Mitochondrial import of PKCε is mediated by HSP90: a role in cardioprotection from ischaemia and reperfusion injury (original) (raw)
Abstract
Aims
Protein kinase C epsilon (PKCε) is critical for cardiac protection from ischaemia and reperfusion (IR) injury. PKCε substrates that mediate cytoprotection reside in the mitochondria. However, the mechanism enabling mitochondrial translocation and import of PKCε to enable phosphorylation of these substrates is not known. Heat shock protein 90 (HSP90) is a cytoprotective protein chaperone that participates in mitochondrial import of a number of proteins. Here, we investigated the role of HSP90 in mitochondrial import of PKCε.
Methods and results
Using an ex vivo perfused rat heart model of IR, we found that PKCε translocates from the cytosol to the mitochondrial fraction following IR. Immunogold electron microscopy and mitochondrial fractionation demonstrated that following IR, mitochondrial PKCε is localized within the mitochondria, on the inner mitochondrial membrane. Pharmacological inhibition of HSP90 prevented IR-induced interaction between PKCε and the translocase of the outer membrane (Tom20), reduced mitochondrial import of PKCε, and increased necrotic cell death by ∼70%. Using a rational approach, we designed a 7-amino acid peptide activator of PKCε, derived from an HSP90 homologous sequence located in the C2 domain of PKCε (termed ψεHSP90). Treatment with this peptide (conjugated to the cell permeating TAT protein-derived peptide, TAT47–57) increased PKCε–HSP90 protein–protein interaction, enhanced mitochondrial translocation of PKCε, increased phosphorylation and activity of an intra-mitochondrial PKCε substrate, aldehyde dehydrogenase 2, and reduced cardiac injury in ex vivo and in vivo models of myocardial infarction.
Conclusion
Our results suggest that HSP90-mediated mitochondrial import of PKCε plays an important role in the protection of the myocardium from IR injury.
Keywords: Protein kinase C epsilon, Mitochondria, Protein–protein interaction, Ischaemia reperfusion, Heat shock protein 90
1. Introduction
Protein kinase C epsilon (PKCε) activation is required and sufficient to protect the heart from ischaemia and reperfusion (IR) injury.1–3 We recently identified mitochondrial aldehyde dehydrogenase 2 (ALDH2) as an intra-mitochondrial substrate of PKCε,4,5 whose phosphorylation and activation by PKCε is required to confer cardioprotection.4 Additional mitochondrial substrates of PKCε include cytochrome c oxidase subunit IV (COIV),6 a PKCε substrate in cardiac myocytes,6 neuronal cells,7 and the lens.8 PKCε activation also prevents opening of the mitochondrial permeability transition pore (MPTP)9 and can promote mitochondrial ATP-sensitive K+ channel (mitoKATP) opening at the inner mitochondrial membrane (IMM),10 although whether this reflects PKCε-mediated phosphorylation of mitoKATP awaits identification of the native channel protein.
A hallmark of PKC activation is translocation of the active enzyme from the cytosol to the cell particulate fraction and a variety of cytoprotective stimuli that activate PKCε, including ischaemic preconditioning,11 ethanol,4,5 urocortin,12 or transgenic expression of constitutively active PKCε,13 result in elevated mitochondrial levels of PKCε. However, not all studies support a role for mitochondrial PKCε in cardioprotection.14,15 Further, although PKCε substrates have been shown to reside within mitochondria, the mechanism enabling mitochondrial import of PKCε has not yet been described. Mitochondrial proteins encoded by nuclear DNA are imported into the mitochondria in a co-translational process,16 using a 20–50-amino acid residue mitochondrial targeting signal that is recognized by the mitochondrial import receptor, Tom20. PKCε does not contain a mitochondrial targeting sequence. However, proteins that lack this sequence can be imported into mitochondria in a process mediated by the stress chaperone protein, heat shock protein 90 (HSP90).17,18 Here, we examined the role of HSP90 in mediating mitochondrial translocation of PKCε and its effect on cardiac ischaemia/reperfusion.
2. Methods
2.1. Ex vivo model of cardiac ischaemia–reperfusion
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). All protocols were approved by the Stanford University Institutional Animal Care and Use Committee. Hearts from male Wistar rats (275–300 g) were perfused via the aorta at 10 mL/min with oxygenated Krebs–Henseleit buffer at 37°C. Hearts were subjected to 35 min global, no-flow ischaemia followed by 15 min reperfusion. HSP90 inhibitors, geldanamycin (GA; 1 µM) or radicicol (RAD; 1 µM), were perfused during the entire reperfusion period (Figure 1A). Cardiac damage was assessed by creatine phosphokinase (CPK) release into the perfusate (Equal Diagnostics, CT, USA). The ψεHSP90 peptide (1 µM) was perfused for 10 min prior to ischaemia and during the reperfusion period, in the absence and presence of GA (1 µM; Figure 5A).
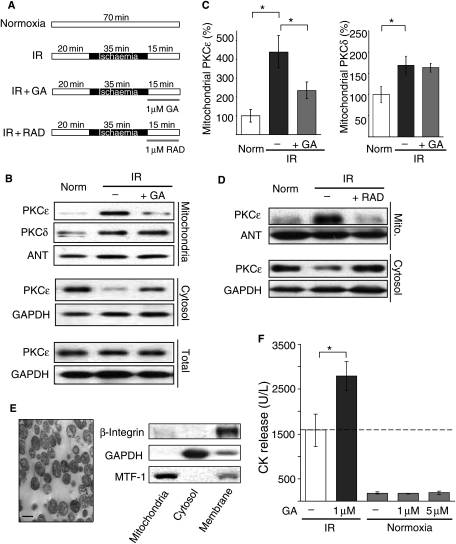
Figure 1.
Inhibition of HSP90 reduces IR-induced PKCε translocation to cardiac mitochondria. (A) Perfused heart protocols. (B) Western blot analysis of mitochondrial PKCε and PKCδ in hearts exposed to normoxia or IR (in the absence and presence of 1 µM GA). Also shown are cytosolic (middle panels) and total PKCε levels (lower panels). (C) Quantification of mitochondrial PKCε and PKCδ levels, normalized to adenine nucleotide translocase (ANT); mean ± SEM (n = 5, *P < 0.05). (D) IR-induced mitochondrial translocation of PKCε in the presence of another HSP90 inhibitor, RAD (1 µM). (E) Purity of the mitochondrial preparation confirmed by electron microscopy (scale bar = 1 µm) and western blotting for marker proteins of the cytosol (GAPDH), the mitochondria (MTF-1), or the plasma membrane (β-integrin). (F) GA effect on CPK release under normoxic and IR conditions; mean ± SEM (n = 5–7, *P < 0.05).
