HIF-2α deletion promotes Kras-driven lung tumor development (original) (raw)
Abstract
Non-small cell lung cancer (NSCLC) is the leading cause of cancer deaths worldwide. The oxygen-sensitive hypoxia inducible factor (HIF) transcriptional regulators HIF-1α and HIF-2α are overexpressed in many human NSCLCs, and constitutive HIF-2α activity can promote murine lung tumor progression, suggesting that HIF proteins may be effective NSCLC therapeutic targets. To investigate the consequences of inhibiting HIF activity in lung cancers, we deleted _Hif-1_α or _Hif-2_α in an established KrasG12D-driven murine NSCLC model. Deletion of _Hif-1_α had no obvious effect on tumor growth, whereas _Hif-2_α deletion resulted in an unexpected increase in tumor burden that correlated with reduced expression of the candidate tumor suppressor gene Scgb3a1 (HIN-1). Here, we identify Scgb3a1 as a direct HIF-2α target gene and demonstrate that HIF-2α regulates Scgb3a1 expression and tumor formation in human KrasG12D-driven NSCLC cells. AKT pathway activity, reported to be repressed by Scgb3a1, was enhanced in HIF-2α-deficient human NSCLC cells and xenografts. Finally, a direct correlation between _HIF-2_α and SCGB3a1 expression was observed in approximately 70% of human NSCLC samples analyzed. These data suggest that, whereas HIF-2α overexpression can contribute to NSCLC progression, therapeutic inhibition of HIF-2α below a critical threshold may paradoxically promote tumor growth by reducing expression of tumor suppressor genes, including Scgb3a1.
Keywords: hypoxia, inducible mouse model, non-small cell lung cancer, tumor suppressor
Despite the access of normal pulmonary epithelia to atmospheric O2 levels, tumor hypoxia has been observed in human non-small cell lung cancer (NSCLC) and correlates with poor prognosis and decreased disease-free survival (1, 2). Cells can overcome hypoxic stress through multiple mechanisms, including the stabilization of hypoxia inducible factor (HIF) transcriptional regulators. HIFs consist of an oxygen labile α-subunit and a constitutively expressed β-subunit (also called ARNT), which together bind hypoxia response elements (HREs) to activate a large number of target genes encoding proteins that mediate adaptive responses to hypoxic stress (3, 4). Two distinct subunits, HIF-1 and HIF-2, have been demonstrated to regulate partly overlapping sets of target genes in hypoxic cells, although each protein also fulfills unique essential functions (5–7).
Many HIF target genes encode proteins important for tumor growth and progression, including glycolytic enzymes, VEGF, matrix metalloproteinase-2 (MMP2), transforming growth factors α and β (TGF-α and TGF-β), and numerous others (4, 8). Collectively, these factors modulate cancer cell metabolism and can promote angiogenesis, invasion, and metastasis, suggesting that HIF proteins may represent suitable targets for antitumor therapies (9–11). It is interesting to note that both HIF-1α and HIF-2α proteins are overexpressed in approximately 50% of human NSCLCs (12), further suggesting they may contribute directly to NSCLC progression.
In a recent report, ectopic expression of a nondegradable HIF-2α promoted growth of KrasG12D-driven murine lung tumors (13). Relative to KrasG12D controls, KrasG12D lung tumors expressing stabilized HIF-2α were larger, displayed evidence of enhanced angiogenesis and epithelial-mesenchymal transition (EMT), and resulted in decreased survival (13). These phentoypes were correlated with increased expression of multiple HIF-2α target genes including VEGFR2, Snail, and others (13). Collectively, these results demonstrated that increased HIF-2α activity can drive NSCLC growth and underscore the potential utility of developing targeted therapies to inhibit HIF proteins in general, and HIF-2α in particular, for NSCLC and renal cell carcinomas (RCC).
To determine the effects of eliminating HIF-1α and HIF-2α expression in NSCLC, we used conditional “floxed” alleles to delete HIF-1α or HIF-2α in lung tumors using the murine KrasG12D NSCLC model (13). Deletion of HIF-1α in these tumor cells had no detectable effect on tumor growth but, surprisingly, deletion of HIF-2α actually increased tumor number and size. This phenotype correlated with reduced expression of Scgb3a1 (14) also known as high in normal-1 (HIN-1), a candidate tumor suppressor gene implicated in lung, breast, pancreatic, and other cancers (15–17). We demonstrate here that Scgb3a1 is a direct HIF-2α target gene and that HIF-2α deficient lung tumor xenografts are characterized by enhanced AKT signaling, consistent with previous observations that Scgb3a1 suppresses AKT activity in human breast cancer cells (18). We further demonstrate that ectopic expression of Scgb3a1 suppresses the growth of HIF-2α–deficient lung tumor xenografts, concomitant with reduced AKT signaling. Finally, a direct correlation between _Hif-2_α and Scgb3a1 expression was observed in human NSCLC cells and primary NSCLC tumors. These data suggest that, although HIF-2α overexpression can contribute to NSCLC progression, therapeutic inhibition of HIF-2α below a critical threshold may actually promote tumor growth by repressing Scgb3a1 and other HIF-2α target genes.
Results
HIF-2α Deletion Promotes KrasG12D-Induced Lung Tumor Growth.
