Elucidating the Chromosome 9 Association with AS; CARD9 is a Candidate Gene (original) (raw)
. Author manuscript; available in PMC: 2011 Mar 1.
Published in final edited form as: Genes Immun. 2010 May 13;11(6):490–496. doi: 10.1038/gene.2010.17
Abstract
Ankylosing spondylitis (AS) is polygenic with contributions from the immunologically relevant genes HLA-B*27, ERAP1 and IL23R. A recent genome-wide association study (GWAS) identified associations (_p_~0.005) with the non-synonymous single nucleotide polymorphisms (nsSNPs), rs4077515 and rs3812571, in CARD9 and SNAPC4 on chromosome 9q that had previously been linked to AS. We replicated these associations in a study of 730 AS patients compared to 2879 historic disease controls, (rs4077515 p = 0.0004 odds ratio (OR) (95% confidence interval) = 1.2 (1.1-1.4); rs3812571 p = 0.0003 OR = 1.2 (1.1-1.4)). Meta-analysis revealed strong associations of both SNPs with AS, rs4077515 p = 0.000005 OR = 1.2 (1.1-1.3) and rs3812571 p = 0.000006 OR = 1.2 (1.1-1.3). We then typed 1604 AS cases and 1020 controls for 13 tagging SNPs; 6 showed at least nominal association, 5 of which were in CARD9. We imputed genotypes for 13 additional SNPs but none was more strongly associated with AS than the tagging SNPs. Finally, interrogation of an mRNA expression database revealed that the SNPs most strongly associated AS (or in strong linkage disequilibrium) were those most associated with CARD9 expression. CARD9 is a plausible candidate for AS given its central role in the innate immune response.
Keywords: spondyloarthropathy, innate immunity, genetic association
Introduction
AS is a chronic inflammatory arthritis that predominantly affects the axial skeleton. The immune response gene HLA-B27 makes a major genetic contribution but there is clear evidence of additional genetic effects. Previous studies, including a meta-analysis of whole genome linkage scans, highlighted a region on chromosome 9q33.1-9q33.2 linked to AS [1, 2]. A previously published genome-wide association scan (GWAS) in AS by the Wellcome Trust Case Control Consortium (WTCCC) identified two non-synonymous SNPs, rs4077515 and rs3812571, separated by ~8.8kb on 9q34.3 that showed association with AS (p < 0.004 and p < 0.005, respectively) [3]. One of these SNPs is in a functional candidate gene CARD9 (caspase recruitment domain-containing protein 9) and the second is in the adjacent gene SNAPC4 (small nuclear RNA-activating complex polypeptide 4).
CARD9 has a central role in the regulation of the innate immune system. It functions as a cytosolic signal transduction protein with several distinct roles. It acts downstream of the antifungal pattern recognition receptor (PRR), dectin 1 and splenic tyrosine kinase (SYK) [4, 5]. Activation of SYK leads to signaling through CARD9 [4, 5] which acts with BCL10 (B-cell lymphoma 10) and MALT1 to induce pro-inflammatory signals via the canonical NF-κB pathway and the stimulation of the p38 MAP kinase and JNK pathways [5, 6]. CARD9 also has a role in the detection of intracellular pathogens via cytoplasmic PRRs of the NOD family. For example, CARD9 has a critical function in NOD2-mediated activation of p38 and JNK in innate immune responses to intracellular pathogens [7]. Stimulation of CARD9 induces the maturation of dendritic cells into antigen-presenting cells that can prime naïve T cells to become IFN-γ-producing Th1 cells and/or IL-17 producing Th17 cells [8]. The latter have already been implicated in AS because of the association of IL23R with AS [3] and its role in Th17 cell propagation [9]. Thus CARD9 has a role in coupling the innate and adaptive immune responses.
SNAPC4 is a subunit of the SNAP complex, a multi-subunit complex of proteins that promotes basal levels of transcription of RNA polymerase II and III snRNAs [10]. A role for this gene in susceptibility to AS seems relatively unlikely and it is more likely that the SNAPC4 SNP is in linkage disequilibrium (LD) with a _CARD9_-associated SNP.
