Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling (original) (raw)
Abstract
The small airways of the human lung undergo pathological changes in pulmonary disorders, such as chronic obstructive pulmonary disease (COPD), asthma, bronchiolitis obliterans and cystic fibrosis. These clinical problems impose huge personal and societal healthcare burdens. The changes, termed ‘pathological airway remodeling’, affect the epithelium, the underlying mesenchyme and the reciprocal trophic interactions that occur between these tissues. Most of the normal human airway is lined by a pseudostratified epithelium of ciliated cells, secretory cells and 6–30% basal cells, the proportion of which varies along the proximal-distal axis. Epithelial abnormalities range from hypoplasia (failure to differentiate) to basal- and goblet-cell hyperplasia, squamous- and goblet-cell metaplasia, dysplasia and malignant transformation. Mesenchymal alterations include thickening of the basal lamina, smooth muscle hyperplasia, fibrosis and inflammatory cell accumulation. Paradoxically, given the prevalence and importance of airway remodeling in lung disease, its etiology is poorly understood. This is due, in part, to a lack of basic knowledge of the mechanisms that regulate the differentiation, maintenance and repair of the airway epithelium. Specifically, little is known about the proliferation and differentiation of basal cells, a multipotent stem cell population of the pseudostratified airway epithelium. This Perspective summarizes what we know, and what we need to know, about airway basal cells to evaluate their contributions to normal and abnormal airway remodeling. We contend that exploiting well-described model systems using both human airway epithelial cells and the pseudostratified epithelium of the genetically tractable mouse trachea will enable crucial discoveries regarding the pathogenesis of airway disease.
Introduction
Basal cells (BCs), so-named for their proximity to the underlying basal lamina, are a common feature of stratified and pseudostratified epithelia throughout the body. These include the conducting airways of the human lung, which are lined with a pseudostratified epithelium containing between 6–30% BCs, depending on location (Mercer et al., 1994; Boers et al., 1998; Nakajima et al., 1998; Evans et al., 2001; Zhang et al., 2009). The abundant cytoskeletal, junctional and adhesive proteins of BCs help to anchor the epithelium to the matrix and insulate the underlying stroma from the external environment. There is now good experimental evidence indicating that airway BCs are a population of multipotent stem cells that drives both homeostasis of the normal epithelium and its orderly regeneration after injury (discussed below). This justifies a much more detailed analysis of BC function than has been afforded so far (Jetten, 1991; Randell et al., 1991; Boers et al., 1998; Hong et al., 2004b; Hackett et al., 2008; Rock et al., 2009).
In addition to their role in epithelial homeostasis, airway BCs probably contribute to disease susceptibility, initiation and progression. For example, disruption of the normal balance between BC proliferation and differentiation can lead, at two extremes, to BC hyperplasia or epithelial hypoplasia. Changes in the lineage choice of BCs or their undifferentiated daughters might contribute to the mucous cell hyperplasia, metaplasia or squamous metaplasia seen in many respiratory disorders. And because BCs are a stem cell population, alterations in their genomes through mutations or epigenetic modifications induced by environmental agents might affect the long-term susceptibility of individuals to respiratory disease. Thus, a greater understanding of BC behavior is potentially of clinical relevance. For example, therapies aimed at regulating BC proliferation and directing their differentiation towards specific lineages might help to restore a normal phenotype in a disease context. Because BCs are a long-lived population, gene or cellular replacement therapies targeting them are likely to provide sustained rather than transient remediation. In addition, monitoring genetic polymorphisms, mutations or epigenetic changes in BCs might help to predict an individual’s susceptibility to the disease-inducing effects of early exposure to pathogenic agents. Finally, as long-term multipotent stem cells, BCs are the ideal starting population for the creation of bioengineered human airways. The clinical use of such reconstructed tissue for a patient with airway stenosis has been recently demonstrated (Macchiarini et al., 2008). However, optimizing the expansion of autologous or donor cells and their efficient regeneration of a functional epithelium will probably require a better understanding of normal BC biology.
In this Perspective, we summarize what is known about BCs of mouse and human pseudostratified airway epithelia. We review literature that collectively suggests that BCs of human airways, like those in the mouse trachea, are a population of long-lived, multipotent classical stem cells. We provide evidence that they are important in the initiation and progression of airway disease, and a potential point of intervention for future therapies. Lastly, we posit that use of well-described in vitro methods and genetically tractable mouse models will greatly enhance our understanding of pathological airway remodeling.
Differences between the mouse and human lung with respect to airway structure and composition
The respiratory system is composed of a tree-like system of branched tubes that carry air to and from the alveoli, where gas exchange takes place. This basic design is conserved among vertebrates, but there are important differences between mouse and human lungs – presumably a result of the very large differences in body size (Fig. 1).
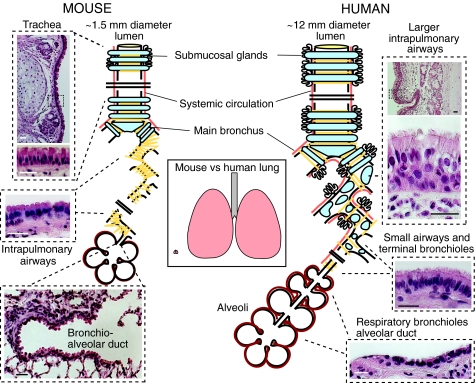
Fig. 1.
Schematic comparison of the structure and epithelial organization of rodent and human lungs. Left panel: mouse lung. The trachea, ∼1.5 mm internal diameter, is lined by a pseudostratified epithelium with about 55% ciliated cells, 30% BCs, secretory cells and sparse neuroendocrine cells. In the rat (but not apparently in the mouse), there are more ciliated cells in the epithelium overlying intercartilage segments versus cartilage (Toskala et al., 2005). The submucosal glands are typically restricted to the four most proximal intercartilage regions. In the mouse, there are ∼six to eight generations of intralobar branches (intrapulmonary airways), which have a stereotypical branching pattern (Metzger et al., 2008). In these airways, the epithelium is simple and columnar, and is made up of ∼48% ciliated cells and the remainder of secretory and neuroendocrine cells. Most of the secretory cells have electron-dense cytoplasmic granules and domed apical surfaces that project into the lumen and express high levels of the secretoglobin SCGB1A1. They are therefore defined as Clara cells (Mercer et al., 1994). There are no BCs and, in laboratory mice, few goblet cells in these intrapulmonary airways. Smooth muscle (yellow lines) surrounds the airways, but there are no cartilage plates. The terminal bronchioles leading into the bronchio-alveolar duct have fewer ciliated cells (∼26%) compared with more proximal airways. Right panel: human lung. The average human trachea has an internal diameter of ∼12 mm. There are more generations of intrapulmonary branches than in the mouse, and cartilage plates and smooth muscle surround the intrapulmonary airways deep into the lung. A pseudostratified epithelium with ∼30% BCs, 30% ciliated and 30% secretory cells lines these airways. The latter are predominantly goblet cells with a few Clara cells (Mercer et al., 1994). The respiratory bronchioles are lined by a simple cuboidal epithelium. The precise identity and gene expression profiles of these cells are poorly understood but they do not seem to express SCGB1A1 [for a detailed description see ten Have-Opbroek et al. (ten Have-Opbroek et al., 1991)]. Scale bars: 25 μm.