The inducible LSL-KrasG12D murine genetic model generates lung tumors that faithfully model human lung adenocarcinoma initiation and progression (19). To evaluate the effect of HIF-2α loss-of-function in lung tumor progression, we crossed mice carrying conditional floxed Hif-2_α_fl or null _Hif-2_αΔ alleles (20) to mice carrying the LSL-KrasG12D allele. The resulting experimental KrasG12D/Hif-2_α_fl/Δ and control KrasG12D/_Hif-2_α+/Δ animals were treated with adenovirus encoding the Cre recombinase by intranasal instillation. Cre-mediated deletion of the Lox-Stop-Lox (LSL) gene cassette in the LSL-KrasG12D allele produced _KrasG12D_-driven lung tumors as previously described (19) and in this case also deleted the conditional Hif-2_α_fl allele. As the control KrasG12D/_Hif-2_α+/Δ animals harbor a wild-type _Hif-2_α allele, HIF-2α function was consequently retained in tumor tissue (Fig. S1_A_). Southern blot analysis confirmed efficient deletion of the Hif-2_α_fl allele specifically in tumors but not surrounding lung tissue (Fig. S1_B_).
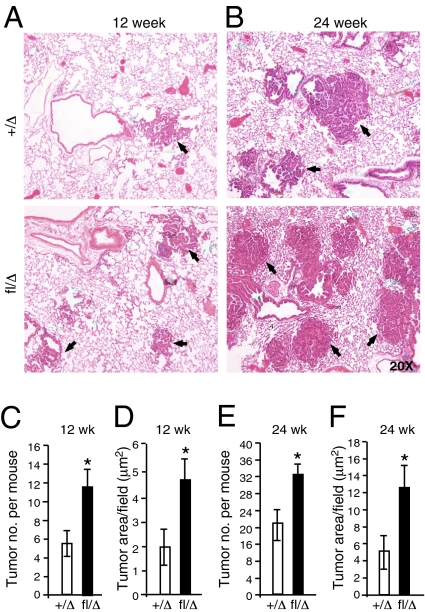
To evaluate the effect of HIF-2α deletion on lung tumor progression, animals were analyzed 12 wk and 24 wk after adenoviral Cre delivery (Fig. S1_C_). Based on the recent report that HIF-2α gain-of-function promotes lung tumor progression and correlates with poor survival in this model (12, 13), we predicted that deletion of HIF-2α would reduce tumor burden and progression. Surprisingly, KrasG12D/Hif-2_α_fl/Δ mutant mice displayed a significant increase in tumor number and size at 12 wk (Fig. 1 A, C, and D) and 24 wk (Fig. 1 B, E, and F) postinfection, as compared with their control littermates. Notably, deletion of a floxed Hif-1_α_fl allele (21) in parallel experiments had no detectable effect on tumor number or volume in KrasG12D lung tumors (Fig. S2 A_–_F).
Fig. 1.
_Hif-2_α deletion increases tumor burden. (A_–_F) KrasG12D/_Hif-2_α+/Δ (designated +/Δ), or KrasG12D/Hif-2αfl/Δ (designated fl/Δ) mice were infected with Adeno-Cre virus and killed 12 (A) or 24 (B) wk postinfection (w.p.i.). H&E staining of murine lung sections indicate increased tumor burden in _Hif-2_α deleted cohorts. Tumor number and area were measured using an automated Axiovision system (Materials and Methods). (C–F) At 12 and 24 w.p.i, fl/Δ animals presented significantly increased tumor number (C and E) and size (D and F) compared with +/Δ controls. (n = 9–11 in each group). Error bars (C_–_F) represent SEM. *P < 0.05.
_Hif-2_α Deletion Promotes Tumor Progression.
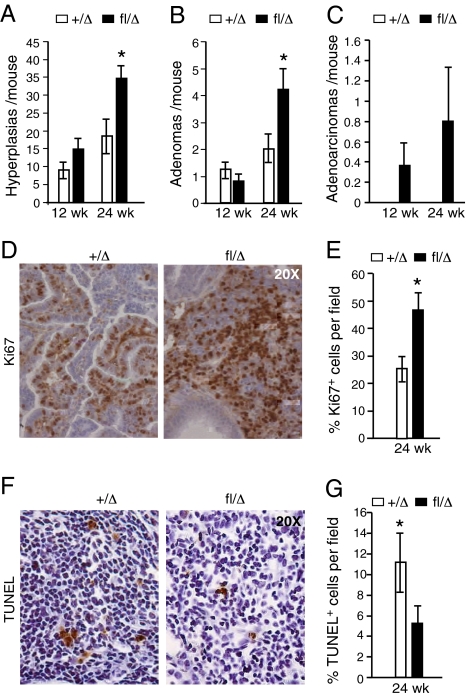
Lung tumors generated in LSL-KrasG12D mice display distinct types of progressive lesions including epithelial hyperplasia, adenomas, and adenocarcinomas (19). HIF-2α deficient KrasG12D lung tumors displayed a significant increase in hyperplastic lesions at 24 wk (Fig. 2_A_), and a similar trend at 12 wk. Similarly, KrasG12D/Hif-2_α_fl/Δ animals displayed significantly increased numbers of adenomas (Fig. 2_B_) at 24 wk compared with control animals. A similar trend toward increased numbers of adenocarcinomas was observed at 24 wk but did not achieve statistical significance (Fig. 2_C_). Collectively, these data indicate that loss of HIF-2α accelerates tumor progression in this context. Interestingly, deletion of HIF-1α in parallel experiments did not alter disease kinetics (Fig. S2 G_–_I), underscoring the differential effects of HIF-1α and HIF-2α in these tumors.
