VANDETANIB, DESIGNED TO INHBIT VEGFR2 AND EGFR SIGNALLING, HAD NO CLINICAL ACTIVITY AS MONOTHERAPY FOR RECURRENT OVARIAN CANCER AND NO DETECTABLE MODULATION OF VEGFR2 (original) (raw)
. Author manuscript; available in PMC: 2011 Jan 1.
Abstract
Purpose:
To evaluate clinical activity and target modulation of vandetanib in women with recurrent ovarian cancer.
Experimental Design:
A phase II trial of orally administered vandetanib 300mg daily was designed to include analyses of target inhibition through paired biopsies and dynamic imaging. Core 18g needle biopsies and dynamic contrast-enhanced (DCE) MRI were obtained prior to initiation of therapy and 6wk into therapy. Biopsy samples were subjected to reverse-phase protein lysate array endpoint analysis. Cytokine concentrations were measured by ELISA in serially collected plasma samples.
Results:
Twelve patients entered the study and accrual terminated in first stage due to lack of response or disease stabilization beyond 6 months. Adverse events included rash, diarrhea, and QTc prolongation, but not hypertension. Exploratory analyses showed that EGFR phosphorylation was reduced in the 8 paired biopsy sets obtained; VEGFR2 phosphorylation was not consistently affected, nor were DCE-MRI permeability and flow parameters. Serial plasma VEGF concentrations were variable, and did not significantly change in the 11 patients assessed.
Conclusions:
Vandetanib 300 mg daily monotherapy had no significant clinical benefit in this disease setting. Proteomic analysis of paired biopsies detected both phosphorylated-EGFR and phosphorylated-VEGFR2 in ovarian tumor tissue, but only phosphorylated-EGFR measurably inhibited by vandetanib.
Keywords: ovarian cancer, vandetanib, EGFR, molecular targets, proteomics
INTRODUCTION
Survival and quality of life in women with ovarian cancer has improved over the last decade, although cure remains elusive for those diagnosed with advanced stage disease (1). New approaches to treatment have focused on molecular targets identified in ovarian cancer. We previously reported on-target activity, but lack of clinical benefit in ovarian cancer, of single-agent gefitinib, an EGFR kinase inhibitor (2). Lack of substantial benefit has been confirmed with gefitinib and other EGFR-selective agents in ovarian cancer (3). This approach to treatment of ovarian cancer may have failed due to lack of necessity for the target or alternative compensatory pathways sustaining the cancer cells.
Vascular tumor support has been validated as a molecular target in ovarian cancer and other carcinomas (4, 5). Bevacizumab, a neutralizing monoclonal antibody against VEGF, has single agent activity in recurrent ovarian cancer (6) and is presently undergoing evaluation in a randomized trial for treatment of newly diagnosed patients. Our group tested the possibility of combining bevacizumab with sorafenib, an inhibitor of VEGFR2 and Raf kinases (7). Bevacizumab and sorafenib approach target inhibition at sequential points in the signaling cascade through VEGFR2. A phase II study of this combination is ongoing for women with recurrent ovarian cancer.
We hypothesized that blocking two targets in parallel signaling pathways also would give greater benefit than individual target modulation. This would complement our strategy of inhibiting one signaling pathway at two points in series. We sought to target both tumor growth and vasculature by blockade of both EGFR and VEGFR2. EGFR is present and activated in ovarian cancer, although we and others have demonstrated that selective inhibition of EGFR is insufficient for response in ovarian cancer (2, 3). The promise of anti-VEGF therapy in ovarian cancer suggested that a combination of agents targeting EGFR and VEGFR, or a single molecule with parallel targets, should be tested. Vandetanib has been shown to inhibit both VEGFR2 and EGFR in preclinical studies (8), and has demonstrated activity in lung cancer when given as a single agent or used in combination with chemotherapy (9-11). Here we report our results in patients with predominantly platinum-resistant recurrent ovarian cancer where vandetanib monotherapy (300 mg/day) did not meet the primary objective of demonstrating objective response or SD >6 months in the first 12 patients recruited. Exploratory translational studies suggested that vandetanib inhibited EGFR signaling in the tumor, but did not provide evidence of VEGFR2 signaling inhibition in these ovarian cancers.
PATIENTS AND METHODS
Patients
Women with recurrent, refractory, or persistent epithelial ovarian cancer and disease amenable to percutaneous core biopsy, adequate end organ function were eligible. Patients previously treated with anti-VEGF therapy were permitted on study; women were ineligible if they had received prior EGFR or VEGFR inhibitor therapy. Patients could have received no more than 4 prior treatment regimens. At least 2 sites of disease were required so that sites of disease could be independently targeted for biopsy and imaging. All patients had histopathologically proven epithelial ovarian, fallopian tube, or primary peritoneal cancer. Other criteria include an Eastern Cooperative Oncology Group performance status of 0-2 and patients must have been at least 4 weeks from their most recent therapeutic intervention and at least 6 weeks from carboplatin therapy due to the potential for sustained or delayed bone marrow suppression. Patients with evidence of central nervous system involvement, a history of cardiac disorders, recent GI bleed, gross hematuria, deep venous thrombosis or pulmonary embolism, or who required ongoing anticoagulation or medication that may cause QTc prolongation were ineligibile. The study was approved by the Institutional Review Board of the National Cancer Institute (Bethesda, MD) and written informed consent was obtained from all patients before enrollment.