In mice, the largest airway, the trachea, has an internal diameter of ∼1.5 mm, equivalent to the diameter of the small peripheral airways in the human lung (Fig. 1). In mice, cartilage rings are only present in the extrapulmonary airways but, in humans, cartilage extends for several bronchial generations into the lung. Submucosal glands, which produce mucins and other factors, are restricted to only the proximal trachea in the mouse but penetrate deep into the human lung. This is significant because glands might provide a particularly protected niche for BCs within their ducts (see later). Striking interspecies differences are also found with respect to the cellular composition of the airways, which normally varies along the proximal-distal axis. In all species, the pseudostratified epithelium contains basal, ciliated, secretory (goblet, serous and Clara cells), neuroendocrine and less well categorized ‘indeterminate’ or ‘intermediate’ cells (Mercer et al., 1994). In humans, mucin-secreting goblet cells are relatively abundant but, in adult mice maintained under laboratory conditions, these cells are rare. Importantly, in humans, the BC-containing pseudostratified epithelium extends distally to terminal bronchioles of about 0.5 mm diameter and only the respiratory bronchioles are lined by a simple cuboidal epithelium lacking BCs. By stark contrast, a pseudostratified epithelium is largely restricted to the mouse trachea, and the transition to a simple columnar epithelium without BCs occurs in the mainstem bronchi (Fig. 1). Thus, the cellular composition and organization of the mouse intrapulmonary airway epithelium essentially resembles only the most distal portions of the human conducting airways, whereas the mouse trachea is much more similar to most airway generations of the human lung. From this, we contend that basic studies of stem cell biology that are relevant to the pseudostratified human airway epithelium are best performed in the mouse trachea and mainstem bronchi.
Characteristics of mouse and human airway BCs
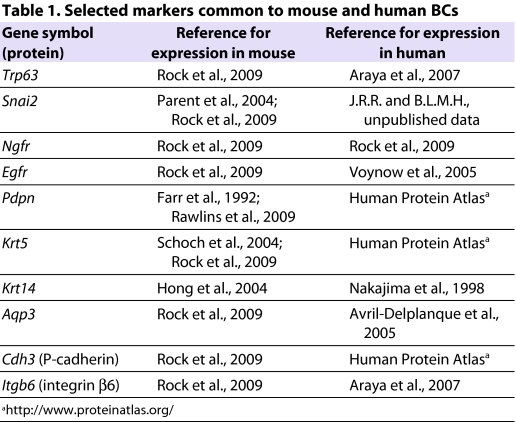
In the adult mouse trachea, BCs constitute ∼30% of the total epithelial population (Hong et al., 2004b; Rock et al., 2009). Transcriptional profiling and immunohistochemistry of BCs have illuminated their gene expression patterns, surface characteristics and interactions with the underlying matrix. Table 1 lists some genes and proteins that are common to both mouse and human BCs. One characteristic of BCs is high expression levels of the transcription factor transformation-related protein 63 (TRP63). Indeed, the development of BCs, which is completed postnatally, is dependent on Trp63, and _Trp63_-null mice lack BCs in the tracheal epithelium (Daniely et al., 2004). Among cytoskeletal proteins, cytokeratins 5 and 14 (KRT5 and KRT14, respectively) are typically expressed in BCs. Interestingly, there is differential expression of these proteins in airway BCs. At steady state, most mouse tracheal BCs express KRT5, whereas only a subset expresses KRT14 (Fig. 2C,D). However, during repair after naphthalene-induced injury, KRT14 expression is upregulated in the BC population (Hong et al., 2004a; Hong et al., 2004b). KRT14 also has a more restricted expression pattern than KRT5 in the human airway, but is expressed in TRP63+ BCs in regions of squamous metaplasia (Fig. 3). The functional significance of differential cytokeratin expression at steady state, after injury and in pathological remodeling requires further investigation.
Table 1.
Selected markers common to mouse and human BCs
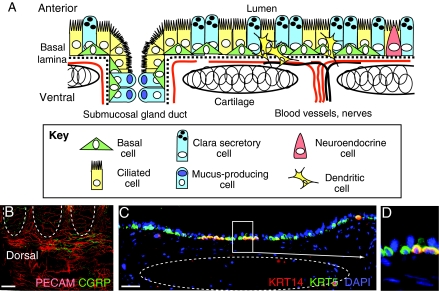
Fig. 2.
Schematic diagram of the mouse trachea showing BCs and their dynamic niche. (A) Schematic representation of BCs in the proximal mouse trachea, in which submucosal glands are present. The surface epithelium consists of basal, ciliated and secretory cells in roughly equal proportions, with relatively few neuroendocrine cells. In laboratory mice, mucus-producing goblet cells are largely restricted to the submucosal glands. Immune cells, including dendritic cells, reside transiently within the epithelium. (B) Whole-mount immunohistochemistry of a mouse trachea. Staining for CD31 (also known as PECAM-1; red) shows dense vascularization of the trachea, especially dorsally and in the intercartilage regions ventrally. These blood vessels are from the systemic circulation. Staining for calcitonin gene-related peptide (CGRP; green) reveals nerves. (C) Immunohistochemistry of a section of mouse trachea showing that most BCs express cytokeratin 5 (KRT5, green), whereas only a subset co-expresses KRT14 (red). DAPI stains nuclei (blue). (B,C) Outlines of cartilage rings are in dotted lines. (D) Higher magnification of boxed region in C. Scale bars: 600 μm (B); 20 μm (C).
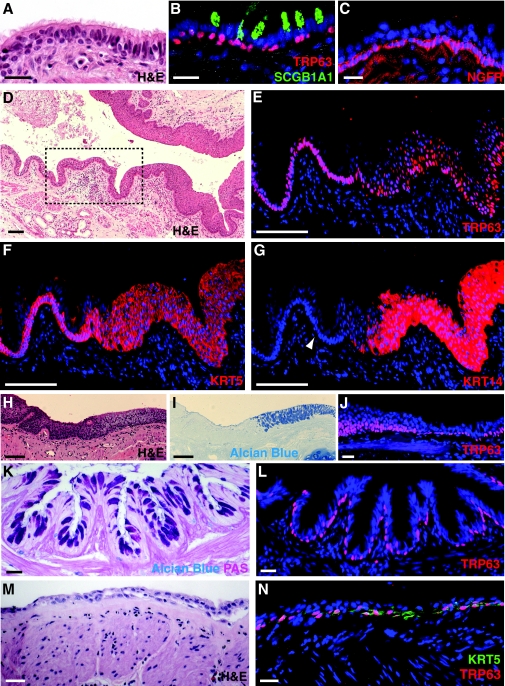
Fig. 3.
BCs in normal and diseased human small airways. (A–C) Sections of a normal human airway (∼2 mm diameter) from a non-smoker that were obtained after lobectomy for a non-lung-cancer metastasis. BCs of the normal pseudostratified airway epithelium are identified by morphology and proximity to the basal lamina in a hematoxylin and eosin (H&E) stained section (A) or by expression of the transcription factor TRP63 (B) and NGFR (C) in adjacent sections. Presence of SCGB1A1 in B marks Clara secretory cells. (D) H&E stained section of an airway from a smoker with COPD. The boxed region shows relatively normal epithelium transitioning into squamous metaplasia. (E–G) High magnification of sections adjacent to the boxed region in D showing expansion of TRP63+ (E, red), KRT5+ (F, red) and KRT14+ (G, red) cells in regions of squamous metaplasia. Note that only rare BCs in normal airway are stained by a mouse monoclonal antibody against KRT14 (arrowhead in G). (H–J) Regions of mucous hyperplasia and squamous metaplasia in an airway of a smoker with COPD stained with H&E (H), Alcian Blue (I) and anti-TRP63 (J, red). Note the sharp transition from squamous metaplasia to mucous hyperplasia. (K,L) Small airway from an organ donor with moderate asthma stained with Alcian-Blue–periodic-acid–Schiff and anti-TRP63 (red, L). (M) Airway from a patient with post-transplant bronchiolitis obliterans syndrome stained with H&E, anti-TRP63 (red, N) and anti-KRT5 (green, N). Nuclei were stained with DAPI (blue) in fluorescent images. Scale bars: 25 μm (A–C,J–N); 100 μm (D–I).
