Cloning, functional characterization, and co-expression studies of a novel aquaporin (FaPIP2;1) of strawberry fruit (original) (raw)
Abstract
In strawberry, the putative participation of aquaporins should be considered during fruit ripening. Furthermore, the availability of different firmness cultivars in this non-climacteric fruit is a very useful tool to determine their involvement in softening. In a previous work, the cloning of a strawberry fruit-specific aquaporin, FaPIP1;1, which showed an expression profile associated with fruit ripening was reported. Here, FaPIP2;1, an aquaporin subtype of PIP2 was cloned and its functional characterization in Xenopus oocytes determined. The Fa_PIP2;1 gene encodes a water channel with high water permeability (P_f) that is regulated by cytosolic pH. Interestingly, the co-expression of both FaPIP subtypes resulted in an enhancement of water permeability, showing P_f_ values that exceeds their individual contribution. The expression pattern of both aquaporin subtypes in two cultivars with contrasting fruit firmness showed that the firmer cultivar (Camarosa) has a higher accumulation of _Fa_PIP1 and _Fa_PIP2 mRNAs during fruit ripening when compared with the softer cultivar (Toyonoka). In conclusion, not only FaPIP aquaporins showed an expression pattern associated with fruit firmness but it was also shown that the enhancement of water transfer through the plasma membrane is coupled to the presence/absence of the co-expression of both subtypes.
Keywords: Aquaporin, fruit ripening, PIP, strawberry, water transport
Introduction
In recent years, more attention has been paid to the possible role played by aquaporins in fruit water status and its relevance for fruit physiology.
Water movements are crucial during ripening for at least two key events: (i) the rapid expansion of fruit achieved by the accumulation of large amounts of water during the developmental process (Coombe, 1976), and (ii) the loss of turgor associated with fruit ripening after solute accumulation in the apoplast (Wada et al., 2008, 2009). Knowledge about the participation of water movements in these events gives good reasons for the current investigation on the specific involvement of aquaporins in ripening (Chervin et al., 2008; Fouquet et al., 2008; Mut et al., 2008). The relevance of water channels in cell physiology arise from the fact that aquaporins can give to the cell rapid and reversible changes in its hydraulic conductance by modulating membrane water permeability. For instance, anoxia modifies cytosolic pH with the consequent closure of plasma membrane aquaporin activity, causing a fast reduction of the hydraulic conductance of Arabidopsis roots (Tournaire-Roux et al., 2003). The plasma membrane water permeability is likely to allow cells to equilibrate within seconds in response to changes in apoplastic water potential, in contrast to a fruit development process, that takes days to weeks. However, it has been proposed that, at the tissue and organ levels, many non-steady-state physiological processes involve water transport through membranes and that even when the kinetics of water equilibration of whole organs can be of the order of hours or days, these time constants would be even longer if aquaporins did not contribute to transmembrane water flow (Tyerman et al., 1999).
Many studies on fruit ripening have focused on the analysis of aquaporin gene expression and their participation at different stages of fruit development has been suggested (Hu et al., 2003; Chervin et al., 2008; Fouquet et al., 2008). However, the functional characterization of fruit aquaporins is still scarce in comparison with data available from gene expression analysis.
In a previous work, a full-length sequence of a PIP1 subtype aquaporin was cloned (FaPIP1;1, accession number GQ390798.1) whose expression increased during strawberry fruit ripening and was negatively regulated by auxins (Mut et al., 2008). However, overexpression of FaPIP1;1 in Xenopus oocytes failed to contribute to water transport through the plasma membrane unless it was co-expressed with AtPIP2;3 (Mut et al., 2008). This particular result could be explained by the mechanism of PIP trafficking (Chaumont et al., 2000; Zelazny et al., 2007, 2009). Two different aquaporin subtypes are involved in water transport through the plasma membrane: PIP1 and PIP2. These subtypes displayed different transport activity when expressed alone in Xenopus oocytes: while PIP2 increased oocyte membrane water permeability, PIP1 displayed no or low water permeability (Chaumont et al., 2000). This differential behaviour seems to be related to PIP trafficking, PIP2 is able to reach the plasma membrane whereas PIP1 seems to be retained in the endoplasmic reticulum (Zelazny et al., 2007, 2009). These functional features of PIP1 and PIP2 seem to be shared by all plant PIPs studied to date, with the exception of some Arabidopsis PIP1s that show moderate water transport (Tournaire-Roux et al., 2003) and a few PIP2s that seems to be unable to increase water permeability when expressed alone in the oocyte plasma membrane (Zhou et al., 2007; Azad et al., 2008).
The fact that FaPIP1;1 is able to reach the oocyte membrane only if a PIP2 aquaporin (in our case AtPIP2;3) is also present (Mut et al., 2008), suggested that a FaPIP2 should be expressed in the same tissue as FaPIP1;1. Unfortunately, the Fragaria×ananassa complete genome sequence is not available yet, so the full number and types of aquaporins present in strawberry are still unknown. EST libraries show that PIP2 aquaporins are expected to be expressed in strawberry fruit (http://www.bioinfo.wsu.edu/gdr/index.php).
In this work, the cloning of the first-full length sequence of a PIP2 from strawberry fruit is reported, and the expression pattern of FaPIP subtypes during ripening of two cultivars with contrasting softening rate has been analysed. The study also includes the functional characterization of _Fa_PIP2;1 and its interaction with _Fa_PIP1;1.
Materials and methods
Plant material
Strawberry (Fragaria_×_ananassa, Duch.) fruit were obtained from local producers (La Plata, Buenos Aires Province, Argentina). Fruit from Camarosa (high firmness) and Toyonoka (low firmness) cultivars were harvested at different ripening stages and classified according to the external coloration degree: large green (LG), white (W), 25% red (25% R), 50% red (50% R), and 100% red (100% R). Fruits were washed, drained, and after calyx and peduncle removal, they were cut apart, frozen in liquid nitrogen, and stored at –20 °C until use.
