A Chromatin-remodeling Protein Is a Component of Fission Yeast Mediator (original) (raw)
Abstract
The multiprotein Mediator complex is an important regulator of RNA polymerase II-dependent genes in eukaryotic cells. In contrast to the situation in many other eukaryotes, the conserved Med15 protein is not a stable component of Mediator isolated from fission yeast. We here demonstrate that Med15 exists in a protein complex together with Hrp1, a CHD1 ATP-dependent chromatin-remodeling protein. The Med15-Hrp1 subcomplex is not a component of the core Mediator complex but can interact with the L-Mediator conformation. Deletion of med15+ and hrp1+ causes very similar effects on global steady-state levels of mRNA, and genome-wide analyses demonstrate that Med15 associates with a distinct subset of Hrp1-bound gene promoters. Our findings therefore indicate that Mediator may directly influence histone density at regulated promoters.
Keywords: Chromatin, Chromatin Immunoprecipitation (ChIP), Chromatin Remodeling, RNA Polymerase II, Transcription Coactivators, Mediator Complex
Introduction
Mediator is required for basal and regulated expression of nearly all RNA pol2 II-dependent genes in Saccharomyces cerevisiae (1, 2). According to a generally accepted model, Mediator conveys regulatory information from enhancers and other control elements to the promoter (3, 4). Functional activities identified for Mediator include stimulation of basal transcription, support of activated transcription, and enhancement of phosphorylation of the C-terminal domain of pol II by the transcription factor II H kinase (5, 6).
Low resolution structure analysis of core S. cerevisiae Mediator (S-Mediator) has identified three structural submodules, the head, middle, and tail domains (7). A second, larger form of Mediator (L-Mediator) has been isolated from many eukaryotes, including mammalian cells and Schizosaccharomyces pombe (4). L-Mediator is characterized by the presence of an additional protein module, the Cdk8 module, which contains four conserved proteins (Med12, Med13, CDK8, and CycC) (8, 9). The Cdk8 module sterically blocks pol II interactions with Mediator, and L-Mediator can therefore not form a holoenzyme complex with pol II (10).
The Mediator head and middle domains contain a number of highly conserved subunits that can interact directly with pol II, leading to the formation of a pol II holoenzyme. In S. cerevisiae, the tail domain has been shown to interact with and be important for the function of many gene-specific activators, e.g. Gcn4 and Pdr1 (11–13). S. pombe Mediator complexes contain orthologues to components of the S. cerevisiae Mediator head and middle region but lack counterparts to the proteins found in the S. cerevisiae tail region (Med2, Med3, Med5, Med15, and Med16) (14, 15). In support of this notion, structural analysis of the S. pombe S-Mediator only reveals the existence of a head and middle domain (10). However, even if there is no tail domain in S. pombe Mediator, the genome does encode for a putative Med15 homologue (SPBC146.01) (16). A role for fission yeast Med15 in Mediator function remains to be established.
In addition to the Mediator complex, transcription is also regulated by a series of mechanisms affecting chromatin structure. The N-terminal tails of histone can be modified in numerous ways, including phosphorylation, acetylation, and methylation. Many of these post-translational modifications have functional consequences for chromatin function, e.g. dimethylation of histone H3 lysine 4 (H3K4me2) makes chromatin permissive to transcription, and trimethylation of the same residue (H3K4me3) is associated only with actively transcribed genes (17).
The CHD (chromo-helicase/ATPase DNA binding)-remodeling factors are highly conserved and distinguished from other remodeling factors by their double chromodomains. Drosophila Chd1 (dChd1) is believed to play a role in transcriptional regulation and has been detected at actively transcribed coding regions (18). The dChd1 protein affects nucleosome spacing (19) and is required for deposition of the histone variant H3.3 into chromatin in vivo (20). Human and yeast Chd1 has been proposed to bind H3K4me3 via its tandem chromodomain (21, 22), but fluorometric titration experiments have argued against this and suggest that only human Chd1 can bind specifically to methylated H3K4 (23). In support of this conclusion, structural analysis with NMR in combination with sequence comparison of Chd1 homologues demonstrated that S. cerevisiae Chd1 lacks several amino residues crucial for H3K4me3 binding (24). Studies of the CHD1 paralogues Hrp1 and Hrp3 in fission yeast have demonstrated that CHD1-remodeling factors are specifically colocalized to promoter regions, where they help to remove nucleosomes near the transcription start site (25). Hrp1 and Hrp3 also regulate nucleosome density in coding regions, where they have redundant roles in stimulating transcription.
In this report, we establish a crucial role for fission yeast Med15 in activated gene transcription. We furthermore demonstrate that Med15 is present in a complex with Hrp1 and that the Med15-Hrp1 complex associates with L-Mediator but not with the purified S-Mediator complex. Genome-wide studies of chromatin binding and gene transcription support a close functional relationship between the Hrp1 and Med15 proteins. Our study reveals a link between Mediator and remodeling of chromatin structure at specific promoters. We propose that Mediator may influence gene chromatin structure by directly influencing the activity of the Hrp1 protein. It should be noted that the structure and function of Mediator appear conserved in fungi and metazoan cells, and to simplify comparisons with other experimental systems, throughout this study, we use the recently proposed unifying Mediator nomenclature (26).
EXPERIMENTAL PROCEDURES
Strains and Cell Culture
The genotypes of the S. pombe strains used in this study are presented in Table 1.
TABLE 1.
Strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| CG71 | h+med15+::myc-kanMX6 ade6-M210 leu1–32 ura4-D18 | This study |
| CG132 | h_−_med15::natMX4 | This study |
| CG197 | h+med15+::2flag-kanMX6 hrp1::natMX4 med13+::TAP-kanMX6 | This study |
| Hu808 | h_−_hrp1::leu2 leu1–32 | Walfridsson et al. (25) |
| HU764 | h+hrp1+::myc-kanMX6 ade6-M216 leu1–32 ura4-D18 | Walfridsson and co-workers (27) |
| L972 | _h_− | |
| TP46 | h+med7+::TAP-kanMX6 ade6-M216 | Elmlund et al. (10) |
| TP68 | h+med13+::TAP-kanMX6 ade6-M216 | Samuelsen et al. (9) |
| TP161 | h+med7+::TAP-ade6–216 med13::kanMX6 | Elmlund et al. (10) |
| TP211 | h_−_med15+::flag-kanMX6 ade6-M216 | This study |
| TP314 | h+med13+::TAP-kanMX6 cdk8:: ura4+ | Baraznenok et al.a |
Chromatin Immunoprecipitation
Cells were grown to a mid-log phase at 30 °C in YES medium (5 g/liter yeast extract, 0.1 g/liter each of adenine, leucine, histidine, uracil, lysine, arginine, and glutamine, and 20 g/liter glucose). For ChIP-on-chip experiments, 2 × 108 cells were fixed in 1% formaldehyde for 30 min, quenched in 125 mm glycine, harvested, and washed twice in ice-cold PBS. Cells were resuspended in lysis buffer (150 mm NaCl, 0.1% SDS, 1% Triton X-100, 0.1% sodium deoxycholate, 1 mm EDTA, and 50 mm HEPES-KOH, pH 7.5) with protease inhibitors and lysed in a FastPrep machine (FP120, BIO101 Savant) with 1 volume of glass beads. The extracts were sonicated (Bioruptor UCD-200, Diagenode) to chromatin fragments of ∼200–1,000 bp. Antibodies used for chromatin immunoprecipitation were α-H3 (ab1791, Abcam) and α-Myc (M4439, Sigma). These antibodies were used with 30 μl of protein A-Sepharose slurry (17-5280-01, GE Healthcare) or 50 μl of protein A beads (P-3391, Sigma). Cross-links were reversed by overnight incubation at 65 °C, after which the immunoprecipitated DNA was treated with proteinase K and purified by phenol-chloroform extraction followed by ethanol precipitation. After RNase A treatment, 2 μl out of a total volume of 40 μl was used as a template for real time quantitative PCR analysis (LightCycler 2.0, Roche Applied Science). Quality of ChIP was assessed against a negative control IP performed without antibody. The enrichment was quantified as a fraction of input DNA.
DNA Amplification, Labeling, and Hybridization
For ChIP-on-chip analysis, 500 ng of amplified IP and input DNA was labeled using Cy3-dCTP or Cy5-dCTP, and both samples were hybridized together to an array (Eurogentec) containing intergenic regions (IGR) corresponding to the 500 bp immediately upstream of the translation start and open reading frames (ORF) sequences corresponding to the last 500 bp of each coding region in the fission yeast genome, as described previously. Additional IGR probes for genes that have larger intergenic regions were included in the array as well as probes for centromere and flanking regions (27). Two to three independent ChIP-on-chip experiments were performed in each case, with dye swap carried out to correct for dye bias.
Microarray Data Analysis
Data analysis using Genespring GX software (v.7.3, Agilent Technologies) was carried out as follows. Measurements less than 0.01 were set to 0.01, and data were normalized to the 50th percentile and filtered on flags to remove data from poor quality spots before further analysis. A cutoff of 2 was used to define sites with high or low histone H3 density, whereas median percentile ranking was carried out for the binding data (cutoffs: Hrp1 binding IGR 0.90 and ORF 0.92; Med15 binding IGR 0.93 and ORF 0.94) (28). Gene lists were compared for similarity using hypergeometric distribution tests. Gene ontology terms were determined using GOMiner (29).
Gene expression was analyzed on the Affymetrix Yeast Genome 2.0 platform with help of the ExpressionStat algorithm followed by per gene normalization to median. Data were filtered on flags to remove spots that did not show any signal for either the WT or the mutant samples to exclude them from expression analysis. Up- or down-regulated genes were chosen using a 2-fold cutoff when compared with wild type expression levels. Microarray data have been deposited in the GEO database under accession number GSE10079.
Protein Purification
Yeast extract preparations for TAP and FLAG purification were performed as described earlier (10). Cultures of 7.5 liters were grown to an _A_600 of ∼3.0. Cells were harvested by centrifugation (JLA 8.100 Beckman Coulter, 4,000 rpm at +4 °C) and washed once with cold water. Cells were ground in liquid nitrogen using the freezer/mill 6850 (SPEX CertiPrep) (five cycles, 2 min of grinding, 2 min of rest, rate 14). 20 ml of 3× lysis buffer (200 mm KOH-HEPES, pH 7.8, 15 mm KCl, 1.5 mm MgCl2, 0.5 mm EDTA, 15% glycerol, 1.5 mm DTT, and protease inhibitors) was added to 40 g of thawing disrupted cells together with 30 ml of 1× lysis buffer. All the following manipulations were performed at +4 °C. The supernatant was precleared by centrifugation (JLA10.500 Beckman Coulter, 9,000 rpm, 15 min), and one-ninth of the volume of 2 m KCl was added and stirred for 15 min before ultracentrifugation (Ti-45 Beckman Coulter, 42,000 rpm, 30 min). 40 ml of supernatant was incubated with 250 μl of FLAG-M2 resin (Sigma) (50% slurry in binding buffer (10 mm Tris-Cl, pH 8.0, 150 mm NaCl)) for 40 min. The beads were washed once with 25 ml of binding buffer containing 0.5 mm DTT and protease inhibitors, transferred to 1.5-ml tubes, and then washed twice with 1.5 ml of wash buffer (10 mm Tris-Cl, pH 8.0, 150 mm NaCl, 0.5 mm DTT, 0.5 mm EDTA, 0.05% Nonidet P-40, and protease inhibitors). The FLAG-Med15 was eluted twice with 300 μl of elution buffer (wash buffer containing 0.8 mg/ml FLAG peptide). TAP purification was performed as described previously (10).