In the uppermost mouse trachea, BCs form a mostly continuous monolayer, but they are found in clusters or as individual cells more distally. In the larger human airways, BCs also form a continuous monolayer but similarly become distributed into clusters and individual cells in the small terminal bronchioles (Nakajima et al., 1998). In both the mouse and human, BCs form desmosomal contacts with neighboring columnar cells and are anchored to the basal lamina via hemi-desmosomes and other highly expressed adhesion molecules. The subjacent lamina propria contains mesenchymal cells, such as fibroblasts and immune cells (e.g. macrophages). In addition, the mouse trachea and human intrapulmonary airways are richly endowed with blood vessels and nerves, which enter between the cartilage rings and encircle the airway (Liebow, 1965) (Fig. 2B). Systemic blood vessels of the bronchial circulation supply many generations of intrapulmonary airways in humans. By contrast, in the mouse, these vessels do not penetrate beyond the mainstem bronchi, even after pathological stimulation of angiogenesis (Mitzner et al., 2000). Therefore, despite notable differences between mouse and human airways, BCs of the pseudostratified airway epithelia in both species are molecularly and histologically very similar.
Evidence that BCs are multipotent adult tissue stem cells
By definition, a stem cell can self-renew and generate differentiated progeny of one or more cell types. An adult tissue stem cell should also be able to repopulate a complete tissue and maintain it over the long term. Assays for stem cell function ideally include genetic lineage tracing to follow the fate of small populations or individual candidate stem cells, either in the intact tissue in vivo or after transplantation. Although only a few long-term lineage-tracing and transplantation studies have been reported for the mouse trachea to date, they provide good evidence that at least a subset of BCs in the mouse trachea and mainstem bronchi functions as a stem cell population. Relevant studies have been reviewed (Randell, 2006; Rawlins and Hogan, 2006) and are summarized below.
In vitro culture and xenograft assays with rodent BCs
Early DNA-precursor pulse-labeling experiments showed that both BCs and nonciliated columnar epithelial cells of the rodent trachea proliferate in the steady state and in response to injury (Evans et al., 1986; Breuer et al., 1990). Other early studies demonstrated that adult rat tracheal BCs sorted by flow cytometry have colony-forming ability in culture and can regenerate a fully differentiated mucociliary epithelium when seeded in denuded tracheas (host tracheas that have been stripped of their epithelium) and transplanted subcutaneously in nude rats (Randell et al., 1991; Liu et al., 1994). The BC-depleted fraction in these studies had a lower colony-forming efficiency in vitro compared with the BC-enriched fraction, but was still able to generate a mucociliary epithelium in the heterotopic graft assay. This result might have been due to minor BC contaminants in the lumenal fraction that survived and efficiently proliferated when co-seeded with non-basal cells in the supportive environment of the grafts. Alternatively, some lumenal cells might have the capacity to regenerate BCs under these conditions. Preliminary evidence that mouse lumenal cells expressing secretoglobin, family 1A, member 1 (Scgb1a1; also known as uteroglobin) can generate BCs with very low efficiency after epithelial injury was obtained from in vivo lineage-tracing studies (Rawlins et al., 2009). Support for the idea that a subset of lumenal cells can give rise to BCs after epithelial injury comes from recent transplantation and regeneration studies in the mouse prostate (Wang et al., 2009). There is also evidence from other mammalian organ systems that daughters of stem cells can revert to stem cells after injury (Nakagawa et al., 2010).
Recently, our group developed a three-dimensional culture system to test the regenerative capacity of airway epithelial cells. In this simple and convenient system, individual sorted (TRP63+;NGFR+;KRT5+) BCs from the mouse trachea self-renew and give rise to clonal ‘tracheospheres’ containing both ciliated and secretory cells. Serial subculture showed that these BCs can be maintained for multiple passages, although they do eventually seem to lose the ability to differentiate. Significantly, in this assay, which does not include mesenchymal cells, NGFR− lumenal cells do not form tracheospheres (Rock et al., 2009).
In vivo lineage-tracing studies with mouse BCs
Pioneering in vivo lineage-tracing experiments with BCs used a human KRT14_-promoter–_CreER transgene to follow the fate of cells in the mouse trachea and mainstem bronchi following epithelial injury. These BCs generated lineage-labeled basal, ciliated and Clara cells (Hong et al., 2004b; Hong et al., 2004a). Because the expression of the lineage tag was not induced until after the epithelial injury (when KRT14 expression is upregulated), these studies did not assess the contribution of the population of KRT14+ BCs under steady-state conditions. The ability of KRT14+ BCs to self-renew and differentiate was assessed for only 43 days, so their longer-term behaviors remain undefined. We used a KRT5-CreER transgene to follow the fate of KRT5+ BCs in vivo during postnatal growth, during steady state in the adult trachea and during repair after injury (Rock et al., 2009). In these studies, BCs gave rise to both ciliated and Clara cells that were maintained for at least 14 weeks and were not replaced by the progeny of an unlabeled population. By contrast, lineage-labeled SCGB1A1+ cells in the trachea proliferated and generated ciliated cells but as a population were replaced over time by unlabeled progenitor cells, presumably BCs (Rawlins et al., 2009). This suggests that, in airway epithelia containing BCs, the Clara cells do not function as stem cells over the long term under normal conditions.
Are there subpopulations of BCs with different potentials?
Although there is good evidence that some BCs are stem cells in vivo, more work is necessary to determine the spectrum of self-renewal potential and differentiation capacity in the population. Indeed, heterogeneity within the BC population is expected on the basis of recent in vivo lineage-tracing analysis of other well-studied epithelial stem cell systems. For example, in the mouse tail and skin, the interfollicular epidermis, and hair follicle shaft and bulge all contain subpopulations of BCs that are either ‘committed progenitors’ (Clayton et al., 2007) or stem cells. The latter group includes cells that are relatively quiescent and become activated during injury and repair, as well as other stem cells that periodically leave the bulge to participate in the regeneration of the hair follicle (Clayton et al., 2007; Zhang et al., 2009; Snippert et al., 2010). Quiescent and cycling epithelial stem cell populations in mouse intestinal crypts have also been described (Batlle, 2008). There is already evidence for heterogeneity among BCs of the mouse trachea. Subsequent to rounds of repeated injury and repair, BCs that retain BrdU (a thymidine analog that is incorporated into DNA during replication) are restricted to the intercartilage regions, including the ductal epithelium of the submucosal glands where they are present (Borthwick et al., 2001). Furthermore, a subset of BCs isolated on the basis of high expression of a KRT5-EGFP (enhanced green fluorescent protein) transgene was enriched for the ability to form large colonies in air-liquid interface cultures (Schoch et al., 2004). Additional approaches, including novel antibodies and alleles for the identification, localization and purification of BC subpopulations are needed. In addition, experiments to follow the fate of individual lineage-labeled BCs in different locations and to quantify clone size and numbers of cell divisions would be highly informative.
Human airway BCs
Evidence that BCs function as stem cells in the human airways is compelling but, by necessity, less direct. Early studies with epithelial cells isolated from human airways demonstrated the presence of a population capable of multi-lineage differentiation in denuded tracheal xenografts (Engelhardt et al., 1995). More recently, a ‘side population’ of Hoescht dye effluxing TRP63+;KRT5+ BCs was isolated from normal human airways (Hackett et al., 2008). These cells, estimated to constitute ∼0.01% of the total airway epithelium, proliferate and generate ciliated and secretory cells in culture at the air-liquid interface. Descriptions of this technique and transcriptional analysis of airway epithelial cells that differentiated at the air-liquid interface have been published (Ross et al., 2007; Dvorak et al., 2010). Similar studies with BCs isolated from the human nasal turbinate and fetal trachea demonstrated the capacity of these BCs to proliferate and differentiate in vitro (Avril-Delplanque et al., 2005; Hajj et al., 2007b). Among human tracheo-bronchial epithelial cell types, TRP63+;NGFR+ BCs uniquely form ‘bronchospheres’ in a three-dimensional culture assay, demonstrating their capacity to self-renew and generate ciliated and secretory cells (Rock et al., 2009). Collectively, these data support the concept that BCs of the pseudostratified epithelium of the human lung, similar to those of the mouse trachea, are classical stem cells. However, it remains to be determined whether there are significant differences in the proliferative and differentiation potential of different subsets of BCs from human trachea, bronchi and bronchioles.