Cloning of FaPIP2;1
The first cloning step of FaPIP2;1 was made with degenerated primers designed by means of CODEHOP online program (Rose et al., 1998) using PIP2 subtype aquaporin sequences available in the GenBank. A cDNA library (Stratagene, La Jolla, CA, USA) constructed from 25–75% R strawberry fruit (cv. Chandler) was used as a template (Civello et al., 1999). Amplification products were cloned into pGEM-T Easy vector (Promega) according to the manufacturer's instructions and sequenced on both strands (Macrogen, Inc., Seoul, Korea). Specific primers designed within the 5′ and 3′ end of cDNAs corresponding to several PIP2 were used in combination with T3 and T7 primers, respectively. PCR products were cloned into pGEM-T Easy vector and sequenced to obtain the full PIP2 sequence. Finally, the specific primers (5′-GGGAGATCTATGGCGAAAGACGTTG-3′) and (5′-GGGACTAGTTTAAGCATTGCTCCTGAAAGACC-3′), both including _Bg_lII and _Spe_I restriction sites, were used for the last cloning step, and the ORF of _Fa_PIP2;1 was inserted into the _Bgl_II and _Spe_I sites of a pT7Ts derived vector carrying 5′ and 3′ untranslated sequences of a β-globin gene from Xenopus (Agre et al., 1999). The FaPIP2;1 sequence was deposited in GenBank under the accession number GQ390799.
Sequence analysis
Analysis of _Fa_PIP2;1 sequence and its comparison with known sequences was carried out using NCBI Blast server (Altschul et al., 1997). The ClustalW program (Thompson et al., 1997) was used for sequence alignment. ESPript was used to generate a PostScript output from aligned sequences (Gouet et al., 1999). Phylogenetic analyses and trees were done using MEGA version 3.0 (Tamura et al., 2007).
In vitro synthesis and translation
Capped complementary RNAs (cRNA) encoding for _Fa_PIP2;1 and _Fa_PIP1;1 were synthesized in vitro using the mMESSAGE mMACHINE T7 High Yield Capped RNA Transcription Kit (Ambion, Austin, Texas, USA) and using _Eco_RI linearized pT7Ts derived vector carrying the corresponding PIP as the template. _At_PIP2;3 (Daniels et al., 1994) was synthesized using mMESSAGE mMACHINE T3 Kit (Ambion Austin, Texas, USA). The synthesized products were suspended in RNAse-free water for performing the oocyte microinjection. The quantification of cRNA was done by means of ethidium bromide staining after 0.8% agarose gel electrophoresis. Comparison of band intensities was performed with a marker previously measured by spectrophotometry. The absence of unincorporated nucleotides in all cRNA was also checked by agarose gel electrophoresis and ethidium bromide staining.
Oocyte transport studies
_P_f assays:
Defollicled Xenopus oocytes were injected with 3–50 ng of cRNA dissolved in RNAase free water. Injected oocytes were incubated for 3 d at 18 °C in ND96 medium (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM HEPES pH 7.5; ∼200 mOsmol kg−1 H20) supplemented with 1 μg ml−1 gentamicin sulphate. Osmotic water permeability (P_f_) was determined by measuring the rate of oocyte swelling induced by a hypo-osmotic shock of 160 mOsm kg−1. Changes in cell volume were video-monitored by a VX-6000 colour video-camera (Microsoft, CA, USA) attached to a zoom stereo-microscope (Olympus SZ40, Olympus Co., Tokyo, Japan).
The cell swelling was video-captured in still images (each 20 s during 180 s) using the AMCaP version 9.20 (http://noeld.com/programs.asp?cat=video#AMCap) and then the images were analysed by treating each oocyte image as a growing sphere whose volume could be inferred from its cross-sectional area (Image Tool version 3, http://ddsdx.uthscsa.edu/dig/itdesc.html). The osmotic water permeability (_P_f) was calculated according to Zhang and Verkman (1991) and Agre et al. (1999).
As a negative control, non-injected oocytes were used after checking that they did not show significant differences with water-injected oocytes (data not shown). AtPIP2;3 was used as the positive control in every experiment.
P_f_ inhibition assays:
For pH inhibition experiments, the oocyte internal (cytosolic) or external pH was modified as previously described (Tournaire-Roux et al., 2003). Briefly, the oocyte internal or external pH was acidified by pre-incubating them for 15 min in 50 mM sodium acetate (for internal pH modification) or NaCl (for only external pH modification), 20 mM MES pH 6.0 and mannitol until the desired osmolality was achieved (∼200 mOsmol kg−1 H2O). In order to reach pH 7.5, MES was replaced by HEPES in the solution described above. The swelling response was performed by transferring the oocyte to the same solution diluted 5-fold with distilled water.
In all treatments, negative controls were performed by submitting non-injected oocytes to the same protocol and the percentage inhibition was calculated using the formula:
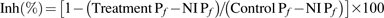
Solute transport measurement:
Solute transport measurement was performed as previously described in Soto et al. (2008). Briefly, Xenopus oocytes were transferred from ND96 solution (200 mosmol kg−1) to a 5-fold diluted medium supplemented with the corresponding solute to be tested (boric acid, glycerol, ammonia or urea) up to 200 mOsmol kg−1 (Hansen et al., 2002; Beitz et al., 2004). Thus, this final solution behaves as an isotonic solution unless the aquaporin is permeable to the solute to be tested. Under these conditions, the increase in oocyte volume is expected to be a consequence of water influx driven by the osmotic gradient caused by the initial solute uptake (chemical gradient).
Solute permeabilities were compared by analysing the initial swelling rates (d(_V/V_0)/dt). As a control, the correct expression and functional activity of the aquaporin (as a water channel) was previously tested in the same batch of oocytes used in the experiments described above.
RNA isolation and Northern blotting assays
Approximately 30 fruits at each ripening stage were harvested, cut in quarters, and immediately frozen. Two independent isolates were made of total RNA from each pool of frozen fruits using the hot borate method (Wan and Wilkins, 1994). For Northern blotting assays, total RNA (10 μg) was electrophoresed on 1.2% (w/v) formaldehyde denaturing agarose gel. To ensure that equal amounts of RNA per lane were loaded, samples were stained with ethidium bromide and individual lanes were evaluated for comparable fluorescence levels upon exposure to a UV light source. After running, the RNA was transferred to Hybond-N+ nylon membrane (Amersham-Pharmacia Biotech, UK), and cross-linked with an UV-Stratalinker Model 1800 (Stratagene, Texas, USA). Membranes were prehybridized with 25 ml of a solution containing 50% (v/v) formamide, SSPE buffer (50 mM NaCl, 12 mM NaH2PO4, 1 mM EDTA, pH 7.4), 5× Denhart's solution, 150 μg ml−1 denatured salmon sperm DNA, and 0.5% (w/v) SDS at 42 °C for 4 h and then hybridized overnight at 42 °C with the denatured radiolabelled probe. The membranes were washed once at 42 °C and twice at 50 °C for 30 min in 25 ml of SSC buffer (15 mM sodium citrate, 150 mM NaCl, pH 7.0) with 0.1% (w/v) SDS. The blot was exposed to X-ray film (X-OMAT AR, Kodak) with an intensifying screen at –80 °C.