Co-immunoprecipitation
Anti-Hrp1 polyclonal antibodies were coupled to NHS-activated Sepharose (GE Healthcare) according to the manufacturer's recommendations. 0.25 g of frozen disrupted cells was thawed in 700 μl of cold Nonidet P-40 buffer (binding buffer with 0.1% Nonidet P-40 and protease inhibitors) and spun for 5 min at 13,000 rpm. The extract was combined with 100 μl of anti-Hrp1 beads (50% slurry in binding buffer) and incubated at +4 °C for 90 min. The beads were washed four times with 400 μl of Nonidet P-40 buffer using Ultrafree filter units (Millipore). The bound fraction was eluted twice with 50 μl of TES (50 mm Tris-Cl, pH 8.0, 1.5% SDS, 10 mm EDTA) at 65 °C for 30 min, resolved on a 10% PAGE gel, and subjected to immunoblot analysis using FLAG antibody (Sigma).
inv1+ Gene Induction
Yeast was grown at 30 °C in YES (5 g/liter yeast extract, 0.1 g/liter each of adenine, leucine, histidine, uracil, lysine, glutamine, and arginine) containing 8% glucose. After the cells had reached mid-log phase, they were spun down and resuspended in YES with supplements and 4% sucrose as a carbon source to induce inv1+ expression. To repress inv1+ transcription, glucose was added to the medium to a final concentration of 3%. For isolation of RNA, 20 ml of the culture was spun down, and the pellets were immediately frozen in liquid nitrogen. Total RNA was isolated using a hot phenol extraction protocol (30), cleaned up using the RNeasy extraction kit (Qiagen), and analyzed by quantitative PCR or primer extension analysis. The quality and quantity of RNA was assessed by a 2100 Bioanalyzer (Agilent Technologies Ltd.). 1 μg of total RNA was used for reverse transcription with random primers, using a 1st strand cDNA synthesis kit (31) according to the manufacturer's recommendations. The reaction mixture was diluted 10 times, and 2 μl was used for real time quantitative PCR using specific primers for inv1+ ORF (Inv1B) or act1+ ORF (Table 2). The level of inv1+ mRNA was normalized to the level of mRNA of act1+ at each time point. For mRNA primer extension analysis, 10 μg of total RNA was used. A primer (5′-TGC TGA CTT CAG TGA TGT TGG AAG-3′) complementary to bases 113–136 of inv1+ coding sequence was γ-32P-labeled with T4 polynucleotide kinase (New England Biolabs). 20 fmol of 5′-labeled primer was annealed at 58 °C for 20 min and then incubated at room temperature for 10 min. The primer extension reaction was performed at 42 °C for 1 h using 30 units of Avian Myeloblastosis Virus reverse transcriptase (Promega, M5101). The extension products were resolved on a 4% polyacrylamide, 7 m urea denaturing gel and visualized by radiography. 5′-RLM-RACE mapping of inv1+ transcript was performed with 10 μg of total RNA using the FirstChoice RLM-RACE kit (Ambion).
TABLE 2.
Primers used in this study
| Gene | Forward | Reverse |
|---|---|---|
| inv1 A | 5′-TAGCCATCATACTGCGATCTTAGACA-3′ | 5′-CGGAGATAAGATTCGGCAAGTTT-3′ |
| inv1 B | 5′-CCTCGTCTCTCTCTTATCATCTACAAAC-3′ | 5′-TGCTGACTTCAGTGATGTTGGAA-3′ |
| inv1 C | 5′-TGCTGACTTCAGTGATGTTGGAAG-3′ | |
| inv1 RACE D | 5′-CTGCACTGCCACTAAATGGGAG-3′ | |
| act1 | 5′-CCTCGTCTCTCTCTTATCATCTACAAAC-3′ | 5′-TGCTGACTTCAGTGATGTTGGAA-3′ |
| cox8 | 5′-CAAGGAAAACTCACCGCACTA-3′ | 5′-GTTGGGAGTTGGTAAGTTTGCTGCTAGC-3′ |
| gti1 | 5′-CGCTCTCTTGCAATCCAATCAATAC-3′ | 5′-GGAAGACGCTTGAGAGTAGAAGAAAA-3′ |
| SPBPB2B2.18 | 5′-CGTGATATGAATCTTGATGGTGGAT-3′ | 5′-GAGAAAGTCACTAGGTCAGAAAGCAA-3′ |
ChIP Analysis of the Inv1+ Gene
The samples for ChIP were collected at time points 0, 15, 30, and 45 min after induction of the inv1+ gene, transcription was repressed, and two additional samples were taken after 15 and 30 min of repression. DNA was purified using phenol extraction followed by ethanol precipitation. H3 density was examined 200 bp upstream from the transcription start site, and the amount of each IP sample was normalized to input DNA from the same extract at each time point.
RESULTS
Med15 Is a Component of the L-Mediator Complex and Interacts with the Chromatin-remodeling Factor Hrp1
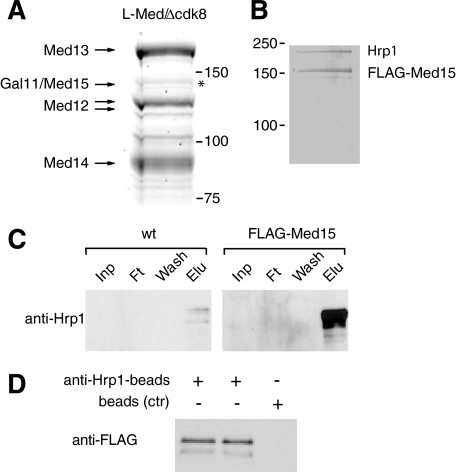
We have previously reported that homogeneous S. pombe L-Mediator devoid of pol II can be purified with a TAP tag on the Cdk8 module component Med13 (9). During our purification of L-Mediator, we noted the presence of a band in the 140-kDa size range (Fig. 1A, asterisk). In comparison with other Mediator subunits, we judged the protein to be present in about 10–15% of all L-Mediator complexes. We employed MALDI-TOF mass fingerprinting (data not shown) and identified the protein as Med15.
FIGURE 1.
