Temporal Profiling of the Secretome during Adipogenesis in Humans (original) (raw)
Abstract
Adipose tissue plays a key role as a fat-storage depot and as an endocrine organ. Although mouse adipogenesis has been studied extensively, limited studies have been conducted to characterize this process in humans. We carried out a temporal proteomic analysis to interrogate the dynamic changes in the secretome of primary human preadipocytes as they differentiate into mature adipocytes. Using iTRAQ-based quantitative proteomics, we identified and quantified 420 proteins from the secretome of differentiated human adipocytes. Our results revealed that the majority of proteins showed differential expression during the course of differentiation. In addition to adipokines known to be differentially secreted in the course of adipocyte differentiation, we identified a number of proteins whose dynamic expression in this process has not been previously documented. They include collagen triple helix repeat containing 1, cytokine receptor-like factor 1, glypican-1, hepatoma-derived growth factor, SPARC related modular calcium binding protein 1, SPOCK 1, and sushi repeat-containing protein. A bioinformatics analysis using Human Protein Reference Database and Human Proteinpedia revealed that of the 420 proteins identified, 164 proteins possess signal peptides and 148 proteins are localized to the extracellular compartment. Additionally, we employed antibody arrays to quantify changes in the levels of 182 adipokines during human adipogenesis. This is the first large-scale quantitative proteomic study that combines two platforms, mass spectrometry and antibody arrays, to analyze the changes in the secretome during the course of adipogenesis in humans.
Keywords: secretome, quantitative proteomics, adipokine, protein microarrays, metabolism
Short abstract
The secretome of adipocytes is regulated during the differentiation of preadipocytes into adipocytes. Using iTRAQ-based quantitative proteomics, we identified and quantified 420 proteins. Additionally, we employed antibody arrays to quantify changes in the levels of 182 adipokines. This is the first large-scale quantitative proteomic study that combines two platforms, mass spectrometry and antibody arrays, to analyze the changes in the secretome during the course of adipogenesis in humans.
Introduction
Adipocytes play a central role in the control of energy balance and lipid homeostasis. As the primary storage depot for triglycerides (TG), adipose tissue is the most efficient source of energy reserve in humans as TG is anhydrous and therefore is stored at a maximum caloric density of ∼9 kcal/g.(1) Obesity, resulting from both hyperplasia and hypertrophy of adipocytes, is tightly linked to type 2 diabetes and cardiovascular diseases; as such, it is a major health risk in the world.(2) Aside from being a fat-storage depot, adipocytes are also endocrine cells that actively regulate signaling pathways responsible for energy balance and lipid homeostasis through the secretion of hormones, cytokines, growth factors and other proteins, collectively termed adipokines.(3) Among the well-studied adipokines are adiponectin, leptin, resistin, plasminogen activated inhibitor 1 (PAI-1), and proinflammatory cytokines IL-6 and TNF-α. Dysregulation in the circulating levels and actions of adipokines are causally linked to the pathogenesis of insulin resistance, diabetes and obesity.
The differentiation of preadipocytes into adipocytes is a complex process that is tightly regulated and orchestrated by a network of transcription factors acting in a precisely controlled temporal fashion to coordinate the expression of the machinery required to specify a fully functional, mature adipocyte.(4) The transcription factor, peroxisome proliferator-activated receptor-gamma (PPAR-gamma), plays a central role in driving the adipogenic program; ectopic expression of PPAR-gamma in nonadipogenic mouse fibroblasts is sufficient to recapitulate much of the adipocyte phenotype.(5) The important role of PPAR-gamma in adipogenesis is supported by many in vitro and in vivo studies.6,7 Major advances in understanding the molecular underpinning of adipogenesis were made possible by the establishment of a fibroblast cell line (3T3-L1) highly capable of differentiating into mature adipocytes filled with lipid droplets.(8) This in vitro system has allowed investigators to employ molecular biology techniques to identify specific genes induced during adipocyte differentiation in culture, allowing the establishment of temporal gene expression patterns that specify sequential events in this process. Although microarray-based approaches have been extensively and successfully used to analyze changes in gene expression during adipogenesis, only a limited number of studies have been carried out to evaluate alterations in protein content, due primarily to the greater technical challenge.(9)
Recently, several mass spectrometry-based proteomics studies have been reported in primary mouse adipose tissue or in vitro differentiated 3T3-L1 mouse adipocytes.10−13 These studies demonstrate that, during differentiation, the entire secretory proteome (termed the secretome) of 3T3-L1 adipocytes changes dramatically with the most prominent changes involving the extracellular matrix components, cytokines, antioxidants, and complement factors. One mass spectrometry study has also been carried out on primary rat adipocytes.(14) To date, two groups have characterized the secretome of differentiated human adipocytes.15,16 A major limitation of these studies is the use of 2-dimensional gels to separate proteins prior to identification by mass spectrometry, thus precluding a greater depth of analysis. A second limitation relates to the scope; by restricting the analysis of the secretome to preadipocytes versus mature adipocytes, the investigators were not able to capture the dynamic temporal changes in protein expression throughout the differentiation process. To overcome these limitations, we have previously described a 5-plex SILAC strategy to quantify temporal changes of the secretome during mouse 3T3-L1 adipocyte differentiation in culture;(12) however, a similar study has not been carried out in humans.
