Patient-Reported Barriers to Colorectal Cancer Screening: A Mixed-Methods Analysis (original) (raw)
. Author manuscript; available in PMC: 2011 May 1.
Abstract
Background
Barriers experienced by patients influence the uptake of colorectal cancer (CRC) screening. Prior research has quantified how often patients encounter these challenges but has generally not revealed their complex perspective and experience with barriers.
Methods
A two-part, mixed-methods study was conducted of primary care patients recruited from Virginia Ambulatory Care Outcome Research Network practices. First, in June–July 2005 a survey was mailed to 660 patients aged 50–75 years posing an open-ended question about “the most important barrier” to CRC screening. Second, beginning in October 2005 seven gender- and largely race-specific focus groups involving 40 patients aged 45–75 years were conducted. Beginning in October 2005, survey verbatim responses were coded and quantitatively analyzed and focus group transcripts were qualitatively analyzed.
Results
Responses to the open-ended survey question, answered by 74% of respondents, identified fear and the bowel preparation as the most important barriers to screening. Only 1.6% of responses cited the absence of physician advice. Focus group participants cited similar issues and other previously reported barriers, but their remarks exposed the intricacies of complex barriers, such as fear, lack of information, time, the role of physicians, and access to care. Participants also cited barriers that have little documentation in the literature, such as low self-worth, “para-sexual” sensitivities, fatalism, negative past experiences with testing, and skepticism about the financial motivation behind screening recommendations.
Conclusions
Mixed-methods analysis helps to disaggregate the complex nuances that influence patient behavior. In this study, patients explained the web of influences on knowledge, motivation, and ability to undergo CRC screening, which clinicians and policymakers should consider in designing interventions to increase the level of screening.
Background
Colorectal cancer (CRC) is the second-leading cause of cancer deaths in the U.S.1 In 2002, the U.S. Preventive Services Task Force recommended that adults aged ≥50 years should receive regular CRC screening by one of four modalities: fecal occult blood testing (FOBT), flexible sigmoidoscopy, colonoscopy, or barium enema.2,3 However, only 60.8% of U.S. adults aged ≥50 years report recent screening.4
Among the factors that account for inadequate levels of CRC screening, barriers perceived and encountered by patients figure prominently. These include the failure of physicians to recommend screening, scheduling difficulties, cost, lack of insurance coverage, gaps in knowledge, fear, embarrassment, pain, and a lack of symptoms.5–11
This list of barriers is useful but limited in several respects. First, while studies have directly asked patients to describe barriers,6,7,12–18,20–25 many studies have included only those without prior screening. Second, selected studies provided qualitative context to understand how these barriers were defined or experienced by patients; however, for simplicity, investigators collapsed similar reasons for not being screened (e.g., fear of cancer, fear of embarrassment) into larger, overarching categories (e.g., fear), potentially obscuring important nuances and distinctions.12,16,19,20 Third, minorities or disadvantaged patients (e.g., low-income) have been insufficiently studied. Since 1997, approximately ten qualitative studies have included vulnerable populations.7,8,13,15,16,20–24 Finally, the literature is dated, coming largely from the pre-colonoscopy era, when FOBT and (primarily rigid) sigmoidoscopy were the main tests.
To build on the findings of previous studies, a two-part mixed-methods study of primary care patients was conducted to understand current perspectives on CRC screening. First, the quantitative portion of the study involved the analysis of an open-ended question placed in a questionnaire mailed to patients. Second, the qualitative portion, which involved focus groups conducted with patients from three practices. As Creswell and others have noted, the use of sequential mixed methods can provide a powerful lens for understanding behavioral issues26–30 and were well suited for the broader purpose of the current study. Findings from this mixed-method study were later used to develop a comprehensive questionnaire assessing barriers to four nationally recommended CRC screening modalities, which was subsequently completed by 3,357 patients.31 This paper reports findings from the mixed-methods study, which addressed the following questions: (1) What does a diverse group of patients identify as the most important barrier to CRC screening when asked in an open-ended survey question? (2) What barriers are identified for CRC screening generally and for each of four recommended screening tests when patients discuss barriers in a focus group setting?
Methods
Study Population and Data Collection
Participants were patients from primary care practices affiliated with the Virginia Ambulatory Care Outcomes Research Network, a practice-based research network. The IRBs of Virginia Commonwealth University and Riverside Medical Group approved the study.
Postal Survey
In June–July 2005, the Health Assessment Survey (HAS) was mailed to 660 randomly selected adults aged 50–75 years who attended two family medicine practices, located in downtown Richmond (VA) and Fairfax (VA), a suburb of Washington, DC. Patients who had completed a HAS within the past year were excluded. Questionnaires were sent using a modified Dillman sequential mailing protocol32,33 with a $2 incentive. A reminder postcard was mailed 1 week after the initial questionnaire; 3 weeks after the first mailing, nonrespondents were mailed a second copy of the questionnaire. Among other items, the survey included questions on past CRC screening experience as well as the following open-ended question about perceived barriers: “Screening for colorectal cancer is recommended for all adults aged ≥50 years. What would you say is the most important reason people do not have these screening tests? _(Please write your answer in your own words.)_” The open-ended format allowed patients to describe any barrier they deemed important, rather than restricting them to fixed response options, and captured the natural language patients used to describe the barriers. Their wording was later examined to develop the comprehensive CRC screening barriers instrument.