Med15 interacts with chromatin modifier Hrp1. A, L-Mediator IgG purified from TAP-Med13 cells polypeptides was resolved on 10% SDS-PAGE, stained with Coomassie Blue, and identified by fingerprint MALDI-TOF. The high molecular mass region is presented. B, extract prepared from FLAG-Med15 cells was purified over M2-agarose. Proteins are separated on 10% SDS-PAGE and stained with Coomassie Blue. C, untagged and FLAG-Med15 cell extracts were purified over M2-agarose. Fractions of input (Inp), flow-through (Ft), wash, and eluate (Elu) were separated on 10% SDS-PAGE and immunoblotted with antibodies against Hrp1. D, extract from FLAG-Med15 cells was incubated with anti-Hrp1 beads or empty beads as a control (ctr). Immunoblot of bounded fractions with anti-FLAG antibody is presented.
Med15 is a conserved component of Mediator complexes isolated from many different species. A Med15 orthologue is also encoded for by the fission yeast genome, but the protein has previously not been identified as a component of the Mediator complex. To better understand the function of Med15, we fused a cassette encoding a tandem FLAG tag in-frame with the med15+ gene. We next used anti-FLAG M2-agarose and purified FLAG-Med15 from whole cell S. pombe extract. FLAG-Med15 isolated in this way did not associate with other Mediator subunits (data not shown). Instead, analysis of the purified material by SDS-PAGE and staining with Coomassie Brilliant Blue revealed two major polypeptides, suggesting that Med15 is present in a heterodimeric protein complex (Fig. 1B). We used mass fingerprinting analysis and found that the protein migrating with an apparent mass of 150 kDa was Med15. The second protein, migrating with an apparent mass of ∼200 kDa, was identified as Hrp1, a fission yeast homologue to the ATP-dependent chromatin remodeler Chd1. We used a polyclonal antibody directed against Hrp1 and observed strong enrichment of Hrp1 in the M2-agarose purified material from the FLAG-Med15 strain when compared with material purified from a wild type control strain (Fig. 1C). We also performed the reciprocal experiment and immunoprecipitated Hrp1 from the FLAG-Med15 whole cell extracts with polyclonal anti-Hrp1 antibodies coupled to NHS-Sepharose. Immunoblot analyses revealed that FLAG-Med15 bound to the antibody Sepharose but not to control beads (Fig. 1D). Based on these findings, we concluded that Med15 interacts with Hrp1 in yeast cell extracts.
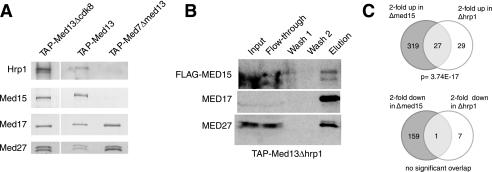
Our data so far were puzzling because we could not observe Mediator subunits in the FLAG-Med15 purified material. In addition, we had previously failed to identify Med15 as a subunit of purified S-Mediator. To clarify these issues, we employed polyclonal antibodies raised in rabbit against the Med15 and Hrp1 proteins to monitor the association of the endogenous proteins with Mediator. In agreement with previous findings, we could not observe Med15 or Hrp1 in S-Mediator preparations (14, 32). However, we could observe both Med15 and Hrp1 in L-Mediator preparations (Fig. 2A). The interaction with L-Mediator was not dependent on Cdk8 because Hrp1 and Med15 remained associated with L-Mediator purified from a Δ_cdk8_ strain. The presence of both Hrp1 and Med15 in the same Mediator preparations strongly supported our finding that these two proteins are present together in a distinct subcomplex, which may associate with the L-Mediator. We also purified L-Mediator from Δ_hrp1_ cells to monitor effects on Med15. Hrp1 was not required for Med15 interactions with L-Mediator because we could observe Med15 in whole cell extracts and also in the purified Mediator complex (Fig. 2B), even if some Med15 was lost during purification.
FIGURE 2.
Med15 and Hrp1 proteins are components of L-Mediator and functionally related. A, Mediator complexes were IgG-purified, resolved on 10% SDS-PAGE, and immunoblotted with antibodies against indicated proteins. B, FLAG-tagged Med15 remains associated with L-Mediator in Δ_hrp1_ cells. L-Mediator was analyzed as in panel A. C, Med15 and Hrp1 targets form an extensively overlapping set. A Venn diagram depicting the overlap of Med15- and Hrp1-dependent genes from the microarray expression analysis is shown. Target gene expression increased at least 2-fold in Δ_med15_ and Δ_hrp1._
Hrp1 Influences the Expression of a Subset of Med15-dependent Genes
To further explore the functional relationship between Med15 and Hrp1, we used Affymetrix transcript profiling. Neither med15+ nor hrp1+ is essential for cell growth, and expression analysis has been used previously to discern S. pombe Mediator organization (16). Deletion of hrp1+ had a milder effect on global gene transcription, but the data revealed a highly significant overlap (p = 3.74 × 10−17) between genes up-regulated 2-fold or more in the Δ_hrp1_ and Δ_med15_ strains (Fig. 2C). We could conclude that Hrp1 and Med15 are functionally related.
