Elowitz et al., 2002; Ozbudak et al., 2002; Paulsson, 2004; Raser and O’Shea, 2004). The concern is that the level of β-catenin would vary from cell to cell, as a result of the stochastic variation of the numerous other proteins in the pathway.
While the level of β-catenin was shown to be fine-tuned, our analysis revealed that another measure of the response of the pathway is robust to most perturbations in the Wnt pathway: the fold-change in β-catenin, defined as the ratio of the β-catenin level after Wnt stimulation to the level of β-catenin before Wnt stimulation (Figure 1A, D). We confirmed this prediction experimentally where we perturbed components of the pathway in mammalian cells, by overexpression and pharmacological inhibitors. In this system we could measure accurately the pre-Wnt level, the post-Wnt level, and the fold-change in β-catenin in response to Wnt. To probe further the biological relevance of this behaviour, we turned to a quantitative study of Wnt signaling in Xenopus embryos. The anatomical phenotype in the early embryo correlated with the Wnt-induced fold-change in β-catenin, and not with the absolute levels of β-catenin. Perturbations that affected the final level of β-catenin and the fold-change altered the phenotype; perturbations that similarly affected the final level but not the fold-change did not alter the normal phenotype. Therefore, fold-change in the level of β-catenin induced by Wnt, and not the final level itself, should potentially be a precise reporter of Wnt signal that is read by the downstream transcriptional system (Figure 1E).
The robustness of the fold-change response holds over a limited range (approximately a fourfold range) of perturbation of the internal components of the pathway; this would be expected to cover the range of naturally occurring noise in protein levels found in many systems (Alon, 2007 and the references therein). Extreme perturbations such as knockdowns and knockouts should overwhelm this buffering, as they do. For this reason it would probably difficult to detect this property previously, unless one were expecting it and testing for it. For example, we show that Wnt stimulation gives a constant fold-change response to the commonly used GSK-3 inhibitors (lithium chloride and BIO), but this was only revealed when we examined concentrations of inhibitors lower than usually used. Experiments in Xenopus embryos commonly use concentrations as high as 0.3M LiCl, which exceeds the buffered range, whereas at lower concentrations, LiCl significantly perturbs the level of β-catenin without affecting the fold-change or the embryonic morphology.
Having the output of the Wnt signaling circuit in the form of fold-change may explain its robustness. Nevertheless, it raises questions about how the transcriptional circuits downstream could respond to fold-changes (Figure 1E). The downstream transcriptional response system must first remember the level of β-catenin before the Wnt signal, and then compare it to the level of β-catenin after the Wnt signal. In other words, responding to fold-changes requires that the transcriptional machinery measure a temporal ratio of the basal state to the stimulated state. We show that in Xenopus embryos the natural target gene circuits are constructed to respond to fold-changes in β-catenin, and not the absolute levels, while artificial promoters comprising only Tcf-Lef/β-catenin binding sites do not show this property. In the following paper, in collaboration with Uri Alon and Oren Shoval, we show how a common transcriptional motif, the incoherent feed forward loop has a remarkable ability to be a fold-change detector.
Modeling analysis predicts that the Wnt induced fold-change in β-catenin is insensitive to variation in most pathway parameters
To study the robustness of the Wnt pathway to variation in its internal parameters, we started with a published mathematical model (Figure 2A) (Lee et al., 2003), describing detailed kinetics of the cytosolic interactions in the Wnt pathway. The model was shown to capture well the effects of various perturbations in Xenopus extracts (e.g., immunodepletion, overexpression, and drug addition, on the flux of β-catenin degradation). Detailed measurements of the rate constants, equilibrium constants, concentrations, and fluxes in the Xenopus extracts permitted evaluation of all parameters within the model.
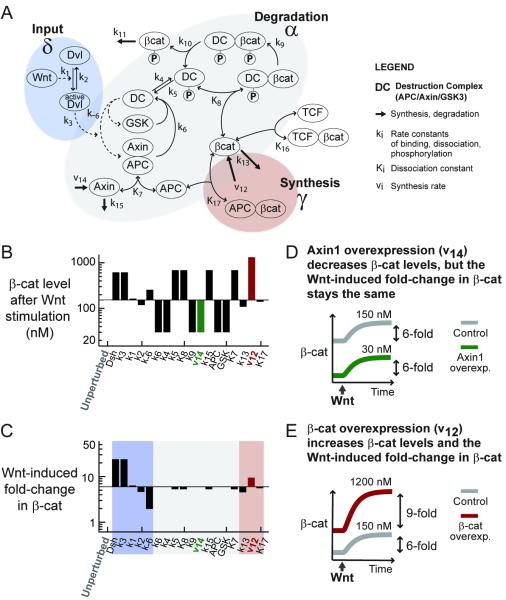
Figure 2. Fold-change in β-catenin, not absolute levels, is predicted to be insensitive to variation in cellular parameters.
(A) A kinetic model of the Wnt pathway (Lee et al., 2003).
(B-C) Parameter sensitivity analysis. The parameters measured in the Xenopus extracts are used as the “unperturbed setpoint” (the first column). From this setpoint, each parameter was increased by 5-fold, one at a time (corresponds to each column). For each parameter set, we simulated the accumulation of β-catenin induced by a step Wnt stimulation, and recorded the level of β-catenin after Wnt stimulation (B) and fold-change in β-catenin induced by Wnt (C).
(D-E) For example, when Axin1 synthesis rate is increased by 5-fold (D) and the system is then stimulated with Wnt, the Wnt-induced level of β-catenin is 30 nM and the Wnt-induced fold-change in β-catenin is 6-fold. When β-catenin synthesis rate is increased by 5-fold (E), and the system is then stimulated with Wnt, the Wnt-induced level of β-catenin is 1200 nM and the Wnt-induced fold-change in β-catenin is 9-fold. Please see Figure S1 for a more complete sensitivity analysis.
When we set up the model and perturbed the Xenopus parameters one at a time, mimicking natural variation in biochemical parameters, we found that the basal level of β-catenin, the β-catenin level after Wnt stimulation, the difference between the basal and final level, the response time, the integrated level, the integrated difference and the integrated fold-change varied extensively with parameters (Figures 2B and S1). By contrast, the Wnt-induced fold-change in β-catenin was insensitive to perturbation in most parameters (Figure 2C). Note that the free, non-GSK3-phosphorylated pool is plotted here, but the results hold when total pool of β-catenin (the measurable quantity) is plotted instead (Figure S2).