About 15% of AS cases have inflammatory bowel disease (IBD) and 10% have psoriasis, both of which are, like AS, associated with the IL23R gene [11, 12]. It is therefore of considerable interest that a recent comprehensive study of immune genes in IBD has identified an association between CARD9 (rs1080077) and both ulcerative colitis (UC) and Crohn's disease (CD) [13].
Robust validation of the results from GWAS is essential to exclude false positives. There are 4 parts to the current study. (1) To replicate the WTCCC study we have genotyped a new patient sample for rs4077515 and rs3812571. We have compared the allele frequencies to historic disease controls previously typed by the WTCCC [3] and performed a meta-analysis of these and published data. (2) We then genotyped additional SNPs in the region and tested them for association with AS to refine the association. (3) Using our genotyping data and that from HapMap [14] we have imputed genotypes for 13 additional SNPs in CARD9 and carried out a logistic regression analysis of imputed and formally genotyped SNPs to identify the primarily associated SNP(s). We also carried out a conditional logistic regression analysis of imputed and formally genotyped SNPs and of formally genotyped SNPs alone, conditioned on the most strongly associated SNP. (4) We interrogated an RNA expression database (mRNA by SNP browser 1.0.1) [15] for CARD9 SNPs that have a _cis_-acting role in gene expression.
Results
1. Replication of WTCCC nsSNP results and meta-analysis of association with rs4077515 and rs3812571
The SNPs rs4077515 in CARD9 and rs3812571 in the neighbouring gene SNAPC4 were genotyped in 730 new AS cases; allele frequencies were compared to those in 3 other diseases previously genotyped in the WTCCC nsSNP GWAS as controls (3Dis), see Table 1. The association between both SNPs and AS was replicated, (rs4077515 p = 0.0004 OR = 1.2 (1.1-1.4) and for rs3812571 p = 0.0003 OR = 1.2 (1.1-1.4)). From HapMap data, r2 between these SNPs is 0.87 which suggests that these SNPs are not independent of each other, see supplementary Table 1.
Table 1.
Analysis of SNPs in the CARD9/SNAPC4 region. Significant associations are highlighted in bold. Positions are taken from Ensembl Genome Browser (www.ensembl.org) (August 2009). NS non-synonymous with amino acid change in brackets, S synonymous, US upstream, DS downstream, UTR untranslated region.
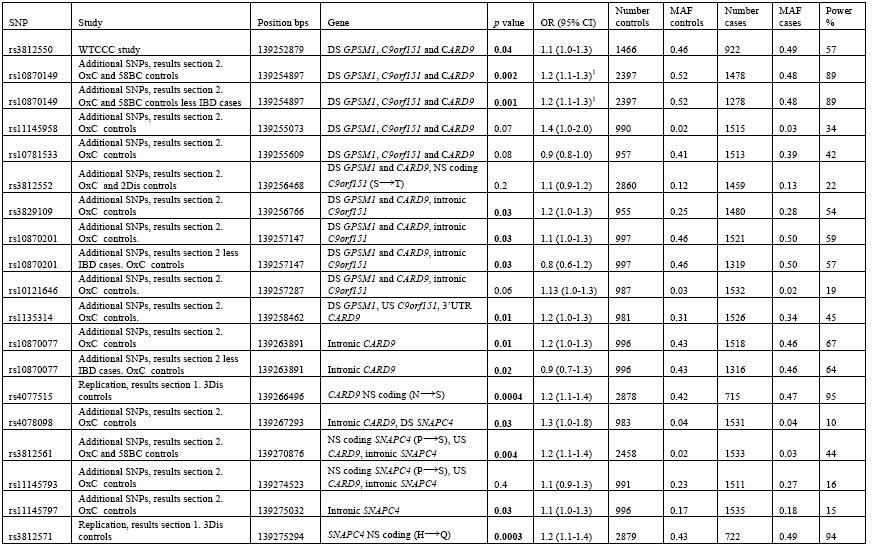
The strength of the association was increased in a meta-analysis of the combined data from this study and WTCCC GWAS (rs4077515 p = 0.000005 OR = 1.2 (1.1-1.3) and for rs3812571 p = 0.000006 OR = 1.2 (1.1-1.3)), supplementary Table 2. Forest plots are shown in Figure 1. The Cochran Q statistic was not significant; I2, a measure of inconsistency, cannot be calculated for analyses combining only 2 studies. Power calculations were made using the numbers of cases and controls, allele frequencies and odds ratios shown in Table 1 and supplementary Table 2. The replication study for rs4077515 and rs3812571 had greater than 80% power to detect an OR of 1.25 (at α = 0.05 with linkage disequilibrium between marker and disease-associated variant of D′ = 1), increasing to ~100% in the meta-analyses, supplementary Table 2.