The BC niche or microenvironment
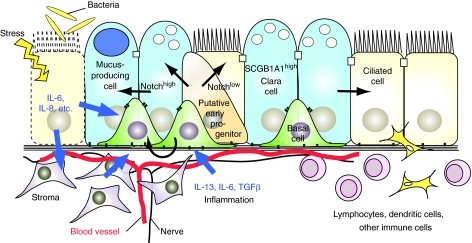
In airways in which BCs are present, there is currently little evidence that there are specific anatomically well-defined ‘niches’ resembling the hair follicle bulge or intestinal crypt base. In the mouse, BCs in the ducts of the submucosal glands and intercartilage regions retain BrdU label after a pulse and long chase, suggesting that these regions are enriched in quiescent BCs. However, as discussed earlier, appropriate lineage-tracing studies that support this hypothesis have not yet been reported. Rather, it seems that the niche of most BCs includes their immediate epithelial neighbors (lumenal cells and perhaps other basal cells) as well as underlying smooth muscle, fibroblasts, blood vessels and nerves (Fig. 2A). Additionally, bone-marrow-derived immune cells, including dendritic cells (Sertl et al., 1986), reside within or in close proximity to the airway epithelium, and their numbers increase during injury and repair. Therefore, the putative niche is highly dynamic, and probably involves cytokines, chemokines and other signaling factors that are derived from multiple cell populations (summarized in Fig. 4).
Fig. 4.
Schematic model of how paracrine signals from the niche influence BC behaviors. A model for how signals from the niche – which includes neighboring epithelial cells, vasculature, nerves, stroma and immune cells – influence BCs and their progeny. In pathological conditions, including bacterial infection and inflammation, epithelial cells and the surrounding stroma produce factors that might modulate BC behaviors. Evidence from other stem cell systems suggests that expression levels of Notch signaling components (which are known to regulate the balance of ciliated and secretory cells in human and embryonic mouse airways) can be modulated by paracrine signals, including cytokines such as IL-6. How these signals are transduced to affect cell biology (e.g. polarity, attachment), proliferation and cell fate decisions, and how they contribute to airway remodeling, such as squamous and mucous metaplasia, are not known.
Pathological airway remodeling in human respiratory disease
Pathological changes (or remodeling) in the composition and organization of the airway epithelium are observed in various conditions, including chronic obstructive pulmonary disease (COPD) (Randell, 2006), bronchiolitis obliterans, cystic fibrosis (CF) and chronic asthma. We focus here on epithelial remodeling in human lung bronchioles that are 1–4 mm in diameter. Normally, these small airways are lined by a well-organized pseudostratified epithelium, which is composed of ciliated, non-ciliated and neuroendocrine cells and a single layer of individual TRP63+;KRT5+;NGFR+ BCs (Fig. 3A–D). The non-ciliated cell population includes mucin-producing goblet cells as well as serous and Clara cells, and currently unclassifiable ‘indeterminate’ or ‘intermediate’ cells (Mercer et al., 1994). Why is disease in these airways so detrimental? Given that they lack supporting cartilage, these narrow tubes are collapsible, and excessive thickening of the airway wall in combination with mucus overproduction and cilia loss can lead to mucus plugging, airflow obstruction, and cycles of bacterial infection and inflammatory damage. Once they reach a critical level, these changes will, in turn, seriously exacerbate clinical symptoms caused by concomitant loss of lung elastic recoil in emphysema, or airway smooth muscle hyper-reactivity in asthma. Typical pathological abnormalities in small airways and the common disorders in which they are found are listed in Table 2, and examples are provided in the sections below.
Table 2.
Pathological abnormalities in common airway disorders
COPD
COPD is a leading cause of disability that affects millions of patients worldwide, and is predicted by the World Health Organization (WHO) to become the third leading cause of death worldwide by 2030 (www.who.int/respiratory/copd/en/index.html). It is a complex disorder that includes chronic bronchitis and emphysema. It is associated with an inflammatory response to toxic gases or particles and with airflow limitation that is not fully reversible and is usually progressive. COPD develops mainly due to smoking but can also occur in non-smokers exposed to burning fuel for heating and cooking, or other exposures (www.goldcopd.com). Smokers with COPD are at higher risk of developing lung cancer than smokers without COPD (Mannino et al., 2003). In the affected subset, progression from epithelial hyperplasia and metaplasia to dysplasia, carcinoma in situ and subsequent malignant transformation is considered the usual course, although direct evidence of this is still lacking (Banerjee, 2009). In regions of squamous metaplasia (Fig. 3E–J), there are multiple layers of TRP63+;KRT5+ cells that, unlike most BCs in the normal epithelium, are also positive for KRT14. Areas of mucous cell (goblet) hyperplasia are often admixed with squamous metaplasia. Indeed, there can be a sharp boundary between the two, with a continuous population of TRP63+ cells underlying both areas (Fig. 3H–J).
Cystic fibrosis
Epithelial remodeling is a feature of several other respiratory disorders that are also associated with recurrent infection and/or inflammation. CF is caused by mutations in the cystic fibrosis transmembrane conductance regulator gene (CFTR). The primary defect observed in the lungs of patients with this disease is disrupted ion transport in columnar epithelial cells (Boucher, 2007). The resulting decrease in airway surface hydration contributes to impaired mucus clearance, chronic bacterial infection, secretion of pro-inflammatory cytokines, severe and sustained leukocyte influx, and tissue damage and repair. Histological changes include goblet cell hyperplasia, BC hyperplasia and airway wall tissue destruction that can lead to bronchiectasis (dilation of the airways). An informative study showed that hyperproliferative airway epithelial cells in patients with CF expressed KRT5, and/or KRT14 and epidermal growth factor receptor (EGFR), which are markers of BCs (Voynow et al., 2005).
Asthma
Asthma is a prevalent disease that is increasing in epidemic proportions, especially in certain populations, such as disadvantaged inner-city children. It is the leading cause of school absenteeism owing to a chronic condition and, according to the Center for Disease Control (CDC), caused over 3000 deaths in the USA in 2007. Hallmarks of asthma are goblet cell hyperplasia and mucus overproduction, and the chronic response is thought to include repeated epithelial shedding, thickening of the basal lamina, airway wall fibrosis, smooth muscle hypertrophy, angiogenesis and hyperplasia of the submucosal gland (Tang et al., 2006). BCs are present in the areas of goblet cell hyperplasia (Fig. 3K,L), and it is therefore possible that changes in their behavior and lineage choice contribute to epithelial shedding and goblet cell hyperplasia (discussed in more detail below). These changes can, in turn, disrupt the reciprocal trophic interactions between the epithelium and mesenchyme, and exacerbate submucosal remodeling.
Bronchiolitis obliterans
It is widely considered that bronchiolitis obliterans results from epithelial denudation and subsequent fibrotic obliteration by in-migrating lamina propria cells that are no longer held in check by an intact epithelium. This can occur following toxic or viral injury and is a principal manifestation of chronic rejection of transplanted lungs, contributing to the fact that patients that receive a lung transplant have the lowest survival rate of all solid organ transplants [∼50% after 5 years (Christie et al., 2008)]. The lesions in patients with bronchiolitis obliterans typically occur in small airways, and denudation might be preceded by abnormal differentiation and hypoplasia (Fig. 3M,N). In contrast to the hyperplastic lesions observed in CF and COPD, epithelial loss in bronchiolitis obliterans might result from a stem cell deficiency that means that homeostasis in the context of injury, inflammation and disrupted epithelial-mesenchymal crosstalk cannot be properly maintained. Understanding underlying mechanisms and developing novel strategies to prevent bronchiolitis obliterans is crucial for improving the success of lung transplantation.