Focus Groups
Beginning in October 2005, 40 adults aged 45–75 years were recruited from three practices (the two sites involved in the postal survey and a rural practice in Front Royal [VA]) to participate in focus groups. Recruitment posters were displayed in participating offices. Interested volunteers called a toll-free number and a research nurse determined eligibility to maximize diversity in demographic characteristics, screening status, and insurance coverage. Seven gender- and largely race-specific focus groups were conducted December 2005 to June 2006. The groups were divided by gender and race because of evidence that such groups may have different perspectives on health, health care, and testing and may be more candid about sensitive topics in homogeneous groups. Focus groups were not restricted by screening status because it was thought that those with previous screening also experience barriers and that discussions would be enhanced if people had varying screening experiences. Participants were paid $50 for participation. The sessions lasted 2 hours, were audiotaped, and were moderated by experienced gender-concordant qualitative researchers. Responses were also used to develop the comprehensive CRC screening barriers instrument. Further focus group protocol details are provided in Appendix A (available online at www.ajpm-online.net).
Data Analysis
Postal Survey
Verbatim responses to the open-ended question were read by two reviewers and coded independently, using a code list developed inductively from the survey question responses with particular attention to staying true to the natural language used by respondents. Given this descriptive coding technique, in which the assigned codes mirrored the precise wording of the respondents,34 both investigators assigned virtually identical codes on their initial review, requiring little arbitration. Along with simple frequency counts of themes cited by survey respondents, multiple and stratified regression analyses were performed, respectively, to determine differences in patient-reported barriers by screening status and demographic covariates (gender, age group, and/or race). SAS/STAT ® software (version 9.1.3, SAS Institute Inc, Cary, NC, 2007) was used for all calculations beginning in October 2005.
Focus Groups
Audiotape recordings of the focus groups were transcribed and coded in Atlas.ti.35 Beginning in June 2006 transcripts were analyzed primarily by the moderators, with input from co-investigators. Appendix A (available online at www.ajpm-online.net) provides further details about the descriptive code list development,34,36 dual review, and arbitration of discordant coding.27,34,37,38
Results
Postal Survey
The HAS was returned by 427 recipients (65%), and 317 respondents (74%) returning a questionnaire answered the open-ended question about barriers to CRC screening. Two thirds (65%) were white, 66% were female, 29% had not graduated from high school, and 18% reported an annual household income of less than $20,000. Overall, 68% reported screening for CRC according to prevailing guidelines (i.e., FOBT in the last year, flexible sigmoidoscopy in the last 5 years, colonoscopy in the last 10 years, or barium enema in the last 5 years).2,3 Responders to the open-ended survey question and nonresponders were similar according to gender. However, nonresponders were more likely than responders to be African-American (42.1% vs 31.6%, _p_=0.05) and aged ≥65 years (43.0% vs 27.8%, _p_=<0.01), and less likely to have some college education (72.1% vs 85.6%, _p_=<0.01).
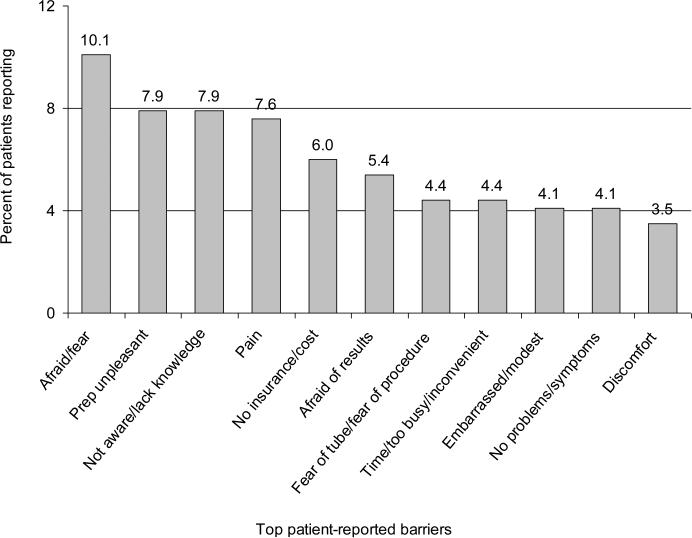
Figure 1 depicts the leading reasons cited by respondents to explain why “people” (not necessarily themselves) might defer CRC screening. These included: fear, apprehension about the bowel preparation (“prep”) that precedes endoscopy and barium enema, being unaware or lack of knowledge, pain, and concerns about insurance and cost. Fear (10%), the bowel prep (8%), and being unaware or lack of knowledge were the leading barriers identified by 26% of respondents. Approximately 20% of respondents cited some type of fear as the most important barrier. Three of the eleven top barriers were specific to endoscopy. Only five (1.6%) responses specifically alluded to the failure of a physician to recommend screening. Table 1 shows the differences in prevalence of the top five reported barriers (see Figure 1) by gender, age, and race. Women were more likely than men to cite fear (14% vs 4%, _p_=<0.01) and the bowel prep (12% vs 1%, _p_=<0.01) as barriers. Men were more likely than women to cite lack of knowledge (12% vs 6%, _p_=0.05). Responses of never-screened participants did not differ significantly after adjustment from those of people who had ever been screened or who reported adherent screening (data not shown).