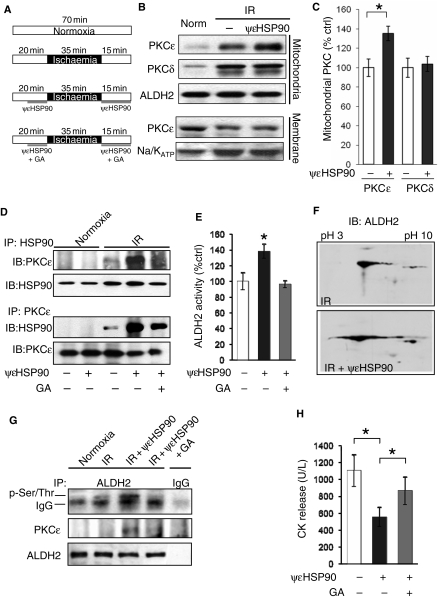
Figure 5.
ψεHSP90 enhances mitochondrial PKCε translocation, increases ALDH2 activity, and reduces cardiac damage by ischaemia/reperfusion, ex vivo. (A) IR protocols in the presence or absence of ψεHSP90 peptide (1 µM) and GA (1 µM). (B) ψεHSP90 peptide increased IR-induced mitochondrial translocation of PKCε but was without effect on mitochondrial translocation of PKCδ. (C) Quantification of data in (B); mean ± S.E.M (n = 7, *P < 0.05). (D) Co-IP of mitochondrial HSP90 and PKCε in normoxic and IR hearts in the absence and presence of ψεHSP90 peptide or GA. (E) Mitochondrial ALDH2 activity in hearts exposed to IR in the absence and presence of ψεHSP90 and GA. (F) Shift of immunoreactive ALDH2 on 2D IEF gels towards acidic pI by ψεHSP90 when compared with IR alone, suggesting increased ALDH2 phosphorylation. (G) ALDH2 phosphorylation detected by immunoblotting the ALDH2 immunoprecipitate with anti-phospho Ser/Thr antibody (upper band on blot, distinct from IgG). PKCε co-IP was also assessed by immunoblotting the ALDH2 immunoprecipitate (middle lane). (H) CPK release following IR injury, in hearts treated with and without ψεHSP90 and GA; mean ± SEM (n = 6, *P < 0.05).
2.2. Subcellular fractionation
Heart ventricles were homogenized in ice cold mannitol–sucrose (MS) buffer using a Polytron homogenizer. The heart homogenate was filtered through gauze then centrifuged at 700 g × 5 min. The resultant supernatant was filtered then centrifuged at 10 000 g × 10 min to pellet mitochondria. Mitochondrial pellets were washed three times and resuspended in 200 µL MS buffer. Mitoplasts were generated as described.19,20 Mitochondria (50 µL of 10 µg/µL) were resuspended in 450 µL hypotonic buffer (5 mM Tris–HCl and 1 mM EDTA, pH 7.4) and incubated on ice for 15 min before centrifugation at 20 000 g for 10 min at 4°C. Mitoplasts were then resuspended in 450 µL hypotonic buffer and sonicated on ice to disrupt the IMM. The solution was then spun at 100 000 g for 40 min with the resultant pellet containing the IMM-enriched fraction and the supernatant containing the matrix-enriched fraction. Submitochondrial particles (SMPs) were generated as described.19,21 Mitochondria (10 mg/mL in MS buffer) were sonicated 3 × 2 min on ice with 1 min intervals. The solution was spun at 10 000 g for 10 min to pellet unbroken mitochondria and the resultant supernatant spun at 100 000 g for 30 min to pellet SMPs.
2.3. Western blotting
Ten micrograms of protein were separated by SDS–PAGE and then transferred to nitrocellulose. Membranes were blocked with 5% milk in Tris-buffered saline (pH 7.5) containing 0.05% Tween (TBS-T), incubated with primary antibody overnight, washed three times in TBS-T, and then incubated with IgG secondary antibody linked to horseradish peroxidase. Protein bands were visualized using chemiluminescence and quantified using ImageJ (NIH).
2.4. Immunoprecipitation
Five hundred micrograms of protein were suspended in 1 mL of immunoprecipitation (IP) buffer and incubated with 2 µg PKCε, HSP90, Tom20, or ALDH2 antibodies (Santa Cruz Biotechnology) for 2 h, followed by overnight incubation with Protein A/G beads at 4°C. Beads were washed three times in IP buffer and immunoprecipitated proteins detected by western blotting.
2.5. Immunogold electron microscopy
Isolated mitochondria were fixed in 4% paraformaldehyde/0.025% gluteraldehyde and 80 nm sections mounted on Ni grids. Mitochondria were incubated with blocking solution, followed by PKCε antibody (1:100 in blocking solution), followed by goat anti-rabbit IgG conjugated to 10 nm gold particles (Ted Pella Inc.) (1:100 in blocking solution). Mitochondria were imaged using a JEOL 1230 electron microscope. Incubation with secondary antibody (IgG) alone served as controls.
2.6. In vitro mitochondrial translocation assay
Isolated mitochondria were incubated with recombinant PKCε with or without diacylglycerol and phosphatidylserine (DAG/PS; 1 mM; PKC activators), hydrogen peroxide (H2O2; 50 µM), or rabbit reticulocyte lysate (RRL; source of HSP90), and with or without 1 µM ψεHSP90. Mitochondrial translocation of recombinant PKCε was assessed by western blotting.
2.7. Sequence alignments
Sequences of the human PKC family members were aligned using ClustalW. Human PKCε (accession no.: NP_005391.1) was aligned with human HSP90α (accession no.: NP_005339) and HSP90β (accession no.: NP_031381) using L-ALIGN, using the Blosum 80 scoring matrix.