As an example of the effects of perturbation in the model, when we moderately overexpress Axin1 (v14) in the model, the basal level of β-catenin decreases (Figure 2D). The unperturbed and the Axin1-overexpressing system with a lower level of β-catenin respond equally to Wnt stimulation: they give the same fold-change in β-catenin. On the other hand, overexpressing β-catenin (v12) in the model also increases the basal level of β-catenin (Figure 2E). However, now cells with a higher level of β-catenin respond excessively to Wnt stimulation: they give a higher fold-change in β-catenin. This numerical sensitivity analysis serves as an illustration; the thorough multi-parameter sensitivity will be addressed by the analytical solution presented next.
To understand how the Wnt-induced fold-change in β-catenin, and not the absolute levels of β-catenin, can buffer variation in parameters, we analyzed the model further. As illustrated by the colour shadings in Figure 2, the parameters group in a meaningful way. For example, the fold-change in β-catenin is sensitive to most parameters, all of which characterize the large degradation machinery (grey-shaded in Figures 2A and C). We can derive this grouping formally using dimensional analysis. This is an analytical tool where each variable is scaled, such that the normalized, dimensionless variable averages around 1. As this technique is widely used in physics and engineering, and involves relatively straightforward albeit tedious algebra, we present the detailed derivation in the supplement. Note that this analysis involves no further assumptions or simplification of the model; the full model is simply rearranged into its most concise form.
Performing the scaling procedure, the numerous parameters in the steady-state model automatically group into 3 terms: α, δ, and γ (Figure 2A). The majority of the parameters fall into the α group, which includes the 12 parameters describing the degradation machinery. The δ group characterizes the strength of ligand stimulation. And the small γ group contains the synthesis rate of β-catenin and the weak binding between β-catenin and APC (Lee et al., 2003; Rubinfeld et al., 1995). Effectively, the parameter grouping suggests that there are 3 independent ways (“effective rates”) to increase the steady-state β-catenin level: by increasing synthesis, inhibiting degradation, and ligand stimulation. Despite the many independent biochemical parameters, there are only 3 independent ways to perturb β-catenin level. Many parameters cluster and affect the system in the same way. Notably, APC was recently shown to have a dual role in Wnt signaling (Takacs et al., 2008). Adding this extra interaction in the model simply leads to extra parameters in α, and no changes in the subsequent analysis (eqns. 31-32 in the supplement).
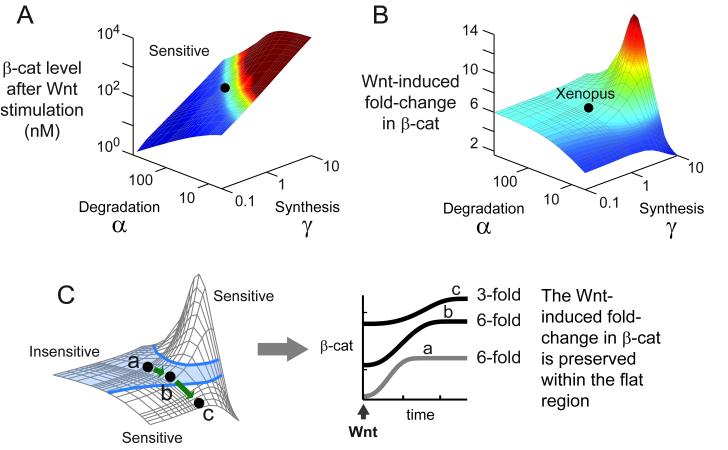
One advantage of understanding the 3 grouping of the parameters is: with a 3-dimensional plot, we can visualize the entire behaviour of the Wnt pathway, for all possible values of parameters. Analytically solving the model, we find that the level of β-catenin after Wnt stimulation is always sensitive to parameters (Figure 3A). The detailed analytical solution is presented in the supplement. As shown in Figure 3A, the level of β-catenin varies continuously with the parameters in the large degradation machinery (α and the parameters in the group γ.
Figure 3. There is a region of parameters where the Wnt-induced fold-change in β-catenin is insensitive to variation in parameters.
(A-B) Analytical solutions for the level of β-catenin after Wnt stimulation (A) and the Wnt-induced fold-change in β-catenin (B). These surfaces were computed analytically using equations 29-30 derived in the supplement. The black dot denotes the Xenopus parameters (α = 66, γ= 1). (C) The light blue shading indicates the region where the fold-change in β-catenin varies by less than ±10%. Suppose the cells reside within this region (point a), and we gradually inhibit degradation (α), thereby moving from point a to b to c. At each point, we stimulate the cells with Wnt, shown in the right panel. The absolute levels of β-catenin vary immediately, but the fold-change in β-catenin is identical for points a and b, which reside within the blue-shaded region.
Next we solve for the fold-change in β-catenin induced by Wnt stimulation. As shown in Figure 3B, there is a parameter region where the fold-change in β-catenin withstands variation in parameters (the flat region). As long as the cells operate in this parameter region, varying most parameters, such as inhibiting GSK3β or overexpressing Axin1, will not affect the way cells respond to Wnt stimulation (i.e., fold-change in β-catenin stays constant)—even though the absolute levels of β-catenin itself has varied (Figure 3C). We also analyzed the effects of the parameters in δ; as expected, the higher the Wnt concentration, the higher the fold-change in β-catenin (Figure S3).
In the simplest model of synthesis and degradation, with ligand affecting the rate of degradation, the Wnt-induced fold-change would stay constant despite variation in synthesis or degradation parameters. In fact, the numerous and nonlinear interactions in the Wnt pathway effectively behave in this simple manner within the flat region in Figure 3B. The exit from this flat region represents a departure from a simple synthesis/degradation balance. It is not that many interactions drop out, but many interactions are wired to make possible the simple behaviour.
Moreover, it is not trivial to be in the parameter region where the simple behaviour is realized. It requires maintaining a very active destruction complex (a rapid cycle of binding, phosphorylation, and dissociation). This insight was obtained by applying a separation of time scale, presented in the supplement. A very potent destruction complex is indeed a hallmark of the Wnt pathway. As shown next, it appears that the simple behaviour is not fully realized in cells.
The Wnt-induced fold-change in β-catenin is insensitive to variation in the degradation machinery
Do cells operate in the parameter region where the Wnt-induced fold-change in β-catenin is insensitive to variation in many parameters? As the first evidence, we note that the parameters measured in the Xenopus system place the Wnt pathway in the region where the fold-change in β-catenin is insensitive to the degradation parameters (Figure 3B). This is a nontrivial coincidence, as the model was constructed without any assumptions that it should exhibit such a region, and the parameters were measured independently. Curiously, the Xenopus parameters lie close to the peak region, such that the fold-change in β-catenin is relatively sensitive to β-catenin synthesis rate (plotted later). To verify these theoretical predictions, we perturbed these parameters and several others experimentally in mammalian cells. The RKO colorectal cell line was chosen because it is an established model for Wnt signaling and its analysis is simplified by the lack of cadherin-bound pool of β-catenin (Figure 4A) (Breen et al., 1993; Giannini et al., 2000). Note again, that the term fold-change always refers to the change in the level of β-catenin induced by Wnt, and not by any other means.