Treatment Plan
Vandetanib was administered orally once daily at 300mg/d for 28 day cycles, based on the maximum tolerated dose in the phase I study (12). Treatment continued until progression, unacceptable toxicity, or withdrawal. Patients were seen every four weeks for history, examination, and laboratory tests, including CA125. Response was assessed by imaging studies every other cycle and was scored according to the Response Evaluation Criteria in Solid Tumors 1.0. Toxicity was assessed by using the National Cancer Institute Common Toxicity Criteria 3.0. Symptomatic management was provided to patients with gastrointestinal and dermatologic toxicities. Dose reduction of 100mg/d was indicated for recurrent grade 2 or initial grade 3 adverse events, or altered QTc after resolution to grade 1 or better. Patients were allowed 2 dose reductions. Patients were not eligible to resume vandetanib if the time to resolution was > 3 weeks.
Translational Endpoints
Sample acquisition
Plasma was collected in EDTA prior to therapy and then monthly, aliquotted, and stored at −80°C until assayed using commercially available ELISA kits (R&D Systems, Minneapolis, MN). Percutaneous 18g core needle biopsies were obtained under imaging guidance prior to therapy and at 6 weeks by an interventional radiologist (BW), and were cryopreserved immediately in OCT (optimum cutting temperature) compound then frozen in liquid nitrogen until use. Sections were evaluated for tumor quality and quantity by a pathologist (KC); samples with predominant necrosis or lymphatic infiltration were not used. Reverse Phase Tissue Array (RPTA) Tissue (30mm2 × 8μm) was extracted in 15μL Tissue Protein Extraction Reagent Buffer (Pierce, Rockford, IL) diluted 1:1 with sample buffer and printed onto nitrocellulose glass-based arrays in replicates, as described (13). Arrays were prepped and stained with indicated titer-optimized antibodies (13). The first and ninth slides were stained with colloidal gold to quantify total protein load and consistency in loading (r2=0.852). Stained arrays were digitized, spot intensities quantified (ImageQuant v5.2; Molecular Dynamics, Sunnyville, Calif), then expression signals normalized to total protein content and standardized to a control printed on each slide to yield normalized intensity values (Table S1 and Figure S1).
Dynamic contrast enhanced-MRI (DCE-MRI)
One sentinel lesion was chosen on CT images, based on RECIST criteria for measurable disease (14). DCE-MRI using a 1.5T magnet conducted on a sentinel lesion was performed prior to enrollment, after 3 days of therapy, and after 6 weeks of therapy. Conventional T1 and T2 weighted images of the target lesion were obtained and a T1 map generated. This was followed by a series of 3D gradient echo T1 weighted dynamic sequence which was acquired before, during, and after the administration of 0.1mm/kg gadolinium chelate contrast. Data were analyzed using a general kinetic model by the Clinical Imaging Processing Service (CIPS) in the Diagnostic Radiology Department (Bethesda, MD). This model generates permeability parameters Ktrans and Kep, analyzed as continuous variables. Vascular fraction (Ve) was also assessed.
The Functional Assessment of Cancer Therapy – Ovary (FACT-O)
FACT-O, v4 quality of life scale was administered at baseline and at clinic visits after 1, 2, 4 and 8 cycles. Changes in patient-reported outcomes of physical, social, emotional, and functional well being and overall quality of life were determined.
Statistical Analysis
The primary end point was objective response (OR; complete or partial response) or disease stabilization lasting greater than or equal to 6 months (SD). Complete response, partial response or stable disease was defined by RECIST criteria (14). CA125 was measured every cycle, but was not used in assessing disease progression or response to vandetanib. A Simon two-stage design was performed with a requirement for 3 or more OR or SD at 6 months occurring in the first 12 patients to move to stage 2 (total 35 patients). This design would rule out an event rate of 10% in favor of 30%. The probability of early termination was 66% under the null hypothesis.
Prospectively defined secondary endpoints were measures of change of activation of target proteins (total and phospho[p]-VEGFR2, EGFR, AKT, and ERK, and if adequate tissue, p27, p38, and cleaved PARP), change in concentration of circulating VEGF, IL-6, and IL-8, measure of tumor vascular permeability using DCE-MRI, and assessment of quality of life. All secondary endpoints were analyzed with exploratory intent using non-parametric analysis. Relative change from baseline (i.e. (post treatment value – pretreatment value)/pretreatment value) was assessed. A Wilcoxon signed rank-test evaluated whether relative changes between posttreatment and pretreatment means differed from zero. Continuous data between two groups were compared using an exact Wilcoxon rank sum test and trends in parameter values across levels of an ordered categorical parameter were evaluated using an exact Jonckheere-Terpstra trend test. Spearman correlation analysis was used to determine the correlation between two continuous parameters. Results are considered exploratory and a p-value of <0.05 suggestive of a trend.