2.8. In vivo model of cardiac ischaemia–reperfusion
Male Wistar rats (275–300 g) were anaesthetized with isoflurane and myocardial ischaemia was induced by ligation of the left anterior descending (LAD) coronary artery for 35 min, followed by 24 h of reperfusion. Control TAT or ψεHSP90 peptides (1 mg/kg in 400 µL saline) were injected intraperitoneally 15 min prior to LAD ligation and 5 min before reperfusion onset. Fractional shortening was determined by echocardiography. Area at risk (AAR) was assessed by re-occlusion of the LAD at the previous suture site, followed by intravenous injection of Evans Blue (1.0 mg/kg). The heart was sectioned into transverse slices, which were incubated with 1% trimethyl tetrazolium chloride (TTC), weighed, and photographed by a digital camera. AAR (negative for Evans Blue) and infarct area (negative for TTC) were quantified using ImageJ and infarct size was calculated as (infarct area/AAR of infarction) × 100 (%).
2.9. Statistical analysis
All data are expressed as mean ± SEM. Statistical analyses between two groups was performed using the unpaired Student's _t_-test. Statistical analysis of more than two groups was performed using one-way ANOVA with Dunnett's multiple comparisons post hoc test. A _P_-value of <0.05 was considered statistically significant.
3. Results
3.1. HSP90 activity is required for IR-induced mitochondrial translocation of PKCε
Thirty-five-minute global ischaemia followed by 15 min reperfusion induced translocation of PKCε and PKCδ to the mitochondrial fraction (Figure 1A, B, and D; n = 5; P < 0.05). HSP90 inhibition with GA (1 µM), during reperfusion (Figure 1A), attenuated IR-induced mitochondrial translocation of PKCε by 54% (Figure 1B and C, left panel, n = 5; P < 0.01), but not mitochondrial translocation of PKCδ (Figure 1B and C, right panel, n = 5). GA had no effect on total PKCε levels (Figure 1B), indicating that reduced mitochondrial PKCε translocation was not due to increased PKCε degradation. HSP90 inhibition with RAD (1 µM), which is structurally unrelated to GA,22 also blocked IR-induced mitochondrial translocation of PKCε (Figure 1D). Mitochondrial purity was confirmed by electron microscopy and western blot analysis with protein markers of cytosolic (GAPDH), plasma membrane (β-integrin), and mitochondrial fractions (mitofusion-1) (Figure 1E). GA treatment during reperfusion also increased CPK release by ∼70% when compared with IR alone (Figure 1F; P < 0.05; n = 7), but caused no damage in normoxic perfused hearts, even when used at five-fold higher concentration (Figure 1F).
3.2. Mitochondrial translocation of PKCε proceeds via the HSP90–Tom20 import system
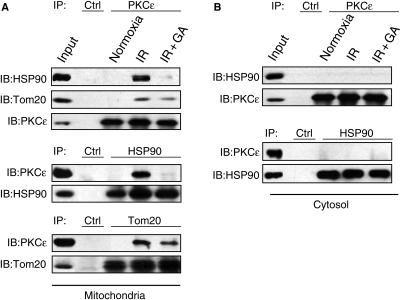
Under normoxic conditions, there was no physical association between PKCε and HSP90 or between PKCε and Tom20 at the mitochondria (Figure 2A). However, PKCε co-immunoprecipitated with both HSP90 and Tom20 in mitochondria after IR. IR-induced co-IP was observed when immunoblotting the PKCε immunoprecipitate for the presence of HSP90 or Tom20, and confirmed by the presence of PKCε in the HSP90 or Tom20 immunoprecipitate. Importantly, IR-induced physical association between PKCε–HSP90 and PKCε–Tom20 was inhibited by GA, suggesting that HSP90 activity was required. No association between PKCε and HSP90 was found in the cytosol under any conditions (Figure 2B), suggesting that IR-induced PKCε–HSP90 interaction occurs following mitochondrial translocation of PKCε.
Figure 2.
IR-induced co-IP of mitochondrial PKCε with HSP90 or Tom20 requires HSP90 chaperone activity. Co-IP of mitochondrial (A) and cytosolic (B) fractions from hearts exposed to normoxia or IR with and without GA (1 µM); representative of five experiments. The presence of HSP90 or Tom20 in the PKCε immunoprecipitate (top) was confirmed by the presence of PKCε in the HSP90 (middle) or Tom20 (bottom) immunoprecipitates. None of the proteins could immunoprecipitate with IgG beads alone. Input (mitochondrial lysate) controls are also provided.
3.3. Mitochondrial PKCε is bound to the IMM
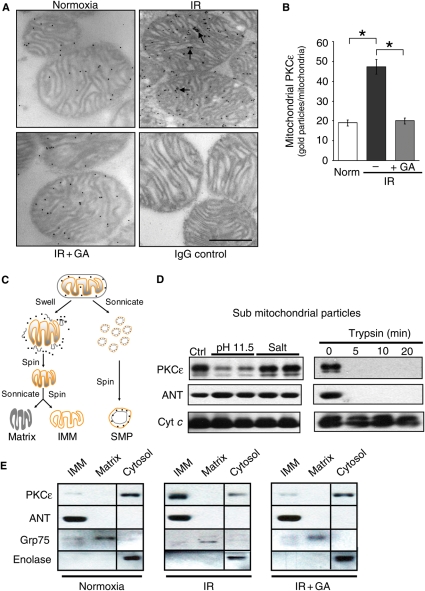
Mitochondrial PKCε location was examined by immunogold electron microscopy (EM) and mitochondrial subfractionation (Figure 3). Gold particles (representing bound PKCε antibody) were found predominantly at the IMM (Figure 3A, right upper panel). Mitochondrial PKCε levels increased by 2.3-fold when compared with normoxic conditions (Figure 3A, IR vs. normoxia, and Figure 3B, n = 3; analysing 60 mitochondria/per group, P < 0.05). The IR-induced increase in PKCε levels was attenuated by GA (Figure 3A, IR + GA and Figure 3B, n = 3, P < 0.05). There was a complete absence of staining when mitochondria were incubated with anti-rabbit IgG conjugated to immunogold, without prior incubation with the PKCε antibody (IgG control), excluding non-specific binding due to IgG.
Figure 3.