Isobaric tags for relative and absolute quantification (iTRAQ) can be used for multiplexed quantitation of proteins by tandem mass spectrometry.17,18 In this study, we employed an iTRAQ-based strategy to specifically characterize the secretory proteome and to profile the temporal changes during human adipogenesis. In addition to identifying many proteins previously known to be secreted by adipocytes such as adiponectin and adipsin, we also uncovered proteins not known to be present in the secretome during adipogenesis. Further, we employed a high-throughput antibody array method to validate some of our proteomic data and to profile the secretome for additional proteins not originally detected by mass spectrometry. Quantitation of the secretome during adipogenesis revealed dynamic expression patterns of these adipokines that were underappreciated in proteomics studies in humans. Our study represents the largest proteomic analysis of the primary human adipocyte secretome carried out to date.
Experimental Procedures
Differentiation of Human Primary Preadipocytes to Adipocytes
The differentiation of human primary preadipocytes to adipocytes was carried out essentially as previously described.(19) The Adipocyte Core of The Boston Obesity Nutrition Research Center (BONRC) provided the preadipocytes. Briefly, subcutaneous fat tissue was obtained from subjects (females aged 41.6 ± 5.4 years and a BMI of 36 ± 7.6) undergoing panniculectomy surgery. The fat tissue then underwent collagenase digestion and the preadipocytes were separated from adipose tissue contaminants such as macrophages and vascular cells by techniques previously described.(20) Then these preadipocytes were grown in preadipocyte media (DMEM/F12 supplemented with 15 mM HEPES, 15 mM NaHCO3, 2 mM l-glutamine, 10% NuSerum, 0.16 mM penicillin and 0.06 mM streptomycin) for seven days while media were changed every three days. Five subjects’ samples were pooled on the basis of having equivalent rates of differentiation based on lipid content indicated by oil red O staining and the percentage of cells containing lipid droplets. Cells were seeded at confluence after cellular proliferation ended so that each subject was equally represented in the pooled cell population. Pooled preadipocytes were cultured in differentiating media (DMEM/F12 supplemented with 15 mM NaHCO3, 17 μM pantothenate, 10 mg/L transferin, 33 mM biotin, 1 g/L Fetuin, 0.16 mM penicillin and 0.06 mM streptomycin) and induced by a cocktail inducer (0.5 μM human insulin, 0.1 μM dexamethasone, 2 nM T3, 1 μM ciglitazone and 540 μM IBMX) to differentiate into adipocytes during a 12-day period while media was changed every 3 days. After 8 days, the cocktail inducer was removed and cells were maintained in differentiating media. Preadipocytes or differentiating adipocytes were washed extensively with PBS and cultured overnight in serum-free media for harvesting the conditioned media.
iTRAQ Labeling of Secretome
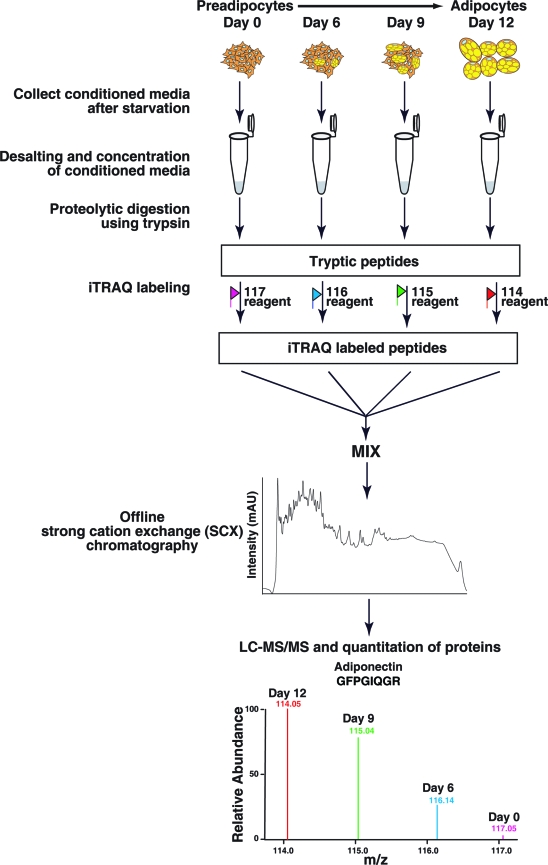
After normalization by cell number, the conditioned media were resolved by SDS-PAGE. Proteins in the gel were stained by a silver staining protocol. The conditioned media were desalted using Amicon centrifugal filters with a 3-kDa cutoff and concentrated. The concentrated conditioned media were normalized and subjected to iTRAQ labeling (Applied Biosystems) according to the manufacturer’s instructions. Briefly, the conditioned media were incubated with the reducing agent (tris(2-carboxyethyl) phosphine (TCEP)) at 60 °C for 1 h, alkylated with the cysteine blocking reagent, methyl methanethiosulfonate (MMTS)) for 10 min at room temperature and digested using sequencing grade trypsin (Promega) (1:10) for 12 h at 37 °C.(17) The resulting peptides were labeled with iTRAQ reagents (day 0, 117; day 6, 116; day 9, 115; and day 12, 114). After iTRAQ labeling, the peptides were mixed and fractionated by a strong cation exchange (SCX) chromatography on PolySULFOETHYL A column (PolyLC, Columbia, MD) (100 × 2.1 mm, 5 μm particles with 300 Å pores) using Agilent HPLC connected to an automated sample collection system. Forty-three SCX fractions were collected from a 0−350 mM KCl gradient in the presence of 10 mM potassium phosphate buffer (pH 2.85), containing 25% acetonitrile. The fractions were dried and analyzed by LC−MS/MS as described below.