Figure 1.
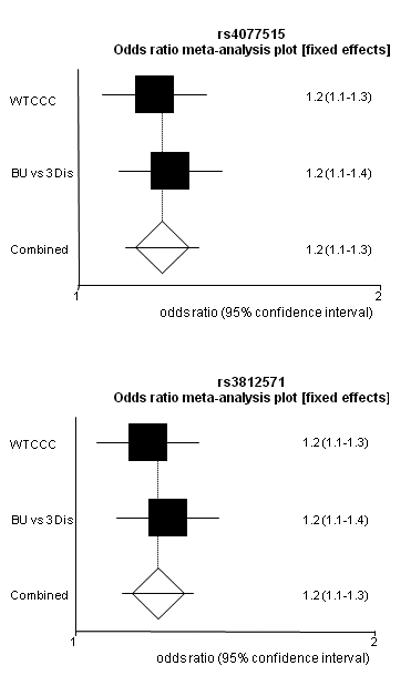
Forest plots of the meta-analysis of CARD9 and SNAPC4 SNPs.
WTCCC is data from the WTCCC GWAS and BU vs 3 Dis is the replication study using data from the 3 other diseases that were genotyped WTCCC as controls.
2. Analysis of additional SNPs at 9q33.2
We genotyped 13 additional tagging SNPs that were identified from HapMap data in 1604 AS cases and in our independent sample of 1020 controls (OxC). There was a large overlap between cases in this part of the study, the WTCCC study and the replication study (77% of the 1604 AS cases were also in the WTCCC GWAS and replication study and 76% of the WTCCC GWAS and replication samples were in the 1604 cases genotyped for these additional SNPs). For rs10870149, we identified additional control data from the 1958 Britsh Birth Cohort (58BC) which was combined with our control data for the association testing. The results are shown in Table 1. SNPs across CARD9 (rs10870149, rs10870201, rs10870077 and rs3829109) show nominal association with AS (p < 0.002 - 0.03), with rs10870149 being the most strongly associated and having > 80% power to detect an odds ratio of ~1.2. There is strong LD between rs10870149, rs10870201 and rs10870077 (r2 = 0.7 - 0.8 for HapMap data and r2 = 0.59 - 0.79 calculated for this data) suggesting that these SNPs are not independently associated. Because rs1080077 is associated with IBD, we repeated the analysis for this SNP and those in LD with it (rs10870149, rs10870201) after removing those AS cases known to also have IBD. This had little effect on the strength of association (see Table 1), suggesting that the AS association is independent of the IBD association.