RESULTS
Patients
Twelve patients with recurrent epithelial ovarian or fallopian tube cancer were enrolled between February 2007 and September 2008 (Table 1). Most patients had platinum-resistant disease (10 of 12 patients, 83%), tumors with serous histological features (92%), and stage III disease (67%). Patients had good performance status and a median of 3.5 prior therapies.
Table 1.
Patient Characteristics and Clinical Outcome
| Patient Characteristic | No. of patients (N=12) |
|---|---|
| Median age (range), y | 58.4 (44-78) |
| ECOG performance status | |
| 0 | 1 |
| 1 | 10 |
| 2 | 1 |
| Stage | |
| II | 3 |
| III-B | 1 |
| III-C | 7 |
| IV | 1 |
| Histologic Subtype | |
| Serous | 10 |
| Endometriod | 1 |
| Serous and transitional features | 1 |
| No. of prior therapies received | |
| 1 | 3 |
| 2 | 0 |
| 3 | 3 |
| 4 | 6 |
| Tumor type | |
| Epithelial ovarian | 10 |
| Fallopian tube | 2 |
| Clinical Outcome | |
| Best response | |
| Stable disease (≥16 wks) | 4 |
| Progressive disease | 8 |
| Time to progression (weeks) | |
| < 8 | 4 |
| 8-12 | 4 |
| 16 | 1 |
| 22 | 3 |
Clinical outcome and toxicity
The primary objective of the study was demonstration of objective response or SD of ≥6 months. The study closed after the first stage of accrual due to inadequate early activity (Table 1) with 32 cycles of administered treatment (median 2; range: 1-6). Four patients had stable disease after 4 cycles, but progressed by 6 cycles on study. One of the two patients with platinum-sensitive ovarian cancer had stable disease as best response (confirmed SD after 4 cycles), and the other had progressed at first restaging (after 2 cycles). CA125 was measured monthly but was not used for determining progression of disease.
Toxicity overall was limited. One patient required dose reduction for grade 3 diarrhea, and one patient for grade 3 QTc prolongation. One patient developed grade 3 dyspnea, possibly related to co-morbid illness, and discontinued vandetanib with concurrent progressive disease. Vandetanib-related toxicities, outlined in Table 2, were most commonly diarrhea, skin rash, elevated AST, and prolonged QTc.
Table 2.
Adverse Events Attributed to Vandetanib
| Toxicity Grade (No of Patients) | |||||
|---|---|---|---|---|---|
| Category | Toxicity | Grade 1 | Grade 2 | Grade 3 | % |
| Cardiac | Prolonged QTc | 1 | 4 | 2 | 5 |
| 8 | |||||
| Constitutional | Fatigue | 6 | 5 | ||
| 0 | |||||
| Weight Loss | 1 | 1 | 17 | ||
| Dermatologic | Acneiform Rash | 3 | 2 | 42 | |
| Skin desquamation | 2 | 3 | 42 | ||
| Hand-foot skin reaction | 2 | 17 | |||
| Dry skin | 2 | 17 | |||
| Gastrointenstinal | Anorexia | 4 | 1 | 4 | |
| 2 | |||||
| Diarrhea | 6 | 1 | 2 | 75 | |
| Abdominal distention/bloating | 1 | 1 | 17 | ||
| Nausea | 4 | 33 | |||
| Lab/Metabolic | Hypoalbuminemia | 6 | 5 | ||
| 0 | |||||
| Elevated alk phos | 2 | 1 | 25 | ||
| Elevated ALT | 3 | 4 | 58 | ||
| Elevated AST | 7 | 2 | 75 | ||
| Hypermagnesemia | 2 | 17 | |||
| Hypomagnesemia | 4 | 33 | |||
| Hyponatremia | 2 | 17 | |||
| Respiratory | Dyspnea | 1 | 1 | 1 | |
| 7 | |||||
| Voice changes: hoarseness | 2 | 17 |
Translational studies
Proteomic profiling
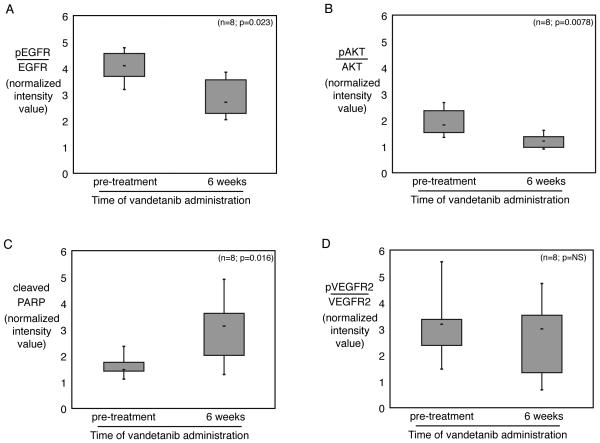
RPTA was used to assess multiple signaling endpoints in paired tissue biopsies. Eight patients had adequate tissue samples from which to perform a comparison between pre- and post-treatment expression profiles. Clinical characteristics of these 8 patients did not differ from the other 4 patients enrolled. Reasons for an incomplete biopsy set included: no matched biopsy and/or inadequate tissue. All patients had progressive disease by 6 months, precluding analysis of the relation between biochemical parameters and response. Statistically significant targeted inhibition of EGFR and AKT was observed in 7/8 paired samples (Figure 1A and B, p= 0.023 and 0.0078, respectively). Cleaved PARP was significantly increased (Figure 1C, p= 0.016). No significant changes occurred in activated VEGFR2 in tissue or in circulating VEGF concentration (Figure 1D and Figure 2A, p=NS). There was no significant change in the level of total VEGFR2 in the tumor samples (data not shown, p=NS). There was no correlation between baseline parameters or changes of parameters with time on study or clinical outcome (data not shown).