LC−MS/MS and Data Analysis
LC−MS/MS analysis of the sample was carried out using reversed phase liquid chromatography interfaced with an LTQ-Orbitrap XL ETD mass spectrometer (Thermo Scientific). The reversed phase system consisted of a trap column (3 cm ×75 mm, C18 material 5−10 mm, 120 Å) and an analytical column (10 cm × 75 mm, C18 material 5 mm, 120 Å). The peptides were separated by acetronitrile gradient (0−60%) containing 0.1% formic acid. The MS spectra were acquired in a data-dependent manner targeting the nine most abundant ions in each survey scan. The settings used were: (1) Precursor scans (FTMS) from 350−1600 m/z at 60,000 resolution; (2) PQD fragmentation of the most intense 9 ions (isolation width, 2.50 m/z; normalized collision energy, 32.5%; activation Q = 0.450; activation time = 0.350 ms). Proteome Discoverer version 1.1 (Thermo Scientific) was used for identification and quantitation of proteins. The data were searched against human RefSeq database version 35 containing 39 380 protein entries. The fixed modifications included iTRAQ 4plex on N-terminal and lysine residues and methylthiolation of cysteine residues. The variable modifications included oxidation of methionine residues and deamination of asparagine or glutamine residues; digestion enzyme, trypsin; maximum number of missed cleavages, 2; peptide mass tolerance, 20 ppm; fragment mass tolerance, 0.5 Da. Quantitation was carried out whenever there were one or more iTRAQ reagent ratios for a peptide.
Relative Quantitation of Adipokines Using an Antibody Array
Relative quantitation of adipokines in the secretome was also carried out using an adipokine antibody array (RayBiotech, Norcross GA) according to the manufacturer’s instructions. Briefly, the conditioned media normalized by cell number was dialyzed overnight against PBS at 4 °C and incubated with biotin-labeling reagents to biotinylate the primary amine of the proteins in the media. The biotin-labeled samples were added to the glass chip and incubated at 4 °C overnight. After washing, the array was incubated with fluorescent dye-conjugated streptavidin. The signal was visualized by Axon Genescanner and the data analyzed using GenePix Pro (ver 6.0) as per the manufacturer’s instructions.
Clustering of Identified Proteins
We used fuzzy c-means (FCM) clustering to classify the proteins based on their changes in abundance during differentiation.(21) FCM clustering gives each protein a membership value of belonging to a category. The membership value indicates the quality of the category assignment for that particular protein. We used the R e1071 package to implement the algorithm. Proteins having missing abundances were dropped from the analysis. The abundance ratios instead of the protein concentrations were used so that the values are on the same scale. The abundance ratios on day 0, day 6 and day 9 were calculated for each protein using the abundance on day 12 as the baseline. We set the degree of fuzzification to 2 (the default value in the implementation) and explored different number of clusters. We found that 3-cluster setting gives a reasonably good result. Setting a higher number of clusters simply split the existing categories into sub categories.
Results and Discussion
Mass Spectrometric Analysis of the Adipocyte Secretome during Differentiation
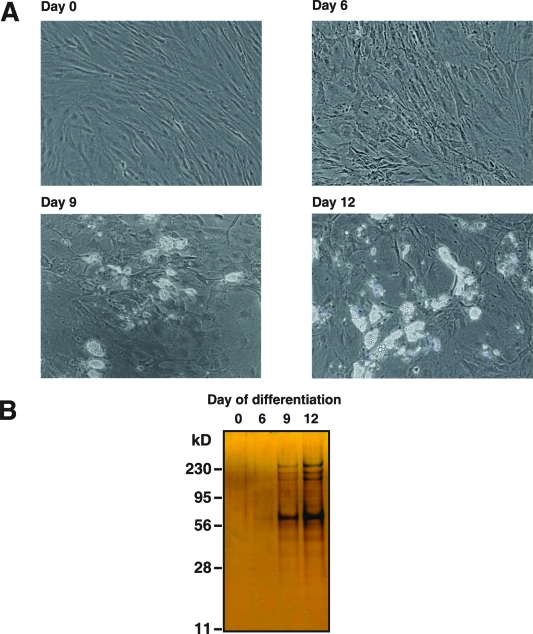
Subcutaneous human preadiopocytes were derived from adipose tissue that was harvested during panniculectomy surgery. After culturing in differentiation media for 12 days, the conversion of human preadipocytes to mature adipocytes was confirmed by visualizing the presence of lipid droplets using phase-contrast microscopy. Figure 1A shows preadipocytes (Day 0) and adipocytes at different stages of differentiation (Days 6, 9 and 12). Proteins in the conditioned media from the same number of cells at different stages of differentiation were resolved by SDS-PAGE and visualized by silver-staining. Figure 1B clearly shows that adipocytes at late stages of differentiation (Days 9 and 12) secrete more proteins than preadipocytes (Day 0) or adipocytes at an earlier stage of differentiation (Day 6). After normalization by cell numbers, the secretomes during the course of adipocyte differentiation were compared using an iTRAQ-based quantitative proteomics approach, as illustrated in Figure 2. After iTRAQ labeling, peptides derived from different stages of differentiation were mixed and fractionated into 43 fractions by strong cation exchange chromatography. Analysis of these fractions by LC−MS/MS on an LTQ-Orbitrap Fourier transform mass spectrometer generated 15 807 MS/MS spectra. We identified 420 proteins using an FDR cutoff of 1% at the peptide level.
Figure 1.
Differentiation of human preadipocytes to mature adipocytes. (A) Representative bright field images of subcutaneous fat depot-derived preadipocytes (Day 0) and day-6, 9, 12 differentiated adipocytes. (B) Proteins in the conditioned media were resolved by SDS-PAGE and visualized by silver staining.