How might changes in BCs contribute to abnormal airway remodeling in respiratory disease?
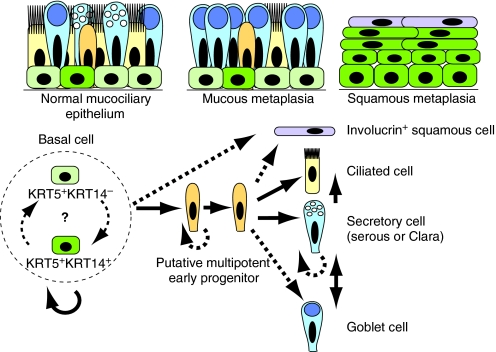
In the normal human airway epithelium, a tight balance between BC self-renewal and generation of physiologically appropriate proportions of secretory and ciliated cells is required (Fig. 5). This is mediated by the behaviors of stem cells and potential multipotent intermediate progenitors (as discussed below). Changes in BCs or their progeny could theoretically lead to pathological airway remodeling in a number of different ways. For example, excessive self-renewal at the expense of differentiation could lead to BC hyperplasia, whereas inappropriate cell fate choices might lead to goblet cell hyperplasia or metaplasia at the expense of ciliated cells. Likewise, the generation of stratified suprabasal cells at the expense of lumenal cells might lead to squamous metaplasia, whereas a failure to proliferate, increased apoptosis or inappropriate commitment to differentiation at the expense of self-renewal could all contribute to epithelial hypoplasia.
Fig. 5.
Tentative working model for the self-renewal and differentiation of basal stem cells in mouse and human airways. The upper panels schematize a normal human pseudostratified mucociliary epithelium (left), goblet cell (mucous) metaplasia (center) and squamous metaplasia (right). In the mouse trachea there are more Clara than goblet cells and BCs are mostly discontinuous. Note that only a subset of BCs in the normal airway expresses both KRT5 and KRT14. The lower panel illustrates probable lineage relationships in the adult pseudostratified epithelium. The relationships between Trp63+ BCs that differ in cytokeratin expression and other properties is not yet clear. As a population (within broken circle), BCs self-renew over the long term and generate ciliated, secretory cells (including SCGB1A1+ Clara cells) and goblet cells. We predict the existence of a multipotent early progenitor that also proliferates. SCGB1A1+ cells can proliferate and give rise to ciliated cells (Rawlins et al., 2009) and, based on studies in the mouse intrapulmonary airways, reversibly give rise to goblet cells (Chen et al., 2009). The origin of squamous cells that express involucrin is not clear.
Such changes in the behavior of BCs and/or their putative multipotent progeny might result from intrinsic (including epigenetic) alterations in their transcriptional and regulatory programs, which in turn affect proliferative and differentiation potential. Alternatively, or in addition, the changes might result from altered paracrine signaling within the dynamic and complex BC niche (Fig. 4). In either case, epithelial changes that alter epithelial-mesenchymal interactions further contribute to disease by exacerbating pathological changes in the mesenchyme, including inflammation, fibrosis and smooth muscle hyperplasia. Although these ideas are plausible, there is currently little supportive experimental evidence. One example, however, comes from studies of squamous metaplasia in COPD and cultured human airway cells. The data suggest that hyperproliferating BCs secrete cytokines, such as interleukin-1β (IL-1β), that promote airway wall fibrosis (Araya et al., 2007). We discuss below other studies of pathological airway remodeling and provide a perspective on how evidence for a role for BCs might be obtained.
Challenges in exploring the role of BCs in pathological airway remodeling
One challenge is to determine the extent to which airway epithelial remodeling involves reversible changes in progenitor cells in response to signals from their dynamic niche versus genetic or epigenetic alterations that result from exposure to environmental agents, such as cigarette smoke. One way to distinguish between these alternatives would be to isolate cells from abnormal tissue and grow them under conditions that are conducive for normal self-renewal and differentiation – heritable changes would probably result in cells retaining their abnormal behavior. Several studies have used this approach to study airway epithelium. In one case, the ability of cells from patients with CF and from healthy controls to regenerate the airway epithelium was compared in a denuded tracheal xenograft model. The patients’ cells had reduced regenerative capacity and gave rise to a histologically abnormal epithelium (Hajj et al., 2007a). This raises the possibility that epithelial remodeling in vivo is influenced by intrinsic changes in the secretion of cytokines and metalloproteinases by _CFTR_-mutant cells, in addition to being influenced by the effects of prior bacterial infection and inflammation. In another case, evidence was obtained for an intrinsic ‘asthma epithelial cell phenotype’ that persists after passage of cells in vitro, away from the inflamed environment found in vivo (Kicic et al., 2006).
Although this approach is attractive, it is complicated by multiple factors. These include the heterogeneity of patient populations, variability in the course of disease progression and shortcomings in current methods for collecting primary human cells in the clinic. For example, investigating the etiology of squamous metaplasia will require the isolation of cells from regions of both squamous and normal epithelium. However, techniques used in routine bronchial brushing might not sufficiently distinguish between normal and diseased regions of the airway, and only very small amounts of tissue are obtained from endobronchial biopsies. Furthermore, choosing ‘normal’ control samples could be complicated if predisposing genetic lesions are widely distributed in the airway and oral epithelium (Slaughter et al., 1953; Spira et al., 2007). Last, in the long run, it will be important to compare pure populations of BCs, other intermediate cell types and differentiated cells, all free from fibroblast contamination. Identifying and isolating these different populations will require characterization of more specific markers and the generation of antibodies that recognize them to enable sorting of pure populations.
Another major challenge is to obtain more information about regional patterns of gene expression in BCs and other specific cell types in human airways under normal and pathological conditions. Recent advances in massively parallel sequencing technologies will facilitate transcriptional profiling of mRNA and microRNA, but well-coordinated and sophisticated bioinformatics approaches to mine and share the data efficiently will also be required (Lonergan et al., 2010). In addition, it will be important to characterize DNA copy number, mutations and polymorphisms, as well as patterns of DNA methylation and chromatin modifications – all molecular ‘signatures’ that potentially influence cell behavior.
Finally, we urgently need a conceptual framework within which we can better understand the lineage relationships and intercellular signaling pathways that regulate the proliferation and differentiation of BCs in the adult human airway epithelium, as well as those that regulate cellular behaviors, such as planes of cell division, apical-basal polarity and adhesion. We posit below that such a framework can be built from experiments using both cultured human and mouse airway cells and other epithelial stem cell systems, and can be tested using genetic tools and injury-repair models in the mouse trachea in vivo.
A model for BC self-renewal and differentiation in the human airway
By analogy with other well-studied adult stem cell systems – for example, the intestinal stem cell of the Drosophila midgut (Conder and Knoblich, 2009) and the mammary gland (Bouras et al., 2008) – we propose that airway BCs that differentiate initially give rise to a multipotent progenitor or ‘early lumenal progenitor’. This cell would downregulate genes expressed in BCs (e.g. TRP63, NGFR and KRT5) and begin to express lumenal cell markers, including KRT8 (Fig. 5). This progenitor might still proliferate (in which case it would be considered a transit-amplifying cell) and give rise to either ciliated or secretory cells. The existence of such an early progenitor population is still hypothetical, but these cells might correspond to some of the ‘indeterminate’ or ‘intermediate’ epithelial cells that have been described in human airways (Mercer et al., 1994).