Figure 1. Changes in paired tumor tissue proteins with vandetanib administration.
(A) The relative level of phosphorylated EGFR decreased after 6 weeks of vandetanib administration in paired tumor biopsies from 8 patients. Total EGFR and phosphorylated EGFR were measured on reverse-phase tissue lysate array (RPTA), and normalized to total protein level. The relative level of EGFR phosphorylation was estimated by calculating the ratio between the two normalized values for each sample. Mean value and range of expression for the group is plotted for each of the indicated timepoints. (B) The relative level of phosphorylated AKT similarly decreased in the same patients. AKT phosphorylation was calculated as EGFR in (A). (C) Cleaved PARP was significantly increased in tumor tissue following vandetanib administration, as measured by RPTA. (D) The relative level of phosphorylated VEGFR2, the other main target of vandetanib, was not significantly changed in paired tumor samples with vandetanib administration.
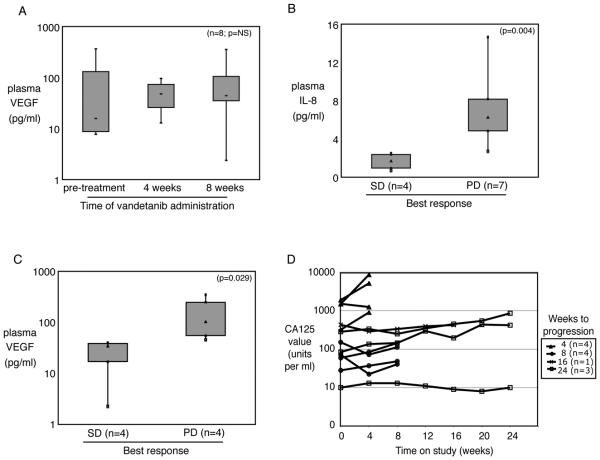
Figure 2. Levels of circulating cytokines and tumor marker CA125.
(A) Plasma VEGF was not significantly changed in 11 patients on study for 4 weeks or 8 patients who remained on study 8 weeks or longer. Median fold change from baseline was 1.45 at 4 weeks and 0.97 at 8 weeks (p=NS). (B) Plasma IL-8 level was higher at baseline in patients who went on to disease progression before 4 months of vandetanib administration. (C) Plasma levels of VEGF at 8 weeks on study were higher in patients whose disease progressed before 4 months of vandetanib administration. (D) The CA125 measurements were variable, and tended rise faster in patients with progressive disease in the first cycle.
Molecular signaling changes were associated with clinical toxicity
The extent of adverse events observed was analyzed based on molecular pathway activation as measured by RPTA at baseline and 6 weeks of vandetanib. The pre-treatment fraction of pVEGFR2 showed an inverse trend to vandetanib-related toxicity (Table 3, p=0.029). After 6 weeks of treatment, tumor tissue from those patients with grade 2 skin rash exhibited higher levels of cleaved PARP and a lower fraction of pAKT (Table 3, p=0.036). A decrease in relative pAKT between 0 and 6 weeks was linked to increased overall toxicity (Table 3, p=0.029), whereas the opposite trend occurred with relative change in pERK (Table 3, p=0.011).
Table 3.