Figure 1.
Top patient-reported barriers; open-ended questionnaire response of the most important reasons why people do not have CRC screening
Barriers were not grouped into seemingly similar categories. Rather, particular attention was paid to staying true to the natural language used by respondents in open-ended question verbatim.
Table 1.
Top five barriers cited in open-ended survey question: differences by gender, age and race. (M % [95% CI]).a
| Gender differences | |||
|---|---|---|---|
| Women | Men | _p_-value | |
| (_n_=201) | (_n_=103) | ||
| Afraid/Fear (nonspecified)b | 13.9 (9.7, 18.2) | 3.7 (0.0, 9.7) | <0.01 |
| Unpleasant preparation | 12.1 (8.4, 15.8) | 0.1 (0.0, 4.5) | <0.01 |
| Not aware/Lack of knowledge | 5.6 (1.9, 9.2) | 10.7 (5.6, 15.9) | 0.11 |
| Pain (nonspecified)c | 8.4 (4.8, 12.0) | 5.3 (0.2, 10.4) | 0.33 |
| No insurance/Cost | 7.9 (4.6, 11.3) | 3.4 (0.0, 8.2) | 0.13 |
| Age differences | |||
| 50– > 65 years | ≥ 65 years | _p_-value | |
| (_n_=229) | (_n_=88) | ||
| Afraid/Fear (nonspecified)b | 8.7 (4.8, 12.7) | 13.6 (7.3, 20.0) | 0.20 |
| Unpleasant preparation | 8.3 (4.8, 11.8) | 5.7 (0.1, 11.3) | 0.44 |
| Not aware/Lack of knowledge | 7.1 (3.6, 10.6) | 9.1 (3.5, 14.7) | 0.55 |
| Pain (nonspecified)c | 7.5 (4.1, 11.0) | 8.0 (2.4, 13.5) | 0.90 |
| No insurance/Cost | 6.3 (3.2, 9.4) | 5.7 (0.7, 10.7) | 0.84 |
| Racial differences | |||
| White | Other | _p_-value | |
| (_n_=207) | (_n_=110) | ||
| Afraid/Fear (nonspecified)b | 9.4 (4.8, 14.0) | 11.3 (4.8, 17.7) | 0.68 |
| Unpleasant preparation | 10.2 (6.2, 14.2) | 3.0 (0.0, 8.6) | 0.06 |
| Not aware/lack of knowledge | 7.3 (3.2, 11.4) | 8.3 (2.6, 14.0) | 0.80 |
| Pain (nonspecified)c | 6.4 (2.3, 10.4) | 9.9 (4.3, 15.5) | 0.36 |
| No insurance/Cost | 5.6 (2.0, 9.2) | 7.0 (2.0, 12.1) | 0.68 |
Focus Groups
Forty-three percent of focus group participants were African-American and 62% were women. While only 5% had not graduated from high school, 38% had an annual household income less than $20,000. About half reported not being screened (43%) or not knowing their screening status (5%), and almost a quarter (23%) were uninsured or on Medicaid; the majority were covered under private insurance (60%) or Medicare (17%).
The focus group participants discussed barriers to CRC screening that the open-ended survey and the literature had identified and that are largely self-explanatory (Table 2). Here, the focus is on barriers with deeper nuances and those that have not been reported previously. Abridged remarks by participants are presented. The full statements, which add context, appear in Appendix B (available online at www.ajpm-online.net) along with the demographic profile and specific focus group of the speaker.
Table 2.
Commonly reported general barriers to colorectal cancer screening derived from focus groups
| • Logistics: not understanding what to do |
|---|
| • Lack of time, inconvenience, and lack of transportationa |
| • Distasteful, prolonged bowel preparation (“prep”)a |
| • Embarrassing/humiliatinga |
| • Invasivea |
| • Painful/uncomfortable/discomforta |
| • Cost (e.g., unaffordable copayment/deductible)/lack of insurance coveragea |
| • Taboo topic, uncomfortable to discuss, not discussed openly in public like prostate and breast cancer screening |
Established Barriers With Deeper Nuances
Previous studies have examined the frequency with which patients encounter barriers to CRC screening. The focus group participants identified some previously reported barriers but their comments also disaggregated these barriers to expose layers of complexity that previous research has not elucidated (Table 3). For example, whereas the literature regularly cites lack of awareness/information as one barrier to screening, the focus groups identified nine domains for which lack of awareness (Table 3) is problematic and which require different remedies. For example, explaining that CRC is a common disease (prevalence) requires different information than explaining what insurance will cover.
Table 3.