Bands corresponding to _Fa_PIP1 and _Fa_PIP2 expression from each ripening stage of both cultivars were analysed by densitometry (Gel Pro Analizer v 3.0). Relative expression (RE) from two independent samples was analysed as follows: RE= [(_Fa_PIP1 BIX/rRNA BIX)/(_Fa_PIP1 BITLG/rRNA BITLG)] and [(_Fa_PIP2 BIX/rRNA BIX)/(_Fa_PIP2 BITLG/rRNA BITLG)], where BIX represents the band intensity for each ripening stage, and BITLG represents the band intensity for the Toyonoka LG ripening stage.
Probe preparation
Probes were prepared by restriction of plasmids T7Ts containing _Fa_PIP1;1 and _Fa_PIP2;1 ORFs with endonucleases _Bg_lII and _Spe_I (Promega, USA). After restriction, the inserts (873 bp and 858 bp for _Fa_PIP1;1 and _Fa_PIP2;1, respectively) were used as templates in a random primer labelling reaction using [32P] dATP. After checking probe specificity by dot-blot assays (see Supplementary Fig. S1 at JXB online), they were used for Northern blotting experiments.
General analytical methods
Osmolarities of all solutions were determined using a vapour pressure osmometer (5520C Wescor, Logan, UT). Chemicals were purchased from Sigma (St Louis, MI, USA) unless otherwise indicated.
Statistical analysis
Data for P_f_ values were analysed by Student's t test at a significance level of 0.05. The heterologous expression of FaPIP1;1 and FaPIP2;1 was performed at least three times with independent batches of oocytes. The figures show one typical experiment, indicating in the legend the number of oocytes employed for each treatment.
Results
Cloning and analysis of FaPIP2;1
An aquaporin was cloned from a library of Fragaria_×_ananassa fruit and named FaPIP2;1. Its nucleotide sequence (858 bp) code a protein of 285 amino acids with theoretical PI and MW of 8.6 and 30.2 kDa, respectively, similar to PIP aquaporins from other species (Gomes et al., 2009).
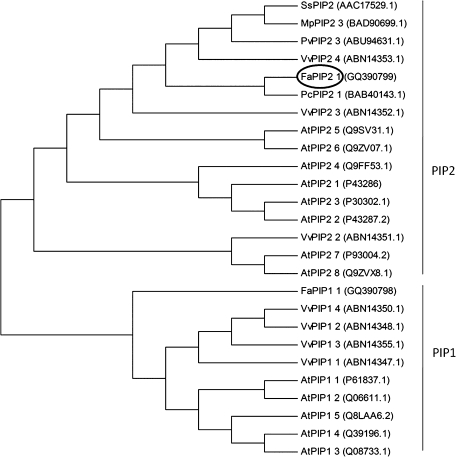
The cloned aquaporin was classified as a PIP2 based on BLASTN sequence analysis (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and named _Fa_PIP2;1 following the nomenclature proposed for plant aquaporins (Johanson et al., 2001). A phylogenetic tree was constructed by including _Fa_PIP2;1 and PIPs from Arabidopsis thaliana, Vitis vinifera, and other species. As shown in Fig. 1, _Fa_PIP2;1 is clustered with all PIP2.
Fig. 1.
Phylogenetic analysis of full-length deduced amino acid sequences of plant aquaporins, including our clones, FaPIP2;1 and FaPIP1;1. Deduced amino acid sequences from full-length plant aquaporins encoding genes from Arabidopsis thaliana, Vitis vinifera, and other plant PIP2s with high identity to FaPIP2;1 were compared using Clustal W. Phylogenetic analyses were conducted using MEGA version 4 (Tamura et al., 2007).
The predicted amino acid sequence of FaPIP2;1 was aligned and compared with aquaporins from different sources (Fig. 2). The criteria used to select sequences for alignment was to choose not only PIPs with the highest identity to FaPIP2;1, but also PIPs from Arabidopsis thaliana and Zea mays reported in functional studies. According to BLASTP analysis of sequence identity, the higher hit (91% identity) for FaPIP2;1 was obtained with Pyrus communis PIP2;2 (BAB40143.1), that belongs to the same taxonomic family than Fragaria×ananassa (Rosaceae) and that is also expressed in its fruit. FaPIP2;1 also presents high identity percentage (>89%) with other PIP2 aquaporins, for example, with Samanea saman (PIP2, AC17529.1), Vitis vinifera (VvPIP2;4, ABN14353.1), and Phaseolus vulgaris (PvPIP2;3, ABU94631.1).
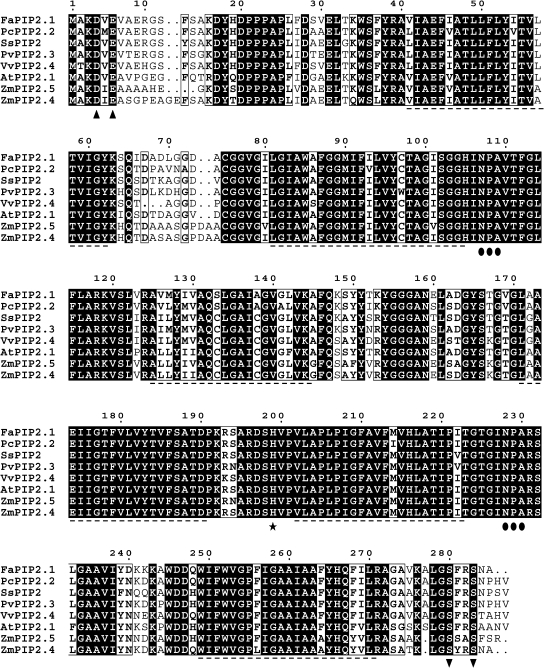
Fig. 2.
Alignment of predicted amino acid sequence of Fragaria×ananassa aquaporin (FaPIP2;1) with other aquaporins (ClustalX). The predicted amino acid sequence of FaPIP2;1 was compared with aquaporins from different sources (aquaporins with the higher identity with FaPIP2;1 or very well studied in the literature). Transmembrane domains are shown with a dashed line below the alignment; triangles indicate a potential diacidic motif (putative ER signal); circles indicate the NPA selectivity filter; a star indicates His199, and inverted triangles putative phosphorylated Ser residues.