Med15, but Not Hrp1, Is Required for Transcriptional Activation of the inv1+ Gene
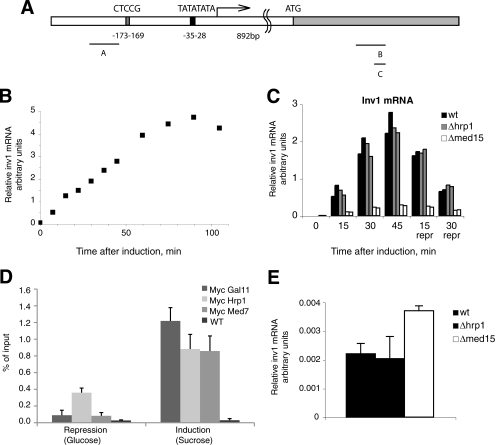
In budding yeast, Med15 is required for galactose induction of some Gal4-responsive genes, including GAL1 (33). We wanted to see whether Med15 was required for rapid induction of transcription in fission yeast and whether Hrp1 contributed to this effect. To test this, we analyzed the induction of the inv1+ gene, which encodes an invertase that catalyzes the hydrolysis of sucrose into glucose and fructose upon glucose starvation. inv1+ is transcriptionally controlled by the zinc finger transcription repressor Scr1 (Fig. 3A) (34). The subcellular localization of Scr1 is regulated, and the protein is quickly exported from the nucleus to the cytoplasm immediately after glucose starvation. Consequently, a shift from glucose to sucrose-based culture medium leads to a very rapid induction of the inv1+ promoter. We used quantitative PCR analysis and found that the inv1+ gene activation led to a linear increase of transcript levels during the first hour of gene activation; thus measurements in this time window likely reflect relative levels of transcription of the inv1+ gene (Fig. 3B). Under induction, the total amount of stable inv1+ mRNA even exceeded the levels of the abundant actin transcript (data not shown). The increased levels of transcripts (more than 1,000-fold) could be rapidly reversed upon the addition of glucose to the medium (Fig. 3C).
FIGURE 3.
Inv1+ induction. A, the inv1+ transcript has a 5′-UTR of 892 nucleotides. The Scr1 binding site is located about 170 bp upstream of the transcription start site. Positions of primers used for ChIP analysis are indicated. B, total RNA was isolated upon induction of inv1+ expression. inv1+ mRNA levels were assessed by quantitative RT-PCR and normalized to the act1+ mRNA levels at each time point. C, changes in mRNA levels in WT, Δ_hrp1_, and Δ_med15_ cells. The mRNA levels were quantified as in panel B. D, ChIP analysis reveals enrichment of Hrp1, Med7 and Med15 under derepressing conditions. E, deletion of med15+ impairs glucose repression of inv1+ gene transcription. Levels of inv1+ mRNA levels in WT, Δ_hrp1_, and Δ_med15_ cells in the presence of glucose are shown. Error bars in panels D and E indicate S.D.
Primer extension analysis identified the start site for transcription at a position 892 bp upstream from the translation start site and confirmed that transcriptional activation of the endogenous inv1+ gene originates from a unique start site (data not shown). The localization of the transcription start site is clearly distinct from what others have predicted using bioinformatics tools (35) but appears logical because it places the promoter in the vicinity of a conserved Scr1 binding site. Importantly, induction at the endogenous chromosomal locus is easily assayed to measure physiologically relevant transcriptional activation in the context of native chromatin. Taken together, these properties establish the endogenous inv1+ gene as a useful model for studying transcriptional mechanisms governing an inducible S. pombe gene.
We used ChIP analysis to analyze recruitment of Med15, Hrp1, and the core Mediator component Med7 to the inv1+ promoter region. We noticed a robust recruitment of all these proteins under derepressing conditions, demonstrating that Hrp1 may be recruited to promoters in parallel with Med15 and core Mediator (Fig. 3D). We next monitored inv1+ gene transcription in the Δ_med15_ and Δ_hrp1_ strains. After gene induction (in the absence of glucose), the wild type and Δ_hrp1_ strains showed similar levels of transcriptional activation, whereas Δ_med15_ displayed about 10-fold less transcription levels when compared with wild type (Fig. 3C). After 45 min, we added glucose to the medium and observed a rapid drop in transcript levels in both the wild type and the Δ_hrp1_ strain. We could conclude that S. pombe Med15 is required for full inv1+ gene activation. In spite of being recruited to the inv1+ promoter during gene induction, Hrp1 does not appear to be required for activation or repression of transcription. Interestingly, the Med15 protein is required for full glucose repression, and we observed a 2-fold increase of transcription in the Δ_med15_ strain in repressed conditions, i.e. in the presence of glucose (Fig. 3E).
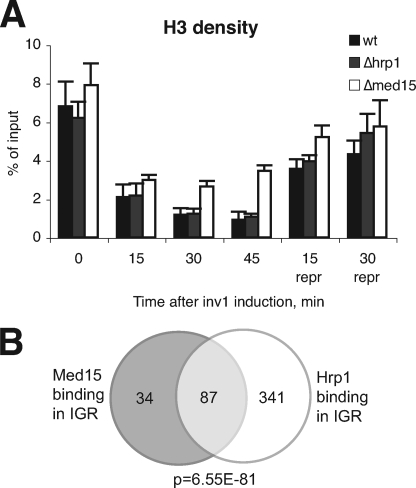
Genome-wide studies have established Hrp1 as an important regulator of nucleosome density and positioning (25). We therefore investigated effects on histone H3 occupancy over the inv1+ promoter before and after gene activation (Fig. 4A). In the WT strain, we observed a 7–8-fold reduction of H3 density already 15 min after glucose depletion and switching to sucrose-containing medium, and the levels remained low during gene activation. After transfer to repressive conditions (glucose), we observed a gradual increase of H3 occupancy. The overall H3 density profile for Δ_hrp1_ was similar to that seen in the wild type strain. We also noted that histone H3 density in the Δ_med15_ did not decrease to wild type levels during induction of gene transcription, and we interpret this as a secondary effect due to the reduced levels of gene transcription seen in this mutant.
FIGURE 4.
A, changes in H3 density in response to sucrose induction and subsequent glucose repression of the inv1+ gene. H3 density was assessed by ChIP analysis. Error bars indicate S.D. B, Med15 and Hrp1 bind to the same genomic regions. The Venn diagram presents the overlap in binding of Med15 and Hrp1 to intergenic regions.
Hrp1, Med15, and the Mediator Complex Are Found at the Same Genomic Locations
Our studies so far had established a physical and functional link between Med15 and Hrp1. The S. cerevisiae orthologue of Hrp1, Chd1, associates with the chromatin-modifying SAGA and SLIK complexes (22). In addition, we have shown that S. pombe Hrp1 associates with the Nap1 histone chaperone and contributes to nucleosome remodeling (25). We were therefore curious to see whether the Med15-associated fraction of Hrp1 displayed any specific characteristics on a genome-wide scale. To investigate this, we monitored the genome-wide profile of Hrp1 binding. ChIP was performed on cells grown to mid-logarithmic phase in rich medium. Input and immunoprecipitated samples were amplified and then applied to a microarray slide spotted in duplicate with 4,960 PCR products corresponding to a region of about 500 bp immediately upstream of almost all fission yeast ORFs (27). These IGRs therefore contain both promoter and upstream activating sequences. The microarray also contained 4,976 ORF fragments in duplicate. Binding targets were defined using the median percentile ranking approach (28).