Two SNPs in SNAPC4 (rs3812561, rs11145797) also showed nominal association with AS (p = 0.004 and p = 0.03, respectively). The absence of LD between these SNPs (r2 = 0.14 for HapMap data and 0.13 for this data) suggests that these associations are independent of each other. Values for r2 and D′, (calculated from HapMap data and form the genotyping data presented here), between all the associated SNPs are shown in supplementary Table 1. Data from 2 of the 3 diseases other than AS genotyped by WTCCC GWAS were available for rs3812552 (2Dis controls) and were combined with our control data for analysis. This SNP was also genotyped by WTCCC but a meta-analysis was not performed as 77% of case samples were present in both studies However, neither study showed association with AS. One other SNP, rs3812550 in GPSM1 adjacent to CARD9, had been typed by WTCCC and showed marginal association with AS, p < 0.04 OR = 1.1 (1.0-1.3). There are also SNPs throughout the region (rs11145958, rs10781533, rs3812552, rs10121646 and rs1135314 in CARD9 and rs11145797 in SNAPC4) that show no association with AS in this study, (see Table1). The relative positions of the SNPs and genes in this region of chromosome 9 are indicated in Figure 2.
Figure 2.
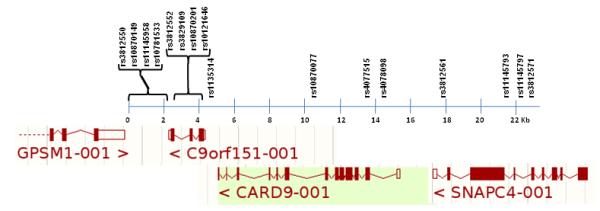
Relative positions of the SNPs used in this study and the genes in this region of chromosome 9. Positions are from Ensembl Genome Browser (www.ensembl.org) (August 2009).
Analysis of imputed SNPs
We used imputation to generate genotype data for 17 additional SNPs in the CARD9 region. Four of these imputed SNPs were excluded from further analysis because they had MAF < 5%. Cochrane-Armitage p value, OR and 95% CI for these SNPs are shown in supplementary Table 3. Imputed SNP genotypes were combined with the formally genotyped SNPs and subjected to logistic regression analysis, imputed data for rs4077515 and rs3812571 was used as the replication data set used different controls. The p values from this analysis are shown in supplementary Table 3. The imputed SNP rs10781496 had the most significant effect (p = 0.007). The next 6 most significant SNPs (rs10781499, rs4077515, rs3812558, rs10781500, rs11794847 and rs10781507) are all in strong LD, and have p values between 0.008 - 0.01. Conditional logistic regression analysis of both genotyped and imputed SNPs, conditioned on the most strongly associated genotyped SNP (rs10870149), showed no overall trend between SNPs. Similarly no overall trend was seen when the analysis was repeated with genotyped SNPs alone.
Comparison with _CARD9 cis_-SNP expression
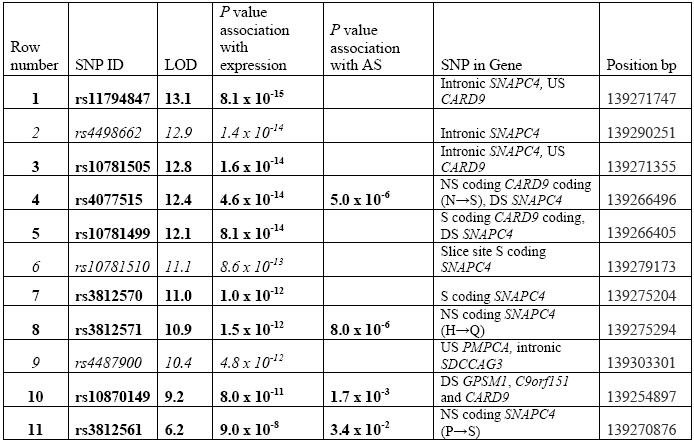
The case control association data were compared with CARD9 expression data from a previous study [15]. Only 3 well-powered SNPs in this case control study had also been analysed in the expression study. Two of these, rs4077515 and rs3812571, tag each other (r2 = 0.87) and are in the top 10 SNPs associated with expression levels (p = 4.6 × 10−14 and p = 1.5×10−12, respectively) detected by the probe 220162_s_at which covers the transcribed region of the CARD9 gene. This has a heritability of 0.53, mean expression level 5.7 and variance of 0.18. These two SNPs tag a further 5 of the top 10 expression-associated SNPs (rs11794847, rs10781505, rs10781499, rs3812570 and rs3812571) see Table 2. A third SNP, rs10870149, which is associated with AS independent of any IBD association, is also in the top 10 SNPs most strongly associated with expression detected by the same probe (see Table 2). A second probe 228272_at, containing sequence in C9orf151 and upstream of CARD9, highlights rs3812561 (in SNAPC4) as associated with expression p = 9.0 × 10−8, heritability 0.7, mean expression level 6.0 and variance 0.26. These results clearly demonstrate that the expression of CARD9 is under significant _cis_-regulation. In contrast no significant _cis_-regulation is evident on interrogating the RNA expression database for SNPs associated with SNAPC4 expression.