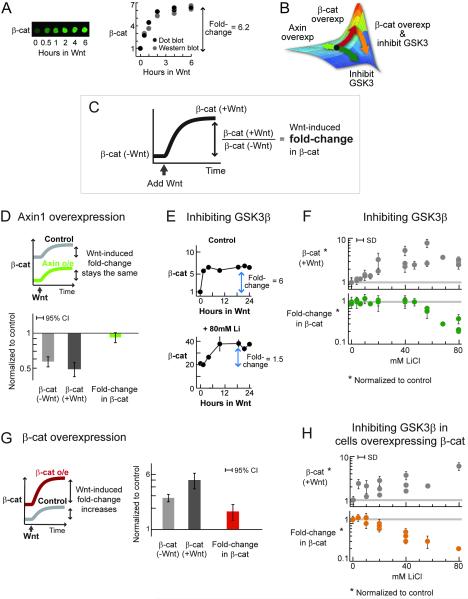
Figure 4. The Wnt-induced fold-change in β-catenin is insensitive to perturbations in the degradation machinery.
In human RKO cells, most β-catenin is cytoplasmic due to absence of cadherin (Breen et al., 1993; Giannini et al., 2000). In each experiment, 2-3 biological replicates were examined, each analyzed using 2-4 dot blots. SD = standard deviation.
(A) A typical Wnt stimulation in RKO cells (i.e., incubation with Wnt3A-conditioned media). Level of β-catenin was quantified using dot blot.
(B) Redrawn from Figure 3B. This figure predicts how the Wnt-induced fold-change in β-catenin would respond to various perturbations, depicted by the arrows.
(C) Definition of the quantities measured in the experiments.
(D) Cells overexpressing Axin1 were stimulated with Wnt. Three independent experiments were performed. 95%CI = 1.96*SD.
(E-F) Cells were pre-treated with different doses of lithium (3h), and then stimulated with Wnt in the presence of lithium. Figure E shows representative profiles of Wnt-induced β-catenin accumulation in the control and treated cells. Figure F shows results from 4 independent experiments. Please see Figure S4 for a detailed experimental protocol, and Figure S6 for similar results using BIO, another GSK3β inhibitor.
(G) Cells overexpressing β-catenin were stimulated with Wnt. Two independent experiments were performed. 95%CI = 1.96*SD.
(H) Cells overexpressing β-catenin were pre-treated with different doses of lithium (3h), and then stimulated with Wnt in the presence of lithium. Three independent experiments were performed.
The Wnt-induced fold-change in β-catenin is insensitive to perturbations in the degradation process in RKO cells
If cells operate within the insensitive region, then the Wnt-induced fold-change in β-catenin should be unaffected by moderate perturbations in the degradation machinery (green arrow, Figure 4B). We enhanced degradation by Axin1 overexpression, a scaffold in the destruction complex. Moderate Axin1 overexpression decreased the basal β-catenin level by half (0.49±0.06, Figure 4D). Remarkably, upon Wnt stimulation, both control and Axin-overexpressing cells gave nearly the same fold-change in β-catenin (0.92±0.07, Figure 4D).
A similar precision in responding to Wnt stimulation was observed when the degradation rate was decreased (Figures 4E-F). We inhibited GSK3β, a key kinase in the destruction complex, using lithium and BIO (Klein and Melton, 1996; Meijer et al., 2003). The basal and Wnt-induced level of β-catenin increased in a dose-dependent manner to lithium treatment (Figure S5, Figure 4F). Instead of being saturated, cells with three times the basal level of β-catenin responded to Wnt stimulation just like the control cells. The fold-change in β-catenin (post-Wnt level / pre-Wnt level) was preserved within 20% (Figure 4F). At higher doses of inhibitor (>40mM), the Wnt-induced fold-change eventually decreased (cells responded less and less to Wnt stimulation). Similarly, the Wnt-induced fold-change in β-catenin was initially unaffected by another GSK3β inhibitor, BIO (Figure S6).
The Wnt-induced fold-change in β-catenin is sensitive to perturbations in synthesis rate in RKO cells
The model predicts both a flat region and an adjacent peak. Though Wnt-induced fold-change in β-catenin in RKO cells is robust to perturbations in the degradation machinery (Figure 3C), the model predicts a peak region, where Wnt stimulation can give rise to a higher fold-change in β-catenin. It also predicts that we should be able bring the cells to this hyper-responsive region by overexpressing β-catenin (red arrow, Figure 4B). Cells overexpressing β-catenin (by 2.8±0.3 times, Figure 4G) are more responsive to Wnt stimulation (Figure 4G). That is, cells overexpressing β-catenin gave a higher fold-change in β-catenin compared to the control cells (by 1.8±0.4, Figure 4G). Thus in the peak region, constancy of fold-change was broken.
The model explains that the weak binding between APC and β-catenin (K17~1000 nM) is responsible for this hyper-responsiveness. Overexpressing of β-catenin (increasing γ, red arrow in Figure 4B) can bring the system into a parameter region where this binding now becomes strong enough to act as a positive feedback: β-catenin sequesters APC, synergizing with Wnt in inhibiting the destruction complex. Note that inhibiting the destruction complex (decreasing α; green arrow in Figure 4B) also increases the level of β-catenin, but the positive feedback is never sufficiently realized if we take this route (the surface simply slopes down). Of course, it all depends on where we start. The model suggests that how we increase β-catenin matters because the effect of an interaction depends on a nonlinear combination of parameters, manifested in a non-monotonic surface manifold (Figure 4B).
In the hyper-responsive peak region, cells gave a higher fold-change in β-catenin compared to control cells. The model predicts that from this setpoint, the fold-change in β-catenin should now be more sensitive to perturbation in the degradation machinery (orange arrow in Figure 4B). It is not that cells are saturated more quickly, because they actually respond more to Wnt stimulation than the control cells (Figure 4G). When lithium treatment was repeated on cells overexpressing β-catenin, the Wnt-induced fold-change in β-catenin decreased more precipitously (Figure 4H). Please also see Figure S7, where the two treatments (lithium treatment and β-catenin overexpression) were performed side-by-side.
In summary, we can observe directly how the fold-change in β-catenin withstands perturbations in the degradation machinery, and how it fails to buffer specific perturbations. That this was predicted by the model suggests that no new molecular mechanisms need to be invoked. The Wnt pathway, as modelled in the present study, can already explain the observed behaviour.