Fig. 2.
_Hif-2_α deletion promotes tumor proliferation and progression. H&E stained lung sections prepared from KrasG12D/_Hif-2_α+/Δ (+/Δ) or KrasG12D/Hif-2_α_fl/Δ (fl/Δ) animals were graded for hyperplastic lesions (A), adenomas (B), and adenocarcinomas (C). (D and E) Control and mutant tumors were assessed 24 w.p.i. for proliferation by Ki67 staining (n = 6). (F and G) Enhanced cell death in +/Δ tumors as compared with Δ/Δ mutant tumors (n = 6) as assessed by a TUNEL assay. Sections were imaged at 20×. Error bars represent SEM. *P < 0.05.
The increased size of HIF-2α–deficient tumors correlated with elevated cell proliferation (Fig. 2 D and E) and decreased apoptosis (Fig. 2 F and G), which was not observed in HIF-1α–deficient tumors (Fig. S2 J and K). We also noted increased numbers of infiltrating leukocytes in HIF-2α–deleted tumors (Fig. S3 A and B), and granulocytes in particular (Fig. S3 C and D), suggesting that inflammatory responses may differ between _Kras_G12D/_Hif-2_αΔ/Δ, and _Kras_G12D/_Hif-2_α+/Δ lung tumors.
Identification of Scgb3a1 as a HIF-2α Target.
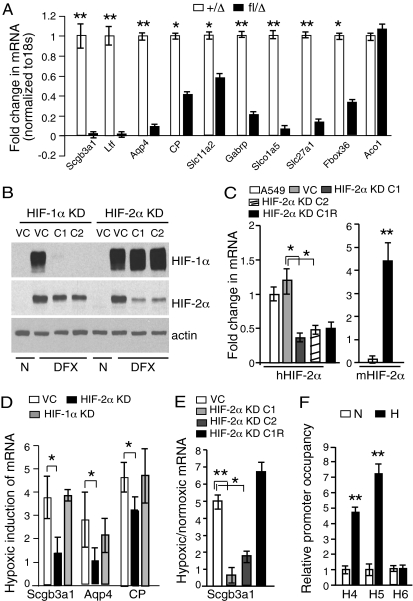
To investigate molecular mechanisms underlying HIF-2α’s tumor suppressive effects, we conducted global gene expression profiling on individual tumors from KrasG12D/Hif-2a fl/Δ or control _KrasG12D/Hif-2_α+/Δ mice. Specifically, RNA was isolated from tumors from experimental and control animals (n = 7 for each group) 28 wk after infection and analyzed independently. Comparisons between the genotypes identified a small set of genes (Table S1) whose expression was significantly and reproducibly reduced in HIF-2α–deficient tumors, whereas expression levels of most genes were unchanged. Subsequent quantitative RT-PCR (qRT-PCR) analysis on the same tumor RNAs revealed dramatic down-regulation of multiple transcripts, including those encoding Scgb3a1, lactotransferrin, aquaporin 4, and ceruloplasmin (Fig. 3_A_). In contrast, transcript levels of the HIF-1α target gene aconitase (Aco1) were unaffected by _Hif-2_α deletion (Fig. 3_A_). The reduced expression of Scgb3a1 was particularly intriguing as (i) Scgb3a1 is expressed primarily in epithelial organs including lung, mammary gland, trachea, prostate, pancreas, and salivary gland (22); (ii) Scgb3a1 expression is silenced in a variety of human cancers including lung, breast, pancreas, and prostate (15, 16); and (iii) down-regulation of Scgb3a1 is a significant independent predictor of poor clinical outcome in early stage NSCLC (17).
Fig. 3.
HIF-2α regulates Scgb3a1 tumor suppressor gene expression. (A) A qRT-PCR analysis of mRNAs from _KrasG12D/Hif-2_α+/Δ (+/Δ) or KrasG12D/Hif-2_α_fl/Δ (fl/Δ) tumors (n = 7) harvested 28 w.p.i. Probe sets were selected from microarray analysis (Table S1). (B and C) A549 human NSCLC cells transduced with retroviral shRNA constructs targeting HIF-1α (HIF-1α KD) or HIF-2α (HIF-2α KD) or with an empty viral control (VC). (B) Independent clones (C1 and C2) were assessed for HIF-1α and HIF-2α protein levels. n = normoxia (21% O2). DFX = deferoxamine (100 μM) treatment to stabilize HIF-α subunits. (C). Endogenous human _HIF-2_α transcript levels were reduced in HIF-2α KD cells (hHIF-2α, Left). HIF-2α rescue cells (HIF-2α KD C1R) were engineered through stable expression of murine HIF-2α mRNA in HIF-2α KD C1 cells (mHIF-2α, Right). (D) Hypoxic induction of Scgb3a1, Aqp4, and CP transcript levels was reduced in pooled HIF-2α KD cells but not HIF-1α KD cells. (E) Scgb3a transcript levels were reduced in hypoxic HIF-2α KD cell clones C1 and C2 (0.5% O2, 16 h) but restored in hypoxic HIF-2α KD C1R cells. (F) Nuclear extracts (n = 3) were isolated from uninfected hypoxic A549 cells (0.5% O2, 16 h), sonicated, and immunoprecipitated with HIF-2α antibodies. DNA was amplified using primers for HRE regions 4, 5, and 6 (designated H4, H5, and H6) and normalized to isotype matched IgG antibody. Error bars (C_–_F) represent SD. *P < 0.05; **P < 0.005.