Figure 2.
iTRAQ-based quantitative proteomics. In the course of differentiation from human preadipocytes to adipocytes, conditioned media were harvested, desalted, and concentrated. The protein samples were subjected to reduction, alkylation, and tryptic digestion. After labeling with iTRAQ labeling reagent, the resulting peptides were mixed and fractionated by strong cation exchange liquid chromatography. Each fraction was analyzed by LC−MS/MS on an LTQ-Orbitrap Fourier transform mass spectrometer.
Dynamic Changes in Adipocyte Secretome during Adipogenesis
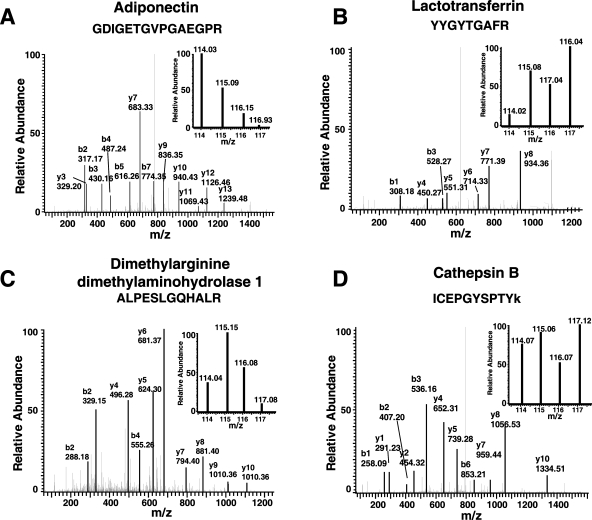
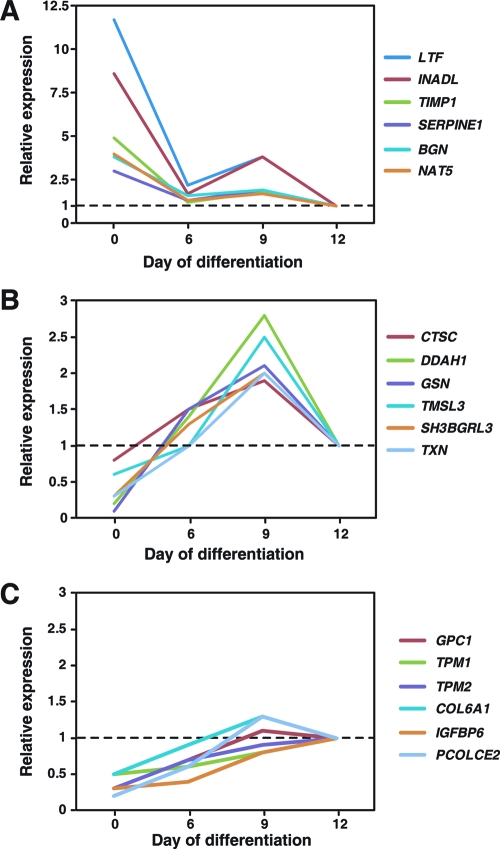
iTRAQ-based quantitation of proteins in the secretome revealed the expression of many secreted proteins during the course of adipocyte differentiation in humans (Supplementary Table 1, Supporting Information). Comparison between Day 0 and Day 12 demonstrated that the majority of proteins (94%) showed an overall trend of increased secretion while 20 proteins (5%) seemed to show a trend of reduced expression during adipogenesis. However, the expression levels obtained from iTRAQ-based quantitation revealed that conventional ‘downregulation’ and ‘upregulation’ terms cannot accurately and adequately describe the temporal expression patterns of these proteins throughout adipogenesis. The representative MS/MS spectra of peptides from 4 proteins-adiponectin, lactotransferrin, dimethylarginine dimethylaminohydrolase 1, and cathepsin B - are shown in Figure 3 with each showing a characteristic profile. Thus, we used a clustering method to search for patterns in the temporal changes of the secretory proteome during adipogenesis. From this analysis, we categorized proteins into three major protein expression patterns in the secretome during adipogenesis. The changes in the expression levels of representative proteins from each pattern are shown in Figure 4. We will discuss these patterns and associated proteins in greater detail below.
Figure 3.
MS/MS spectra of iTRAQ labeled peptides from representative differentially secreted proteins identified in this study. (A) Adiponectin; (B) Lactotransferrin; (C) dimethylarginine dimethylaminohydrolase 1; and (D) Cathepsin B. (Insets) Reporter ions generated during MS/MS that were used for quantitation.
Figure 4.
Dynamic expression patterns of proteins identified from the secretome of adipocytes during the course of differentiation. (A−C) Examples of proteins belonging to these distinct patterns.
Figure 4A represents a cluster of secreted proteins expressed at high levels at the beginning of adipogenesis that decreased during the process of differentiation despite some fluctuations on Days 6 or 9. For example, lactotransferrin, a member of the transferrin family, an iron-binding glycoprotein, has recently been shown to suppress adipogenesis.22,23 Indeed, our results showed a decrease in secreted lactotransferrin levels as preadipocytes differentiate into mature adipocytes, consistent with its proposed inhibitory role in adipogenesis. Another protein whose levels decreased in the course of adipocyte differentiation is the PDZ domain-containing protein, InaD-like protein (INADL), thought to play a role in tight junctions. This protein has never been described in the context of adipocyte biology. Although lacking a conventional signal peptide, INADL has been detected in serum.(24) The exact function of this protein in adipocytes is currently unknown. Nevertheless, our study illustrates the power of mass spectrometry to identify novel adipocyte secreted proteins in an unbiased fashion. Another interesting molecule exhibiting this pattern is a classic adipokine, plasminogen activator inhibitor-1 (PAI-1), induced by proinflammatory cytokines TNF-α in adipocytes and adipose tissue.(25) Serum PAI-1 levels are positively correlated with tissue insulin resistance and the risk for developing cardiovascular diseases.(26) Consistent with a previous study, we observed that secreted PAI-1 protein levels decreased during primary human adipocyte differentiation in culture. Biglycan, a small leucine-rich proteoglycan, plays a role in the assembly of collagen fibrils and muscle regeneration. In humans, biglycan is also involved in osteogenesis.(27) We have previously shown that during mouse adipogenesis, secreted biglycan levels dramatically increased ∼8 fold from day 0 to day 3, then decreased back to the baseline levels by the end of differentiation.(12) In contrast, secreted human biglycan level in the secretome decreased at the initiation of adipocyte differentiation, without exhibiting an early phase induction, indicating subtle differences between primary human adipocytes and the 3T3-L1 mouse adipocyte systems.