Previously reported but nuanced barriers to colorectal cancer screening derived from focus groups
| • Lack of awareness |
|---|
| ○ Unawareness of the prevalence of colorectal cancer |
| ○ Unawareness of the benefits/harms of colorectal cancer screening |
| ○ Unawareness of the arguments for screening that apply to the patient personally |
| ○ Unwareness of what happens when colorectal cancer screening is performed |
| ○ Unawareness of how patients should care for themselves before/after screening |
| ○ Unawarenes of the pros and cons of each test |
| ○ Unawareness of what insurance will cover |
| ○ Unawareness of what modern early colorectal cancer treatment entails, and its level of success |
| ○ Unawareness of survival rates for screen-detected colorectal cancer |
| • Lack of a recommendation from the physician |
| ○ Lack of clear, direct advice to get tested |
| ○ Lack of emphasis of its importance or the rationale for screening |
| ○ Lack of personalization of the argument for the individual patient |
| ○ Failure to present the options for screening, or the details of what they entail |
| • Fear |
| ○ Fear of cancer (being diagnosed, being treated) |
| ○ General fear of medical tests |
| ○ Fear of being sedated by anesthesia |
| ○ Fear of complications from screening test procedure (e.g., colonic perforation) |
| ○ Fear of learning of an abnormal test result or of late-stage disease |
| ○ Fear of burden on family/friends (economically, psychologically, physically) |
| • Better to find out later |
| ○ Better to find out later because would not want to concern family/friends |
| ○ Better to find out later because would not want to undergo treatment |
| • Fatalism |
| • Time |
| ○ Time to build motivation to undergo test |
| ○ Time to arrange test |
| ○ Time needed to undergo or complete test |
| • Lack of social support from family and close friends |
| • Competing demands |
| • Concern that some screening modalities are outdated |
Participants reported that pat messages from physicians or the media to promote screening failed to personalize the argument for why they, as individuals, required testing. As expressed by one participant, “[Doctors] don't say if it's relatively likely or unlikely that I—given who I am—am likely to have these things....” Although physicians are important in promoting CRC screening,4,5,7,11,13,17,21,39–41 simply advising patients to get screened is, according to some participants, insufficient motivation by itself. They urged greater effort by physicians to convey the importance of screening in their message and tone, articulate a compelling rationale, and outline test options and what they entail.
Respondents discussed familiar access barriers, such as not having a source of primary care or being uninsured, but also highlighted more subtle challenges. Access can be limited, even at free clinics, when out-of-pocket costs make screening unaffordable or when health plans restrict coverage of tests. Further, confusion exists about insurance coverage and the details of what the various screening options entail. As stated by one woman, “...I think most physicians would recommend one of these tests, and not tell you that if that test didn't work for you there is another that might be more doable for you. Insurance may have something to do with this.”
Three fear-related categories (~20% of responses) were most commonly cited in the open-ended survey question. In the focus groups, it was found that fear can take on different meanings—fear of cancer, an invasive procedure, complications, test results, or family ramifications—each of which involve different issues. The fear expressed by some participants, especially disadvantaged patients, was that screen-detected CRC would be too advanced for a favorable prognosis. Some participants harbored misconceptions, such as the belief that most people with CRC require a colostomy or that CRC is incurable. Their enthusiasm for detecting CRC was dampened by adverse experiences among family and friends and by the burdens their diagnosis imposed on relatives. Consequently, some said they would prefer to delay the diagnosis or not know (“Ignorance [is] bliss”). Respondents also invoked themes of fatalism (“you got to go—sometime”) or faith (“Only God knows what the plan is for each of us”) as counterarguments to screening.
Time, when described as a barrier to CRC screening, typically refers to the time sacrificed for testing (e.g., missed work), but participants also spoke of time needed to build motivation to undergo screening, study options, resolve concerns, and arrange testing. They faulted the unrealistic expectation that patients will act immediately once advice is given. Focus group participants who had been screened reported initially delaying screening as they attempted to get information and deal with their concerns.
Some cited competing factors as barriers, such as the demands imposed by coexisting diseases (e.g., diabetes) or a spouse's illness. Some discussed social support in terms of encouragement to undergo testing or assistance going to the doctor's office: “[Being alone] is a barrier to me....”
Some participants dismissed tests other than colonoscopy, such as flexible sigmoidoscopy or barium enema, which they perceived as outmoded technology. “...I don't understand why [you] would have [flexible sigmoidoscopy] done because you could miss something higher up. If you're going to go through the prep and the procedure, it just seems to me you should have the whole shebang...you may need additional testing such as a colonoscopy anyway.”
Newly Elucidated Barriers
Participants also discussed barriers that are rarely reported in previous literature (Table 4). Participants described past experiences with CRC tests as barriers to retesting; they described pain with sigmoidoscopy, discomfort with barium retention, anesthesia reactions, and “cold” or rude physicians and/or technicians. Some participants spoke of deferring screening in anticipation of a forthcoming test that might be more palatable, or more effective. “...Is this [colonoscopy] another one of those screening things that you'll hear about 20 years from now that, oh, you didn't really need to do that.”
Table 4.
Rarely reported and newly elucidated general barriers to colorectal cancer screening derived from focus groups
| • Bad experience with (or stories of) previous colorectal cancer screening tests or insensitive professionals performing them |
|---|
| • Para-sexual issues, such as homosexual sensitivities and history of sexual abuse |
| • Inadequate sense of self-worth |
| • Mistrust...feeling that cheaper (i.e., lower-quality) tests are being recommended or physicians have a conflict of interest (i.e., ordering tests that are beneficial to them given reimbursement) |
| • Confusing an in-office fecal occult blood test (FOBT) with recommended home FOBT colorectal cancer screening |
| • Waiting for a new test that may be easier to undergo (i.e., virtual colonoscopy) |
Some participants mentioned “para-sexual” issues, such as homosexual sensitivities among male patients. As stated by one man, “I think it's problematic for men...especially homophobic men...they think you let someone do that to you, you ain't a real man...” For one woman, a history of sexual abuse posed a psychological barrier: “I just told my doctor I wasn't going to do it. But that was based on, I've got PTSD for childhood trauma and, and I knew it would trigger a major reaction...”