To extend our studies to human NSCLC, we introduced a retroviral shRNA gene construct targeting human _Hif-2_α mRNA (23) into human A549 lung adenocarcinoma cells, which harbor an activating Kras mutation (24). Despite extensive screening, only partial HIF-2α knockdown was observed in multiple cell clones, as revealed by Western blot and qRT-PCR analyses (Fig. 3 B and C). Nevertheless, pooled HIF-2α knockdown cells (Fig. 3_D_), as well as independent knockdown cell clones HIF-2α KD C1 and HIF-2α KD C2 (Fig. 3_E_), exhibited a significant reduction in Scgb3a1 transcript levels (approximately 2.5-fold), consistent with our previous observations. A similar reduction was observed for transcripts encoding aquaporin 4 (Aqp4), ceruloplasmin (CP), and VEGF (Fig. 3_D_ and Fig. S4 A and B) but not the HIF-1α specific target PGK1 (Fig. S4_B_). As a control for functional specificity, we introduced a murine cDNA encoding HIF-2α into the human HIF-2α KD C1 knockdown cells (designated HIF-2α KD C1R, Fig. 3_C_), which in turn restored Scgb3a1 transcript levels (Fig. 3_E_). Interestingly, HIF-1α depletion in A549 cells (Fig. 3_B_) did not alter transcript levels of Scgb3a1 or other genes identified in the microarray experiment (Fig. 3_D_ and Fig. S4_C_).
Our results indicate that HIF-2α regulates Scgb3a1 expression in lung adenocarcinoma cells in a cell autonomous manner and that this activity is not shared by HIF-1α. We next investigated the possibility that HIF-2α regulates Scgb3a1 directly. Analysis of human and murine Scgb3a1 gene sequences revealed multiple putative HREs spanning the upstream promoter and enhancer regions (Fig. S4_D_). ChIP assays revealed HIF-2α occupation of two HREs (H4 and H5) in the Scgb3a1 promoter in A549 cells, which increased (4- to 7-fold) under hypoxic conditions (Fig. 3_F_). Not all HREs in the Scgb3a1 promoter appear functional, as H6 fails to bind HIF-2α (Fig. 3_F_). Moreover, HIF-1α failed to bind H4 and H5 (Fig. S4_E_). Collectively, these data demonstrate that Scgb3a1 is a direct HIF-2α target gene.
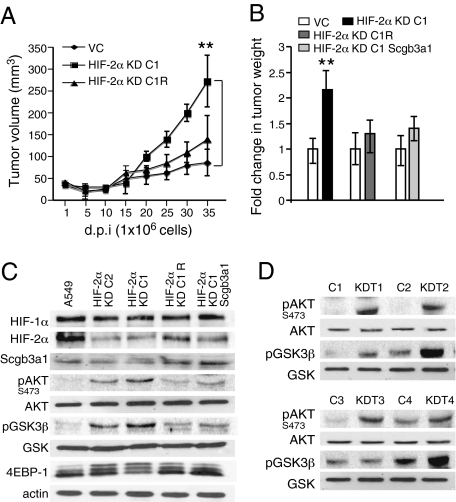
To test the effects of HIF-2α knockdown in tumor formation by the A549 cells, we implanted 5 × 106 HIF-2α KD C1 or HIF-2α KD C2 cells s.c. in immunocompromised mice to generate xenograft tumors. Consistent with our results from the autochthonous HIF-2α deficient lung tumors, xenograft tumors from HIF-2α KD C1 cells displayed a modest but significant growth advantage over A549 control tumors and a similar trend was also observed for HIF-2α KD C2 cells (Fig. S5 A and B). As the recipient mice had to be killed between 25 and 35 d due to the large size of these xenografts, the experiment was repeated using fewer (1 × 106) HIF-2α KD C1 cells (Fig. 4_A_). In this experiment, HIF-2α KD C1 xenografts displayed a clear and significant growth advantage over the vector control xenografts, and restored HIF-2α expression (HIF-2α KD C1R cells) suppressed tumor growth to levels essentially indistinguishable from the vector control tumors (Fig. 4 A and B). Importantly, ectopic expression of human Scgb3a1 in the HIF-2α KD C1 cells (Fig. S5_B_) also effectively suppressed tumor growth, implicating Scgb3a1 as a critical mediator of HIF-2α activity in this setting (Fig. 4_B_).
Fig. 4.