PKCε associates with the IMM. (A) Electron micrographs of PKCε immunogold labelling in cardiac mitochondria. Localization on the IMM is indicated by arrows (scale bar = 0.5 µm). Also included is an IgG control example. (B) Quantitation of mitochondrial PKCε immunogold staining; mean ±SEM (n = 3 animals, *P < 0.05). (C) Schematic of protocols used to fractionate mitochondria (SMPs). (D) SMPs from IR hearts were exposed to 200 mM Na2CO3 (pH 11.5), 400 mM KCl (salt), or trypsin digestion and levels of PKCε, the IMM protein, ANT, and the IMS protein, cytochrome c, were determined by western blotting. (E) IMM and mitochondrial matrix-enriched and cytosolic fractions were probed for PKCε, ANT (a marker of IMM), glucose response protein (Grp75; a marker of matrix fraction), and enolase (a cytosolic marker) from hearts subjected to normoxia, IR, or IR in the presence of 1 µM GA.
To further investigate the localization of PKCε in the mitochondria, SMPs were prepared from hearts subjected to IR. SMPs were generated by sonication, creating inside-out mitochondrial vesicles, exposing IMM-associated proteins that face the matrix, and sequestering proteins that face the inner mitochondrial space, like cytochrome c, within the inverted mitochondrial vesicle21 (Figure 3C). Exposure to a 200 mM Na2CO3 carbonate wash at pH 11.5 (which removes strongly bound, but non-integral, membrane proteins) removed PKCε from the IMM, whereas exposure to 400 mM KCl high-salt wash (which removes loosely associated proteins from membranes) did not dislodge PKCε from this fraction (Figure 3D, upper left panel). Trypsin, which cannot cross membranes, completely removed PKCε from these inside-out mitochondrial vesicles (Figure 3D, top right panel). That trypsin could access PKCε suggests that PKCε is present on the exposed (matrix) side of the IMM in the SMP preparation.21 In contrast, the levels of cytochrome c, present in the space between the inner and the outer mitochondrial membranes (and therefore resides inside the SMP vesicles), were unaffected by trypsin digestion (Figure 3D, lower right panel), whereas the adenine nucleotide translocase (ANT), an integral IMM protein, showed a similar sensitivity to trypsin digestion as PKCε (Figure 3D, middle right panel).
Mitochondria from hearts exposed to normoxia or IR were also subfractionated to yield IMM- and matrix-enriched components (Figure 3C, right scheme, and Figure 3E), which were then probed for PKCε (Figure 3E, upper panels). [Purity was confirmed using antibodies against ANT (a marker of IMM), Grp75 (a mitochondrial matrix marker), and enolase (a cytosolic marker).] Similar to the EM analysis, PKCε in the IMM-enriched fraction increased following exposure to IR and was reduced by GA.
3.4. A rationally designed peptide activator of HSP90 and PKCε interaction (ψεHSP90) increases PKCε translocation to cardiac mitochondria
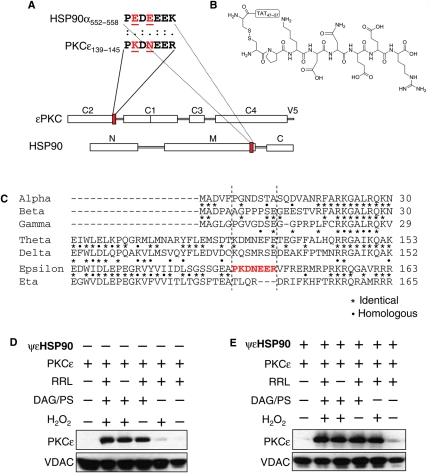
We previously identified peptide activators of PKCε that induce protein–protein interaction between PKCε and its anchoring protein, εRACK.3 Specifically, an 8-amino acid peptide corresponding to a sequence in the C2 domain of PKCε that is homologous to a sequence in εRACK3 (hence termed pseudo-εRACK or ψεRACK peptide) was found to be an allosteric agonist of PKCε. ψεRACK interferes with the auto-inhibitory _intra_-molecular interaction within PKCε, stabilizing PKCε in a conformational state in which the εRACK-binding site on PKCε is available for _inter_-molecular interaction with εRACK.3,23 The observation that PKCε only binds HSP90 upon activation (Figure 2), as is the case with PKCε-εRACK binding, led us to reason that the HSP90-binding site on PKCε may also participate in an auto-inhibitory _intra_-molecular interaction when PKCε is inactive. Using L-ALIGN sequence alignment software, we identified PKDNEER at the end of the C2 domain of PKCε (amino acids 139–145; Figure 4A) to be homologous to PEDEEEK, found at the end of the middle domain of HSP90 (amino acids 552–558 on HSP90α and 544–550 on HSP90β; Figure 4A). There are two charge differences between these homologous sequences (Lys140 and Asn142 on PKCε compared with Glu553 and Glu555 on HSP90α; red and underlined in Figure 4A). Such charge differences between the sequences were found in each of the other previous pseudo-site-derived peptides.24,25 Further, the HSP90 homologous sequence on PKCε is not found in any other members of the PKC family (Figure 4C), but is evolutionary conserved in PKCε in a variety of species, including human, rat, mouse, and Xenopus. We synthesized a peptide corresponding to the HSP90 homology region in PKCε (termed ψεHSP90) and conjugated it, via cysteine–cysteine bond, to the cell permeating TAT protein-derived peptide, TAT47–5725 (Figure 4B).
Figure 4.
A rationally designed peptide activator of HSP90 and PKCε interaction (ψεHSP90) increases mitochondrial PKCε translocation. (A) Homologous sequence between PKCε and HSP90 displaying charge differences (Lys140 and Asn142 in human PKCε and Glu553 and Glu555 in human HSP90α). Also shown is a scheme indicating the location of the homologous sequences (red) on PKCε and HSP90. (B) ψεHSP90 peptide conjugated to cell penetrating TAT47–57 peptide. (C) Alignment of human PKC isozymes, indicating that the ψεHSP90 sequence is unique for the PKCε isozyme. Identical and homologous sequences are indicated by asterisks and filled circles, respectively. Mitochondrial association of recombinant PKCε after activation with DAG/PS (1 mM) and/or H2O2 (50 µM) and with RRL in the absence (D) and presence (E) of 1 µM ψεHSP90. VDAC is used as a loading control.