Some participants described low self-worth as a barrier. For example, one African-American woman said, “You have to feel like you're worth it...worth taking care of...We get so many messages from so many places in our society that we're not worthy.”
Participants suspected that some tests (e.g., FOBT) are promoted because they are “cheap” and that financial considerations underlie physicians’ recommendations (“[colonoscopy] is suggested because it's a money maker”) or by health plans: “I'm sure the insurance companies are looking at all that stuff [costs and benefits]...I mean, they're trying to make money.”
Misunderstandings about in-office FOBT may pose a barrier to FOBT performed at home. In-office FOBT, commonly included in the rectal/vaginal examination performed by physicians, was misinterpreted by some patients as a recommended screening test. “I've had you know like the rectal and the stool testing [in the office]...and I always felt like if they found something there then it would go farther.” An African-American woman said, “I've never had one of these [FOBT home kits] given to me, but I have one done every time I have my annual.”
Test-Specific Barriers
Barriers associated with specific tests (Table 5) were generally inutitive (“_adults don't play with poop_”), but others were not. For example, FOBT, often seen by providers as the simplest option, was described by some participants as disgusting or unsanitary as well as complex and confusing; they questioned their ability to perform the test correctly and avoid misleading results. Some participants did not favor FOBT because abnormal results require a colonoscopy. For some, being awake during flexible sigmoidoscopy or barium enema was a barrier. Pain was cited as a barrier for flexible sigmoidoscopy and barium enema as well as colonoscopy. The bowel prep, a notorious barrier for all three tests, was perceived as less worth the sacrifice when performed for flexible sigmoidoscopy and barium enema, because these tests require a second prep and colonoscopy if an abnormality is detected, and sigmoidoscopy visualizes less of the colon.
Table 5.
Test-specific barriers to colorectal cancer screening derived from focus groupsa
| Fecal Occult Blood Test |
|---|
| • Disgusting or unsanitary connotations of handling stool |
| • Logistics of sampling and storage (e.g., shipping stool samples through mail) |
| • Confusion about instructions or concerned about doing test correctly |
| • Need for follow on testing if positive result |
| Flexible Sigmoidoscopy |
| • Awake during procedure |
| • Examines only distal colon |
| Colonoscopy |
| • Risks/undesirability of anesthesia and sedation |
| • Relatively greater risk of complications (e.g., perforation) |
| Barium Enema |
| • Awake during procedure |
| • Cannot biopsy; need colonoscopy if lesion detected |
Discussion
In this mixed-methods study, a diverse group of patients—including a substantial portion of African Americans and those with low education and income levels—exposed a complex and nuanced set of barriers to CRC screening that previous research has not elucidated. Important implications of these results exist for public health, policymakers, physicians, and patients. The barriers related to (1) knowledge, a factual understanding of what to do and what it entails; (2) motivation, a willingness to obtain the test, despite reservations; and (3) ability, the means to obtain what one wants. Barriers to CRC screening exist in each area, as the literature documents, but this study sheds light on their nature and subtleties.
Knowledge
Lack of awareness and inadequate knowledge and information, documented barriers to screening, provide the rationale for efforts to educate the public about CRC and to encourage physicians to promote screening among eligible patients. The current data suggest, however, that merely advising people to get screened may not satisfy their information needs. Specifically, focus group participants articulated a need for more details in nine information domains (Table 3), ranging from disease prevalence to insurance coverage. The value of a personalized rationale statement for each individual was emphasized.
The central role of physician advice comports with the literature,4,5,7,11,13,17,21,39–41 which identifies such advice as a key motivator for CRC screening, and with responses to a fixed-response survey administered to 6,100 patients following (and informed by) this project. In that survey, the absence of physician advice ranked among the top five barriers to screening.31 A contrary finding was observed in the open-ended survey question, answered by 317 patients: Only 1.6% of respondents mentioned physician advice. This discrepancy may suggest that people may not attribute their behaviors to physicians unless cued to do so by response options. Additionally, many patients in this study saw a doctor within the last 2 years and had been screened for CRC.
Focus group participants of all social backgrounds expressed a need for more information about CRC screening and exhibited confusion, even after completing a worksheet about each test. FOBT, a test considered simple by clinicians, was described as confusing. Many patients harbored misconceptions. In the open-ended survey question, the absence of symptoms ranked among the top ten reasons for not being screened, a misconception restated in the focus groups, where some participants also implied that people without a family history of CRC need not be screened. Some participants confused in-office FOBT with home FOBT, thought colonoscopy—a procedure performed under anesthesia—was painful, and suspected that CRC was largely incurable and required a colostomy. Having four test options was considered desirable but complex, a problem undoubtedly amplified by the recent introduction of new screening technologies (e.g., virtual colonoscopy, stool DNA testing). Such confusion underscores the need, voiced by participants, to offer patients more complete information, both to overcome knowledge barriers and to foster more informed choices.