The data suggest that an insensitive region (Figure 3C) predicted by the model indeed exists, and that RKO cells operate within this region. Independent measurements of the model parameters performed in Lee et al., 2003 suggest that the Xenopus system also operates within the insensitive region. These argue for the biological relevance of this region, where the Wnt-induced fold-change in β-catenin buffers perturbations. Moreover, we can alter the behaviour of the Wnt pathway in the specific ways predicted by the model. The data suggest that the fold-change in β-catenin is a more precise reporter of Wnt stimulation than the absolute level of β-catenin because it can be insensitive to most parameters that naturally vary from cell to cell.
Phenotypic outcomes of Wnt signaling in embryonic development are robust to variation in the degradation machinery
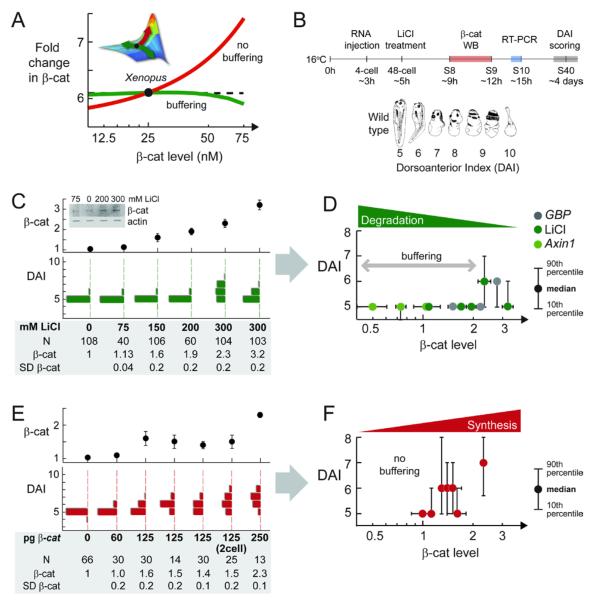
The relative importance of fold-changes versus absolute levels of β-catenin can be addressed in a complex embryonic system. In Xenopus, the canonical Wnt signaling regulates the formation of dorsal-anterior structures. Ectopic Wnt or β-catenin expression causes duplication of dorsoanterior structures (i.e., twin-headed tadpoles) (Funayama et al., 1995; McMahon and Moon, 1989), while blocking Wnt or β-catenin expression eliminates dorsoanterior structures (Heasman et al., 1994; Tao et al., 2005). Globally blocking degradation of β-catenin leads to a global increase of β-catenin (Schneider et al., 1996) and enlarged dorsoanterior structures.
From the Xenopus setpoint, the model predicts that the Wnt-induced fold-change in β-catenin should be relatively insensitive to variations in the large degradation machinery, and sensitive changes in β-catenin expression (Figure 5A, please see also Figure S8 for more details). If fold-change in β-catenin, rather than absolute level of β-catenin, dictates the phenotypic outcome of Wnt signaling, then embryonic development should be relatively insensitive to degradation parameters, and sensitive to β-catenin overexpression.
Figure 5. Phenotypic Outcome of Wnt Signaling in Xenopus Patterning Is Insensitive to Perturbations in the Degradation Machinery.
(A) Model prediction. To facilitate comparison with experimental data, we plot the Wnt-induced fold-change in β-catenin against the basal level of β-catenin. (Please also see Figure S8 to help visualize this.) From the Xenopus setpoint, perturbing degradation (α, green) will vary β-catenin level without initially affecting the Wnt-induced fold-change in β-catenin. However, increasing synthesis (γ, red) will increase both β-catenin level as well as the Wnt-induced fold-change.
(B) RNA was injected to all four cells at 4-cell stage. Lithium treatment was performed at 48-cell stage for 5 min. Dorsoanterior phenotype was scored using the DAI index (Kao and Elinson, 1988). Cadherin-free pool of β-catenin was quantified using western blot on stage 8, around the onset of target gene expression.
(C) Lithium treatment.
(D) Plotting median DAI versus measured level of β-catenin for lithium treatment, GBP RNA injection, and Axin1 RNA injection.
(E and F) β-catenin injection. (F) Plotting median DAI versus measured level of β-catenin for β-catenin RNA injection.
Note: Phenotypic scoring, β-catenin quantitation, and RT-PCR (in Figure 6) were performed in sibling embryos. SD of β-catenin is from two to four western blots. For more raw data and controls, see Table S1 . Sixty percent of the DAI 7/8 embryos developed as Janus twins (Kao and Elinson, 1988). Image in (B) is reprinted from Kao and Elinson, 1988.
To test this experimentally, various perturbations were made by protein expression or addition of inhibitors (Figure 5B). Specifically, RNA was injected at the 4-cell stage in each blastomere; lithium exposure was at 48-cell stage. The cadherin-free pool of β-catenin was quantified just before gastrulation, when embryonic induction occurs; transcription measurements were made at gastrulation; final ascertainment of morphology was performed at day 4 when the dorsal-ventral differences are easiest to score (see also Figures S9-10). To facilitate scoring the morphology, we used the dorsal-anterior index, which goes from 1 to 10 (DAI, Figure 5A) (Kao and Elinson, 1988). DAI 1 is the totally ventralized morphology, observed when Wnt signaling is completely blocked (Heasman et al., 1994; Tao et al., 2005). DAI 10 is the totally dorsalized morphology, observed when Wnt signaling is strongly activated everywhere (Kao and Elinson, 1988). DAI 5 is the wild-type morphology.
The embryonic phenotype is robust to perturbations in the degradation machinery
To inhibit degradation of β-catenin, we overexpressed GSK3β binding protein (GBP) or treated the embryos with lithium (inhibitor of GSK3β (Klein and Melton, 1996; Yost et al., 1998). Inhibiting GSK3β with lithium raised the overall level of cadherin-free β-catenin in a dose-dependent manner (Figure 5C). However, despite a 2-fold increase in the level of β-catenin in the embryo, the embryos maintained the wild-type morphology (Figure 5C). Eventually, at a higher dose of inhibitor (300mM), ≥40% of the embryos showed dorsalization (Figure 5C), in agreement with published studies. The phenotypic buffering at ≤200 mM lithium is highly reproducible (Figure S11).
The embryos also remained wild type when the degradation rate was decreased by GBP overexpression or when the degradation rate was increased by Axin1 overexpression (Figure 5D). To facilitate comparison across independent experiments, we plotted the median DAI versus the measured β-catenin level (Figure 5D). Again, the overall cadherin-free β-catenin level in the embryo varied with RNA dose in a dose-dependent manner. Despite a 2-fold increase (due to GBP injection) or a 2-fold decrease (due to Axin1 injection) in the level of β-catenin, the embryos remained wild type (Figure 5D). Higher doses of GBP or Axin1 RNA eventually led to dorsoanterior defects, as expected from published studies (Kao and Elinson, 1988; Kofron et al., 2001; Yost et al., 1998). As shown later in Figure 6, the “extra” β-catenin is transcriptionally active; hence the observed phenotypic buffering is not simply due to regulation of nuclear entry.