The analysis of the FaPIP2;1 amino acid sequence shows that the protein shares the following features with other reported aquaporins: (i) a hydrophobic profile consistent with six alpha-helical transmembrane domains and five inter-helical loops (predicted by TMHMM, http://www.cbs.dtu.dk/services/TMHMM/), (ii) two highly conserved NPA (Asn-Pro-Ala) and the ar/R (Phe-His-Thr-Arg) motifs, both proposed as defining the specificity of the water pore (Forrest and Bhave, 2007), (iii) the Lys3 and Glu6 amino acids, that were shown to undergo methylation in _At_PIP2;1 (Santoni et al., 2006), (iv) the Ser280 and Ser283 determined as phosphorylated in _At_PIP2;1 (Prak et al., 2008), (v) the pH sensor, in this case His199 (Tournaire-Roux et al., 2003), and (vi) the motif DIE (Asp-Ile-Glu) in position 4–6, recently proposed to have a putative role in endoplasmic reticulum export signalling (Zelazny et al., 2009).
Functional studies of FaPIP2;1 in Xenopus laevis oocytes
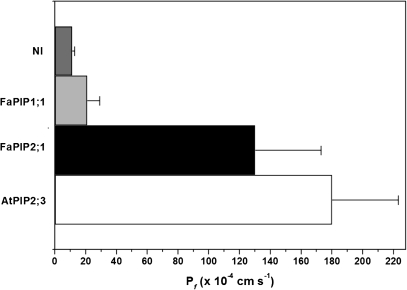
To analyse water transport capacity, Xenopus oocytes expressing FaPIP2;1 were exposed to a hypo-osmotic shock of 160 mOsm kg−1. Expression of this aquaporin in Xenopus oocytes led to an almost 10-fold increase of the swelling rate compared with the negative control oocytes (Fig. 3). From the calculated P_f_ values (130±43×10−4 cm s−1), FaPIP2;1 can be characterized as a water channel with a high water permeability, like the well-known aquaporin AtPIP2;3 (positive control; P_f_ 180±43×10−4 cm s−1). As expected, when FaPIP1;1 was expressed alone in the same batch of oocytes, the osmotic water permeability remained very low (P_f_ 21±8×10−4 cm s−1).
Fig. 3.
Functional expression of FaPIP2;1 in Xenopus oocytes. Calculated mean water permeabilities (P_f_±SEM) of oocytes under hypo-osmotic conditions for NI (non-injected, negative control) or expressing 25 ng of FaPIP1;1, FaPIP2;1, and AtPIP2;3 (used as positive control) are shown. The number of measured oocytes for each condition is 5 to 8. P_f_ of oocytes expressing both FaPIP2;1 and AtPIP2;3 present significant differences from negative control (p _<_0.05).
The transport of small, uncharged solutes across the oocytes expressing FaPIP2;1 was also tested. Urea, glycerol, boric acid, and ammonia permeability were analysed in iso-osmotic swelling assays with an inwardly directed gradient of solute (Beitz et al., 2004; Soto et al., 2008). Oocytes injected with 25 ng of FaPIP2;1 cRNA showed no significant swelling rates for any of the solutes tested (Table 1).
Table 1.
FaPIP2;1 does not transport solutes when expressed in Xenopus oocytes
| Solute tested | d(V/_V_0)/dt (10−4 s−1) |
|---|---|
| Urea | –0.15±0.20 (_n_=7) |
| Boric acid | –0.44±0.12 (_n_=6) |
| Ammonia | –0.26±0.14 (_n_=7) |
| Glycero 1 | 0.10±0.19 (_n_=10) |
Co-expression of FaPIP in Xenopus laevis oocytes
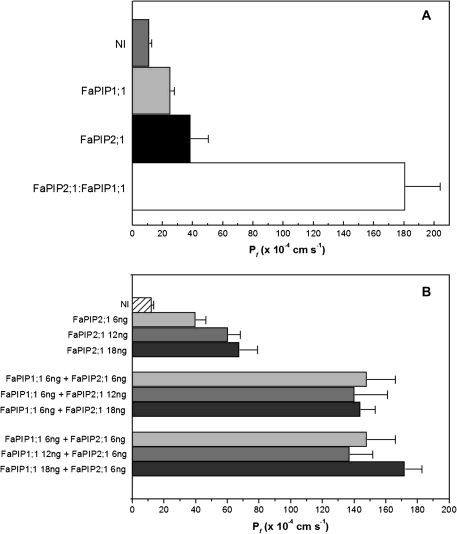
Our next step was to test if FaPIP co-expression enhances water permeability. This approach requires a small amount of the PIP with high P_f_ (in our case FaPIP2;1) to be co-injected with a larger amount of the PIP1. Figure 4A clearly shows that small amounts of FaPIP2;1 cRNA co-injected with FaPIP1;1 cRNA (mass ratio 1:4, in mass values 3 ng:12 ng of FaPIP2;1:FaPIP1;1) increased P_f_ to 181±23×10−4 cm s−1. This result represents an increment of 79% compared with the P_f_ obtained by the injection of 3 ng of FaPIP2;1 alone (39±11×10−4 cm s−1).
Fig. 4.
Co-expression of FaPIP2;1 and FaPIP1;1. (A) Co-expression of 3 ng of cRNA of FaPIP2;1 with 12 ng of cRNA of FaPIP1;1 is shown (white bar). As a control, 3 ng of FaPIP2;1 (black bar) and 12 ng of FaPIP1;1 (light grey bar) were injected separately. The co-expression increased significantly the water permeability six times compared with the expression of FaPIP2;1 alone (p <0.05). Data are expressed as mean P_f_±SEM, n_=5 or 6 oocytes. NI are non-injected oocytes (grey bar). (B) Increasing cRNA mass of FaPIP2;1 injected alone in oocytes (from 6 ng to 18 ng) shows a increasing P_f. This relationship is not observed in FaPIP2;1-FaPIP1;1 co-expressing oocytes. P_f remains high but constant no matter the cRNA mass ratio injected. Data are shown as mean P_f ±SEM, _n_=6–8 oocytes.
It is interesting to note that, while there is a correlation between the cRNA mass of FaPIP2;1 injected alone and the resulting oocyte P_f_, this correlation is lost when FaPIP2;1:FaPIP1;1 are co-expressed. Figure 4B shows that oocyte P_f_ remained high and similar for all injected cRNA mass ratios.