Our analysis revealed binding of Hrp1 to 428 IGR spots. Interestingly, statistical analysis of these Hrp1 targets showed a highly significant (hypergeometric probability p = 6.55 × 10−81) overlap with the 121 IGR targets of Med15 identified in an identical manner (Fig. 4B). This overlap was also seen in the coding region, although to a lesser extent (p = 1.22 × 10−14, data not shown). From our analysis, we could conclude that Med15 and Hrp1 are found at the same promoter regions in vivo, strongly supporting our previous analysis of Med15-Hrp1 interactions. Furthermore, in agreement with Hrp1 being a component of other, non-Mediator complexes, we found a large group of genes bound by Hrp1 but not Med15. We investigated the enrichment of specific gene ontology terms to analyze whether the promoters bound by both Hrp1 and Med15 were functionally related. We observed that genes involved in nucleosome assembly were specifically affected, with binding of Hrp1 and Med15 to a number of genes encoding histone H2B, H3, and H4 (Table 3). We could not observe any significant changes in the transcription of these genes in either the Δ_hrp1_ or the Δ_med15_ strain, and because we were unable to establish a viable Δ_hrp1/Δ_med15 double mutant strain, we do not know whether the concerted action of Hrp1 and Med15 may affect histone gene transcription. Genes bound by both Hrp1 and Med15 for which the levels of transcription are changed in either the Δ_hrp1_ or the Δ_med15_ strain are listed in the supplemental material.
TABLE 3.
Statistically significant GO terms
| Gene Ontology category | Total genes | Bound genes | Enrichment | p value | Genes |
|---|---|---|---|---|---|
| Histone H4: SPBC1105.12 and SPBC8D2.03c | |||||
| GO:0000786: nucleosome | 11 | 4 | 27.08 | 9.17 × 10−6 | Histone H3: SPBC8D2.04; histone 2B, SPCC622.09 |
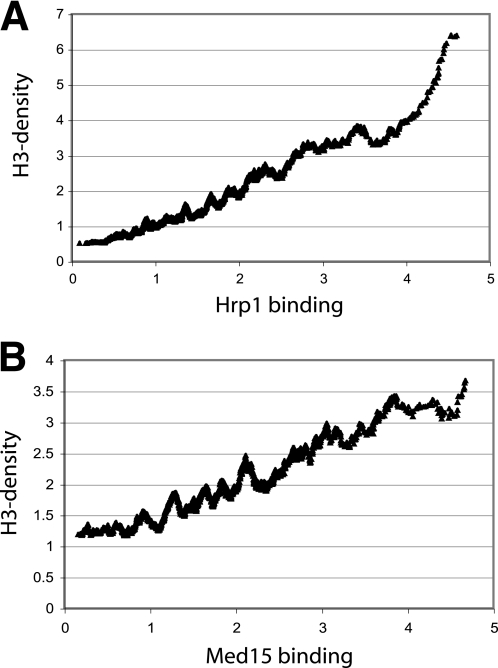
We have previously reported that Hrp1 contributes to the removal of nucleosomes near transcriptional start sites and that nucleosome density increases in an Δ_hrp1_ mutant background (25). We now performed ChIP-on-chip experiments to examine histone H3 density as a function of Med15 binding. We noted a strong correlation between high histone H3 density and high Hrp1 binding (Fig. 5A). Surprisingly, we noted a similar trend with Med15 binding, which displayed an almost linear relationship with increased histone H3 density (Fig. 5B). We therefore concluded that genome-wide Med15 and Hrp1 binding correlates with high histone H3 density.
FIGURE 5.
Med15 (A) and Hrp1 (B) occupancy correlates with genome-wide histone H3 density. A moving average window size of 100 was used.
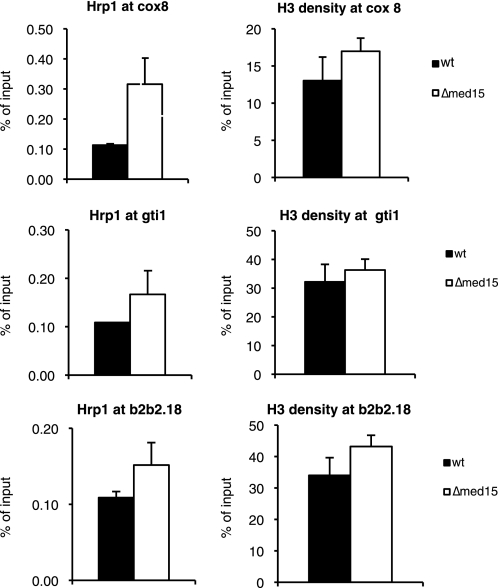
Next, we investigated whether deletion of med15+ could influence Hrp1 and histone H3 density at specific target genes. To this end, we selected three genes, whose expression was increased in Δ_med15_ deletion strain and that were bound by both Hrp1 and Med15 in genome-wide ChIP-on-chip analysis. Interestingly, deletion of med15+ caused an increase in Hrp1 levels at the cox8+, gti1+, and SPBP_B2B2.18_+ promoters (Fig. 6). Histone H3 levels at the affected locations were not significantly altered in Δ_med15_. Our experiments thus demonstrated that Hrp1 is effectively recruited to promoters even in the absence of Med15 and instead suggested that Med15 may play a role in removing Hrp1 from specific target promoters.
FIGURE 6.
Deletion of med15+ leads to increased Hrp1 occupancy at a number of Med15 target promoters. The error bars represent one standard deviation.