HIF-2α suppresses A549 xenograft growth and AKT signaling. (A) Nude (Nu/Nu) mice were injected s.c. with 1 × 106 HIF-2α KD C1 cells. A549 cells expressing the empty vector (VC) injected in contralateral flank served as controls, and individual tumor volumes were measured (mm3) at indicated days postimplantation (d.p.i). HIF-2α KD C1 cells (n = 10) formed significantly larger tumors compared with control cells (n = 10). Tumors formed by mHIF-2α expressing HIF-2α KD C1R cells (n = 6) were comparable to control tumors (n = 6). Pooled VC tumor volumes from two simultaneously conducted independent experiments [(VC; HIF-2α KD C1) and (VC; HIF-2α KD C1R)] were used for computation. (B) Ectopic expression of HIF-2α or Scgb3a1 in HIF-2α KD C1 cells inhibits tumor growth. Xenografts (n = 10) were generated as in A and tumor weights measured at 35 d. (C) A549 wild-type cells, HIF-2α KD C1, HIF-2α KD C2, HIF-2α KD C1R, and HIF-2α KD C1 cells expressing Scgb3a1 were cultured under hypoxic conditions (0.5% O2) for 20 h and whole cell extracts analyzed for AKT pathway activation by Western blot analysis. Actin served as the loading control. (D) HIF-2α KD C1 [n = 4 (KD T1-4)] and contralateral A549 control [n = 4 (C1-4])] xenograft tumors were processed for whole cell protein extraction and analyzed by Western blot. Error bars represent SEM. **P < 0.005.
HIF-2α Deficiency Reduces AKT Signaling.
Enforced Scgb3a1 expression was shown to inhibit AKT pathway signaling associated with decreased AKT phosphorylation at Ser473 in human breast cancer cells (18). We therefore hypothesized that HIF-2α knockdown in A549 cells would increase AKT pathway activity. Exposure of HIF-2α KD C1 and HIF-2α KD C2 cells to hypoxia significantly increased levels of phopshorylated AKT at Ser473 (Fig. 4_C_). Moreover, levels of phosphorylated AKT downstream effectors including pGSK-3β and 4EBP-1 were higher compared with control cells (Fig. 4_C_). Notably, restoring either HIF-2α or Scgb3a1 expression in the HIF-2α KD C1 cells reduced AKT, GSK-3β, and 4EBP-1 phosphorylation to nearly control levels (Fig. 4_C_). A similar trend was observed in the xenograft tumors, as Western blot analysis revealed that HIF-2α KD C1 tumors displayed increased pAKT and pGSK3β levels compared with control tumors (Fig. 4_D_).
_HIF-2_α Levels Correlate with SCGB3a1 in Human NSCLC.
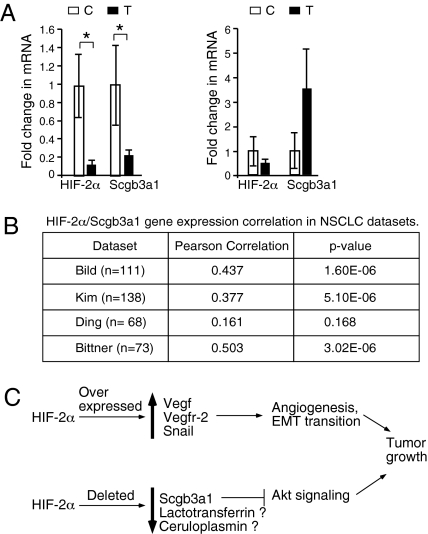
The role of HIF-2α in regulating Scgb3a1 led us to investigate whether _HIF-2_α and SCGB3a1 transcript levels correlate in human NSCLC. The qRT-PCR analysis of 15 independent human adenocarcinoma biopsies revealed that in 73% (11/15), _HIF-2_α mRNA levels were significantly reduced relative to normal tissue, which correlated directly with reduced SCGB3a1 mRNA expression (Fig. 5_A_). In contrast, tumors that retained normal _HIF-2_α mRNA levels did not display statistically significant changes in SCGB3a1 mRNA levels. To further investigate the connection between _HIF-2_α and SCGB3a1 expression, we evaluated lung cancer datasets available through the Oncomine dataset repository (www.oncomine.org). Filtering specifically for NSCLC tumor datasets containing HIF-2α and SCGB3a1 probes, we found significant correlation between _HIF-2_α and SCGB3a1 expression in three of four datasets (Fig. 5_B_).
Fig. 5.
_HIF-2_α and SCGB3a1 levels correlate in NSCLC samples. (A and B) RNA was isolated from fresh frozen or formaldehyde/paraformaldehyde fixed human NSCLC tumors (designated T) (n = 15), used to generate cDNA for quantitative RT-PCR analysis. Uninvolved adjacent lung tissue (designated C) served as control. Transcipt levels were normalized to 18s RNA; 73% (11 out of 15) NSCLC samples analyzed demonstrated significantly decreased levels of both _HIF-2_α and SCGB3a1 mRNA compared with adjacent control tissue (Left). Samples with normal SCGB3a1 expression (n = 4) did not display any significant change in _HIF-2_α level (Right). Error bars represent SEM. *P < 0.05. (B) Significant correlation between _HIF-2_α and SCGB3a1 mRNA expression was observed in three (of four) human NSCLC datasets obtained from Oncomine. (C) Schematic representation of the opposing roles of HIF-2α in tumorigenesis, wherein both HIF-2α overexpression through activation of protumorigenic target genes, and HIF-2α deletion through loss of expression of candidate tumor suppressor genes, including Scgb3a1, could promote tumor growth. Other HIF-2α targets such as lactotransferrin and ceruloplasmin may also influence tumor outcome, but their role in tumor suppression is as yet unclear.