_P_f inhibition assays
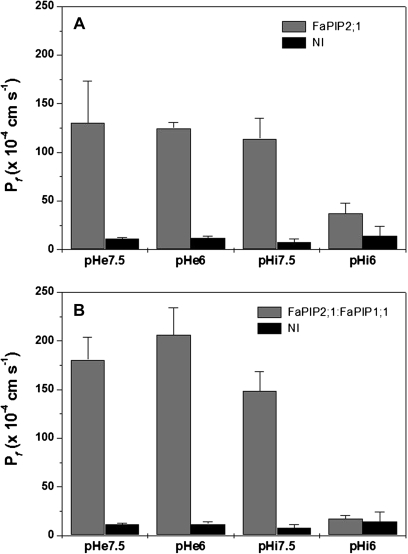
Highly conserved histidines have been described to trigger a pH inhibitory response, shutting down PIPs when cytosolic medium is acidified (Tournaire-Roux et al., 2003). In order to test if FaPIP2;1 also shows a functional blockage of its activity, oocytes expressing FaPIP2;1 were exposed to different pH values. The results shown in Fig. 5A confirms that FaPIP2;1 partially shuts down water permeability when the oocyte internal pH was acidified. The percentage inhibition when three independent inhibition experiments from different batches of oocytes were pooled is ∼52%. External pH acidification does not modify FaPIP2;1 activity.
Fig. 5.
P_f_ inhibitory response after cytosolic acidification. (A) FaPIP2;1 expressing oocytes were exposed to different external (pHe) or internal (pHi) pH conditions. Negative controls (NI) are non-injected oocytes. P_f_ for oocytes expressing FaPIP2;1 exposed to internal acidification is statistically different from its control (i.e. pHi=7.5), while treatment with pHe=6.0 does not result in a significant inhibition (_p <_0.05) when compared with its control (pHe=7.5). (B) FaPIP2;1-FaPIP1;1 was co-expressed in Xenopus oocytes and exposed to different external (pHe) or internal (pHi) pH conditions. Negative controls (NI) are non-injected oocytes. Data are shown as mean P_f_±SEM, _n_=8–10 oocytes.
The pH inhibitory response on FaPIP co-expression was also tested and, interestingly, in this condition the inhibition was complete (98%; Fig. 5B). Thus, the partial blockage displayed by FaPIP2;1 alone is replaced by a complete reduction in water transport when both types of aquaporins are present in the oocyte plasma membrane. A similar observation has recently been reported in Beta vulgaris PIP (Bellati et al., 2010).
Expression of FaPIP2 and FaPIP1 during strawberry fruit ripening
After checking probe specificity, by dot-blot assays (see Supplementary Fig. S1 at JXB online), complete _Fa_PIP1;1 and _Fa_PIP2;1 ORFs were used as probes in Northern blot experiments. Due to the high identity between different PIP1 isoforms from a single species, it cannot be confirmed that _Fa_PIP1;1 is distinguishable from other unknown PIP1; then, this probe was considered as a general probe against PIP1. The same was considered regarding the PIP2 subtype and its isoforms.
The expression of both mRNAs was analysed in fruit at different ripening stages from two cultivars: one that produces firm fruit (Camarosa) and another whose fruit are very delicate and soft (Toyonoka) (Fig. 6; see Supplementary Fig. 2 at JXB online). The expression of both aquaporins was clearly lower in Toyonoka than in Camarosa during fruit ripening.
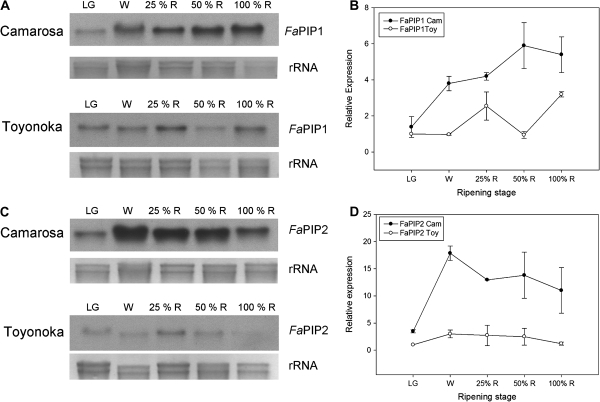
Fig. 6.
_Fa_PIP1 and _Fa_PIP2 expression pattern during strawberry fruit ripening. (A, C). Northern blot showing the accumulation of _Fa_PIP mRNA in different ripening stages of strawberry fruits: large green (LG), white (W), 25% red (25%R), 50% red (50%R), and 100% red (100%R) for two different cultivars, a firm (Camarosa) and a softer one (Toyonoka). (B, D) Quantification of _Fa_PIP1 and _Fa_PIP2 expression relative to Toyonoka LG stage is shown for Camarosa and Toyonoka cultivars (Gel Pro Analizer v 3.0 was used). Data are shown as mean ±SEM, _n_=2.
The expression of _Fa_PIP1 in Camarosa was low in the LG stage, increased at W and kept increasing until the end of ripening (100% R). In the case of Toyonoka, the expression of this gene was low in LG, W, and 50% R stages, and increased at 25% R and 100% R stages (Fig. 6A, B).
In the case of _Fa_PIP2, the expression in Camarosa increased markedly from the LG to the W stage, reaching the highest level, and decreasing progressively until the end of ripening (Fig. 6A, B). In Toyonoka, the expression increased slightly in the W stage and then remained approximately constant during ripening (Fig. 6C, D).
Discussion
To our knowledge, FaPIP2;1 is the first full-length PIP2 aquaporin described in Fragaria×ananassa, after the characterization of a root-specific TIP (Vaughan et al., 2006) and FaPIP1;1 (Mut et al., 2008). FaPIP2;1 is a water channel which promotes very high membrane P_f_ (Fig. 3), and is unable to transport some solutes already described for other plant aquaporins: urea, ammonia, boric acid or glycerol (Table 1). Although it cannot be dismissed that FaPIP2;1 might be involved in the transport of other non-tested solutes or even ions, this aquaporin seems to be mainly involved in water transport.