The effect of ψεHSP90 on mitochondrial PKCε translocation was initially determined, in vitro. Isolated cardiac mitochondria were incubated with recombinant PKCε in the absence or presence of the PKC activators, DAG/PS (1mM), and/or with hydrogen peroxide (H2O2; 50 µM Figure 4D and E). Unstimulated recombinant PKCε did not associate with the mitochondria. However, on activation with DAG/PS, there was substantial mitochondrial PKCε association (Figure 4D). H2O2 induced only a limited PKCε translocation and did not increase the mitochondrial PKCε translocation induced by DAG/PS (Figure 4D). RRL, used as an exogenous source of HSP90,26 did not increase PKCε association with the mitochondria, suggesting that HSP90 is already present at the mitochondria. This was also demonstrated by the data in Figure 2A (see also Figure 5D), in which HSP90 was detected at the mitochondria under normoxic conditions, and in agreement with previous data.27 In the presence of ψεHSP90 peptide (1 µM, Figure 4E), the H2O2-induced mitochondrial association of PKCε was enhanced (Figure 4E, second to last lane), suggesting that ψεHSP90 enhances oxidative stress-induced mitochondrial translocation of PKCε.
3.5. ψεHSP90 promotes mitochondrial PKCε translocation, activates mitochondrial ALDH2, and reduces IR injury
We determined whether ψεHSP90 peptide increased mitochondrial translocation of PKCε in ex vivo hearts. Mitochondria isolated from hearts subjected to IR in the presence of 1 µM ψεHSP90 (see protocols in Figure 5A) had a 35 ± 7% increase in PKCε (n = 5, P < 0.05; Figure 5B and C), when compared with the IR alone group. Mitochondrial PKCδ translocation was unaffected by ψεHSP90 treatment (Figure 5B and C), indicating specificity for PKCε. Further, ψεHSP90 did not affect PKCε translocation to the plasma membrane (Figure 5B, lower panels), indicating a selective increase in mitochondrial PKCε. There was no effect of the TAT carrier peptide alone on mitochondrial translocation of PKCε or CK release (see Supplementary material online, Figure S1). Treatment with ψεHSP90 also enhanced IR-induced physical interaction between HSP90 and PKCε and the ψεHSP90-induced increase in PKCε–HSP90 association was substantially reduced by pharmacological inhibition of HSP90 with GA (Figure 5D).
We previously found that selective PKCε activation results in ALDH2 phosphorylation and increases ALDH2 activity,4 and that ALDH2 activity correlates with cardioprotection from IR [_R_2 = 0.97].4 When compared with control-treated hearts, ψεHSP90 treatment caused a 38 ± 9% increase in ALDH2 activity (Figure 5E; n = 6, P < 0.05), an effect that was blocked by HSP90 inhibition with GA (Figure 5E; n = 6, P < 0.05). To determine whether increased ALDH2 activity was due to increased ALDH2 phosphorylation, we performed two-dimensional gel immunoelectrophoresis (2D IEF) followed by immunoblotting with anti-ALDH2 antibodies. Similar to our study using ψεRACK,4 immunoreactive ALDH2 shifted to more acidic pI ranges after ψεHSP90 treatment when compared with IR alone (Figure 5F), suggesting increased ALDH2 phosphorylation. This was also confirmed directly by immunoblotting the ALDH2 immunoprecipitate with anti-phospho Ser/Thr antibody (Figure 5 G). The ψεHSP90-induced increase in ALDH2 phosphorylation and activity was prevented by GA, demonstrating the requirement of HSP90. In addition, ψεHSP90 treatment increased ALDH2-PKCε co-IP in these samples (Figure 5G, lane 3). Finally, we determined the effect of ψεHSP90 peptide on cardiac damage following IR. Hearts treated with ψεHSP90 peptide had ∼50% reduced IR-induced cardiac damage as assessed by CPK release (Figure 5H; P < 0.05, n = 6), which was attenuated by HSP90 inhibition (Figure 5H; P < 0.05, n = 5).
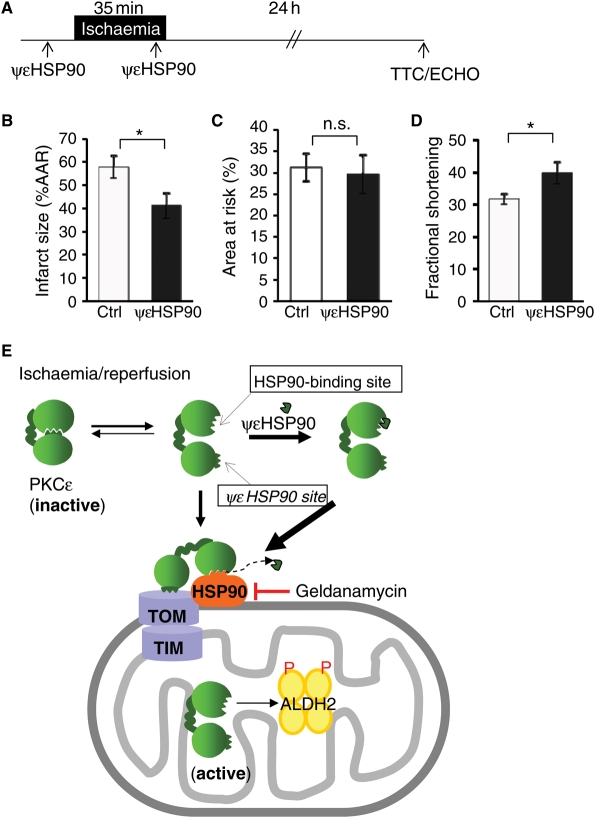
3.6. ψεHSP90 peptide reduces infarct size and increases functional recovery following ischaemia and reperfusion injury, in vivo
We used an in vivo model of acute myocardial infarction in adult male rats that consisted of 35 min ischaemia followed by 24 h reperfusion. Rats were injected intraperitoneally with either ψεHSP90 or with the control carrier peptide, TAT, (1 mg/kg in 400 µL saline, each), which were administered 15 min before LAD occlusion and 5 min before the onset of reperfusion (Figure 6A). [The dose and route of administration were chosen based on our previous work using other TAT-conjugated peptides, such as ψεRACK and εV1–228,29.] Treatment with ψεHSP90 reduced infarct size by ∼30% relative to control animals (Figure 6B; n = 6, P < 0.05), which had equivalent AAR for infarction (Figure 6C). Treatment with ψεHSP90 also led to improvement in cardiac function; fractional shortening increased from 31 ± 2% in TAT control-treated rats to 40 ± 3% in rats treated with ψεHSP90 (Figure 6D; n = 6, P < 0.05).