Protein Parameters and Adverse Events
| Pretreatment Variable | No. of Patients | Mean | SEM | P value* |
|---|---|---|---|---|
| Maximum overall toxicity | ||||
| pVEGFR2wk0/VEGFR2wk0 | ||||
| Grade 0 or 1 | 2 | 0.44 | 0.12 | 0.029 |
| Grade 2 | 3 | 0.33 | 0.01 | |
| Grade 3 | 3 | 0.20 | 0.04 | |
| Posttreatment Variable | No. of Patients | Mean | SEM | P value |
| Maximum skin toxicity | ||||
| Cleaved PARPwk6 | ||||
| Grade 0 or 1 | 5 | 2252.08 | 395.02 | 0.036 |
| Grade 2 | 3 | 4004.99 | 435.78 | |
| pAKTwk6/AKTwk6 | ||||
| Grade 0 or 1 | 5 | 1.29 | 0.08 | 0.036 |
| Grade 2 | 3 | 0.90 | 0.04 | |
| Maximum overall toxicity | ||||
| ΔERK/ERKwk0 | ||||
| Grade 0 or 1 | 2 | −0.19 | 0.02 | 0.011 |
| Grade 2 | 3 | −0.06 | 0.05 | |
| Grade 3 | 3 | 0.20 | 0.14 | |
| (Δ pAKT/AKT)/(pAKT/AKTwk0) | ||||
| Grade 0 or 1 | 2 | −0.05 | 0.03 | 0.029 |
| Grade 2 | 3 | −0.41 | 0.04 | |
| Grade 3 | 3 | −0.49 | 0.05 |
Plasma IL-8 concentrations were related to outcome
Plasma sampling for the measurement of concentrations of proangiogenic cytokines VEGF, IL-6, and IL-8 was planned prospectively. No significant difference was evident in pre- and post-treatment cytokine concentrations (Figure 2A and data not shown). Baseline plasma IL-8 concentrations were lower in patients whose best response was SD at 4 months compared to those who progressed before 4 months (Figure 2B, p=0.004). Similarly, circulating VEGF cycle 2 concentration was lower in patients with SD (Figure 2C, p=0.029). IL-6 concentrations showed no significant association with outcome (data not shown, p=NS). The CA125 measurements were variable, and tended rise faster in patients with progressive disease in the first cycle (Figure 2D).
DCE-MRI permeability parameters did not change with vandetanib administration
There was no significant change from baseline in DCE-MRI kinetic parameters (Ktrans, Kep, Ve) after 3 days of therapy with vandetanib in the eight patients who underwent repeat imaging. There was no correlation between DCE-MRI parameters and outcome, duration of therapy, or toxicity (data not shown).
Quality of life was unaffected by vandetanib administration
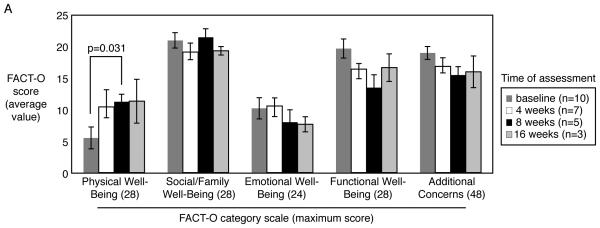
FACT-O scores from baseline were compared to results prior to cycle 2 and cycle 3 in the 9 patients who completed all 3 cycles. No significant change in total FACT-O scores was observed; however, average physical symptom subscale score increased 6.83 points from pretreatment assessment to assessment performed prior to cycle 2 (Figure 3, p =0.031). Subscales in emotional, social/family, and functional well being were examined as well and there were no significant changes during the first 8 weeks of vandetanib therapy. (Figure 3, p=NS).
Figure 3. Quality of life assessment.
Quality of life was estimated by administering the FACT-O questionnaire to patients before starting study treatment and after 4, 8 and 16 weeks of vandetanib administration. No significant changes were noted except for an increase in the physical well being subscale score at 8 weeks compared to baseline.
DISCUSSION
We hypothesized that targeting two active pathways in ovarian cancer would translate to greater clinical benefit than targeting a single pathway. Preclinical studies of vandetanib demonstrated inhibition at nanomolar concentrations of both VEGFR2 and EGFR (8). We selected this agent in order to focus on targets in the tumor microenvironment, the tumor cells, stromal support, and vasculature. Although, vandetanib did not show sufficient clinical activity in the initial cohort and the study was terminated early, the prospectively planned translational endpoints were key in determining potential etiologies for the lack of clinical benefit. We demonstrated reduction in EGFR activation but despite measurable presence of total and activated VEGFR2, no modulation of that vandetanib target could be documented.
We designed this trial to include prospective collection of translational endpoints to allow investigation of proof of mechanism in tumor tissue, in the circulation, and in the affected organs using tissue proteomics, measurement of plasma cytokines, and vascular permeability imaging. The early termination limited the potential power of the translational endpoints. The number of paired tumor samples examined was small (n=8). Nonetheless, execution of these translational endpoints allowed us to query molecular mechanisms responsible for the lack of clinical benefit in this ovarian cancer patient cohort, a disease where angiogenesis inhibitors are showing promise. Unexpectedly, clinical and biochemical parameters confirming VEGFR2 inhibition were negative.
Clinical pharmacodynamic measurements were consistent with lack of VEGFR2 blockade. Anti-VEGF therapy with sunitinib, sorafenib, bevacizumab or VEGF-trap have been documented to induce or augment hypertension (15). In our ongoing clinical trials blocking VEGF signaling with bevacizumab and sorafenib in ovarian cancer, 67% of patients experienced hypertension (7). This expected clinical parameter was not observed in the group of 12 treated ovarian cancer patients reported herein. The lack of hypertension in this study suggests frequency may be less than that reported in lung cancer patients treated with vandetanib, where elevated blood pressure was observed in 12% of patients (11).