Discussion
Many human cancers display a direct correlation between HIF-α overexpression and poor prognosis (4, 8), consistent with the idea that HIF target genes positively regulate critical features of tumor progression, including cancer cell metabolism, survival, movement, and local angiogenesis. This correlation has spurred the development of HIF inhibitors as therapeutic treatments for cancer, as well as other diseases (11). The specific activities of HIF-1α and HIF-2α in tumor progression are quite complex, however, and appear to vary depending on cell type and oncogenic background. For example, although HIF-1α overexpression drives murine mammary tumor progression and metastasis in a murine model (25), it also inhibits the growth of certain von Hippel-Lindau (VHL)-deficient RCCs (26, 27), in part by restricting the activity of the c-Myc oncoprotein (28, 29). In contrast, HIF-2α expression potentiates c-Myc signaling in RCCs and promotes tumor growth (29). Furthermore, a recent report demonstrated that expression of a stabilized (gain-of-function) _Hif-2_α allele similarly promotes tumor progression in the LSL-KrasG12D murine NSCLC model (13). These and other data suggest that inhibiting either HIF-1α or HIF-2α (or both) may be of therapeutic value in specific disease settings.
The data presented here indicate that HIF-2α has an unexpected tumor suppressive role in KrasG12D-driven lung tumors and that reduced expression of the HIF-2α target Scgb3a1 contributes to this effect. This interpretation is supported by the observation that restored Scgb3a1 expression inhibited the growth of HIF-2α–deficient A549 xenograft tumors. Moreover, elevated AKT signaling was observed in HIF-2α–deficient A549 cells and was inhibited by restored expression of either HIF-2α or Scgb3a1. These observations are consistent with previous reports that enforced Scgb3a1 expression inhibits AKT activity in human breast cancer cells (18).
Scgb3a1 was originally identified as a candidate tumor suppressor through gene expression studies comparing human mammary carcinomas to normal mammary epithelial cells (14). It was subsequently shown that Scgb3a1 expression is reduced in human lung, prostate, pancreatic, and nasopharyngeal cancers, and corresponding hypermethylation of the Scgb3a1 promoter was reported for multiple malignancies (15–17, 30). Moreover, decreased Scgb3a1 expression was identified as a primary independent predictor of poor clinical outcome in early stage human NSCLCs (17). Our data indicate that Scgb3a1 expression is directly regulated by HIF-2α in human lung adenocarcinoma cells: this relationship is underscored by the direct correlation between _HIF-2_α and SCGB3a1 mRNA levels observed in approximately 70% of human lung adenocarcinomas analyzed and in three of four NSCLC datasets obtained from the Oncomine database.
Interestingly, our results contrast directly with those from a recent report in which HIF-2α inhibition in A549 cells, as well as in HCT116 colon carcinoma and U87MG glioblastoma cells, actually reduced xenograft tumor growth (31). The nature of these disparate results is not clear, as identical shRNA sequences were used; however, our data are supported by two important controls. First, the phenotypes observed in our HIF-2α deficient A549 cells are reversed by enforced expression of a murine HIF-2α mRNA that escapes shRNA inhibition, confirming the HIF-2α dependent nature of these phenotypes. Second, the HIF-2α deficient A549 xenograft tumors phenocopy directly the behavior of autochthonous tumors generated by _Hif-2_α genetic deletion in the LSL-KrasG12D model.
The tumor suppressive activity of HIF-2α we observed was surprising, as expression of a stabilized HIF-2α in the identical LSL-KrasG12D model promoted tumor growth, and HIF-2α overexpression similarly promotes growth of RCCs (29). In particular, it raises the question of how HIF-2α can behave as both a tumor suppressor and an oncogene in the same NSCLC model. It appears that entirely different sets of HIF-2α target genes mediate these effects in a manner perhaps analogous to the proproliferative and proapoptotic activities of c-Myc (32). Whereas increased HIF-2α function elevates the expression of genes associated with angiogenesis and epithelial-to-mesenchymal transition (EMT) in LSL-KrasG12D NSCLCs (13), HIF-2α deletion in the same model diminishes the expression of a putative tumor suppressor Scgb3a1, among other genes.
Collectively, these results strongly suggest that inhibition of overexpressed HIF-2α in NSCLCs (and perhaps other carcinomas) might have beneficial effects but that reducing HIF-2α function below a critical threshold might also drive tumor progression by inhibiting the expression of specific tumor suppressor genes. This model (Fig. 5_C_) assumes that HIF-2α overexpression either fails to increase Scgb3a1 function above normal levels or that increased Scgb3a1 activity is offset by elevated expression of other HIF-2α targets. It is also interesting to note that the time at which HIF-2α function affects tumor progression differs between the two models. In a previous study (13), expression of stabilized HIF-2α was initiated at the earliest stage in lung tumorigenesis (i.e., Ad-Cre infection). In contrast, HIF-2α protein stabilization in the lung tumors of our control mice probably occurs subsequent to Kras activation, either as a result of tumor hypoxia or additional oncogenic events. The importance of this temporal difference in expression is not yet clear. In general, however, our results caution that inhibition of HIF complexes in cancer treatment may require a narrower therapeutic window than initially imagined.