Motivation
The focus groups also highlighted attitudes that attenuate interest, such as taboos surrounding CRC, fatalism, and fear in its many forms. They articulated barriers that prior literature has not emphasized, such as low self-worth, demands from coexisting illnesses and family life, inadequate social support, “para-sexual” issues, unpleasant past experiences with health care, and cynicism about financial motivations behind recommendations. The elicited spectrum of barriers that other studies have either omitted or ranked differently may reflect sampling artifacts or this study's comprehensive approach to posing questions.
When posed in an open-ended survey format, with no response options as cues, patients named fear—as a general construct—and apprehensions about the bowel prep as the first and second most commonly cited barriers. The three fear-related categories represented in Figure 1 reflect the most common barrier theme in the open-ended survey question (20% of responses). The focus groups deconstructed fear to reveal six subdomains—apart from generic fears—that included fears of the test, anesthesia, CRC, complications, and impact on loved ones. The top ten responses to the open-ended question included fears of pain, “the tube,” the procedure, the results, and embarassment.
Responses differed by gender. In open-ended responses, women were more likely to cite fear as a barrier, whereas men were more likely to cite lack of knowledge. Prior sexual abuse was mentioned as a barrier by one woman in a focus group. Stigma limits reporting of domestic violence, but its estimated prevalence is 20.7%, 42 making such trauma a potentially underrecognized barrier. Machismo and homophobic sensitivities, barriers cited by male focus group participants, have been reported in studies of male minorities.20,21
Attention to pychosocial barriers to screening is important. Patients with coexisting chronic illnesses, little social support, unspoken fears, fatalistic beliefs, or a history of abuse require efforts by clinicians to identify and address these barriers. Patients who defer retesting because of past experiences also require special attention. Psychosocial issues can be addressed in various ways, such as community and patient education programs that “normalize” and discuss these barriers, dialogue about CRC screening options that encourage patients to voice these concerns, and sensitivity among clinicians, when necessary, to address psychosocial issues first before initiating conversations about CRC. The motivation to overcome fears may, according to participants, depend on how strongly physicians advocate screening; they urged physicians to convey importance in both message and tone and to be courteous with patients, who may defer screening to avoid the “coldness” of the clinical encounter. They also noted that patients need time—to build motivation, reach closure, and arrange testing—and cannot be expected to act immediately when screening is recommended.
Participants identified test-specific barriers that color attitudes, such as those evoked by the bowel prep or handling stool. They were dubious about tests they considered outdated or inferior, such as barium enema or FOBT, a sentiment reported elsewhere among veterans15 and African-American church members19,43 FOBT is the only screening test shown in randomized trials to lower CRC mortality.44–47 Modeling studies and national guidelines advocate FOBT and other tests as fully effective alternatives to colonoscopy. Nonetheless, patients discounted these options as “cheap” substitutes for colonoscopy, echoing many physicians’ preferences for the latter. The larger goal of detecting CRC could be undermined by this attitude if patients who defer colonoscopy are not educated by physicians about equivalent alternatives or are misinformed about their effectiveness, which could utlimately result in receiving no form of screening.48
Ability
Regardless of their knowledge or attitudes, patients cannot obtain recommended screening tests or act on results if the necessary resources are lacking. In the open-ended survey, the fifth most common barrier cited by respondents was the costs of tests and inadequate health insurance coverage. Focus group participants also discussed this barrier, noting that access to certain tests is limited by health plans and prohibitive out-of-pocket costs. Those costs place greater financial burdens on disadvantaged patients, who also have diminished access to physicians. Providing universal coverage, with no copayments, for all recommended CRC screening tests are obvious priorities.
This study has several limitations. First, its generalizability is limited; the sample was drawn from established patients of three family medicine practices in Virginia. The study sample may have systematically excluded people with other perspectives about barriers. Patients in other settings and people with unreliable access to physicians or CRC screening (e.g., the uninsured) were under-represented. Approximately half of focus group participants and 68% of respondents to the open-ended survey had been screened for CRC previously. The perceived barriers of those who have been screened (and have overcome barriers) may differ from those who have not been screened, although study data did not reveal this difference. Second, differences in the framing of the open-ended survey question (i.e., most important barrier to “people”) and the focus group questions (i.e., barriers for “people like you”) may have introduced a subtle artifact. Focus group participants tended to answer these questions in reference to themselves or others they knew, rather than for the whole population. Third, the study, having occurred in 2005–2006, did not address recently introduced screening technologies. Finally, the data are cross-sectional and do not permit inference about causal relationships between reported barriers and screening behaviors.
The study has several strengths, however, and adds details and texture to current understanding of the barriers to CRC screening. The open-ended survey question captured unique data by not restricting the response options and giving respondents complete freedom to describe barriers in their own words without external cues. The focus groups were divided by gender and race to encourage participants to speak freely about sensitive issues, and followed a protocol that dealt comprehensively with issues surrounding a range of screening tests. Participants in both studies encompassed a diverse sample from urban, suburban, and rural settings, including many African Americans and low-income patients. Finally, the open-ended survey question and focus groups often produced congruent findings, both in content and relative importance, strengthening the evidence that psychosocial factors influence screening behaviors.