The lack of effective VEGFR2 inhibition in the ovarian cancer patients treated with vandetanib was corroborated by the absence of significant changes in circulating cytokines, including VEGF. VEGF/VEGFR signaling blockade has been shown by us and others to result in increased circulating VEGF due to paracrine feedback mechanisms to the VEGF-secreting cells (7, 16). Vandetanib has been associated with a trend towards increased VEGF secretion in patients with lung cancer (17). The lack of significant induction of VEGF during the course of vandetanib treatment in the present study is consistent with the lack of clinical efficacy.
We have optimized the technique of RPTA to measure selected proteins in small quantities of tumor biopsy material (18). We successfully applied this technique to detect changes in total and phosphorylated VEGFR2 in tumor samples from our ongoing trial with bevacizumab and sorafenib ((19), and Azad et al, manuscript in preparation). Good signals for VEGFR2 and p-VEGFR2 were detected in the tumor biopsy samples from the current trial with vandetanib, yet no change in quantity of total or p-VEGFR2, or relative activation of VEGFR2 was measured in the samples after 6 weeks of daily vandetanib therapy. A positive endothelial cell control was incorporated on each test slide in order to confirm detectability of the signal (Figure S1). Therefore, the inability to detect significant differences in VEGFR2 activity is unlikely to be due to technical reasons.
RPTA of the paired tumor biopsies was able to detect decreased phosphorylation of EGFR and its downstream effector AKT after 6 weeks of vandetanib administration. This reduction in EGFR activation was supported by the expected clinical events, consistent with the molecular target inhibition. Two well described clinical manifestations of anti-EGFR therapy (20), rash and diarrhea, were seen in 42% and 75% of our patients, respectively. This led us to question the role of EGFR in ovarian cancer. Targeted EGFR inhibition has demonstrated clinical efficacy in tumors that are driven by this pathway. Specifically, lung adenocarcinomas harboring activating mutations in the EGFR kinase domain rely on this oncogene, and respond to single agent EGFR inhibitor therapy (21). Ovarian cancers, however, rarely have mutated EGFR, although it is frequently amplified, suggesting there may be some contribution to the pathogenesis of ovarian cancer (22-24). Our prior clinical trial targeting EGFR with gefitinib showed lack of clinical benefit despite biochemical evidence of EGFR inhibition in tumor tissue (2). No EGFR mutations were identified in patients on that trial. In GOG-170C, a phase II trial of gefitinib, one patient had a PR and that patient's tumor was found to have an EGFR mutation. It remains possible that vandetanib may have activity in rare ovarian cancers that harbor an activating mutation of EGFR. Other trials of EGFR inhibitors, selective and HER family inhibitors such as lapatinib, were without activity in ovarian cancer (3); similarly, anti-EGFR therapy with cetuximab achieved minimal activity in this disease setting (25). It has been unclear as to whether EGFR activity is unrelated to ovarian cancer progression or complementary to other pathways. We questioned whether it might be necessary for ovarian cancer growth, but that its blockade was insufficient as a molecular target in isolation.
We strove in this trial to increase responsiveness of ovarian cancer to targeted EGFR therapy by using vandetanib, a small molecule designed to inhibit VEGFR2 in addition to EGFR. The addition of VEGFR2 blockade was logical since anti-VEGF therapy with bevacizumb previously demonstrated 19% response rate and 40% disease stabilization in women with recurrent ovarian cancer (6). Our combination of bevacizumab with sorafenib has yielded over 40% PR in women with advanced recurrent ovarian cancer (7). The patients on the vandetanib study were predominantly platinum-resistant, and had received a median of 3.5 prior treatments with cytotoxic chemotherapy. Current treatments for patients with platinum-resistant ovarian cancer have limited activity, estimated at 15-30% response rate with cytotoxic chemotherapy (26). Retrospective analysis of anti-VEGF therapy with bevacizumab in women with platinum-resistant ovarian cancer showed 28% response and 40% stable disease in patients who had received a median of 5 prior chemotherapy regimens (27). Prospective testing of bevacizumab in 44 patients with relapsed platinum-resistant ovarian cancer attained a 16% response and median PFS of 4.4 months (6). A study of bevacizumab in 62 patients with relapsed ovarian cancer included 26 women with platinum-resistant disease (28). Progression-free survival with bevacizumab was not impacted by sensitivity to platinum, and resulted in a similar (4.7 months) interval as that in the purely platinum resistant setting.