Figure 4B represents a cluster of proteins whose levels increased shortly after the initiation of differentiation (Day 0), reaching their peak levels in the middle of the process (Day 6 or 9), followed by a decrease at Day 12 where adipocytes acquired their mature phenotype. We identified multiple interesting proteins exhibiting this pattern. For example, cathepsin C, a cysteine proteinase belonging to the peptidase C1 family, activates a number of granule-associated serine proteases with pro-inflammatory and immunological roles by cleaving off their inhibitory N-terminal dipeptides.(28) Loss-of-function mutations of cathepsin C in humans cause Papillon-Lefevre syndrome, an autosomal recessive disorder characterized by palmoplantar keratosis and periodontitis.(29) Despite lacking a classical signal peptide, cathepsin C is present in human serum.(30) Indeed, this is the first study to demonstrate cathepsin C as part of the secretome during human adipogenesis, suggesting a potentially important role for this protease in adipocyte differentiation. Dimethylarginine dimethylaminohydrolase 1, which is also observed in this cluster, regulates the cellular concentrations of methylarginine known to inhibit nitric oxide synthase activity. Although generally considered to be an intracellular protein, dimethylarginine dimethylaminohydrolase 1 is present in human cerebrospinal fluid.(31) Our data showing a reduction of dimethylarginine dimethylaminohydrolase 1 in mature primary human adipocytes is consistent with the described role of nitric oxide in suppressing mouse adipocyte differentiation.(32) Another newly identified protein in this cluster is hemicentin 1. The human HMCN1 gene encodes a 600-kDa protein containing 5635 amino acid residues that belongs to the family of the extracellular matrix proteins, fibulins. Recent reports accumulated evidence that variations in HMCN1 play a role in age-related macular degeneration.(33) Most recently, Kim and colleagues found an association of diabetic nephropathy with HMCN1. Here, our data showed that hemicentin 1 exhibited different expression patterns from other fibrilins - fibrilin 1, 2, and 5 - identified in our studies and that the secretion of this protein was increased from Day 0 until Day 9 then decreased on Day 12, suggesting that hemicentin 1 may play a different role from other fibrilins in adipocyte differentiation.
Figure 4C represents the third and largest cluster of secreted proteins whose protein levels consistently increased throughout the entire process of differentiation. We have identified many proteins belonging to this cluster. One such protein is glypican-1, a GPI-anchored cell surface heparan sulfate proteoglycan that is part of a larger family of glypicans. Many GPI-anchored proteins are released into the extracellular milieu by the action of phosphatidylinositol- specific phospholipase C (PI-PLC) that cleave at the GPI anchor, likely accounting for glypican-1 presence in the human adipocyte secretome. A related member of the same family, glypican-3, interacts with the glucose transporter GLUT4 to facilitate glucose uptake into cells.(34) Thus, as with glypican-3, glypican-1 may play a similar role in human adipocytes. Another interesting family of proteins in this cluster is the tropomyosins. We have previously shown a fluctuation of the secretion of tropomyosins during mouse 3T3-L1 adipocyte differentiation where secretion of tropomyosin 1, 3, and 4 was decreased from day 1 to day 2, increased on day 3 or day 4 and then decreased again on day 5 and that the level of tropomyosins on day 1 was higher than days 2−5. However, we observed two different expression patterns of tropomyosins during the course of adipocyte differentiation in humans where the levels of tropomyosin 1 and 2 were continuously increased from day 0 to day 12 and that the levels of human tropomyosin 3 and 4 were increased from day 0 to day 9 and then decreased on day 12. This discrepancy again highlights the difference between primary human adipocyte and the mouse 3T3-L1 adipocyte system. Another interesting family of proteins in this cluster are collagens. Khan and colleagues have previously shown that collagens I, IV and VI are abundantly expressed in mature adipocytes and that knockout of collagen VI in ob/ob mice improved metabolic phenotype.(35) Their results also suggest that collagen VI affects the ability of adipocytes to expand. Interestingly, our data also show that the expression levels of collagen VI and other collagens were increased during the course of the differentiation of preadipocytes to adipocytes in humans. One newly identified protein in this cluster is SPARC related modular calcium binding protein 1 (SMOC1), a 51-kDa secreted glycoprotein. SMOC1 contains five domains: two thyroglobulin-like domains, one Kazal-type serine protease inhibitor domain and two EF-hand calcium-binding domains. SMOC1 has 37% homology to a well-known adipokine, SPARC/osteonectin, at the amino acid level. SPARC has been associated with obesity and secretion of SPARC by adipose tissue is increased by insulin.(36) SMOC1 may also play a similar role in human adipogenesis. Another newly identified protein in this cluster is dermcidin. Dermcidin was originally identified as a broad-spectrum antibiotic peptide secreted by sweat glands.(37) The 110 amino acid long protein dermcidin can be proteolytically processed to generate the C-terminal peptide and N-terminal peptide. As a cancer cell survival factor, the N-terminal peptide (also called proteolysis-inducing-factor or Y−P30 peptide) confers a survival advantage in breast and prostate cancer cells against oxidative stress and hypoxia.38−40 The C-terminal peptide DCD-1 L containing 47 amino acid residues has antibiotic activities and can further be proteolytically processed to shorter fragments by cathepsin D after secretion, which also have antibiotic activities.(41) Interestingly, our proteomics data showed that dermcidin and cathepsin D exhibited similar expression pattern during the course of differentiation, suggesting that dermcidin may play an important role in adipocyte differentiation.