Figure 6.
ψεHSP90 reduces infarct size and improves functional recovery following ischaemia/reperfusion injury in vivo. (A) An in vivo model of myocardial infarction (B) ψεHSP90 reduced infarct size from 57 ± 5% in control (TAT carrier treated) to 41 ± 6% (n = 6, *P < 0.05). (C) AAR was equal in both experimental groups. (D) ψεHSP90 improved fractional shortening from 31 ± 2% in controls, to 40 ± 3% (n = 6, *P < 0.05). Histograms show mean ± SEM (n = 6, *P < 0.05). (E) A proposed scheme of mitochondrial PKCε translocation. On activation, PKCε undergoes a conformational change, exposing the HSP90-binding site, enabling HSP90–PKCε interaction at the mitochondria and PKCε interaction with TOM, promoting mitochondrial import of PKCε. The ψεHSP90 peptide binds to the open form of PKCε, stabilizing the enzyme in a conformation that promotes PKCε–HSP90 interaction. Because the affinity of PKCε for the ψεHSP90 peptide is lower than that of the native PKCε-binding site on HSP90, the peptide is displaced when PKCε binds HSP90 at the mitochondria.
4. Discussion
PKCε is critical for cardioprotection from IR injury. A number of studies have demonstrated that PKCε-mediated protection is due to phosphorylation of mitochondrial proteins.4,6,8,13,30 Here, we report that mitochondrial import of PKCε is mediated by HSP90 and plays a crucial cardioprotective role. Although previous studies have demonstrated PKCε activity within mitochondria,6–8,10,30 to our knowledge, this study is the first description of interaction between PKCε and the mitochondrial import machinery and suggests a possible mechanism for mitochondrial translocation of PKCε. We also describe a peptide, designed based on PKCε–HSP90 protein–protein interaction sites (ψεHSP90), which increases mitochondrial PKCε-HSP90 interaction, promotes mitochondrial translocation of PKCε, and reduces infarct size ex vivo and in vivo.
A number of cardioprotective stimuli have been found to enhance mitochondrial translocation of PKC,4,5,9,11,12,30 whereas other studies did not support a role for mitochondrial PKCε in cardioprotection.14,15 This discrepancy may be explained by differences in stimulation or models used, the time point at which the translocation analyses were performed, or differences in cellular fractionation techniques. Here, we found that IR-induced mitochondrial translocation of both PKCε and PKCδ when analysed 15 min after reperfusion began. HSP90 inhibition during reperfusion attenuated mitochondrial translocation of PKCε, but not that of PKCδ (Figure 1). Electron microscopy and mitochondrial subfractionation analyses confirmed that intra-mitochondrial PKCε levels are increased by IR in an HSP90-dependent manner and demonstrated that mitochondrial PKCε is localized at the matrix side of the IMM (Figure 3). These data are consistent with recent studies reporting PKCε at the IMM,10,30 and with data demonstrating that PKCε phosphorylates a number of intra-mitochondrial proteins.4–6,8,13,30 Because mitochondrial translocation of PKCε occurs rapidly, with a corresponding decline in cytosolic PKCε levels, and since the total cellular PKCε levels do not change (Figure 1B), our data suggest that HSP90 enables dynamic mitochondrial translocation of PKCε in response to IR. HSP90-mediated mitochondrial import of proteins proceeds via the translocase of the outer membrane (TOM) multiprotein complex through recognition of the chaperoned protein by the import receptors, Tom20, Tom22, or Tom70.16 We found that IR induced physical association between PKCε and mitochondrial Tom20, which was prevented by GA. These data suggest that stimulus-induced mitochondrial import of PKCε proceeds via an HSP90-dependent interaction with the TOM import complex. HSP90 inhibition did not affect IR-induced mitochondrial translocation of PKCδ. Other chaperones including HSP70, HSC70, and HSP40 mediate mitochondrial import of proteins;17,31 therefore, it is possible that an another chaperone mediates mitochondrial import of PKCδ.
HSP90 inhibition with GA during reperfusion resulted in a 70% increase in CK release, indicating that HSP90 mediates a cytoprotective function during reperfusion of ischaemic myocardium. However, HSP90 mediates a number of functions, including mitochondrial translocation of other cytoprotective proteins;18 therefore, increased damage due to GA was not exclusively due to effects on mitochondrial PKCε. We therefore sought a means to selectively modulate PKCε–HSP90 interaction. Because PKCε is regulated by multiple _intra_-molecular interactions,23,32 we reasoned that an inhibitory _intra_-molecular interaction may exist between the HSP90-binding site in PKCε and a sequence within PKCε that shares homology with a region on HSP90 (Figure 6E). The ψεHSP90 peptide corresponds to such a 7-amino acid sequence homology between the C2 domain of PKCε, which is homologous with a sequence in the middle domain of HSP90. The C2 domain of PKCε is known to mediate PKCε protein–protein interactions,33 and the corresponding sequence on HSP90 resides within a region that is essential for HSP90 protein–protein interaction.34 Importantly, charge differences exist between these homologous sequences, characteristic of interaction sites within PKC.25,33
Our data demonstrate that ψεHSP90 treatment enhanced IR-induced protein–protein interaction between PKCε and HSP90 (Figure 5D), enhanced mitochondrial PKCε translocation (Figures 4D and E, and 5B_–_D), and decreased cardiac injury (Figures 5H and 6B). The ψεHSP90-induced effects were attenuated by GA, demonstrating that ψεHSP90 requires HSP90 (Figure 5E_–_H). The ψεHSP90 peptide did not affect PKCδ mitochondrial translocation, demonstrating selectivity for PKCε. Since PKCε does not associate with HSP90 until activation with IR, it is likely that PKCε–HSP90 interaction is dependent on a conformational change that occurs upon PKCε activation, which exposes the HSP90-binding site. We propose that ψεHSP90 stabilizes the activated PKCε in a transient conformation that promotes its binding to HSP90, resulting in enhanced mitochondrial import of PKCε (Figure 6E).