Table 2.
Ten CARD9 SNPs most associated with expression as detected by probe 220162_s_at (rows 1-10) and the SNP most associated with expression detected by probe 228272_at (row 11). SNPs in bold are those genotyped in AS case control study or in LD with a typed SNP. SNPs in italics are highly associated with expression but not typed in this study. SNPs rs3812571 and rs4077515 tag the following SNPs: rs11794847, rs10781505, rs10781499, rs3812570 and rs3812571. Positions are from Ensembl Genome Browser (www.ensembl.org) (August 2009). Expression data is from the mRNA by SNP Browser 1.0.1. NS non-synonymous with amino acid change in brackets, S synonymous, US upstream, DS downstream, UTR untranslated region.
Discussion
The association between AS and the immune response gene HLA-B27 is the strongest association seen in any complex disease but it only accounts approximately one-third of the total genetic risk [1]. HLA-B27 is almost a prerequisite for the development of AS but appears to require other genetic influences to be fully penetrant. There has been significant progress recently in identifying AS-associated genes by GWAS [3, 16]. For example, in the WTCCC nsSNP study, two SNPs, one each in SNAPC4 and CARD9 on chromosome 9, showed nominal association with AS. It is interesting to note that this region of chromosome 9 (9q32-q34.23) is paralogous with chromosome 6p21.3, the MHC region [17]. We have generated strong evidence that CARD9, a strong functional candidate, is associated with AS. The strong association observed with a SNP in SNAPC4 is more likely the result of strong LD; a role for this gene in an autoimmune/inflammatory disease is certainly more difficult to envisage. We have replicated the initial genetic associations and employed a fine mapping strategy to refine these. Because of strong LD across the region identification of the primary causative variant is difficult. Deep sequencing of the region to identify rare variants may be useful in pinpointing the causative variant(s).
Expression data identified a major locus on chromosome 9 influencing CARD9 expression. This locus was detected with the probe 220162_s_at with maximal significance at marker rs11794847. The heritability of CARD9 expression traits varied between probes. Maximum heritability was observed for probe 228272_at that also showed significant association with expression peaking at rs3812561, a SNP marginally associated with AS. These data suggest that a number of SNPs may have significant _cis_-acting effect(s) on the expression of CARD9.
Recently in a comprehensive screen of 354 SNPs in 85 innate immune related genes, an association has been demonstrated between rs10870077 in CARD9 and IBD [13]. This SNP is associated with AS in our study independently of any IBD association. To assess if this primarily reflects an increased proportion of subjects with IBD in our cases, we excluded all individuals known to have either CD or UC and re-analyzed the data. A small change in the _p_-values was noted, but the association remained, indicating that the AS association is robust, not merely due to co-existent clinical IBD in our AS cases. Similarly it has been demonstrated that CD, psoriasis and AS are all independently associated with the IL23R gene [3, 11, 18, 19]. We propose that CARD9 is the second gene to share a genetic association with both AS and IBD. A recent GWAS of patients with psoriasis identified a chromosome 9 association but with a SNP (rs1076160) about 2Mb centomeric of CARD9 [20]. A six-marker haplotype near to TNFSF15 has been proposed as the chromosome 9 candidate in a study of spondylarthritis in families and sporadic cases. In this study only 58% of family cases and 48% of sporadic cases had classic AS, the remainder had undifferentiated spondyloarthritis or psoriatic arthritis [21]. As more is learnt about the genetics of human diseases, more examples of such genetic pleiotropy are being identified, in which genetic variants influence susceptibility to several diseases. These extend to several genes involved in many autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, type 1 diabetes, autoimmune thyroid disease and celiac disease. It is unsurprising that similar genetic overlap can be observed in conditions, such as AS, IBD and psoriasis, where familial recurrence is well recognised.