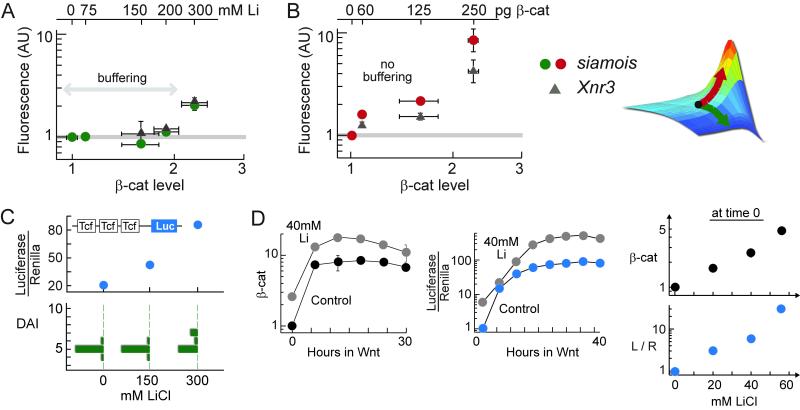
Figure 6. Transcriptional outcome of Wnt signaling is insensitive to variations in the degradation parameters.
(A-B) Quantitative RT-PCR analysis of two direct target genes of the Wnt pathway, siamois and Xnr3, in embryos treated with lithium (A) and injected with β-catenin RNA (B). RT-PCR was performed at stage 10; SD = 2-3 measurements. Cadherin-free pool of β-catenin was quantified at stage 8-9 using quantitative Western blot; SD = 2-4 blots.
(C) Embryos were injected with TopFlash and RL-TK constructs at 4-cells stage, and then treated with lithium. Luciferase activity was assayed at stage 10.
(D) RKO cells stably expressing TopFlash construct was treated with lithium. β-catenin and luciferase profiles were measured in the same experiment.
The embryonic phenotype is sensitive to perturbations in the β-catenin synthesis rate
In contrast to the insensitivity to perturbations of degradation, the dorsoanterior phenotype was sensitive to β-catenin overexpression. At the equivalent overall levels of β-catenin, increasing of synthesis had a measurable effect, whereas decreasing degradation did not. Upon injection of β-catenin RNA, dorsalized embryos readily appear in the population (Figures 5E-F). In response to lithium or GBP injection, β-catenin level increased by ≤2-fold and the embryos stayed wild type. In response to β-catenin injection, β-catenin level increased by ≤2-fold and the embryos were dorsalized.
The lack of buffering to β-catenin overexpression is highly reproducible, though the exact morphologies show a range (Figure 5E shows the results of 4 independent 125 pg injection). Please see Table S1 for more repeats and negative controls. Quantitation of β-catenin protein at different stages does not alter the conclusion (Figure S12). Injection performed earlier (at early 2-cell) led to the same conclusion, suggesting that spread of injected RNA is not limiting (Figure 5E).
These data suggests that the phenotypic outcome of Wnt signaling in Xenopus embryos responds to perturbations in the same way as predicted for the Wnt-induced fold-change in β-catenin (Figures 5A, D, F). The phenotypic outcome of Wnt signaling does not correlate with the absolute levels of β-catenin per se. The data suggests that, just like the Wnt-induced fold-change in β-catenin, the phenotypic outcome of Wnt signaling is buffered against perturbations in the degradation machinery, while sensitive to β-catenin overexpression. Fold-change in β-catenin, and not absolute level of β-catenin, seems to dictate the phenotypic outcomes of Wnt signaling.
Transcriptional responses to Wnt signaling are robust to perturbations in the degradation machinery
The observed phenotypic buffering suggests that either each target gene of β-catenin is buffered, or that multiple target genes of β-catenin interact to give a robust phenotype. The first scheme would place the fold-reading mechanism within the upstream regulation of each target gene. The second scheme would place the fold-reading mechanism within a larger transcriptional or developmental network. Expression of direct target genes of Wnt signaling, measured much earlier than the morphological changes, is also insensitive to perturbation of the degradation parameters (Figure 6A). Two direct target genes of the Wnt pathway, siamois and Xnr3 (Lemaire et al., 1995; McKendry et al., 1997), were analyzed. Despite a 2-fold increase in β-catenin level, expression of siamois and Xnr3 remained at a wild-type level. With a higher dose of lithium (300mM), expression of siamois and Xnr3 finally increased, coinciding with the appearance of dorsalized phenotype in the population. An even stronger lithium treatment leads to a greater increase in target gene expression, confirming that transcription is not saturated (Figure S13). In contrast, expression of siamois and Xnr3 changed readily when β-catenin was increased via β-catenin overexpression (Figure 6B). Like the dorsoanterior phenotype, expression of direct target genes, siamois and Xnr3, appear to correlate with fold-changes in β-catenin, and not absolute levels of β-catenin.
The situation was very different with an exogenous promoter known as the TopFlash reporter, which contains three repeats of Tcf binding sites upstream from a luciferase gene. When the lithium perturbation experiment was repeated in the presence of the TopFlash reporter, the luciferase activity increased steadily with lithium (Figure 6C), mimicking the increase in β-catenin. The correlation between luciferase and β-catenin level, but not with fold-change in β-catenin, was also observed in cultured cells (Figure 6D). A higher level of β-catenin readily translated into a higher transcriptional activity (as read by luciferase activity), but the phenotype and endogenous target genes remained unaltered.
DISCUSSION
To summarize, the data from mammalian cells showed experimentally that the Wnt-induced fold-change in β-catenin is insensitive to perturbations in the degradation machinery (within a range), but increased with β-catenin overexpression. The absolute level of β-catenin, by contrast, shifts readily with all perturbations performed. Together with the measurements from Xenopus (Lee et al., 2002), these data confirm that the there is indeed a region of parameters where the fold-change in β-catenin can withstand naturally occurring variation in cells, and suggest that the RKO cells and Xenopus operate within this parameter region.