The co-injection of cRNA of FaPIP2;1 and FaPIP1;1 significantly raised the oocyte membrane P_f_ (Fig. 4A). Despite a correlation between oocyte P_f_ and the FaPIP2;1 cRNA mass injected (Fig. 4B), this relationship is not observed when FaPIP2;1 and FaPIP1;1 are co-expressed, and P_f_ values remain high and constant in all the different mass ratios assayed. This result could be reflecting ER processing of aquaporins when their cRNA are co-injected in the same oocyte (Zelazny et al., 2007). Co-expressed aquaporins from grape berries show a similar response to the one reported here (Vandeleur et al., 2009).
PIP1–PIP2 interaction has been reported both in Xenopus oocytes and in living plant cells (Fetter et al., 2004; Zelazny et al., 2007) and as a consequence, if PIP2 is not expressed, PIP1 alone cannot enhance water permeability. It is therefore expected that the modification of FaPIP1;1 and FaPIP2;1 expression profiles during ripening reported here could be another mechanism of controlling plasma membrane water transport. That is, high levels of FaPIP1;1 at the red stage (as in the case of Camarosa) does not guarantee the enhancement of water transport unless FaPIP2;1 is present. On the other hand, although FaPIP2;1 can concede high water permeability to the membrane, the presence of FaPIP1;1 can co-operatively trigger much higher values. Isolated vesicles of an enriched fraction of plasma membrane from the 100% red stage fruit show very high water permeability values (Mut et al., 2008), which supports our hypothesis.
When oocytes expressing FaPIP2;1 are subjected to cytosolic acidification, membrane P_f_ is partially shut down (Fig. 5A). These results are in accordance with the presence of the highly conserved His199 in the FaPIP2;1 sequence, a residue shown to be responsible for water transport blockage under cytosolic acidification in other PIP2 aquaporins (Tournaire-Roux et al., 2003). It is interesting to analyse the reduction of water transport under cytosolic acidification detected for FaPIP2;1 (partial inhibition) compared with FaPIP2;1-FaPIP1;1 co-expression (total inhibition). This differential response of water channels, also found for Beta vulgaris aquaporins (Bellati et al., 2010), could reflect the faculty of the cell to turn partial water transport to zero water transport through aquaporins under cytosolic acidification depending on the PIP2–PIP1 relative expression in the plasma membrane. So, not only the co-expression of aquaporins, but also the pH response of the assembly formed by this co-expression widens interestingly the modulating possibilities of water transport cell control.
Aquaporin gene expression during ripening might be associated with the main mechanisms involved in cell division and cell expansion. The water channel expression pattern during ripening has been studied in grape berry, a non-climacteric fruit (Fouquet et al., 2008). In a previous work, it was shown that the expression of FaPIP1;1 varies with the ripening stages of the strawberry cultivar Selva (Mut et al., 2008). Four genes annotated as aquaporins (according to the putative function of its closest NCBI database homologues) have been detected as expressed preferentially in strawberry fruit receptacles, with higher expression in the red stage (three out of four genes studied) compared with the green stage or with a higher expression in turning/white stage (one of four genes studied) (Aharoni et al., 2002).
In ripening, despite cell division and cell expansion, fruit softening is also an important feature. Cell wall disassembly is a key element determining softening; however, softening has also been postulated as a physical consequence of a reduction in cell turgor (Thomas et al., 2006; Saladiè et al., 2007; Wada et al., 2008, 2009). Turgor diminution can be attributed to the water loss that follows apoplastic solute accumulation occurring during ripening (Wada et al., 2008). All this evidence supports the idea that a decline of fruit turgor, in addition to cell wall degradation, could contribute to fruit softening.
In strawberry it has been reported that cell turgor values decline from 250 kPa in green-white fruit to 50 kPa in pink fruit mainly due to high solute accumulation in the apoplast (Pomper and Breen, 1995). In this sense, the osmotic gradient generated by solute accumulation in the apoplast could lead to fruit turgor reduction and aquaporins (if expressed mainly in the receptacle tissue as shown by Aharoni et al., 2002) could speed up water outflow from cells.
Here _Fa_PIP1 and _Fa_PIP2 mRNA accumulation during ripening of firm (Camarosa) and soft (Toyonoka) strawberry cultivars has been evaluated. Gene expression of _Fa_PIP1 and _Fa_PIP2 increase in W or 25% R stages in both cultivars, coincidently with the main firmness decrease that occurs at the early ripening stages (Rosli et al., 2004, 2009; Villarreal et al., 2008) (Fig. 6).
Higher levels of both FaPIP1 and FaPIP2 were detected in the firmer cultivar (Camarosa) than the softer one (Toyonoka). It is worth mentioning that, in strawberry fruit, the extension of cell wall disassembly is cultivar-dependent (Rosli et al., 2004, 2009; Bustamante et al., 2006; Villareal et al. 2008). Some correlations between a higher expression of genes related to cell wall degradation and a higher fruit softening have been found. Among them, the cultivar Toyonoka shows a higher and earlier expression of a polygalacturonase and two expansin genes, compared with the Camarosa cultivar (Dotto et al., 2006; Villarreal et al., 2008). Assuming that both cell wall disassembly and cell turgor contribute to fruit softening, it is possible to hypothesize that loss of cell turgor mediated by aquaporins had a higher contribution to Camarosa softening, while in the Toyonoka cultivar the main source of fruit softening would be cell wall disassembly.
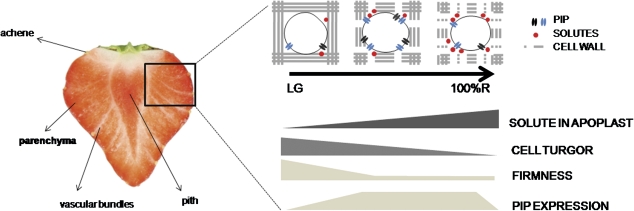
In this work it is speculated that, if the main target tissue of PIPs expression in strawberry fruit is the receptacle parenchyma, fruit softening could be associated not only with cell wall disassembly but also to loss of cell turgor mediated by water flow through aquaporins (Fig. 7).
Fig. 7.
Hypothetical and schematic representation of the role of aquaporins in fruit ripening. Section of a strawberry fruit showing fibrovascular strands (vascular bundles), achenes, the interior of the receptacle (pith), and main parenchyma; all marked tissues are possible targets of PIP expression due to their participation in water and solute balance in strawberry fruit.