The shared association between AS and IBD with CARD9 adds strength to our proposal that CARD9 is the prime positional and functional candidate gene for association with AS in this region of chromosome 9. It has a central role in the innate immune system and has a critical role in responses to both intracellular and extracellular bacterial/fungal components through their respective pattern recognition receptors. Of particular interest, CARD9 is a component of the NOD2 signaling pathway. NOD2 is a PRR for intracellular bacteria and variants in the NOD2 gene are highly associated with Crohn's disease [22, 23]. CARD9 is a highly plausible candidate for susceptibility to AS, particularly since there is some evidence to support the idea that this disease may be triggered by contact with common bacteria. This is supported by the near-global occurrence of AS (reflecting the HLA-B27 distribution) and the observations of an animal model of AS that requires bacterial colonization for arthritis to develop. Thus, transgenic rats over-expressing human HLA-B27 and β2-microglobulin develop inflammatory intestinal, joint, skin and genital lesions. However, if the rats are raised in a germ-free environment the intestinal and joint disease does not develop, indicating that the gut and joint inflammation are related [24]. Current genetic evidence supports this concept with the sharing of variants in IL23R and now in CARD9 having a role in susceptibility to both IBD and AS. CARD9 is a highly conserved gene in mammals which indicates that it fulfills an essential role in the innate immune response. Interference with this role seems to increase susceptibility to at least two related inflammatory conditions. It is very likely that there are other genetic influences common to both these disorders, and also psoriasis, that should be explored in future genetic studies.
Materials and Methods
Ethics statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the National Research Ethics Service, Cambridgeshire 4 Research Ethics Committee, UK (MREC project number 98/5/23). All patients provided written informed consent for the collection of samples and subsequent analysis.
Patients and controls
The subjects with AS were either members of the National Ankylosing Spondylitis Society (UK), attendees at the Nuffield Orthopaedic Centre (Oxford, UK) or referrals from rheumatologists elsewhere in the UK. All patients fulfilled the modified New York criteria for the diagnosis of AS and were of British white European origin (grandparents originated from UK or Ireland). DNA was prepared by standard methods from peripheral blood. Control data used for comparisons came from 3 sources: (1) genotype data from 2879 individuals with either multiple sclerosis, breast cancer or autoimmune thyroid disease from the WTCCC/TASC GWAS [3]; (2) the 1958 British Birth Cohort (58BC) (deposited by P. Deloukas, Wellcome Trust Sanger Institute, Hinxton, Cambridge CB10 1SA, UK) (http://www.b58cgene.sgul.ac.uk/, October 2008); and (3) from 1020 in-house British white European controls from the blood transfusion service or spouses of patients with osteoarthritis. This study makes use of data generated by the WTCCC under award 076113 [3]. The list of investigators who contributed to the data is available from www.wtccc.org.uk.
Genotyping
SNP genotyping performed by the WTCCC was done with the Infinium I assay (Illumina), which is based on allele-specific primer extension (rs3812550, rs3812552, rs4077515 and rs3812571) [3]. Additional genotyping was performed either by iPLEX technology (MassArray, Sequenom, San Diego, USA) (SNPs, rs10870149, rs11145958, rs10781533, rs3812552, rs10870201, rs10121646, rs1135314, rs10870077, rs4078098, rs3812561, rs11145793 and rs11145797), by restriction fragment length polymorphism analysis of PCR products (some samples for rs4077515) with a restriction site engineered using program dCAPS Finder 2.0 [25] or typed by KBiosciences (Hoddesdon, Herts, UK) using KASPar technology (rs3829109).