We recently identified mitochondrial ALDH2 as a PKCε substrate, whose activity correlates with cardioprotection from IR.4 Here, we showed that ψεHSP90 increased phosphorylation and activity of ALDH2. Although the current study focused on ALDH2, PKCε can regulate other mitochondrial functions that mediate cytoprotection, including regulation of mitochondrial respiration and ROS production (mediated by phosphorylation of COIV by PKCε),6,7,30 regulation of mitochondrial K+ flux and mitochondrial matrix swelling,10 and inhibition of MPTP opening.9
In summary, our results demonstrate that mitochondrial import of PKCε is mediated by HSP90 and is required for cardiac protection against IR. Our data suggest a possible mechanism by which PKCε can access cytoprotective substrates located within the mitochondria to confer cardioprotection. We also describe a novel peptide activator of PKCε, ψεHSP90, which promotes mitochondrial PKCε–HSP90 interaction and may have therapeutic use in the treatment of cardiac IR injury.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: D.M.-R. is a founder and shareholder of KAI pharmaceuticals. None of the work was supported by, or performed in collaboration with the company.
Funding
This work was supported by NIH grants AA11147 and HL52141 to D.M.-R. and, in part, by an American Heart Association Western States postdoctoral fellowship to G.R.B. Funding to pay the Open Access publication charges for this article was provided by National Institutes of Health grants AA11147 and HL52141 to D.M.-R.
Supplementary Material
Supplementary Data
References
- 1.Liu GS, Cohen MV, Mochly-Rosen D, Downey JM. Protein kinase C-epsilon is responsible for the protection of preconditioning in rabbit cardiomyocytes. J Mol Cell Cardiol. 1999;31:1937–1948. doi: 10.1006/jmcc.1999.1026. doi:10.1006/jmcc.1999.1026. [DOI] [PubMed] [Google Scholar]
- 2.Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, et al. Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997;81:404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- 3.Dorn GW, II, Souroujon MC, Liron T, Chen CH, Gray MO, Zhou HZ, et al. Sustained in vivo cardiac protection by a rationally designed peptide that causes epsilon protein kinase C translocation. Proc Natl Acad Sci USA. 1999;96:12798–12803. doi: 10.1073/pnas.96.22.12798. doi:10.1073/pnas.96.22.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. doi:10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Churchill EN, Disatnik MH, Mochly-Rosen D. Time-dependent and ethanol-induced cardiac protection from ischemia mediated by mitochondrial translocation of varepsilonPKC and activation of aldehyde dehydrogenase 2. J Mol Cell Cardiol. 2009;46:278–284. doi: 10.1016/j.yjmcc.2008.09.713. doi:10.1016/j.yjmcc.2008.09.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogbi M, Chew CS, Pohl J, Stuchlik O, Ogbi S, Johnson JA. Cytochrome c oxidase subunit IV as a marker of protein kinase Cepsilon function in neonatal cardiac myocytes: implications for cytochrome c oxidase activity. Biochem J. 2004;382:923–932. doi: 10.1042/BJ20040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dave KR, DeFazio RA, Raval AP, Torraco A, Saul I, Barrientos A, et al. Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase C epsilon. J Neurosci. 2008;28:4172–4182. doi: 10.1523/JNEUROSCI.5471-07.2008. doi:10.1523/JNEUROSCI.5471-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnett M, Lin D, Akoyev V, Willard L, Takemoto D. Protein kinase C epsilon activates lens mitochondrial cytochrome c oxidase subunit IV during hypoxia. Exp Eye Res. 2008;86:226–234. doi: 10.1016/j.exer.2007.10.01. doi:10.1016/j.exer.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baines CP, Song CX, Zheng YT, Wang GW, Zhang J, Wang OL, et al. Protein kinase Cepsilon interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ Res. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. doi:10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaburek M, Costa AD, Burton JR, Costa CL, Garlid KD. Mitochondrial PKC epsilon and mitochondrial ATP-sensitive K+ channel copurify and coreconstitute to form a functioning signaling module in proteoliposomes. Circ Res. 2006;99:878–883. doi: 10.1161/01.RES.0000245106.80628.d3. doi:10.1161/01.RES.0000245106.80628.d3. [DOI] [PubMed] [Google Scholar]
- 11.Ohnuma Y, Miura T, Miki T, Tanno M, Kuno A, Tsuchida A, et al. Opening of mitochondrial K(ATP) channel occurs downstream of PKC-epsilon activation in the mechanism of preconditioning. Am J Physiol Heart Circ Physiol. 2002;283:H440–H447. doi: 10.1152/ajpheart.00434.2001. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence KM, Kabir AM, Bellahcene M, Davidson S, Cao XB, McCormick J, et al. Cardioprotection mediated by urocortin is dependent on PKCepsilon activation. FASEB J. 2005;19:831–833. doi: 10.1096/fj.04-2506fje. [DOI] [PubMed] [Google Scholar]
- 13.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, et al. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. doi:10.1161/01.RES.0000012702.90501.8D. [DOI] [PubMed] [Google Scholar]
- 14.Clarke SJ, Khaliulin I, Das M, Parker JE, Heesom KJ, Halestrap AP. Inhibition of mitochondrial permeability transition pore opening by ischemic preconditioning is probably mediated by reduction of oxidative stress rather than mitochondrial protein phosphorylation. Circ Res. 2008;102:1082–1090. doi: 10.1161/CIRCRESAHA.107.167072. doi:10.1161/CIRCRESAHA.107.167072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uecker M, Da Silva R, Grampp T, Pasch T, Schaub MC, Zaugg M. Translocation of protein kinase C isoforms to subcellular targets in ischemic and anesthetic preconditioning. Anesthesiology. 2003;99:138–147. doi: 10.1097/00000542-200307000-00023. doi:10.1097/00000542-200307000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Neupert W, Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem. 2007;76:723–749. doi: 10.1146/annurev.biochem.76.052705.163409. doi:10.1146/annurev.biochem.76.052705.163409. [DOI] [PubMed] [Google Scholar]