Functional Analysis of Identified Proteins
We carried out a literature-based functional analysis by searching the identified proteins against the manually curated Human Protein Reference Database (HPRD)(42) and Human Proteinpedia.43,44 HPRD uses multiple bioinformatic programs to predict the existence of a signal peptide located at the N-termini of proteins. Of the 420 proteins, 164 were predicted to have a signal peptide. HPRD and Human Proteinpedia also provide experimentally verified information concerning the cellular and subcellular localization of proteins. Although many adipokines have already been identified over the years,11,12,14−16,45 we were still able to discover a number of novel adipokines, thus highlighting the power of mass spectrometry to complement other existing approaches to more comprehensively enumerate the secretome of adipocytes. During the preparation of this manuscript, Kim and colleagues published a label-free shotgun proteomics strategy to compare proteins secreted by the stromal vascular cells versus those secreted by differentiated human adipocytes.(46) To the best of our knowledge, 107 proteins that we have identified in the human adipocyte secretome have still not been previously described by other studies including the study by Kim et al. (Supplementary Table 2, Supporting Information). A subset of these novel proteins is listed in Table 1.
Table 1. Subset of Novel Proteins Identified in the Secretome during Human Adipogenesis.
| gene symbol | GI accession | protein | abundance ratio | ||
|---|---|---|---|---|---|
| day 0/day 12 | day 6/day 12 | day 9/day 12 | |||
| DCN | 19743846 | Decorin | 1.4 ± 0.4 | 1.4 ± 0.4 | 1.6 ± 0.3 |
| SPOCK1 | 4759164 | Sparc/osteonectin, cwcv and kazal-like domains proteoglycan 1 | 1.0 ± 0.3 | 1.6 ± 0.2 | 1.3 ± 0.1 |
| HMCN1 | 118572606 | Hemicentin 1 | 0.8 ± 0.4 | 0.7 ± 0.3 | 1.7 ± 0.9 |
| APOD | 4502163 | Apolipoprotein D | 0.6 ± 0.4 | 1.6 ± 0.5 | 1.7 ± 0.04 |
| COL5A3 | 110735435 | Collagen, type V, alpha 3 | 0.6 ± NA | 0.2 ± 0.2 | 0.8 ± 0.3 |
| CTHRC1 | 19923989 | Collagen triple helix repeat containing 1 | 0.6 ± 0.2 | 1.0 ± 0.3 | 1.4 ± 0.04 |
| LAMA2 | 119466532 | Laminin alpha 2 subunit | 0.5 ± 0.1 | 1.3 ± 0.2 | 1.4 ± 0.3 |
| CRLF1 | 4758062 | Cytokine receptor-like factor 1 | 0.5 ± NA | 1.8 ± NA | 1.7 ± NA |
| PRSS1 | 4506145 | Protease, serine, 1 | 0.5 ± NA | 0.5 ± NA | 0.5 ± NA |
| MASP1 | 21264359 | Mannan-binding lectin serine protease 1 | 0.4 ± NA | 1.2 ± NA | 1.1 ± NA |
| FBN2 | 66346695 | Fibrillin 2 | 0.4 ± 0.1 | 1.0 ± 0.02 | 1.1 ± 0.1 |
| SMOC1 | 11545873 | SPARC related modular calcium-binding protein 1 | 0.4 ± NA | 0.5 ± NA | 0.7 ± NA |
| DCD | 16751921 | Dermcidin | 0.3 ± NA | 0.8 ± NA | 1.5 ± NA |
| HDGF | 4758516 | Hepatoma-derived growth factor | 0.3 ± NA | 0.8 ± NA | 1.9 ± NA |
| GPC1 | 4504081 | Glypican 1 | 0.3 ± NA | 0.7 ± NA | 1.1 ± NA |
| TXNRD1 | 33519426 | Thioredoxin reductase 1 | 0.2 ± 0.2 | 0.9 ± 0.2 | 1.8 ± 0.8 |
| ITIH2 | 70778918 | Interalpha globulin inhibitor H2 polypeptide | 0.2 ± 0.1 | 0.7 ± 0.1 | 2.0 ± 0.1 |
| ANPEP | 4502095 | Membrane alanine aminopeptidase | 0.2 ± 0.1 | 0.6 ± 0.03 | 1.1 ± 0.03 |
| LGALS3 | 115430223 | Galectin 3 | 0.2 ± 0.1 | 0.5 ± 0.1 | 1.2 ± 0.3 |
| ITIH4 | 31542984 | Interalpha (globulin) inhibitor H4 | 0.2 ± NA | 0.3 ± NA | 1.3 ± NA |
| AHSG | 4502005 | Alpha-2-HS-glycoprotein | 0.1 ± 0.1 | 0.3 ± 0.1 | 1.7 ± 0.3 |
| PCOLCE2 | 7019483 | Procollagen C-endopeptidase enhancer 2 | 0.2 ± NA | 0.6 ± NA | 1.3 ± NA |
Antibody Arrays for Temporal Analysis of the Adipocytes Secretome
We have previously used Western blot analysis to validate temporal changes of a limited set of proteins identified from the secretome of mouse adipocytes. However, several drawbacks are associated with this approach. First, Western blot-based validation strategy is greatly limited by the availability of existing antibodies that can specifically recognize their target proteins on immunoblots. In general, good antibodies, whether they are from a commercial or noncommercial source, exist only for well-described proteins. Many of the secretory proteins we have identified in the present study are not in this category of such well-studied proteins. Second, the enhanced chemiluminescence detection method often used in conjunction with Western blot analysis is not strictly quantitative. Third, it is time-consuming, manually demanding and cost prohibitive to carry out Western blot analysis for a large number of candidates. Due to these limitations, we decided to use a different antibody array platform to quantify the expression levels of adipokines during adipogenesis.