Statistical analysis
SNPs were tested for adherence to Hardy-Weinberg equilibrium. Allele frequencies of cases and controls were compared by contingency tables using the Cochrane-Armitage test of trend. Meta-analysis was performed using the Mantel-Haenszel test for fixed effects odds ratio using the StatsDirect statistical package. Power calculations were made with the program Quanto using a gene-only log additive model, assuming a disease prevalence of 0.004 and significance level of 0.05 [26], and linkage disequilibrium calculations (r2 and D′) were performed using the program Haploview v4.1 [27]. Additional SNPs were imputed using the Markov Chain Haplotyping software (MACH v1.0) (www.sph.umich.edu/csg/abecasis/mach/index.htm) and phased data from CEPH individuals (Utah residents with ancestry from northern and western Europe) from the HapMap project (www.hapmap.org) as the reference set of haplotypes [14]. We analyzed SNPs that could be imputed with relatively high confidence (R2 > 0.3) and with an imputed minor allele frequency greater than 5%. A logistic regression case-control analysis of genotyped and imputed SNPs was performed using the statistical package SPSS, with genotypes weighted by imputation probabilities to determine statistical significance of association. Conditional logistic regression analysis was performed using the program PLINK v1.07 (http://pngu.mgh.harvard.edu/purcell/plink/) [28].
Comparison with _CARD9 cis_-SNP expression data
A recent study of 400 children (with and without childhood asthma) generated strong evidence that CARD9 expression traits are heritable and under _cis_-acting regulatory control [15]. In that study, individuals were genotyped for > 400,000 SNPs and had transcript levels measured for > 20,000 genes. Associations between genotypes and expression levels were tested for and a comprehensive expression database was produced. We have used this to investigate the correlation of AS-associated SNPs with CARD9 expression. Expression data were available from 2 non-overlapping probes, Affymetrix U133 Plus 2.0 probes, 220162_s_at and 228272_at.
Supplementary Material
1
2
3
Acknowledgements
JP is funded by the NIHR Oxford Biomedical Research Centre ankylosing spondylitis chronic disease cohort (Theme Code:A91202). DH is funded by the National Ankylosing Spondylitis Society (UK). CF is funded by The Thames Valley Comprehensive Local Research Network (TVCLRN) which forms part of the NIHR Comprehensive Clinical Research Network (CCRN). TK is funded by the Henni Mester studentship. This study was funded, in part, by the Arthritis Research UK, award numbers 18797 and 19356, by the Wellcome Trust under award no. 076113.We are grateful to the many patients who contributed samples to these studies and to their physicians for allowing us to study their patients. We also thank the National Ankylosing Spondylitis Society (UK) for additional financial support and their unstinting help in patient recruitment. We thank the Osteoarthritis Group, Oxford for the use of their control samples. This study makes use of data generated by the Wellcome Trust Case Control Consortium. A full list of the investigators who contributed to the data is available from www.wtccc.org.uk. We acknowledge use of genotype data from the British 1958 Birth Cohort DNA collection, funded by the Medical Research Council grant G0000934 and the Wellcome Trust grant 068545/Z/02. Additional laboratory support was provided by the NIHR Oxford Musculoskeletal Biomedical Research Unit. Finally, we thank Lyn-Louise Johnson for iPLEX genotyping and the Bioinformatics and Statistical Genetics groups for support, both of the Wellcome Trust Centre for Human Genetics, Oxford.
Footnotes
Supplementary Information
Supplementary information is available at Genes and Immunity's website
Conflict of interest: None declared.