- 17.Young JC, Hoogenraad NJ, Hartl FU. Molecular chaperones Hsp90 and Hsp70 deliver preproteins to the mitochondrial import receptor Tom70. Cell. 2003;112:41–50. doi: 10.1016/s0092-8674(02)01250-3. doi:10.1016/S0092-8674(02)01250-3. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Sinovas A, Boengler K, Cabestrero A, Gres P, Morente M, Ruiz-Meana M, et al. Translocation of connexin 43 to the inner mitochondrial membrane of cardiomyocytes through the heat shock protein 90-dependent TOM pathway and its importance for cardioprotection. Circ Res. 2006;99:93–101. doi: 10.1161/01.RES.0000230315.56904.de. doi:10.1161/01.RES.0000230315.56904.de. [DOI] [PubMed] [Google Scholar]
- 19.Pallotti F, Lenaz G. Isolation and subfractionation of mitochondria from animal cells and tissue culture lines. Methods Cell Biol. 2007;80:3–44. doi: 10.1016/S0091-679X(06)80001-4. doi:10.1016/S0091-679X(06)80001-4. [DOI] [PubMed] [Google Scholar]
- 20.Kang D, Nishida J, Iyama A, Nakabeppu Y, Furuichi M, Fujiwara T, et al. Intracellular localization of 8-oxo-dGTPase in human cells, with special reference to the role of the enzyme in mitochondria. J Biol Chem. 1995;270:14659–14665. doi: 10.1074/jbc.270.24.14659. [DOI] [PubMed] [Google Scholar]
- 21.Pagliarini DJ, Wiley SE, Kimple ME, Dixon JR, Kelly P, Worby CA, et al. Involvement of a mitochondrial phosphatase in the regulation of ATP production and insulin secretion in pancreatic beta cells. Mol Cell. 2005;19:197–207. doi: 10.1016/j.molcel.2005.06.008. doi:10.1016/j.molcel.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Schulte TW, Akinaga S, Soga S, Sullivan W, Stensgard B, Toft D, et al. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones. 1998;3:100–108. doi: 10.1379/1466-1268(1998)003<0100:arbttn>2.3.co;2. doi:10.1379/1466-1268(1998)003<0100:ARBTTN>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schechtman D, Craske ML, Kheifets V, Meyer T, Schechtman J, Mochly-Rosen D. A critical intramolecular interaction for protein kinase Cepsilon translocation. J Biol Chem. 2004;279:15831–15840. doi: 10.1074/jbc.M310696200. doi:10.1074/jbc.M310696200. [DOI] [PubMed] [Google Scholar]
- 24.Kheifets V, Bright R, Inagaki K, Schechtman D, Mochly-Rosen D. Protein kinase C delta (deltaPKC)-annexin V interaction: a required step in deltaPKC translocation and function. J Biol Chem. 2006;281:23218–23226. doi: 10.1074/jbc.M602075200. doi:10.1074/jbc.M602075200. [DOI] [PubMed] [Google Scholar]
- 25.Chen L, Hahn H, Wu G, Chen CH, Liron T, Schechtman D, et al. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc Natl Acad Sci USA. 2001;98:11114–11119. doi: 10.1073/pnas.191369098. doi:10.1073/pnas.191369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scherrer LC, Hutchison KA, Sanchez ER, Randall SK, Pratt WB. A heat shock protein complex isolated from rabbit reticulocyte lysate can reconstitute a functional glucocorticoid receptor-Hsp90 complex. Biochemistry. 1992;31:7325–7329. doi: 10.1021/bi00147a017. doi:10.1021/bi00147a017. [DOI] [PubMed] [Google Scholar]
- 27.Barksdale KA, Bijur GN. The basal flux of Akt in the mitochondria is mediated by heat shock protein 90. J Neurochem. 2009;108:1289–1299. doi: 10.1111/j.1471-4159.2009.05878.x. doi:10.1111/j.1471-4159.2009.05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inagaki K, Begley R, Ikeno F, Mochly-Rosen D. Cardioprotection by epsilon-protein kinase C activation from ischemia: continuous delivery and antiarrhythmic effect of an epsilon-protein kinase C-activating peptide. Circulation. 2005;111:44–50. doi: 10.1161/01.CIR.0000151614.22282.F1. doi:10.1161/01.CIR.0000151614.22282.F1. [DOI] [PubMed] [Google Scholar]
- 29.Begley R, Liron T, Baryza J, Mochly-Rosen D. Biodistribution of intracellularly acting peptides conjugated reversibly to Tat. Biochem Biophys Res Commun. 2004;318:949–954. doi: 10.1016/j.bbrc.2004.04.121. doi:10.1016/j.bbrc.2004.04.121. [DOI] [PubMed] [Google Scholar]
- 30.Ogbi M, Johnson JA. Protein kinase Cepsilon interacts with cytochrome c oxidase subunit IV and enhances cytochrome c oxidase activity in neonatal cardiac myocyte preconditioning. Biochem J. 2006;393:191–199. doi: 10.1042/BJ20050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhangoo MK, Tzankov S, Fan AC, Dejgaard K, Thomas DY, Young JC. Multiple 40-kDa heat-shock protein chaperones function in Tom70-dependent mitochondrial import. Mol Biol Cell. 2007;18:3414–3428. doi: 10.1091/mbc.E07-01-0088. doi:10.1091/mbc.E07-01-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ron D, Mochly-Rosen D. Agonists and antagonists of protein kinase C function, derived from its binding proteins. J Biol Chem. 1994;269:21395–21398. [PubMed] [Google Scholar]
- 33.Kheifets V, Mochly-Rosen D. Insight into intra- and inter-molecular interactions of PKC: design of specific modulators of kinase function. Pharmacol Res. 2007;55:467–476. doi: 10.1016/j.phrs.2007.04.014. doi:10.1016/j.phrs.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizuno K, Shirogane T, Shinohara A, Iwamatsu A, Hibi M, Hirano T. Regulation of Pim-1 by Hsp90. Biochem Biophys Res Commun. 2001;281:663–669. doi: 10.1006/bbrc.2001.4405. doi:10.1006/bbrc.2001.4405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data