The translational endpoints included on this clinical trial demonstrate that vandetanib inhibited EGFR signaling, but did not affect VEGFR2 signaling in this group of women with relapsed ovarian cancer. The lack of inhibition of VEGFR2 signaling in these patients' tumors suggests a reason for its clinical inactivity. Therefore, it remains unknown whether parallel blockade of primary receptor tyrosine kinase pathways is a viable approach to treat recurrent platinum resistant ovarian cancer. It is still possible that combining VEGFR2 and EGFR targeted agents may be more successful than a single competitive kinase inhibitor with activity at both receptors. Incorporation of similar translational studies to explore the molecular events is necessary. Future studies will continue to address the potential efficacy of simultaneously inhibiting pathways in series or parallel with VEGFR, with the goal of improving outcome in women with ovarian cancer.
Statement of Translational Relevance.
Ovarian cancer often is diagnosed at advanced stage, responds to initial therapy, but ultimately recurs. New therapeutic directions are needed to improve and prolong life. We previously reported lack of patient benefit with EGFR inhibition in ovarian cancer despite proteomic demonstration of target inhibition. We hypothesized that additional signaling pathways inhibited in parallel with EGFR would increase antitumor activity, and designed a trial testing vandetanib, an inhibitor of EGFR and VEGFR2 tyrosine kinases. Vandetanib demonstrated no clinical activity despite translational evidence of EGFR inhibition in paired tumor biopsies from a subset of patients. In contrast, we were unable to demonstrate inhibition of VEGFR2. These exploratory data expand our understanding of vandetanib's molecular profile and stress the importance of determining target presence, molecular activity, therapy effect, and association with clinical behavior.
Supplementary Material
Supplemental Figure 1. RPTA controls.
(A) HMVEC cells were stimulated with 50ng/ml VEGF165 (R&D) for 2 min after overnight serum starvation. Change in p-VEGFR2 was measurable (p=1×10−18). (B) Raw intensity values of total VEGFR2 were measured in the linear range with or without VEGF stimulation. (C) Intensity values of p-VEGFR2 without VEGF stimulation were in the linear range at low dilution. (D) Intensity values of p-VEGFR2 with VEGF stimulation showed linear measurements at low dilution, as well. (E) HeLa cells were treated with 100ng/ml EGF for 10 min after overnight serum starvation, or 100μM etoposide for 3 hrs. All measurements were in the linear range.
2
Acknowledgments
This work was supported by the Intramural Program of the Center for Cancer Research, NCI and is an investigator-sponsored clinical trial under a Cooperative Research and Development Agreement between AstraZeneca and the NCI.
REFERENCES
- 1.Fleming GF, Ronnett BM, Seidman J, Zaino RJ, Rubin SC. Epithelian Ovarian Cancer. In: Barakat RR, Markman M, Randall ME, editors. Principles and Practices of Gynecologic Oncology. 5th ed Lippincott, Williams and Wilkins; Baltimore: 2009. pp. 763–836. [Google Scholar]
- 2.Posadas EM, Liel MS, Kwitkowski V, et al. A phase II and pharmacodynamic study of gefitinib in patients with refractory or recurrent epithelial ovarian cancer. Cancer. 2007;109:1323–30. doi: 10.1002/cncr.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palayekar MJ, Herzog TJ. The emerging role of epidermal growth factor receptor inhibitors in ovarian cancer. Int J Gynecol Cancer. 2008;18:879–90. doi: 10.1111/j.1525-1438.2007.01144.x. [DOI] [PubMed] [Google Scholar]
- 4.Annunziata CM, Azad NS, Hoskins ER. E.C. K. Tumor invasion, angiogenesis and metastasis: biology and clinical application. In: Barakat RR, Markman M, Randall ME, editors. Principles and Practices of Gynecologic Oncology. 5th ed Lippincott, Williams and Wilkins; Baltimore: 2009. pp. 71–84. [Google Scholar]
- 5.Martin L, Schilder R. Novel approaches in advancing the treatment of epithelial ovarian cancer: the role of angiogenesis inhibition. J Clin Oncol. 2007;25:2894–901. doi: 10.1200/JCO.2007.11.1088. [DOI] [PubMed] [Google Scholar]
- 6.Cannistra SA, Matulonis UA, Penson RT, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25:5180–6. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 7.Azad NS, Posadas EM, Kwitkowski VE, et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol. 2008;26:3709–14. doi: 10.1200/JCO.2007.10.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciardiello F, Caputo R, Damiano V, et al. Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase. Clin Cancer Res. 2003;9:1546–56. [PubMed] [Google Scholar]