The data from Xenopus suggest that the phenotypic and transcriptional outcomes of Wnt signaling, just like the model prediction for the Wnt-induced fold-change in β-catenin, are buffered to perturbations in degradation machinery (within a range), but sensitive to β-catenin overexpression. Neither dorsoanterior phenotype nor transcription of target genes appears to correlate with the absolute level of β-catenin in the embryo. In response to inhibiting the degradation machinery, β-catenin level increased by ≤2-fold and the embryos stayed wild type. In response to overexpressing β-catenin, β-catenin level increased by ≤2-fold and the embryos became dorsalized. From the Xenopus setpoint, gradually inhibiting degradation increases β-catenin levels without initially affecting fold-change in β-catenin (i.e., cells respond to Wnt stimulation normally despite higher levels of β-catenin everywhere)—and the embryos initially remained wild type. From the Xenopus setpoint, gradually increasing synthesis increases β-catenin levels as well as fold-change in β-catenin (i.e., cells respond excessively to Wnt stimulation)—and dorsalized embryos appear readily. Fold-changes in β-catenin, and not the absolute levels of β-catenin, appear to be a more precise output of Wnt stimulation that correlates with the functional outcomes of Wnt signaling in vivo.
The Wnt-induced fold-change in β-catenin can withstand cellular variation provided that the parameters in the Wnt pathway are properly tuned. The Wnt pathway, with the fold-change in β-catenin as the output as proposed here, would be robust because its functional outcomes can be insensitive to biochemical parameters that naturally vary from cell to cell (how robustness is currently defined (Alon, 2007; Barkai and Leibler, 1997; Stelling et al., 2004).
The Wnt pathway is merely one step in the complex process of dorsoanterior patterning in Xenopus. We can imagine an alternative world where the dorsoanterior phenotype is robust to perturbations in the internal cellular milieu, including to variation in the components of the Wnt pathway, but the Wnt pathway itself is not robust. In this case there should be a noisy and inaccurate level of β-catenin, which would then translate into a noisy transcriptional outcome of Wnt signalling. To assure a normal phenotypic outcome under these conditions, downstream processes would have to compensate for the noisy Wnt signaling. The present study suggests that there is already a high degree of error checking mechanism within the Wnt pathway itself. This robustness comes at a price, as it imposes constraints on the downstream transcriptional circuits. It is a price that complex and modular eukaryotic transcriptional machinery apparently can afford to pay.
How can a transcriptional system be built so that it is responsive to fold-changes in a transcription factor, and not its absolute level? Intuitively, reading fold-changes in β-catenin requires that cells remember the basal level of β-catenin before Wnt stimulation, and compare it to the level of β-catenin after Wnt stimulation. It suggests that the cell tracks signaling dynamics over time: it not only senses its present state, but actively compares it to some previous state. In an accompanying theory paper, we discuss how a simple and common network motif with a repressive feedforward can accomplish such a feat. The repression might operate in cis-regulation, 3′-end regulation, chromatin modification, or some other yet unknown features. Whereas patterning genes such as siamois and Xnr3 correlate with fold-changes in β-catenin, we would predict that some target genes, such as those induced as signaling feedbacks, may respond to the absolute levels of β-catenin.
Reading fold-changes may be a common feature of biological systems. In an accompanying paper, Cohen-Saidon and colleagues present evidence that in the ERK system, fold-changes in the doubly phosphorylated ERK, and not its absolute levels, are the more reliable response to ligand stimulation. This strategy is analogous to well known properties of sensory systems in physiology. As described by Weber’s law, various sensory modalities (e.g., hearing, vision, weight discrimination) detect the ratio of a new stimulus to the background stimulus (fold difference) over a wide range (Weber, 1996). A novel implication of our study is that such a ratio-sensing feature should also be found at the level of transcription. Response to fold-change, as opposed to absolute level, might be a common strategy in biological sensory systems, from the physiological to the molecular.
MATERIALS AND METHODS
Experiments in cultured cells
RKO cells (ATCC) are human colon carcinoma cells contains no known mutations in the Wnt pathway, lacks E-Cadherin (Breen et al., 1993), and shows no membrane localization of β-catenin (Giannini et al., 2000). Cells were cultured in DMEM + 10% FBS, 10μg/mL L-Glutamine, in a 37°C humidified incubator with 5% CO2. For each condition, temporal profile of β-catenin accumulation was characterized to look for steady state.
Cell lysis
Cells were lysed in 0.5% NP-40, 150mM NaCl, 50mM Tris pH 7.6, 5 mM EDTA, 1 mM PMSF, and Roche protease inhibitor cocktail (#11836153001). 2X Laemmli was added to give a total protein concentration of 4-5 mg/mL, estimated using the Bradford assay.
Dot blot
Far-red fluorescent signal was detected using the Odyssey system (Li-COR). Primary antibodies: mouse monoclonal β-catenin (BD Signal Transduction #610154, 1:500), rabbit polyclonal actin (Sigma A2066, 1:500). Secondary antibodies: goat-anti-mouse IR800CW (LI-COR #926-32210, 1:5000), goat-anti-rabbit 680 (Alexa Fluor A-21076, 1:5000). 3-5 μL of cell lysate was dotted to nitrocellulose membrane, followed by Odyssey staining protocol (http://biosupport.licor.com/support). Actin-normalized β-catenin signal was used in all calculations. Fluorescent signal was linear across a 10-fold range: varying dot volumes (0.3uL-3uL) were measured and a linear fit was obtained (R2=0.99). For each experiment, 2-4 dot blots were performed; within each blot, each sample was dotted 1-3 times. Measurement uncertainty was propagated with standard methods. Sources of uncertainty for estimating a fold-change in β-catenin: A sample dotted 10 times gave fluorescent signal with CV=14%. Blot-to-blot variability: CV~10%. Between biological repeats in each experiment: CV~20%.
Wnt stimulation
Wnt-conditioned medium (WCM) was collected from L-Wnt-3A mouse fibroblast cells (ATCC). The control-conditioned medium (LCM) was collected from L cells, the parental cell line (ATCC). The Wnt-induced fold-change of β-catenin was identical using LCM or DMEM as the control. DMEM was subsequently used as the control medium.
LiCl experiments
Cells were pre-incubated in LiCl for 3 hours, followed by 15min-24hour incubation in LiCl/DMEM or LiCl/WCM. Incubation in 80mM of NaCl for 27 hours did not alter β-catenin level (NaCl/DMEM=0.9±0.3). Pre-incubation of 3 or 6 hours did not make a significant difference.
β-catenin and Axin overexpression
Axin-GFP expression was obtained using uninduced, leaky metallothionein promoter. β-catenin-GFP was constitutively expressed by the CMV promoter. RKO cells were transduced with lentiviruses expressing the constructs. Transduced cells were generous gifts from Ana Hernandez.
Experiments in Xenopus laevis embryos
Standard fertilization protocols were followed (Sive, 2000). Staging was based on Nieuwkoop and Faber (Nieuwkoop, 1975). Unless specified, embryos developed at 16°C. In all experiments, control embryos were the uninjected/untreated siblings from the same fertilization batch.