Our results also raise a number of interesting questions for subsequent work, including (i) to what degree does the suppression of other putative HIF-2α target genes (lactotransferrin, ceruloplasmin, etc.) contribute to the phenotypes we observe; (ii) are there noncell-autonomous effects of HIF-2α deletion that regulate LSL-KrasG12D NSCLC tumor progression (for example, the increase in granulocyte infiltration); and (iii) is the relation between HIF-2α and Scgb3a1 observed only in the context of activating Kras mutations or does it extend to other oncogenic backgrounds? Importantly, how does Scgb3a1 regulate AKT activity and are additional signaling pathways involved in its tumor suppressive effects? To what degree does elevated AKT activity, in concert with other direct and indirect HIF-2α targets, contribute to the proliferative and apoptotic phenotypes we observe in KrasG12D-driven, HIF-2α deficient lung tumors?
In summary, we used both autochthonous and xenograft genetic tumors to reveal a tumor suppressive effect of HIF-2α in NSCLC and identify its direct target gene Scgb3a1 as a downstream effector. This may be a more general phenomenon: for example, a previous report demonstrated that either HIF-1α or HIF-2α expression can limit tumor growth in genetically manipulated GS9L glioma and murine embryonic stem cells by altering angiogenesis and tumor cell apoptosis (33). Determining the cellular conditions and molecular mechanisms that distinguish the tumor promoting, or tumor suppressive, activities of HIF-1α and HIF-2α proteins will clearly require additional investigation.
Materials and Methods
Generation of and Characterization of Inducible LSL-KrasG12D, Conditional _Hif-α_fl Mice, and Lung Tumors.
Mice carrying the previously described inducible (LSL-KrasG12D) knock-in allele (19), the conditional floxed Hif-2_α_fl allele, and the related null _Hif-2_αΔ allele (20) were intercrossed to generate experimental and control animals (SI Materials and Methods). Lung morphometry, histology, and immunohistochemistry were performed as described in SI Materials and Methods.
Cell Culture and Infection.
A549 cells (ATCC) were grown in DMEM with 10% FBS (Gemini), amino acids, and antibiotics (SI Materials and Methods); A549 cells were infected with retroviruses encoding shRNAs targeting HIF-1α (34), HIF-2α (23) (designated HIF-1α KD and HIF-2α KD, respectively), or with the retroviral backbone as the negative viral control (VC).
Western Blot Analysis.
Protein extracts were prepared and analyzed as described in SI Materials and Methods. For hypoxic analysis, cells were cultured under 0.5% O2 and 5% CO2 for 16–20 h in an InVivo2 400 hypoxia workstation with O2 control (Ruskinn Technology Ltd.). Western blots were probed with antibodies against HIF-1α (Cayman for mouse protein, R&D for human protein), HIF-2α (Novus Biologicals), Scgb3a1 (Abcam), AKT, pAKT, GSK-3β, pGSK-3-α/β, p4EBP1, and β-actin (Cell Signaling).
RNA Purification, Reverse Transcription, and Real-Time PCR Amplification.
RNA was extracted with TRIzol (Invitrogen) or RecoverAll Total nucleic Acid Isolation kit (Ambion) and purified on RNeasy spin columns (Qiagen) as described in SI Materials and Methods; cDNA was synthesized using a high capacity c-DNA transcription kit (Applied Biosystems). Transcript levels were normalized to 18S rRNA using an Applied Biosystems 7900HT analyzer and represented as fold change 2−ΔCT (normoxia)/ 2−ΔCT (hypoxia); mean normoxic value was set to 1.
cDNA Microarray and Oncomine Analysis.
Microarray analysis was performed by the University of Pennsylvania Microarray Core facility, and Oncomine data were analyzed as described in SI Materials and Methods.
ChIP Analysis.
A549 cells were cultured either under normoxia or hypoxia, crosslinked with 1% formaldehyde for 20 min at 37 °C, and quenched with 1 M glycine. Nuclear lysates were analyzed as described in SI Materials and Methods.
Xenograft Assays.
We injected 5 × 106 or 1 × 106 (as indicated) viable HIF-2α KD C1 or HIF-2α KD C2 cells in a 1:1 mix of DMEM:growth factor reduced matrigel matrix (BD) s.c. into one flank of 6-wk-old nude (NU/NU) female mice (Charles River Laboratories). The other flank was either injected with wild-type A549 cells or cells carrying the empty vector (VC). Tumor volume (mm3) was determined using a caliper every 5 d postimplantation (d.p.i). Animals were killed 5 wk postimplantation, or earlier (3-4 wk) in case of ulcerating tumors.
Statistics.
P values were determined using the Student's t test; P < 0.05 was considered significant.
Supplementary Material
Supporting Information
Acknowledgments
We thank Shetal Patel and Ania Warczyk (University of Pennsylvania) for assistance with immunohistochemistry and xenograft assays, Hongwei Yu and Alan Wanicur (University of Pennsylvania) for preparation of tissue sections, and William Y. Kim (University of North Carolina) and the Simon laboratory for helpful discussions. This work was supported by the Howard Hughes Medical Institute (M.C.S.) and National Institutes of Health Grant HL66130 (to B.K. and M.C.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE22575).