Notch and other signals that influence BCs and their progeny
Analysis of other epithelial stem cell systems has provided evidence that the evolutionarily conserved Notch signaling pathway probably plays a key role in regulating the fate of airway BCs and their progeny. The Notch signaling pathway regulates adult stem cells in a variety of vertebrate and invertebrate systems, including the skin, mammary gland, nervous system and gut (Blanpain et al., 2006; Ohlstein and Spradling, 2007; Visvader, 2009; Mazzone et al., 2010). There is also good evidence that this pathway regulates lineages in the airway epithelium during embryonic development. For example, studies in the developing and regenerating mouse lung (which does not contain BCs) and in the trachea before BCs have differentiated, suggest that canonical Notch signaling regulates the differentiation of progenitors into secretory and ciliated cells (Guseh et al., 2009; Tsao et al., 2009; Morimoto et al., 2010). Specifically, high levels of Notch promote the differentiation of secretory cells, whereas lower levels promote ciliated cell fate. However, the Notch pathway is notoriously complex and studies in distal airways lacking BCs might not be fully predictive of BC fate in the normal airway and during pathological remodeling. It has recently been shown that expression of components of the Notch pathway is downregulated in the airway epithelium of smokers with COPD (Tilley et al., 2009). Furthermore, treating human air-liquid interface airway epithelial cultures (probably initiated mainly with BCs) with exogenous Notch ligand or IL-13 results in a drastic increase in the number of mucus-producing goblet cells (Guseh et al., 2009). Precisely what these findings mean in terms of how the Notch signaling pathway influences BC proliferation and differentiation in vivo is still not known.
Once core regulatory networks, including Notch, are identified, it will be important to understand how they are coordinated and how microenvironmental signals are integrated to direct homeostasis, repair and pathological remodeling of airways. It is well known that the production of cytokines, such as IL-13, IL-6, IL-1β and tumor necrosis factor α (TNFα), is increased in asthma and other inflammatory conditions (Tang et al., 2006). The inflammatory milieu might affect airway homeostasis by modulating Notch signaling or other pathways in BCs, early progenitors and differentiated cell types (Fig. 4). In a mouse model, Notch-induced goblet cell metaplasia occurred in the absence of signal transducer of activated T cells 6 (STAT6), which mediates IL-13-induced signal transduction (Guseh et al., 2009). However, it is possible that levels of Notch signaling are modulated by pro-inflammatory cytokines, a concept that has been demonstrated in the Drosophila midgut intestinal epithelium (Conder and Knoblich, 2009). Similarly, the transcription factor SPDEF is both necessary and sufficient for specifying goblet cells from Clara cell progenitors in response to allergens or cytokines (Chen et al., 2009). However, it is not currently known whether Notch signaling is upstream of SPDEF and whether SPDEF can drive BCs directly to the secretory lineage. Other paracrine signals from neighboring epithelial cells and mesenchyme – including Wnt, EGF, TGFβ and BMP –undoubtedly influence the behavior of airway BCs and their progeny. Obtaining information about these signals and how they are disrupted in airway disease is essential to our understanding of airway remodeling.
Mouse models for studying BCs and their role in repair and disease
We have argued here that morphological, molecular and functional similarities make BCs of the mouse trachea a strong model for testing hypotheses about pathological airway remodeling in human small airways. Importantly, although there are differences between the two tissues (e.g. relative abundance of SCGB1A1+ Clara and goblet cells), there is currently no alternative genetically tractable in vivo model that can be used to define lineage relationships between airway BCs and their progeny, or the molecular and cellular mechanisms that lead to squamous and mucous metaplasia. As described above, we have used a KRT5-CreER transgene to follow the fate of BCs over a period of at least 14 weeks in vivo during steady state and after injury (Rock et al., 2009), whereas others have used a KRT14-CreER system to assess BC fate after injury (Hong et al., 2004b; Hong et al., 2004a). As markers for more subpopulations of BCs and their early progeny are identified, it will be important to generate new genetic tools to characterize their capacity for self-renewal and differentiation in vivo. In addition to enabling lineage-tracing studies, mouse models allow investigation of the role of specific factors in disease progression in vivo through genetic gain- and loss-of-function approaches: candidate pathways and molecules, identified by transcriptional profiling of normal and pathological human samples, could be activated or inactivated in BCs of the mouse trachea and their progeny.
The mouse is well established as an important tool for studying pathological airway remodeling in allergic asthma. Current models include a regime of sensitization and challenge with ovalbumin, and transgenic mice that either constitutively express the pro-inflammatory cytokine IL-13 in SCGB1A1+ Clara cells or that respond to IL-13 only in Clara cells. These models are characterized by mucous metaplasia, smooth muscle hyperplasia, fibrosis and inflammation. It is believed that Clara cells change their phenotype to either express mucins or chitinases, depending on their position in the airways (Homer et al., 2006), and there seems to be direct, reversible conversion of Clara cells into goblet cells, a change that is correlated with expression of SPDEF and downregulation of another transcription factor, FOXA2 (Chen et al., 2009). These studies focused on the intrapulmonary airways of mice, in which there are no BCs and where SCGB1A1+ Clara cells self-renew over the long term (Rawlins et al., 2009). However, because mucous metaplasia and the expression of SPDEF are observed in human small airways, in which BCs are abundant (Fig. 5) (Chen et al., 2009), it will be important to model this condition in proximal mouse airways in which BCs are found. It is likely that cytokines such as IL-13, IL-6, IL-1β and TNFα directly or indirectly influence the fate of BCs and their immediate progeny (Fig. 4). In support of this idea, treating human air-liquid interface airway epithelial cultures (probably initiated mainly with BCs) with exogenous Notch ligand or IL-13 resulted in a drastic increase in the number of mucus-producing goblet cells (Guseh et al., 2009).
There is currently no in vivo genetic mouse model for squamous metaplasia of the airways. Some chemical treatments have been reported to generate squamous carcinoma, but the underlying molecular mechanisms are not well understood (Yoshimoto et al., 1980; Henry et al., 1981). Airway epithelial cells cultured at an air-liquid interface in the absence of retinoids generate squamous multilayers rather than a mucociliary epithelium; enhanced EGF signaling has been implicated in this context (Jetten, 1991). Although retinoid deficiency is a well-known cause of squamous metaplasia in cultured cells, the mechanisms by which different levels of retinoids and/or EGF-mediated signaling alter BC behavior and the fate of their immediate progeny remains poorly understood. Loss-of-function studies in the mouse have demonstrated that signaling of the cytokine IL-6 through gp130 and STAT3 is required for repair of the bronchiolar epithelium following Clara cell depletion (Kida et al., 2008). In the absence of gp130 or STAT3, reparative cells failed to generate a normal pseudostratified epithelium and, instead, areas of flattened epithelial cells persisted. However, these studies did not characterize repair in the trachea, in which BCs comprise ∼30% of the epithelium. In the future, as candidate pathways contributing to squamous metaplasia are identified through transcriptional, genetic and epigenetic profiling, they can be tested in vivo in the context of the mouse trachea.
Although cellular turnover in the airways is relatively slow, the epithelium of the mouse trachea demonstrates a remarkable capacity for regeneration after injury. Given that repetitive rounds of epithelial injury and regeneration can occur following exposure to environmental agents and smoking, this reparative ability provides a convenient means for studying airway stem cells in the context of wound repair (Crosby and Waters, 2010). One model that can be used is inhalation of SO2, which causes lumenal cell death and sloughing, followed by proliferation of surviving cells and repair. The reproducible phases of this injury model – lumenal cell loss, spreading and motility migration of surviving epithelial cells (which could be considered a form of epithelial-mesenchymal transition), inflammation, proliferation, hyperplasia, and differentiation – provide an excellent setting to identify regulatory mechanisms influencing numerous BC behaviors (Borthwick et al., 2001; Rawlins and Hogan, 2008; Rock et al., 2009). The injury does not extend into the intrapulmonary airways. In the trachea, only BCs and a few Clara cells survive and rapidly spread to cover the exposed basal lamina. Proliferation peaks 24 hours post-injury and is associated with transient infiltration of immune cells. Within 72 hours, the trachea is lined with a stratified epithelium consisting of BCs and undifferentiated lumenal (KRT14−;KRT8+) daughter cells. Two weeks later, a pseudostratified architecture with normal proportions of ciliated cells, Clara cells and BCs is restored. Lineage tracing with a Krt5-CreER allele has shown that surviving BCs throughout the trachea divide and generate lineage-labeled ciliated and secretory cells. Moreover, the KRT5+ basal cells that regenerate the lumenal population are widely distributed throughout the trachea and are not confined to particular sites such as ducts of submucosal glands (Rock et al., 2009).