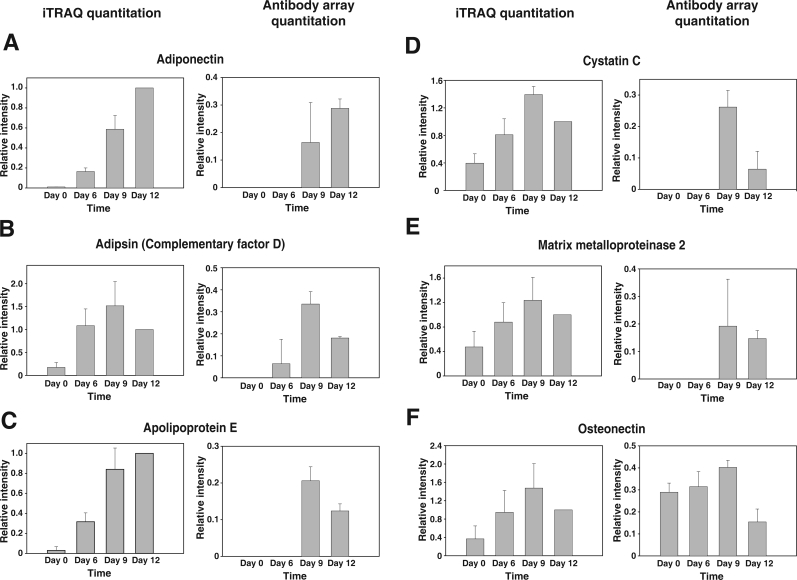
Antibody arrays have been used to quantify the secreted cytokine levels in many biological contexts.47−49 We chose a commercially available adipokine antibody array to validate some of our findings. An advantage of using these arrays is that they complement our mass spectrometric analysis and allow us to profile proteins of low abundance (especially cytokines and chemokines) that might not easily be identified by mass spectrometry. These commercial arrays contain specific antibodies directed against 182 proteins (spotted in triplicate) known to be secreted by adipocytes/adipose tissue and four replicate arrays are spotted on one glass slide. Of these antibodies, 19 were directed against proteins we identified in this study. The proteins from the conditioned media on Days 0, 6, 9, and 12 were biotinylated and incubated with antibody arrays. The bound proteins were detected by fluorescent dye-conjugated streptavidin. The signals visualized from the four time points were normalized and quantified based on a set of common controls spotted across the arrays. Although the arrays were designed to detect picogram levels of proteins, 14 adipokines were not detected in the secretome at any of the four time points (Supplementary Table 3, Supporting Information). Further, the signals for almost half of the spots on the arrays were zero, suggesting that the concentrations of many adipokines in the cultured media were still below the detection limit of the antibody arrays or that there was no specific binding to those antibodies. Among the 19 proteins common to the arrays and mass spectrometric analysis, the expression patterns of nine proteins—adiponectin, adipsin, apolipoprotein E, glutathione peroxidase 3, clusterin, galectin-1, cystatin C, osteonectin and matrix metalloproteinase 2—showed similar trends as that obtained by mass spectrometry. As shown in Figure 5, relative expression of six of nine proteins measured by antibody arrays and mass spectrometry were compared. Five proteins—pentraxin 3, pigment epithelium derived factor, S100 calcium binding protein A10, tissue inhibitor of metalloproteinase 1 and 2—were below the detection limits of the antibody arrays. Five proteins, FABP4, M-CSF, PDGF-D, Thrombospondin 1 and 2, showed expression patterns that were different from those observed by mass spectrometry. Other than these proteins, we were also able to quantify the secreted levels of many other adipokines not detected by our mass spectrometry analysis. Importantly, the secreted levels of many adipokines also differ during the course of adipocyte differentiation. Overall, it is clear that the unique strength of mass spectrometry and antibody arrays are complementary and can provide insights into the dynamic changes in the adipocyte secretome during differentiation.
Figure 5.
Expression patterns of representative proteins commonly quantitated by mass spectrometry and antibody arrays. (A) Adiponectin; (B) Adipsin (Complementary Factor D); (C) Apolipoprotein E; (D) Cystatin C; (E) Matrix metalloproteinase 2; and (F) Osteonectin. In each panel, iTRAQ-based quantitation is shown on the left while antibody array-based quantitation is shown on the right.