Embryo homogenization
Protocol in (Guger and Gumbiner, 2000) was followed with modifications. Ten stage 8 embryos were homogenized with pestle in 100μL lysis buffer (0.5% NP-40, 150mM NaCl, 10mM Hepes-NaOH pH 7.4, 1.5mM NaEDTA, 1mM PMSF, 0.5mM iodoacetamide, 1μg/mL pepstatin A, 10μg/mL antipain, 50□g/mL benzamidine, and Roche protease inhibitor cocktail, #11836153001). Cadherin-bound β-catenin was removed using ConA-Sepharose4B beads (Figure S10). One-quarter volume of 5X Laemmli was added to the final supernatant.
Western blot
Far-red fluorescent signal was detected using the Odyssey system (Li-COR). Primary antibodies: rabbit poyclonal β-catenin (Novus NB100-2141, 1:1000), rabbit polyclonal actin (Sigma A2066, 1:500). Secondary antibodies: goat-anti-rabbit IR800CW (LI-COR # 926-32211, 1:5000), goat-anti-rabbit 680 (Alexa Fluor A-21076, 1:5000). ~1/2 embryo (10-15μL) was loaded on 4-12% Bis/Tris gel (NuPage NP0321BOX), followed by wet transfer. The Odyssey protocol was followed with modifications: 3-hour blocking and >12h 4°C incubation in the primaries. The fluorescent signal was linear over > 3-fold range: varying sample volume was loaded (5-20μL) and a linear fit was obtained (R2=0.96). A sample loaded 6 times in the same gel gives fluorescent signal with 10% CV. For measuring a fold-change, across blots: CV~10%. A 10-fold mistake in dilution allowed us to control against a possible bias due to an a priori expectation of seeing a change (Table S1). 2-4 Western blots were performed in each experiment.
LiCl treatment
48- to 64-cell stage embryos were incubated in LiCl/0.1XMMR for 5 minutes (Kao and Elinson, 1988). See Table S1 for positive and negative controls.
RNA injection
Each blastomere in 4-cell stage embryos was injected (3nL) at the mid-equatorial region (marginal zone). See Table S1 for full injection data. Technical controls: no phenotypes were observed in embryos injected with equal volume of water or 1000pg GFP. Constructs: Full-length Xenopus β-catenin (McCrea et al., 1991), Xenopus GBP (Yost et al., 1998), mouse Axin1 (Zeng et al., 1997) were in pCS2+ vector, and transcribed with mMessage mMachine (Ambion AM1340). A note on the Xbcat clone. The Xbcat M77013 (McCrea et al., 1991) clone has 1 non-silent substitution at nucleotide 1920 when compared to consensus sequences of β-catenin from X. laevis, X. tropicalis, mouse, human, and plakogobin from mouse and human. We used site-directed mutagenesis (Stratagene #200519) to mutate back n1920 (m1920 clone) and injected both transcripts. Both clones are similarly potent.
Quantitative real-time RT-PCR
Embryos were collected from late stage 9, during which siamois and Xnr3 expressions have plateaued (Figure S9). Total RNA was extracted in Trizol and reverse-transcribed using QuantiTect RT (Qiagen #205310). Real-time RT-PCR was performed using the QuantiTect Custom Assays (Qiagen) in BioRad iCycler iQ. Two runs were performed per sample, with 4 technical repeats. See Table S2 for the FAM-probe/primers sequences.
Phenotype scoring
Tadpoles at stage 40-41 were scored using the DAI index (Kao and Elinson, 1988). Since mutants were weaker than their normal siblings, to avoid biasing the phenotype, it was important to avoid overcrowding, remove dead embryos promptly, and score repeatedly from day 3.5 onward. 15-150 embryos/batch were scored. Some batches were photographed entirely and re-scored on other days.
Luciferase assay
Reporter constructs were injected to all 4 cells at 4-cell stage (100 pg pTopFlash, Millipore 21-170 and 20 pg pRL-TK, Promega E2241). Lithium treatment was performed at 32-cell stage. Luminescence was assayed in early gastrulaes (S10) using the dual-luciferase Promega assay.
Supplementary Material
01
ACKNOWLEDGMENTS
We wish to dedicate this paper to Reinhart Heinrich (1946-2006), who opened for us the study of quantitative features of signal transduction and who, in his influential work, extracted many general biological truths by mathematical analysis. He is sorely missed.
The authors thank Uri Alon for insights on the study. For helpful suggestions and critical reading of the manuscript, the authors thank Hao Yuan Kueh, Pedro Bordalo, Roy Kishony, Johan Paulsson, Martin Feinberg, Eric Batchelor, Natalie Andrew, Ran Kafri, Sophie Dumont, Ana Hernandez, Andres Lebehnson, Richard Deibler, Allon Klein, Leon Peshkin, Jean-Baptise Michel, Ethan Lee, and Adrian Salic. For technical help, the authors thank Michael Gage, Robert Freeman, Martin Wuehr, Paul Jorgensen, and Jennifer Gallop. The authors thank Ana Hernandez, Randall Moon, and Ben Major for reagents. LG is a Robert Black Fellow of the Damon Runyon Foundation (DRG-1958-07). This work was supported by R01 HD037277 (MWK) and the Novartis-Harvard-Hebrew University program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon U. An Introduction to Systems Biology. Chapman & Hall/CRC; Boca Raton, FL: 2007. [Google Scholar]
- Bar-Even A, Paulsson J, Maheshri N, Carmi M, O’Shea E, Pilpel Y, Barkai N. Noise in protein expression scales with natural protein abundance. Nat Genet. 2006;38:636–643. doi: 10.1038/ng1807. [DOI] [PubMed] [Google Scholar]
- Barkai N, Leibler S. Robustness in simple biochemical networks. Nature. 1997;387:913–917. doi: 10.1038/43199. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Blake WJ, Balazsi G, Kohanski MA, Isaacs FJ, Murphy KF, Kuang Y, Cantor CR, Walt DR, Collins JJ. Phenotypic consequences of promoter-mediated transcriptional noise. Mol Cell. 2006;24:853–865. doi: 10.1016/j.molcel.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Blake WJ, M KA, Cantor CR, Collins JJ. Noise in eukaryotic gene expression. Nature. 2003;422:633–637. doi: 10.1038/nature01546. [DOI] [PubMed] [Google Scholar]
- Breen E, Clarke A, Steele G, Jr., Mercurio AM. Poorly differentiated colon carcinoma cell lines deficient in alpha-catenin expression express high levels of surface E-cadherin but lack Ca(2+)-dependent cell-cell adhesion. Cell Adhes Commun. 1993;1:239–250. doi: 10.3109/15419069309097257. [DOI] [PubMed] [Google Scholar]
- Elowitz MB, Levine AJ, Siggia ED, Swain PS. Stochastic gene expression in a single cell. Science. 2002;297:1183–1186. doi: 10.1126/science.1070919. [DOI] [PubMed] [Google Scholar]
- Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of beta-catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannini AL, Vivanco M, Kypta RM. alpha-catenin inhibits beta-catenin signaling by preventing formation of a beta-catenin*T-cell factor*DNA complex. J Biol Chem. 2000;275:21883–21888. doi: 10.1074/jbc.M001929200. [DOI] [PubMed] [Google Scholar]