Supplementary Material
01
Acknowledgements
This study was funded with grant number R21 CA100517 from the National Cancer Institute. The authors thank the three family medicine practices in the Virginia Ambulatory Care Outcomes Research Network that participated in the study: Virginia Commonwealth University Family Medicine at Nelson Clinic, Richmond, VA; Fairfax Family Practice, Fairfax, VA; and Front Royal Family Practice, Front Royal, VA. We also thank Michelle Lee, PhD for her assistance as a co-moderator of the women's focus groups.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman D, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the U.S. Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Preventive Services Task Force Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. U.S. Preventive Services Task Force. Ann Intern Med. 2008 2008 Nov 4;149(9):627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. Epub 2008 Oct 6. Summary for patients in: Ann Intern Med 2008 Nov 4;149(9):1–44.
- 4.CDC Use of colorectal cancer tests—U.S., 2002, 2004, 2006. MMWR. 2008;57(10):253–8. [PubMed] [Google Scholar]
- 5.Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst. 1997;89(19):1406–22. doi: 10.1093/jnci/89.19.1406. [DOI] [PubMed] [Google Scholar]
- 6.James AS, Campbell MK, Hudson MA. Perceived barriers and benefits to colon cancer screening among African Americans in North Carolina: how does perception relate to screening behavior? Cancer Epidemiol Biomarkers Prev. 2002;11:529–534. [PubMed] [Google Scholar]
- 7.Reding DJ, Lapper KA, Krueger M, Kolehouse BL, Steneil D, Leer RA. Cancer screening and prevention in rural Wisconsin: the greater Marshfield experience. Wisconsin Medical Journal. 1997;96(8):32–7. [PubMed] [Google Scholar]
- 8.Beeker C, Kraft JM, Southwell BG, Jorgensen CM. Colorectal cancer screening in older men and women: qualitative research findings and implications for intervention. J Community Health. 2000;25(3):263–78. doi: 10.1023/a:1005104406934. [DOI] [PubMed] [Google Scholar]
- 9.Hsia J, Kemper E, Kiefe C, et al. The importance of health insurance as a determinant of cancer screening: evidence from the Women's Health Initiative. Prev Med. 2000;31:261–70. doi: 10.1006/pmed.2000.0697. [DOI] [PubMed] [Google Scholar]
- 10.Cokkinides VE, Chao A, Smith RA, Vernon SW, Thun MJ. Correlates of underutilization of colorectal cancer screening among U.S. adults, age 50 years and older. Prev Med. 2003;36(1):85–91. doi: 10.1006/pmed.2002.1127. [DOI] [PubMed] [Google Scholar]
- 11.Zapka JG, Puleo E, Vickers-Lahti M, Luckmann R. Healthcare system factors and colorectal cancer screening. Am J Prev Med. 2002;23:28–35. doi: 10.1016/s0749-3797(02)00444-0. [DOI] [PubMed] [Google Scholar]
- 12.Finney Rutten LJ, Nelson DE, Meissner HI. Examination of population-wide trends in barriers to cancer screening from a diffusion of innovation perspective (1987–2000). Prev Med. 2004;38:258–68. doi: 10.1016/j.ypmed.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Greiner KA, Born W, Nollen N, Ahluwalia JS. Knowledge and perceptions of colorectal cancer screening among urban African Americans. J Gen Intern Med. 2005;20(11):977–83. doi: 10.1111/j.1525-1497.2005.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogedegbe G, Cassells AN, Robinson CM, et al. Perceptions of barriers and facilitators of cancer early detection among low-income minority women in community health centers. J Natl Med Assoc. 2005;97(2):162–70. [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman MJ, Ogdie A, Kanamori MJ, Cañar J, O'Malley AS. Barriers and facilitators of colorectal cancer screening among Mid-Atlantic Latinos: focus group findings. Ethn Dis. 2006;16(1):255–61. [PubMed] [Google Scholar]
- 16.Greisinger A, Hawley ST, Bettencourt JL, Perz CA, Vernon SW. Primary care patients’ understanding of colorectal cancer screening. Cancer Detect Prev. 2006;30:67–74. doi: 10.1016/j.cdp.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Friedemann-Sánchez G, Griffin JM, Partin MR. Gender differences in colorectal cancer screening barriers and information needs. Health Expect. 2007;10:148–60. doi: 10.1111/j.1369-7625.2006.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lasser KE, Ayanian JZ, Fletcher RH, Good MJ. Barriers to colorectal cancer screening in community health centers: a qualitative study. BMC Fam Pract. 2008;9(1):15. doi: 10.1186/1471-2296-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green AR, Peters-Lewis A, Percac-Lima S, et al. Barriers to screening colonoscopy for low-income Latino and white patients in an urban community health center. J Gen Intern Med. 2008;23(6):834–40. doi: 10.1007/s11606-008-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz ML, James AS, Pignone MP, et al. Colorectal cancer screening among African- American church members: a qualitative and quantitative study of patient–provider communication. BMC Public Health. 2004;4:62. doi: 10.1186/1471-2458-4-62. Available from: http://www.biochmedcentral.com/1471-2458/4/62. [DOI] [PMC free article] [PubMed]
- 21.Tessaro I, Mangone C, Parkar I, Pawar V. Knowledge, barriers, and predictors of colorectal cancer screening in an Appalachian church population. Prev Chronic Dis. 2006;3(4):1–11. Available from: http:// www.cdc.gov/pcd/issues/2006/oct/06_0033.htm. [PMC free article] [PubMed]