References
- 1.Swinson DE, et al. Carbonic anhydrase IX expression, a novel surrogate marker of tumor hypoxia, is associated with a poor prognosis in non-small-cell lung cancer. J Clin Oncol. 2003;21:473–482. doi: 10.1200/JCO.2003.11.132. [DOI] [PubMed] [Google Scholar]
- 2.Le QT, et al. An evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res. 2006;12:1507–1514. doi: 10.1158/1078-0432.CCR-05-2049. [DOI] [PubMed] [Google Scholar]
- 3.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 5.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raval RR, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordan JD, Simon MC. Hypoxia-inducible factors: Central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17:71–77. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8:967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kung AL, Wang S, Klco JM, Kaelin WG, Livingston DM. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat Med. 2000;6:1335–1340. doi: 10.1038/82146. [DOI] [PubMed] [Google Scholar]
- 10.Kung AL, et al. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer Cell. 2004;6:33–43. doi: 10.1016/j.ccr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. HIF-1 inhibitors for cancer therapy: From gene expression to drug discovery. Curr Pharm Des. 2009;15:3839–3843. doi: 10.2174/138161209789649402. [DOI] [PubMed] [Google Scholar]
- 12.Giatromanolaki A, et al. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881–890. doi: 10.1054/bjoc.2001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim WY, et al. HIF2alpha cooperates with RAS to promote lung tumorigenesis in mice. J Clin Invest. 2009;119:2160–2170. doi: 10.1172/JCI38443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krop IE, et al. HIN-1, a putative cytokine highly expressed in normal but not cancerous mammary epithelial cells. Proc Natl Acad Sci USA. 2001;98:9796–9801. doi: 10.1073/pnas.171138398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krop I, et al. Frequent HIN-1 promoter methylation and lack of expression in multiple human tumor types. Mol Cancer Res. 2004;2:489–494. [PubMed] [Google Scholar]
- 16.Shigematsu H, et al. Aberrant methylation of HIN-1 (high in normal-1) is a frequent event in many human malignancies. Int J Cancer. 2005;113:600–604. doi: 10.1002/ijc.20622. [DOI] [PubMed] [Google Scholar]
- 17.Marchetti A, et al. Down regulation of high in normal-1 (HIN-1) is a frequent event in stage I non-small cell lung cancer and correlates with poor clinical outcome. Clin Cancer Res. 2004;10:1338–1343. doi: 10.1158/1078-0432.ccr-1174-03. [DOI] [PubMed] [Google Scholar]
- 18.Krop I, et al. HIN-1, an inhibitor of cell growth, invasion, and AKT activation. Cancer Res. 2005;65:9659–9669. doi: 10.1158/0008-5472.CAN-05-1663. [DOI] [PubMed] [Google Scholar]
- 19.Jackson EL, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruber M, et al. Acute postnatal ablation of Hif-2alpha results in anemia. Proc Natl Acad Sci USA. 2007;104:2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryan HE, et al. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- 22.Porter D, Lahti-Domenici J, Torres-Arzayus M, Chin L, Polyak K. Expression of high in normal-1 (HIN-1) and uteroglobin related protein-1 (UGRP-1) in adult and developing tissues. Mech Dev. 2002;114:201–204. doi: 10.1016/s0925-4773(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 23.Kondo K, Kim WY, Lechpammer M, Kaelin WG., Jr Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1:E83. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sweet-Cordero A, et al. An oncogenic KRAS2 expression signature identified by cross-species gene-expression analysis. Nat Genet. 2005;37:48–55. doi: 10.1038/ng1490. [DOI] [PubMed] [Google Scholar]
- 25.Liao D, Corle C, Seagroves TN, Johnson RS. Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res. 2007;67:563–572. doi: 10.1158/0008-5472.CAN-06-2701. [DOI] [PubMed] [Google Scholar]
- 26.Maranchie JK, et al. The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell. 2002;1:247–255. doi: 10.1016/s1535-6108(02)00044-2. [DOI] [PubMed] [Google Scholar]
- 27.Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG., Jr Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–246. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 28.Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gordan JD, et al. HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell. 2008;14:435–446. doi: 10.1016/j.ccr.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong TS, et al. Promoter hypermethylation of high-in-normal 1 gene in primary nasopharyngeal carcinoma. Clin Cancer Res. 2003;9:3042–3046. [PubMed] [Google Scholar]
- 31.Franovic A, Holterman CE, Payette J, Lee S. Human cancers converge at the HIF-2alpha oncogenic axis. Proc Natl Acad Sci USA. 2009;106:21306–21311. doi: 10.1073/pnas.0906432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eilers M, Eisenman RN. Myc's broad reach. Genes Dev. 2008;22:2755–2766. doi: 10.1101/gad.1712408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acker T, et al. Genetic evidence for a tumor suppressor role of HIF-2alpha. Cancer Cell. 2005;8:131–141. doi: 10.1016/j.ccr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Lum JJ, et al. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21:1037–1049. doi: 10.1101/gad.1529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information