DISCUSSION
In S. cerevisiae, the Mediator tail module interacts with many gene-specific transcription factors (e.g. Gal4 and Gcn4). With the notable exception of Med15, the S. pombe genome does not encode any obvious homologues to the budding yeast tail module components. It is therefore possible that the Med15-Hrp1 complex corresponds to a _S. pombe_-specific tail module, which flexibly associates with the other Mediator components. In support of this interpretation, members of the S. cerevisiae tail module have been shown to be severely substoichiometric relative to other core Mediator components (6), and it is therefore possible that the tail module is found in only a subset of budding yeast Mediator complexes. Others have also demonstrated that the tail module of budding yeast Mediator can function as a separate entity in certain mutant backgrounds (12). It therefore seems plausible that the S. cerevisiae Mediator tail module may interact dynamically with other Mediator components, similar to what we observe for the Med15-Hrp1 complex in S. pombe.
Fission yeast Mediator is important for activated transcription both in vivo and in vitro, but except for the Cdk8 kinase, no direct enzymatic activity has been described for the complex. We here demonstrate direct and functionally relevant interactions between Med15 and a chromatin-remodeling activity, Hrp1. Deletion of hrp1+ has a relatively mild effect on global gene transcription when compared with Δ_med15_, but there is a highly significant overlap in genes up-regulated by these two deletions. A close functional relationship between Hrp1 and Med15 is also confirmed by ChIP-on-chip analysis. In this experiment, Med15 and Hrp1 binding reveals a dramatic overlap. Out of 121 genomic target sites for Med15, Hrp1 also binds no less than 87. This finding strongly supports the notion that Hrp1 and Med15 may be present as a distinct protein complex at certain promoters. Finally, in a Δ_med15_ mutant strain, histone H3 density and Hrp1 occupancy have a tendency to increase at promoters normally bound by Med15. We can therefore conclude that Med15 interacts directly with Hrp1 and that these two proteins together may affect histone H3 density at Med15 target sites.
Recent studies of human Mediator further support a close functional link between Mediator and nucleosomal structure. The GCN5L histone acetyltransferase was reported to stably associate with Mediator together with the TRRAP polypeptide (36). Similar to what we have demonstrated here for Hrp1, TRRAP/GCN5L does not associate with the transcriptionally active core Mediator but rather with Mediator that contains the Cdk8 subcomplex. The complex has important functional consequences because cooperative activity of Cdk8 and GCN5L within Mediator directs tandem phosphoacetylation of histone H3. Meyer et al. (36) have suggested that GCN5L may work together with L-Mediator to coordinate post-translational modifications within histone H3. This process may be initiated well before assembly of the preinitiation complex and also explains why Cdk8 module recruitment correlates with transcriptional activation. As a histone kinase, Cdk8 may work together with other chromatin-modifying/remodeling factors to establish a chromatin environment favorable for transcription.
Similar to GCN5L in mammalian cells, Hrp1 and Med15 are present in S. pombe L-Mediator but not in the smaller S-Mediator complex. We believe that Hrp1 may contribute to chromatin-dependent regulation of transcription. By helping to maintain the chromatin structure at regulated promoters, Hrp1 can fine-tune transcription. Med15 could perhaps function as a regulator of this process and govern interactions between the core Mediator, the Cdk8 module, and Hrp1. Such a model could explain the dual modes of Med15 function, both as a co-activator and as a co-repressor, which has been demonstrated in many different species.
Supplementary Material
Supplemental Data
Acknowledgments
We thank the Affymetrix core facility at Novum, BEA, Bioinformatics and Expression Analysis, which is supported by the board of research at the Karolinska Institute and the research committee at the Karolinska hospital. We thank Tomas Linder, Indranil Sinha, and Carolina Bonilla for helpful discussions.
*
This work was supported by grants from Swedish Cancer Society (to K. E. and C. M. G.), Swedish Research Council (to K. E. and C. M. G.), the European Union (EU) “The Epigenome” Network of Excellence (NoE) network (to K. E.), the EU Marie Curie Early Stage Training Center “Mitomed-Train” (to P. H. W.), and the Göran Gustafsson Foundation (to C. M. G.).
2
The abbreviations used are:
pol
polymerase
IGR
intergenic regions
TAP
tandem affinity purification.