SO2 inhalation destroys lumenal cells non-selectively. However, there is one model in which only a particular lineage is selectively killed. This involves systemic administration of naphthalene, a lipophilic xenobiotic that is metabolized by intracellular cytochrome P450 monooxygenases into toxic derivatives (Stripp et al., 1995). Mature Clara cells are exceptionally rich in these enzymes and are therefore sensitive to naphthalene-induced death. This injury model was used to demonstrate that KRT14-expressing BCs of the mouse trachea and mainstem bronchi self-renew and generate Clara and ciliated cells following Clara cell ablation (Hong et al., 2004b; Hong et al., 2004a). Because this model creates a specific deficit of secretory cells, it will be useful for identifying the pathways that regulate the specification of this lineage. In the future, new models in which only ciliated cells, neuroendocrine cells or BCs in the trachea are killed will provide additional important information.
Conclusions
There is still much more to learn about the behavior of mouse and human airway BCs and their probable heterogeneity in proliferative and differentiation potential in vivo. Understanding the mechanisms that regulate these characteristics in a healthy pseudostratified epithelium, and how they are disrupted in the context of airway diseases, will require the collaboration of several different groups. These include clinicians with access to human samples, bioinformaticians, and scientists exploiting cell culture and ex vivo transplantation models as well as genetically tractable model systems. We have argued here that BCs of the mouse trachea that are molecularly and functionally similar to BCs found in human small airways provide a model system that has great potential for discovery research. It is through such collaborations that new hypotheses will be generated, tested and applied to the treatment of human disease.
Acknowledgments
The authors thank the Cystic Fibrosis Research and Treatment Center at the University of North Carolina at Chapel Hill and Mark Onaitis, MD for human tissue samples, and Christina Barkauskas, MD for critical comments on the manuscript. Some of the work on mouse BCs in the Hogan lab was supported by NIH grant HL071303. Deposited in PMC for release after 12 months.
Footnotes
COMPETING INTERESTS
The authors declare no competing interests.
REFERENCES
- Araya J, Cambier S, Markovics JA, Wolters P, Jablons D, Hill A, Finkbeiner W, Jones K, Broaddus VC, Sheppard D, et al. (2007). Squamous metaplasia amplifies pathologic epithelial-mesenchymal interactions in COPD patients. J Clin Invest. 117, 3551–3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avril-Delplanque A, Casal I, Castillon N, Hinnrasky J, Puchelle E, Peault B. (2005). Aquaporin-3 expression in human fetal airway epithelial progenitor cells. Stem Cells 23, 992–1001 [DOI] [PubMed] [Google Scholar]
- Banerjee AK. (2009). Preinvasive lesions of the bronchus. J Thorac Oncol. 4, 545–551 [DOI] [PubMed] [Google Scholar]
- Batlle E. (2008). A new identity for the elusive intestinal stem cell. Nat Genet. 40, 818–819 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Lowry WE, Pasolli HA, Fuchs E. (2006). Canonical notch signaling functions as a commitment switch in the epidermal lineage. Genes Dev. 20, 3022–3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boers JE, Ambergen AW, Thunnissen FB. (1998). Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am J Respir Crit Care Med. 157, 2000–2006 [DOI] [PubMed] [Google Scholar]
- Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. (2001). Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 24, 662–670 [DOI] [PubMed] [Google Scholar]
- Boucher RC. (2007). Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 261, 5–16 [DOI] [PubMed] [Google Scholar]
- Bouras T, Pal B, Vaillant F, Harburg G, Asselin-Labat ML, Oakes SR, Lindeman GJ, Visvader JE. (2008). Notch signaling regulates mammary stem cell function and luminal cell-fate commitment. Cell Stem Cell 3, 429–441 [DOI] [PubMed] [Google Scholar]
- Breuer R, Zajicek G, Christensen TG, Lucey EC, Snider GL. (1990). Cell kinetics of normal adult hamster bronchial epithelium in the steady state. Am J Respir Cell Mol Biol. 2, 51–58 [DOI] [PubMed] [Google Scholar]
- Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. (2009). SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest 119, 2914–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie JD, Edwards LB, Aurora P, Dobbels F, Kirk R, Rahmel AO, Taylor DO, Kucheryavaya AY, Hertz MI. (2008). Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult lung and heart/lung transplantation report-2008. J Heart Lung Transplant. 27, 957–969 [DOI] [PubMed] [Google Scholar]
- Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, Jones PH. (2007). A single type of progenitor cell maintains normal epidermis. Nature 446, 185–189 [DOI] [PubMed] [Google Scholar]
- Conder R, Knoblich JA. (2009). Fly stem cell research gets infectious. Cell 137, 1185–1187 [DOI] [PubMed] [Google Scholar]
- Crosby LM, Waters CM. (2010). Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 298, L715–L731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, Oren M, Jetten AM. (2004). Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 287, C171–C181 [DOI] [PubMed] [Google Scholar]
- Dvorak A, Tilley AE, Shaykhiev R, Wang R, Crystal RG. (2010). Do airway epithelium air-liquid cultures represent the in vivo airway epithelium transcriptome? Am J Respir Cell Mol Biol. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt JF, Schlossberg H, Yankaskas JR, Dudus L. (1995). Progenitor cells of the adult human airway involved in submucosal gland development. Development 121, 2031–2046 [DOI] [PubMed] [Google Scholar]
- Evans MJ, Shami SG, Cabral-Anderson LJ, Dekker NP. (1986). Role of nonciliated cells in renewal of the bronchial epithelium of rats exposed to NO2. Am J Pathol. 123, 126–133 [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. (2001). Cellular and molecular characteristics of basal cells in airway epithelium. Exp Lung Res. 27, 401–415 [DOI] [PubMed] [Google Scholar]
- Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. (2009). Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development 136, 1751–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TL, Shaheen F, Johnson A, Wadsworth S, Pechkovsky DV, Jacoby DB, Kicic A, Stick SM, Knight DA. (2008). Characterization of side population cells from human airway epithelium. Stem Cells 26, 2576–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajj R, Lesimple P, Nawrocki-Raby B, Birembaut P, Puchelle E, Coraux C. (2007a). Human airway surface epithelial regeneration is delayed and abnormal in cystic fibrosis. J Pathol. 211, 340–350 [DOI] [PubMed] [Google Scholar]
- Hajj R, Baranek T, Le Naour R, Lesimple P, Puchelle E, Coraux C. (2007b). Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells 25, 139–148 [DOI] [PubMed] [Google Scholar]
- Henry CJ, Billups LH, Avery MD, Rude TH, Dansie DR, Lopez A, Sass B, Whitmire CE, Kouri RE. (1981). Lung cancer model system using 3-methylcholanthrene in inbred strains of mice. Cancer Res. 41, 5027–5032 [PubMed] [Google Scholar]
- Homer RJ, Zhu Z, Cohn L, Lee CG, White WI, Chen S, Elias JA. (2006). Differential expression of chitinases identify subsets of murine airway epithelial cells in allergic inflammation. Am J Physiol Lung Cell Mol Physiol. 291, L502–L511 [DOI] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. (2004a). Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol. 164, 577–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. (2004b). In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 286, L643–L649 [DOI] [PubMed] [Google Scholar]