- 22.Coronado GD, Farias A, Thompson B, Godina R, Oderkirk W. Attitudes and beliefs about colorectal cancer among Mexican Americans in communities along the U.S.–Mexico border. Ethn Dis. 2006;16(2):421–7. [PubMed] [Google Scholar]
- 23.Fernandez ME, Wippold R, Torres-Vigil I, et al. Colorectal cancer screening among Latinos from U.S. cities along the Texas–Mexico border. Cancer Causes Control. 2008;19(2):195–206. doi: 10.1007/s10552-007-9085-6. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman-Goetz L, Thomson MD, Donelle L. Reasons for declining colorectal cancer screening by older Canadians: a pilot study. J Cancer Educ. 2008;23(1):32–6. doi: 10.1080/08858190701821188. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro JA, Seeff LC, Thompson TD, Nadel MR, Klabunde CN, Vernon SW. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17:1623–30. doi: 10.1158/1055-9965.EPI-07-2838. [DOI] [PubMed] [Google Scholar]
- 26.Creswell JW. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. 3rd edition Sage; Thousand Oaks, CA: 2009. [Google Scholar]
- 27.Creswell JW, Plano-Clark VO. Designing and Conducting Mixed Methods Research. Sage; Thousand Oaks, CA: 2007. [Google Scholar]
- 28.Pope N, Mays N. Qualitative research in health care. 3rd ed. Wiley Blackwell; 2006. [Google Scholar]
- 29.Creswell JW, Fetters MD, Ivankova NV. Designing a mixed methods study in primary care. Ann Fam Med. 2004;2(1):7–12. doi: 10.1370/afm.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borkan JM. Mixed methods studies: a foundation for primary care research. Ann Fam Med. 2004;2(1):4–6. doi: 10.1370/afm.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones RM, Woolf SH, Cunningham TD, Johnson RE, Krist AH, Rothemich S, Vernon SW. The relative importance of patient-reported barriers to colorectal cancer screening, submitted. Am J Prev Med. doi: 10.1016/j.amepre.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dillman DA. Mail and Internet Surveys: The Total Design Method. 2nd ed. John Wiley and Sons; 1999. [Google Scholar]
- 33.Dillman DA, Carley-Baxter LR. Social and Economic Sciences Research Center technical report #00-12. Washington State University; Pullman, WA: 2000. Structural determinants of response rates to 102 national park satisfaction surveys, 1988–1999. [Google Scholar]
- 34.Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. Sage; Thousand Oaks, CA: 1994. [Google Scholar]
- 35.Muhr T. ATLAS.ti 5.0 [Version 5:] ATLAS.ti Scientific Software Development GmbH; Berlin, Germany: 2004. Available from http://www.atlasti.com/ [Google Scholar]
- 36.Richards L. Handling Qualitative Data: A Practical Guide. Sage; Thousand Oaks, CA: 2005. [Google Scholar]
- 37.Lingard L, Albert M, Levison W. Grounded theory, mixed methods, and action research,”. BMJ. 2008;337:a567. doi: 10.1136/bmj.39602.690162.47. [DOI] [PubMed] [Google Scholar]
- 38.Devers KJ. How will we know “good” qualitative research when we see it? Beginning the dialogue in health services research. Health Serv Res. 1999;34(5 Pt 2):1153–88. Review. [PMC free article] [PubMed] [Google Scholar]
- 39.Rawl SM, Menon U, Champion VL, Foster JL, Skinner CS. Colorectal cancer screening beliefs. Focus groups with first-degree relatives. Cancer Pract. 2000;8(1):32–7. doi: 10.1046/j.1523-5394.2000.81006.x. [DOI] [PubMed] [Google Scholar]
- 40.Seeff LC, Nadel MR, Klabunde C, et al. Patterns and predictors of colorectal cancer test use in the adult U.S. population: results from the 2000 National Health Interview Survey. Cancer. 2004;100:2093–103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 41.Klabunde CN, Vernon SW, Nadel MR, Breen NL, Seeff LC, Brown ML. Barriers to colorectal cancer screening: a comparison of reports from primary care physicians and average-risk adults. Med Care. 2005;43:939–44. doi: 10.1097/01.mlr.0000173599.67470.ba. [DOI] [PubMed] [Google Scholar]
- 42.CDC Adverse Childhood Experiences Study. Prevalence of Individual Adverse Childhood Experiences. http://www.cdc.gov/nccdphp/ace/prevalence.htm.
- 43.Wright M, Corwin S, Brandt H, Hebert J, Friedman D. Assessing psychosocial and cultural barriers to colorectal screening among African Americans: a descriptive study. Journal of Immigrant and Minority Health. In press. [Google Scholar]
- 44.Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screen for fecal occult blood. J Natl Can Inst. 1999;91:434–7. doi: 10.1093/jnci/91.5.434. [DOI] [PubMed] [Google Scholar]
- 45.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. New Engl J Med. 2000;343(22):1603–7. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 46.Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–71. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 47.Jorgensen OD, Kronborg O, Fenger C. A randomized study of screening for colorectal cancer using faecal occult blood testing: results after 13 years and seven biennial screening rounds. Gut. 2002;40:29–32. doi: 10.1136/gut.50.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woolf SH. The best screening test for colorectal cancer – A personal choice. N Eng J Med. 2000;343(22):1641–3. doi: 10.1056/NEJM200011303432211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01