- 9.Heymach JV, Johnson BE, Prager D, et al. Randomized, placebo-controlled phase II study of vandetanib plus docetaxel in previously treated non small-cell lung cancer. J Clin Oncol. 2007;25:4270–7. doi: 10.1200/JCO.2006.10.5122. [DOI] [PubMed] [Google Scholar]
- 10.Heymach JV, Paz-Ares L, De Braud F, et al. Randomized phase II study of vandetanib alone or with paclitaxel and carboplatin as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:5407–15. doi: 10.1200/JCO.2008.17.3138. [DOI] [PubMed] [Google Scholar]
- 11.Natale RB, Bodkin D, Govindan R, et al. Vandetanib versus gefitinib in patients with advanced non-small-cell lung cancer: results from a two-part, double-blind, randomized phase ii study. J Clin Oncol. 2009;27:2523–9. doi: 10.1200/JCO.2008.18.6015. [DOI] [PubMed] [Google Scholar]
- 12.Tamura T, Minami H, Yamada Y, et al. A phase I dose-escalation study of ZD6474 in Japanese patients with solid, malignant tumors. J Thorac Oncol. 2006;1:1002–9. [PubMed] [Google Scholar]
- 13.Espina V, Mehta AI, Winters ME, et al. Protein microarrays: molecular profiling technologies for clinical specimens. Proteomics. 2003;3:2091–100. doi: 10.1002/pmic.200300592. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 15.Izzedine H, Ederhy S, Goldwasser F, et al. Management of hypertension in angiogenesis inhibitor-treated patients. Ann Oncol. 2009;20:807–15. doi: 10.1093/annonc/mdn713. [DOI] [PubMed] [Google Scholar]
- 16.Golshayan AR, Brick AJ, Choueiri TK. Predicting outcome to VEGF-targeted therapy in metastatic clear-cell renal cell carcinoma: data from recent studies. Future Oncol. 2008;4:85–92. doi: 10.2217/14796694.4.1.85. [DOI] [PubMed] [Google Scholar]
- 17.Kiura K, Nakagawa K, Shinkai T, et al. A randomized, double-blind, phase IIa dose-finding study of Vandetanib (ZD6474) in Japanese patients with non-small cell lung cancer. J Thorac Oncol. 2008;3:386–93. doi: 10.1097/JTO.0b013e318168d228. [DOI] [PubMed] [Google Scholar]
- 18.Winters M, Dabir B, Yu M, Kohn EC. Constitution and quantity of lysis buffer alters outcome of reverse phase protein microarrays. Proteomics. 2007;7:4066–8. doi: 10.1002/pmic.200700484. [DOI] [PubMed] [Google Scholar]
- 19.Azad NS, Henning R, Yu M, et al. Translational proof of mechanism for sorafenib with bevacizumab: Endpoint analysis and clinical activity. J Clin Oncol. 2009;27 abstr 3574. [Google Scholar]
- 20.Rudin CM, Liu W, Desai A, et al. Pharmacogenomic and pharmacokinetic determinants of erlotinib toxicity. J Clin Oncol. 2008;26:1119–27. doi: 10.1200/JCO.2007.13.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adamo V, Franchina T, Adamo B, et al. Gefitinib in lung cancer therapy: Clinical results, predictive markers of response and future perspectives. Cancer Biol Ther. 2009:8. doi: 10.4161/cbt.8.3.7465. [DOI] [PubMed] [Google Scholar]
- 22.Lassus H, Sihto H, Leminen A, et al. Gene amplification, mutation, and protein expression of EGFR and mutations of ERBB2 in serous ovarian carcinoma. J Mol Med. 2006;84:671–81. doi: 10.1007/s00109-006-0054-4. [DOI] [PubMed] [Google Scholar]
- 23.Stadlmann S, Gueth U, Reiser U, et al. Epithelial growth factor receptor status in primary and recurrent ovarian cancer. Mod Pathol. 2006;19:607–10. doi: 10.1038/modpathol.3800575. [DOI] [PubMed] [Google Scholar]
- 24.Vermeij J, Teugels E, Bourgain C, et al. Genomic activation of the EGFR and HER2-neu genes in a significant proportion of invasive epithelial ovarian cancers. BMC Cancer. 2008;8:3. doi: 10.1186/1471-2407-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schilder RJ, Pathak HB, Lokshin AE, et al. Phase II trial of single agent cetuximab in patients with persistent or recurrent epithelial ovarian or primary peritoneal carcinoma with the potential for dose escalation to rash. Gynecol Oncol. 2009;113:21–7. doi: 10.1016/j.ygyno.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozols RF. Recurrent ovarian cancer: evidence-based treatment. J Clin Oncol. 2002;20:1161–3. doi: 10.1200/JCO.2002.20.5.1161. [DOI] [PubMed] [Google Scholar]
- 27.Simpkins F, Belinson JL, Rose PG. Avoiding bevacizumab related gastrointestinal toxicity for recurrent ovarian cancer by careful patient screening. Gynecol Oncol. 2007;107:118–23. doi: 10.1016/j.ygyno.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. RPTA controls.
(A) HMVEC cells were stimulated with 50ng/ml VEGF165 (R&D) for 2 min after overnight serum starvation. Change in p-VEGFR2 was measurable (p=1×10−18). (B) Raw intensity values of total VEGFR2 were measured in the linear range with or without VEGF stimulation. (C) Intensity values of p-VEGFR2 without VEGF stimulation were in the linear range at low dilution. (D) Intensity values of p-VEGFR2 with VEGF stimulation showed linear measurements at low dilution, as well. (E) HeLa cells were treated with 100ng/ml EGF for 10 min after overnight serum starvation, or 100μM etoposide for 3 hrs. All measurements were in the linear range.
2