- Jetten AM. (1991). Growth and differentiation factors in tracheobronchial epithelium. Am J Physiol. 260, L361–L373 [DOI] [PubMed] [Google Scholar]
- Kicic A, Sutanto EN, Stevens PT, Knight DA, Stick SM. (2006). Intrinsic biochemical and functional differences in bronchial epithelial cells of children with asthma. Am J Respir Crit Care Med. 174, 1110–1118 [DOI] [PubMed] [Google Scholar]
- Kida H, Mucenski ML, Thitoff AR, Le Cras TD, Park KS, Ikegami M, Muller W, Whitsett JA. (2008). GP130-STAT3 regulates epithelial cell migration and is required for repair of the bronchiolar epithelium. Am J Pathol. 172, 1542–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebow AA. (1965). Patterns of origin and distribution of the major bronchial arteries in man. Am J Anat. 117, 19–32 [DOI] [PubMed] [Google Scholar]
- Liu JY, Nettesheim P, Randell SH. (1994). Growth and differentiation of tracheal epithelial progenitor cells. Am J Physiol. 266, L296–L307 [DOI] [PubMed] [Google Scholar]
- Lonergan KM, Chari R, Coe BP, Wilson IM, Tsao MS, Ng RT, Macaulay C, Lam S, Lam WL. (2010). Transcriptome profiles of carcinoma-in-situ and invasive non-small cell lung cancer as revealed by SAGE. PLoS ONE 5, e9162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, et al. (2008). Clinical transplantation of a tissue-engineered airway. Lancet 372, 2023–2030 [DOI] [PubMed] [Google Scholar]
- Mannino DM, Aguayo SM, Petty TL, Redd SC. (2003). Low lung function and incident lung cancer in the United States: data from the first National Health and Nutrition Examination Survey follow-up. Arch Intern Med. 163, 1475–1480 [DOI] [PubMed] [Google Scholar]
- Mazzone M, Selfors LM, Albeck J, Overholtzer M, Sale S, Carroll DL, Pandya D, Lu Y, Mills GB, Aster JC, et al. (2010). Dose-dependent induction of distinct phenotypic responses to Notch pathway activation in mammary epithelial cells. Proc Natl Acad Sci USA 107, 5012–5017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, Russell ML, Roggli VL, Crapo JD. (1994). Cell number and distribution in human and rat airways. Am J Respir Cell Mol Biol. 10, 613–624 [DOI] [PubMed] [Google Scholar]
- Metzger RJ, Klein OD, Martin GR, Krasnow MA. (2008). The branching programme of mouse lung development. Nature 453, 745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzner W, Lee W, Georgakopoulos D, Wagner E. (2000). Angiogenesis in the mouse lung. Am J Pathol. 157, 93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto M, Liu Z, Cheng HT, Winters N, Bader D, Kopan R. (2010). Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J Cell Sci. 123, 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. (2010). Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science 328, 62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Kawanami O, Jin E, Ghazizadeh M, Honda M, Asano G, Horiba K, Ferrans VJ. (1998). Immunohistochemical and ultrastructural studies of basal cells, Clara cells and bronchiolar cuboidal cells in normal human airways. Pathol Int. 48, 944–953 [DOI] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. (2007). Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science 315, 988–992 [DOI] [PubMed] [Google Scholar]
- Randell SH. (2006). Airway epithelial stem cells and the pathophysiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 3, 718–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randell SH, Comment CE, Ramaekers FC, Nettesheim P. (1991). Properties of rat tracheal epithelial cells separated based on expression of cell surface alpha-galactosyl end groups. Am J Respir Cell Mol Biol. 4, 544–554 [DOI] [PubMed] [Google Scholar]
- Rawlins E, Hogan BLM. (2006). Epithelial stem cells of the lung: privileged few or opportunities for many? Development 133, 2455–2465 [DOI] [PubMed] [Google Scholar]
- Rawlins EL, Hogan BL. (2008). Ciliated epithelial cell lifespan in the mouse trachea and lung. Am J Physiol Lung Cell Mol Physiol. 295, L231–L234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. (2009). The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 4, 525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. (2009). Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 106, 12771–12775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, Dailey LA, Brighton LE, Devlin RB. (2007). Transcriptional profiling of mucociliary differentiation in human airway epithelial cells. Am J Respir Cell Mol Biol. 37, 169–185 [DOI] [PubMed] [Google Scholar]
- Schoch KG, Lori A, Burns KA, Eldred T, Olsen JC, Randell SH. (2004). A subset of mouse tracheal epithelial basal cells generates large colonies in vitro. Am J Physiol Lung Cell Mol Physiol. 286, L631–L642 [DOI] [PubMed] [Google Scholar]
- Sertl K, Takemura T, Tschachler E, Ferrans VJ, Kaliner MA, Shevach EM. (1986). Dendritic cells with antigen-presenting capability reside in airway epithelium, lung parenchyma, and visceral pleura. J Exp Med. 163, 436–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter DP, Southwick HW, Smejkal W. (1953). Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer 6, 963–968 [DOI] [PubMed] [Google Scholar]
- Snippert HJ, Haegebarth A, Kasper M, Jaks V, van Es JH, Barker N, van de Wetering M, van den Born M, Begthel H, Vries RG, et al. (2010). Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327, 1385–1389 [DOI] [PubMed] [Google Scholar]
- Spira A, Beane JE, Shah V, Steiling K, Liu G, Schembri F, Gilman S, Dumas YM, Calner P, Sebastiani P, et al. (2007). Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat Med. 13, 361–366 [DOI] [PubMed] [Google Scholar]
- Stripp BR, Maxson K, Mera R, Singh G. (1995). Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol. 269, L791–L799 [DOI] [PubMed] [Google Scholar]
- Tang ML, Wilson JW, Stewart AG, Royce SG. (2006). Airway remodelling in asthma: current understanding and implications for future therapies. Pharmacol Ther. 112, 474–488 [DOI] [PubMed] [Google Scholar]
- ten Have-Opbroek AA, Otto-Verberne CJ, Dubbeldam JA, Dykman JH. (1991). The proximal border of the human respiratory unit, as shown by scanning and transmission electron microscopy and light microscopical cytochemistry. Anat Rec. 229, 339–354 [DOI] [PubMed] [Google Scholar]
- Tilley AE, Harvey BG, Heguy A, Hackett NR, Wang R, O’Connor TP, Crystal RG. (2009). Down-regulation of the notch pathway in human airway epithelium in association with smoking and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 179, 457–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toskala E, Smiley-Jewell SM, Wong VJ, King D, Plopper CG. (2005). Temporal and spatial distribution of ciliogenesis in the tracheobronchial airways of mice. Am J Physiol Lung Cell Mol Physiol. 289, L454–L459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. (2009). Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 136, 2297–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE. (2009). Keeping abreast of the mammary epithelial hierarchy and breast tumorigenesis. Genes Dev. 23, 2563–2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voynow JA, Fischer BM, Roberts BC, Proia AD. (2005). Basal-like cells constitute the proliferating cell population in cystic fibrosis airways. Am J Respir Crit Care Med. 172, 1013–1018 [DOI] [PubMed] [Google Scholar]
- Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM. (2009). A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 461, 495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto T, Inoue T, Iizuka H, Nishikawa H, Sakatani M, Ogura T, Hirao F, Yamamura Y. (1980). Differential induction of squamous cell carcinomas and adenocarcinomas in mouse lung by intratracheal instillation of benzo(a)pyrene and charcoal powder. Cancer Res. 40, 4301–4307 [PubMed] [Google Scholar]
- Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. (2009). Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell 5, 267–278 [DOI] [PMC free article] [PubMed] [Google Scholar]