References
- 1.Laval SH, Timms A, Edwards S, Bradbury L, Brophy S, et al. Whole-genome screening in ankylosing spondylitis: evidence of non-MHC genetic-susceptibility loci. Am J Hum Genet. 2001;68:918–926. doi: 10.1086/319509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miceli-Richard C, Zouali H, Said-Nahal R, Lesage S, Merlin F, et al. Significant linkage to spondyloarthopathy on 9q31-34. Hum Mol Genet. 2004;13:1641–1648. doi: 10.1093/hmg/ddh179. [DOI] [PubMed] [Google Scholar]
- 3.Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat Genet. 2007;39:1329–1337. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertin J, Guo Y, Wang L, Srinivasula SM, Jacobson MD, et al. CARD9 is a novel caspase recruitment domain-containing protein that interacts with BCL10/CLAP and activates NF-kappa B. J Biol Chem. 2000;275:41082–41086. doi: 10.1074/jbc.C000726200. [DOI] [PubMed] [Google Scholar]
- 5.Hsu YM, Zhang Y, You Y, Wang D, Li H, Duramad O. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol. 2007;8:198–205. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
- 6.Gross O, Gewies A, Finger K, Schafer M, Sparwasser T. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature. 2006;442:651–656. doi: 10.1038/nature04926. [DOI] [PubMed] [Google Scholar]
- 7.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 8.LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 10.Wong MW, Henry RW, Ma B, Kobayashi R, Klages N. The large subunit of basal transcription factor SNAPc is a Myb domain protein that interacts with Oct-1. Molec Cell Biol. 1998;18:368–377. doi: 10.1128/mcb.18.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, et al. A Genome-Wide Association Study Identifies IL23R as an Inflammatory Bowel Disease Gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhernakova A, Festen EM, Franke L, Trynka G, van Diemen CC, et al. Genetic analysis of innate immunity in Crohn's disease and ulcerative colitis identifies two susceptibility loci harboring CARD9 and IL18RAP. Am J Hum Genet. 2008;82:1202–1210. doi: 10.1016/j.ajhg.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The International HapMap Consortium The International HapMap Project. Nature. 2003;426:789–796. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 15.Dixon AL, Liang L, Moffatt MF, Chen W, Heath S, et al. A genome-wide association study of global gene expression. Nat Genet. 2007;39:1202–1207. doi: 10.1038/ng2109. [DOI] [PubMed] [Google Scholar]
- 16.Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danchin E, Vitiello V, Vienne A, Richard O, Gouret P. The major histocompatibility complex origin. Immunol Rev. 2004;198:216–232. doi: 10.1111/j.0105-2896.2004.00132.x. [DOI] [PubMed] [Google Scholar]
- 18.Capon F, Di Meglio P, Szaub J, Prescott NJ, Dunster C, et al. Sequence variants in the genes for the interleukin-23 receptor (IL23R) and its ligand (IL12B) confer protection against psoriasis. Hum Genet. 2007;122:201–206. doi: 10.1007/s00439-007-0397-0. [DOI] [PubMed] [Google Scholar]
- 19.Karaderi T, Harvey D, Farrar C, Appleton LH, Stone MA, et al. Association between the interleukin 23 receptor and ankylosing spondylitis is confirmed by a new UK case–control study and meta-analysis of published series. Rheumatology (Oxford) 2009;48:386–389. doi: 10.1093/rheumatology/ken501. [DOI] [PubMed] [Google Scholar]
- 20.Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, et al. for the Collaborative Association Study of Psoriasis Genome-wide scan reveals association of psoriasis with IL-23 and NF-kB pathways. Nat Genet. 2009;41:199–204. doi: 10.1038/ng.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zinovieva E, Bourgain C, Kadi A, Letourneur F, Izac B, Said-Nahal R, et al. Comprehensive linkage and association analyses identify haplotype, near to the TNFSF15 gene, significantly associated with spondyloarthritis. PLoS Genetics. 2009;5:e1000528. doi: 10.1371/journal.pgen.1000528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 23.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 24.Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neff MM, Turk E, Kalishman M. Web-based Primer Design for Single Nucleotide Polymorphism Analysis. Trends in Genetics. 2002;18:613–615. doi: 10.1016/s0168-9525(02)02820-2. [DOI] [PubMed] [Google Scholar]
- 26.Gauderman WJ, Morrison JM. QUANTO 1.1: A computer program for power and sample size calculations for genetic-epidemiology studies. 2006. http://hydra.usc.edu/gxe.
- 27.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 28.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, et al. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3