In conclusion, oocyte experiments proved (i) that FaPIP2;1 is a highly active water channel able to reduce its water transport activity by cytosolic acidification but insensitive to external (in plants, apoplastic) acidification, and (ii) that FaPIP2;1 could interact with FaPIP1;1 not only modifying final plasma membrane P_f_ but also pH response. In addition, both aquaporins are expressed (at different levels) through all the ripening stages of strawberry in two cultivars with different firmness. These results suggest that a complex regulation of water transport depending on the PIP1 and PIP2 rate expression profile could play a role in fruit physiology. Our results open the field of aquaporin participation in specific ripening events and their coupling with fruit softening. In this context, cell wall degradation and water exchange mediated by aquaporins could be juxtaposed and even integrated events during ripening. Further work will help to clarify how specific target tissues request changes in plasma membrane water transport activity at the cell level to impact on fruit water balance.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. S1. Dot blot assays after hybridization with the _Fa_PIP1;1 and _Fa_PIP2;1 probes.
Supplementary Fig. S2. The expression of both mRNAs in fruit at different ripening stages from the two cultivars, Camarosa and Toyonoka.
Supplementary Material
Supplementary Data
Acknowledgments
This study was financed by PICT07-655, UBACyTM001, and CONICET-PIP5154 grants to GA and by PICT06-01804 and UBACyTM602 grants to KA. Our particular thanks are due to Noel Danjou who improved the AMCAP program (version 9.20) to allow the acquisition of still images as requested, Alex Paladini (INGEBI, CONICET) who designed and provided us with built-in chambers for oocyte experiments, and Mark Daniels (UCSD, USA) for the kind gift of _At_PIP2;3 cDNA. KA, GM, MC, and GA are CONICET research fellows.
References
- Agre P, Mathai JC, Smith BL, Preston GM. Functional analyses of aquaporin water channel proteins. Methods in Enzymology. 1999;294:550–572. doi: 10.1016/s0076-6879(99)94032-6. [DOI] [PubMed] [Google Scholar]
- Aharoni A, Keizer LCP, Van Den Broeck HC, Blanco-Portales R, Muñoz-Blanco J, Bois G, Smit P, De Vos RCH, O'Connell AP. Novel insight into vascular, stress, and auxin-dependent and -independent gene expression programs in strawberry, a nonclimacteric fruit. Plant Physiology. 2002;129:1019–1031. doi: 10.1104/pp.003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad AK, Katsuhara M, Sawa Y, Ishikawa T, Shibata H. Characterization of four plasma membrane aquaporins in tulip petals: a putative homolog is regulated by phosphorylation. Plant and Cell Physiology. 2008;49:1196–1208. doi: 10.1093/pcp/pcn095. [DOI] [PubMed] [Google Scholar]
- Beitz E, Pavlovic-Djuranovic S, Yasui M, Agre P, Schultz JE. Molecular dissection of water and glycerol permeability of the aquaglyceroporin from Plasmodium falciparum by mutational analysis. Proceedings of the National Academy of Sciences, USA. 2004;101:1153–1158. doi: 10.1073/pnas.0307295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellati J, Alleva K, Soto G, Vitali V, Jozefkowicz C, Amodeo G. Intracellular pH sensing is altered by plasma membrane PIP aquaporin co-expression. Plant Molecular Biology. 2010 doi: 10.1007/s11103-010-9658-8. (in press) [DOI] [PubMed] [Google Scholar]
- Bustamante CA, Rosli HG, Añón MC, Civello PM, Martínez GA. β-xylosidase in strawberry fruit: isolation of a full-length gene and analysis of its expression and enzymatic activity in cultivars with contrasting firmness. Plant Science. 2006;171:497–504. doi: 10.1016/j.plantsci.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Jung R, Chrispeels MJ. Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiology. 2000;122:1025–1034. doi: 10.1104/pp.122.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervin C, Tira-Umphon A, Terrier N, Zouine M, Severac D, Roustan JP. Stimulation of the grape berry expansion by ethylene and effects on related gene transcripts, over the ripening phase. Physiologia Plantarum. 2008;134:534–546. doi: 10.1111/j.1399-3054.2008.01158.x. [DOI] [PubMed] [Google Scholar]
- Civello PM, Powell ALT, Sabehat A, Bennett AB. An expansin gene expressed in ripening strawberry fruit. Plant Physiology. 1999;121:1273–1279. doi: 10.1104/pp.121.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombe BG. The development of freshy fruits. Annual Review of Plant Physiology. 1976;27:207–228. [Google Scholar]
- Daniels MJ, Mirkov TE, Chrispeels MJ. The plasma membrane of Arabidopsis thaliana contains a mercury-insensitive aquaporin that is a homolog of the tonoplast water channel protein TIP. Plant Physiology. 1994;106:1325–1333. doi: 10.1104/pp.106.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotto MC, Martínez GA, Civello PM. Expression of expansin genes in strawberry varieties with contrasting fruit firmness. Plant Physiology and Biochemistry. 2006;44:301–307. doi: 10.1016/j.plaphy.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Fetter K, Van Wilder V, Moshelion M, Chaumont F. Interactions between plasma membrane aquaporins modulate their water channel activity. The Plant Cell. 2004;16:215–228. doi: 10.1105/tpc.017194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest KL, Bhave M. Major intrinsic proteins (MIPs) in plants: a complex gene family with major impacts on plant phenotype. Functional Integrative Genomics. 2007;7:263–289. doi: 10.1007/s10142-007-0049-4. [DOI] [PubMed] [Google Scholar]
- Fouquet R, Léon C, Ollat N, Barrieu F. Identification of grapevine aquaporins and expression analysis in developing berries. Plant Cell Reports. 2008;27:1541–1550. doi: 10.1007/s00299-008-0566-1. [DOI] [PubMed] [Google Scholar]
- Gomes D, Agasse A, Thiébaud P, Delrot S, Gerós H, Chaumont F. Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochemistry and Biophysics Acta. 2009;1788:1213–1228. doi: 10.1016/j.bbamem.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Gouet P, Courcelle E, Stuart DI, Metoz F. ESPript: multiple sequence alignments in PostScript. Bioinformatics. 1999;15:305–308. doi: 10.1093/bioinformatics/15.4.305. [DOI] [PubMed] [Google Scholar]
- Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjövall S, Fraysse L, Weig AR, Kjellbom P. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiology. 2001;126:1358–1369. doi: 10.1104/pp.126.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M, Kun JF, Schultz JE, Beitz E. A single, bi-functional aquaglyceroporin in blood-stage Plasmodium falciparum malaria parasites. Journal of Biological Chemistry. 2002;277:4874–4882. doi: 10.1074/jbc.M110683200. [DOI] [PubMed] [Google Scholar]