REFERENCES
- 1.Thompson C. M., Young R. A. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 4587–4590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holstege F. C., Jennings E. G., Wyrick J. J., Lee T. I., Hengartner C. J., Green M. R., Golub T. R., Lander E. S., Young R. A. (1998) Cell 95, 717–728 [DOI] [PubMed] [Google Scholar]
- 3.Kornberg R. D. (2005) Trends Biochem. Sci. 30, 235–239 [DOI] [PubMed] [Google Scholar]
- 4.Björklund S., Gustafsson C. M. (2005) Trends Biochem. Sci. 30, 240–244 [DOI] [PubMed] [Google Scholar]
- 5.Kim Y. J., Björklund S., Li Y., Sayre M. H., Kornberg R. D. (1994) Cell 77, 599–608 [DOI] [PubMed] [Google Scholar]
- 6.Myers L. C., Gustafsson C. M., Bushnell D. A., Lui M., Erdjument-Bromage H., Tempst P., Kornberg R. D. (1998) Genes Dev. 12, 45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asturias F. J., Jiang Y. W., Myers L. C., Gustafsson C. M., Kornberg R. D. (1999) Science 283, 985–987 [DOI] [PubMed] [Google Scholar]
- 8.Borggrefe T., Davis R., Erdjument-Bromage H., Tempst P., Kornberg R. D. (2002) J. Biol. Chem. 277, 44202–44207 [DOI] [PubMed] [Google Scholar]
- 9.Samuelsen C. O., Baraznenok V., Khorosjutina O., Spahr H., Kieselbach T., Holmberg S., Gustafsson C. M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 6422–6427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elmlund H., Baraznenok V., Lindahl M., Samuelsen C. O., Koeck P. J., Holmberg S., Hebert H., Gustafsson C. M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 15788–15793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myers L. C., Gustafsson C. M., Hayashibara K. C., Brown P. O., Kornberg R. D. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 67–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F., Sumibcay L., Hinnebusch A. G., Swanson M. J. (2004) Mol. Cell. Biol. 24, 6871–6886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thakur J. K., Arthanari H., Yang F., Pan S. J., Fan X., Breger J., Frueh D. P., Gulshan K., Li D. K., Mylonakis E., Struhl K., Moye-Rowley W. S., Cormack B. P., Wagner G., Näär A. M. (2008) Nature 452, 604–609 [DOI] [PubMed] [Google Scholar]
- 14.Spåhr H., Samuelsen C. O., Baraznenok V., Ernest I., Huylebroeck D., Remacle J. E., Samuelsson T., Kieselbach T., Holmberg S., Gustafsson C. M. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 11985–11990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Béve J., Hu G. Z., Myers L. C., Balciunas D., Werngren O., Hultenby K., Wibom R., Ronne H., Gustafsson C. M. (2005) J. Biol. Chem. 280, 41366–41372 [DOI] [PubMed] [Google Scholar]
- 16.Linder T., Rasmussen N. N., Samuelsen C. O., Chatzidaki E., Baraznenok V., Beve J., Henriksen P., Gustafsson C. M., Holmberg S. (2008) Nucleic Acids Res. 36, 2489–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kouzarides T. (2007) Cell 128, 693–705 [DOI] [PubMed] [Google Scholar]
- 18.Stokes D. G., Tartof K. D., Perry R. P. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 7137–7142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lusser A., Urwin D. L., Kadonaga J. T. (2005) Nat. Struct. Mol. Biol. 12, 160–166 [DOI] [PubMed] [Google Scholar]
- 20.Konev A. Y., Tribus M., Park S. Y., Podhraski V., Lim C. Y., Emelyanov A. V., Vershilova E., Pirrotta V., Kadonaga J. T., Lusser A., Fyodorov D. V. (2007) Science 317, 1087–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flanagan J. F., Mi L. Z., Chruszcz M., Cymborowski M., Clines K. L., Kim Y., Minor W., Rastinejad F., Khorasanizadeh S. (2005) Nature 438, 1181–1185 [DOI] [PubMed] [Google Scholar]
- 22.Pray-Grant M. G., Daniel J. A., Schieltz D., Yates J. R., 3rd, Grant P. A. (2005) Nature 433, 434–438 [DOI] [PubMed] [Google Scholar]
- 23.Sims R. J., 3rd, Chen C. F., Santos-Rosa H., Kouzarides T., Patel S. S., Reinberg D. (2005) J. Biol. Chem. 280, 41789–41792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okuda M., Horikoshi M., Nishimura Y. (2007) J. Mol. Biol. 365, 1047–1062 [DOI] [PubMed] [Google Scholar]
- 25.Walfridsson J., Khorosjutina O., Matikainen P., Gustafsson C. M., Ekwall K. (2007) EMBO J. 26, 2868–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourbon H. M., Aguilera A., Ansari A. Z., Asturias F. J., Berk A. J., Bjorklund S., Blackwell T. K., Borggrefe T., Carey M., Carlson M., Conaway J. W., Conaway R. C., Emmons S. W., Fondell J. D., Freedman L. P., Fukasawa T., Gustafsson C. M., Han M., He X., Herman P. K., Hinnebusch A. G., Holmberg S., Holstege F. C., Jaehning J. A., Kim Y. J., Kuras L., Leutz A., Lis J. T., Meisterernest M., Naar A. M., Nasmyth K., Parvin J. D., Ptashne M., Reinberg D., Ronne H., Sadowski I., Sakurai H., Sipiczki M., Sternberg P. W., Stillman D. J., Strich R., Struhl K., Svejstrup J. Q., Tuck S., Winston F., Roeder R. G., Kornberg R. D. (2004) Mol. Cell 14, 553–557 [DOI] [PubMed] [Google Scholar]
- 27.Wirén M., Silverstein R. A., Sinha I., Walfridsson J., Lee H. M., Laurenson P., Pillus L., Robyr D., Grunstein M., Ekwall K. (2005) EMBO J. 24, 2906–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buck M. J., Lieb J. D. (2004) Genomics 83, 349–360 [DOI] [PubMed] [Google Scholar]
- 29.Zeeberg B. R., Feng W., Wang G., Wang M. D., Fojo A. T., Sunshine M., Narasimhan S., Kane D. W., Reinhold W. C., Lababidi S., Bussey K. J., Riss J., Barrett J. C., Weinstein J. N. (2003) Genome Biol. 4, R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue Y., Haas S. A., Brino L., Gusnanto A., Reimers M., Talibi D., Vingron M., Ekwall K., Wright A. P. (2004) Yeast 21, 25–39 [DOI] [PubMed] [Google Scholar]
- 31.Lemieux K., Larochelle M., Gaudreau L. (2008) Biochem. Biophys. Res. Commun. 369, 1103–1107 [DOI] [PubMed] [Google Scholar]
- 32.Spåhr H., Bève J., Larsson T., Bergström J., Karlsson K. A., Gustafsson C. M. (2000) J. Biol. Chem. 275, 1351–1356 [DOI] [PubMed] [Google Scholar]
- 33.Suzuki Y., Nogi Y., Abe A., Fukasawa T. (1988) Mol. Cell. Biol. 8, 4991–4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka N., Ohuchi N., Mukai Y., Osaka Y., Ohtani Y., Tabuchi M., Bhuiyan M. S., Fukui H., Harashima S., Takegawa K. (1998) Biochem. Biophys. Res. Commun. 245, 246–253 [DOI] [PubMed] [Google Scholar]
- 35.Iacovoni J. S., Russell P., Gaits F. (1999) Gene 232, 53–58 [DOI] [PubMed] [Google Scholar]
- 36.Meyer K. D., Donner A. J., Knuesel M. T., York A. G., Espinosa J. M., Taatjes D. J. (2008) EMBO J. 27, 1447–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data