- Guger KA, Gumbiner BM. A mode of regulation of beta-catenin signaling activity in Xenopus embryos independent of its levels. Dev Biol. 2000;223:441–448. doi: 10.1006/dbio.2000.9770. [DOI] [PubMed] [Google Scholar]
- Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Krupnik VE, Sokol SY. Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and beta-catenin. Curr Biol. 1998;8:591–594. doi: 10.1016/s0960-9822(98)70229-5. [DOI] [PubMed] [Google Scholar]
- Kao KR, Elinson RP. The entire mesodermal mantle behaves as Spemann’s organizer in dorsoanterior enhanced Xenopus laevis embryos. Dev Biol. 1988;127:64–77. doi: 10.1016/0012-1606(88)90189-3. [DOI] [PubMed] [Google Scholar]
- Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofron M, Klein P, Zhang F, Houston DW, Schaible K, Wylie C, Heasman J. The role of maternal axin in patterning the Xenopus embryo. Dev Biol. 2001;237:183–201. doi: 10.1006/dbio.2001.0371. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Moerer P, van Donselaar E, Huls G, Peters PJ, Clevers H. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- Lee E, Salic A, Kruger R, Heinrich R, Kirschner MW. The roles of APC and Axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol. 2003;1:E10. doi: 10.1371/journal.pbio.0000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TI, Rinaldi NJ, Robert F, Odom DT, Bar-Joseph Z, Gerber GK, Hannett NM, Harbison CT, Thompson CM, Simon I, et al. Transcriptional regulatory networks in Saccharomyces cerevisiae. Science. 2002;298:799–804. doi: 10.1126/science.1075090. [DOI] [PubMed] [Google Scholar]
- Lemaire P, Garrett N, Gurdon JB. Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell. 1995;81:85–94. doi: 10.1016/0092-8674(95)90373-9. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, Zhang Z, Lin X, He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991;254:1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- McKendry R, Hsu SC, Harland RM, Grosschedl R. LEF-1/TCF proteins mediate wnt-inducible transcription from the Xenopus nodal-related 3 promoter. Dev Biol. 1997;192:420–431. doi: 10.1006/dbio.1997.8797. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Moon RT. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989;58:1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- Meijer L, Skaltsounis AL, Magiatis P, Polychronopoulos P, Knockaert M, Leost M, Ryan XP, Vonica CA, Brivanlou A, Dajani R, et al. GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem Biol. 2003;10:1255–1266. doi: 10.1016/j.chembiol.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) North-Hollan Publishing Company; Amsterdam: 1975. [Google Scholar]
- Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J Biol Chem. 1997;272:24735–24738. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- Ozbudak EM, Thattai M, Kurtser I, Grossman AD, van Oudenaarden A. Regulation of noise in the expression of a single gene. Nat Genet. 2002;31:69–73. doi: 10.1038/ng869. [DOI] [PubMed] [Google Scholar]
- Paulsson J. Summing up the noise in gene networks. Nature. 2004;427:415–418. doi: 10.1038/nature02257. [DOI] [PubMed] [Google Scholar]
- Peifer M, Pai LM, Casey M. Phosphorylation of the Drosophila adherens junction protein Armadillo: roles for wingless signal and zeste-white 3 kinase. Dev Biol. 1994a;166:543–556. doi: 10.1006/dbio.1994.1336. [DOI] [PubMed] [Google Scholar]
- Peifer M, Sweeton D, Casey M, Wieschaus E. wingless signal and Zeste-white 3 kinase trigger opposing changes in the intracellular distribution of Armadillo. Development. 1994b;120:369–380. doi: 10.1242/dev.120.2.369. [DOI] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Raser JM, O’Shea EK. Control of stochasticity in eukaryotic gene expression. Science. 2004;304:1811–1814. doi: 10.1126/science.1098641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggleman B, Schedl P, Wieschaus E. Spatial expression of the Drosophila segment polarity gene armadillo is posttranscriptionally regulated by wingless. Cell. 1990;63:549–560. doi: 10.1016/0092-8674(90)90451-j. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Munemitsu S, Polakis P. The APC protein and E-cadherin form similar but independent complexes with alpha-catenin, beta-catenin, and plakoglobin. J Biol Chem. 1995;270:5549–5555. doi: 10.1074/jbc.270.10.5549. [DOI] [PubMed] [Google Scholar]
- Salic A, Lee E, Mayer L, Kirschner MW. Control of beta-catenin stability: reconstitution of the cytoplasmic steps of the wnt pathway in Xenopus egg extracts. Mol Cell. 2000;5:523–532. doi: 10.1016/s1097-2765(00)80446-3. [DOI] [PubMed] [Google Scholar]
- Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, et al. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P. Beta-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev. 1996;57:191–198. doi: 10.1016/0925-4773(96)00546-1. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RN. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2000. [Google Scholar]
- Stelling J, Sauer U, Szallasi Z, Doyle FJ, 3rd, Doyle J. Robustness of cellular functions. Cell. 2004;118:675–685. doi: 10.1016/j.cell.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- Takacs CM, Baird JR, Hughes EG, Kent SS, Benchabane H, Paik R, Ahmed Y. Dual positive and negative regulation of wingless signaling by adenomatous polyposis coli. Science. 2008;319:333–336. doi: 10.1126/science.1151232. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell. 2005;120:857–871. doi: 10.1016/j.cell.2005.01.013. [DOI] [PubMed] [Google Scholar]
- van Leeuwen F, Samos CH, Nusse R. Biological activity of soluble wingless protein in cultured Drosophila imaginal disc cells. Nature. 1994;368:342–344. doi: 10.1038/368342a0. [DOI] [PubMed] [Google Scholar]
- van Noort M, Meeldijk J, van der Zee R, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. J Biol Chem. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- Weber EH. E.H. Weber on the Tactile Senses. 1 edn Psychology Press; 1996. [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Yost C, Farr GH, 3rd, Pierce SB, Ferkey DM, Chen MM, Kimelman D. GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell. 1998;93:1031–1041. doi: 10.1016/s0092-8674(00)81208-8. [DOI] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL, 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01