- Hu CG, Hao YJ, Honda C, Kita M, Moriguchi T. Putative PIP1 genes isolated from apple: expression analyses during fruit development and under osmotic stress. Journal of Experimental Botany. 2003;54:2193–2194. doi: 10.1093/jxb/erg238. [DOI] [PubMed] [Google Scholar]
- Mut P, Bustamante C, Martínez G, Alleva K, Sutka M, Civello M, Amodeo G. A fruit-specific plasma membrane aquaporin subtype PIP1;1 is regulated during strawberry (Fragaria×ananassa) fruit ripening. Physiologia Plantarum. 2008;132:538–551. doi: 10.1111/j.1399-3054.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- Pomper KW, Breen PJ. Levels of apoplastic solutes in developing strawberry fruit. Journal of Experimental Botany. 1995;46:743–752. [Google Scholar]
- Prak S, Hem S, Boudet J, Viennois G, Sommerer N, Rossignol M, Maurel C, Santoni V. Multiple phosphorylations in the C-terminal tail of plant plasma membrane aquaporins: role in subcellular trafficking of AtPIP2;1 in response to salt stress. Molecular Cell Proteomics. 2008;7:1019–1030. doi: 10.1074/mcp.M700566-MCP200. [DOI] [PubMed] [Google Scholar]
- Rose T, Schultz E, Henikoff J, Pietrokovski S, McCallum C, Henikoff S. Consensus-degenerate hybrid oligonucleotide primers for amplification of distantly related sequences. Nucleic Acids Research. 1998;26:1628–1635. doi: 10.1093/nar/26.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosli HG, Civello PM, Martínez GA. Changes in cell wall composition of three Fragaria×ananassa cultivars with different softening rate during ripening. Plant Physiology and Biochemistry. 2004;42:823–831. doi: 10.1016/j.plaphy.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Rosli HG, Civello PM, Martínez GA. Alpha-l-arabinofuranosidase from strawberry fruit: cloning of three cDNAs, characterization of their expression and analysis of enzymatic activity in cultivars with contrasting firmness. Plant Physiology and Biochemistry. 2009;47:272–281. doi: 10.1016/j.plaphy.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Saladié M, Matas AJ, Isaacson T, et al. A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiology. 2007;144:1012–1028. doi: 10.1104/pp.107.097477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni V, Verdoucq L, Sommerer N, Vinh J, Pflieger D, Maurel C. Methylation of aquaporins in plant plasma membrane. Biochemistry Journal. 2006;400:189–197. doi: 10.1042/BJ20060569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto G, Alleva K, Mazzella MA, Amodeo G, Muschietti JP. AtTIP1;3 and AtTIP5;1, the only highly expressed Arabidopsis pollen-specific aquaporins, transport water and urea. FEBS Letters. 2008;582:4077–4082. doi: 10.1016/j.febslet.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Thomas TR, Matthews MA, Shackel KA. Direct in situ measurement of cell turgor in grape (Vitis vinifera L.) berries during development and in response to plant water deficits. Plant, Cell, and Environment. 2006;29:993–1001. doi: 10.1111/j.1365-3040.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu DT, Bligny R, Maurel C. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature. 2003;25:393–397. doi: 10.1038/nature01853. [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Bohnert HJ, Maurel C, Steudle E, Smith JAC. Plant aquaporins: their molecular biology, biophysics and significance for water relations. Journal of Experimental Botany. 1999;50:1055–1071. [Google Scholar]
- Vandeleur RK, Mayo G, Shelden MC, Gilliham M, Kaiser BN, Tyerman SD. The role of plasma membrane intrinsic protein aquaporins in water transport through roots: diurnal and drought stress responses reveal different strategies between isohydric and anisohydric cultivars of grapevine. Plant Physiology. 2009;149:445–460. doi: 10.1104/pp.108.128645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan SP, James DJ, Lindsey K, Massiah AJ. Characterization of FaRB7, a near root-specific gene from strawberry (Fragaria×ananassa Duch.) and promoter activity analysis in homologous and heterologous hosts. Journal of Experimental Botany. 2006;57:3901–3910. doi: 10.1093/jxb/erl185. [DOI] [PubMed] [Google Scholar]
- Villarreal NM, Rosli HG, Martínez GA, Civello PM. Polygalacturonase activity and expression of related genes during ripening of strawberry cultivars with contrasting fruit firmness. Postharvest Biology and Technology. 2008;47:141–150. [Google Scholar]
- Wada H, Shackel K, Matthews M. Fruit ripening in Vitis vinifera: apoplastic solute accumulation accounts for pre-veraison turgor loss in berries. Planta. 2008;227:1351–1361. doi: 10.1007/s00425-008-0707-3. [DOI] [PubMed] [Google Scholar]
- Wada H, Matthews M, Shackel K. Seasonal pattern of apoplastic solute accumulation and loss of cell turgor during ripening of Vitis vinifera fruit under field conditions. Journal of Experimental Botany. 2009;60:1773–1781. doi: 10.1093/jxb/erp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan CY, Wilkins TA. A modified hot borate method significantly enhances the yield of high-quality RNA from cotton (Gossypium hirsutum L.) Analytical Biochemistry. 1994;15:7–12. doi: 10.1006/abio.1994.1538. [DOI] [PubMed] [Google Scholar]
- Zelazny E, Borst JW, Muylaert M, Batoko H, Hemminga MA, Chaumont F. FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proceedings of National Academy of Sciences, USA. 2007;104:12359–12364. doi: 10.1073/pnas.0701180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazny E, Miecielica U, Borst JW, Hemminga MA, Chaumont F. An N-terminal diacidic motif is required for the trafficking of maize aquaporins ZmPIP2;4 and ZmPIP2;5 to the plasma membrane. The Plant Journal. 2009;57:346–355. doi: 10.1111/j.1365-313X.2008.03691.x. [DOI] [PubMed] [Google Scholar]
- Zhang RB, Verkman AS. Water and urea permeability properties of Xenopus oocytes: expression of mRNA from toad urinary bladder. American Journal of Physiology. 1991;260:C26–C34. doi: 10.1152/ajpcell.1991.260.1.C26. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Setz N, Niemietz C, Qu H, Offler CE, Tyerman SD, Patrick JW. Aquaporins and unloading of phloem-imported water in coats of developing bean seeds. Plant, Cell and Environment. 2007;30:1566–1577. doi: 10.1111/j.1365-3040.2007.01732.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data