Conclusions
The mouse 3T3-L1 adipocyte culture system has been widely used as a model to study molecular mechanisms of adipogenesis. A number of groups have used proteomic methods to analyze the secretome during the differentiation of preadipocytes into mature 3T3-L1 adipocytes. Only very recently have studies been conducted to analyze the secretome of human adipocytes. These studies that mainly used 2D gels focused solely on a simple comparison of two cellular state—preadipocytes and differentiated mature adipocytes—thus precluding a dynamic snapshot of the entire differentiation process. The inherent limitations of 2D gels makes it likely that the entire complement of secreted proteins in adipocytes has not been fully enumerated.(50) To overcome these limitations, we used the iTRAQ methodology to monitor and quantify the levels of proteins in the secretome of primary human adipocytes at different stages of differentiation. Our study not only identified and confirmed multiple secreted proteins known to be expressed by human adipocytes, but also revealed several novel adipokines. Our analysis also uncovered three major dynamic expression patterns of adipokines during the differentiation process. Further, we used antibody arrays to complement, validate and extend the temporal analysis of human adipocyte secretome. In summary, this is the first large-scale quantitative proteomic study that combines two orthogonal and complementary platforms to enable a more comprehensive understanding of human adipogenesis.
Acknowledgments
We thank the Boston Obesity Nutrition Research Center Adipocyte Core for the harvest and separation of preadipocytes. We also thank Ada Kane for technical advice. The work is supported by grants DK056690 and DK46200 from the National Institute of Health (B.E.C.), SDG2260721 from the American Heart Association (G.W.W.), D5P60DK079637 from the Baltimore Diabetes Research and Training Center (G.W.W.), DK084171 from the National Institute of Health (G.W.W.), and by a grant S10RR023025 from the High End Instrumentation Program of the National Institutes of Health (A.P.), and an NIH Roadmap grant “Technology Center for Networks and Pathways” U54 RR 020839 (A.P. and J.S.B.).
Supporting Information Available
Supplementary Table 1 shows iTRAQ-based quantitation of proteins identified by mass spectrometry. Supplementary Table 2 provides comparison of proteins identified in our secretomic analysis with other previous secretomics reports. Supplementary Table 3 provides relative quantitation of adipokines using antibody arrays. This material is available free of charge via the Internet at http://pubs.acs.org.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Jensen M. D. Adipose tissue metabolism -- an aspect we should not neglect. Horm. Metab. Res. 2007, 39, 722–5. [DOI] [PubMed] [Google Scholar]
- Guilbert J. J. The world health report 2002 - reducing risks, promoting healthy life. Educ. Health (Abingdon) 2003, 16, 230. [DOI] [PubMed] [Google Scholar]
- Halberg N.; Wernstedt-Asterholm I.; Scherer P. E. The adipocyte as an endocrine cell. Endocrinol. Metab. Clin. North Am. 2008, 37, 753–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer S. R. Transcriptional control of adipocyte formation. Cell Metab. 2006, 4, 263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P.; Hu E.; Spiegelman B. M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 1994, 79, 1147–56. [DOI] [PubMed] [Google Scholar]
- Barak Y.; Nelson M. C.; Ong E. S.; Jones Y. Z.; Ruiz-Lozano P.; Chien K. R.; Koder A.; Evans R. M. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 1999, 4, 585–95. [DOI] [PubMed] [Google Scholar]
- Rosen E. D.; Sarraf P.; Troy A. E.; Bradwin G.; Moore K.; Milstone D. S.; Spiegelman B. M.; Mortensen R. M. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell 1999, 4, 611–7. [DOI] [PubMed] [Google Scholar]
- Green H.; Kehinde O. An established preadipose cell line and its differentiation in culture. II Factors affecting the adipose conversion. Cell 1975, 5, 19–27. [DOI] [PubMed] [Google Scholar]
- Prokesch A.; Hackl H.; Hakim-Weber R.; Bornstein S. R.; Trajanoski Z. Novel insights into adipogenesis from omics data. Curr. Med. Chem. 2009, 16, 2952–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki N.; Jin-no S.; Nakagawa Y.; Asai N.; Arakawa E.; Tamura N.; Tamura T.; Matsuda T. Identification and characterization of microvesicles secreted by 3T3-L1 adipocytes: redox- and hormone-dependent induction of milk fat globule-epidermal growth factor 8-associated microvesicles. Endocrinology 2007, 148, 3850–62. [DOI] [PubMed] [Google Scholar]
- Kratchmarova I.; Kalume D. E.; Blagoev B.; Scherer P. E.; Podtelejnikov A. V.; Molina H.; Bickel P. E.; Andersen J. S.; Fernandez M. M.; Bunkenborg J.; Roepstorff P.; Kristiansen K.; Lodish H. F.; Mann M.; Pandey A. A proteomic approach for identification of secreted proteins during the differentiation of 3T3-L1 preadipocytes to adipocytes. Mol. Cell. Proteomics 2002, 1, 213–22. [DOI] [PubMed] [Google Scholar]
- Molina H.; Yang Y.; Ruch T.; Kim J. W.; Mortensen P.; Otto T.; Nalli A.; Tang Q. Q.; Lane M. D.; Chaerkady R.; Pandey A. Temporal profiling of the adipocyte proteome during differentiation using a five-plex SILAC based strategy. J. Proteome Res. 2009, 8, 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A.; Kumar S. G.; Kim S. W.; Hwang H. J.; Baek Y. M.; Lee S. H.; Hwang H. S.; Shon Y. H.; Nam K. S.; Yun J. W. Proteomic analysis for inhibitory effect of chitosan oligosaccharides on 3T3-L1 adipocyte differentiation. Proteomics 2008, 8, 569–81. [DOI] [PubMed] [Google Scholar]
- Chen X.; Cushman S. W.; Pannell L. K.; Hess S. Quantitative proteomic analysis of the secretory proteins from rat adipose cells using a 2D liquid chromatography-MS/MS approach. J. Proteome Res. 2005, 4, 570–7. [DOI] [PubMed] [Google Scholar]
- Alvarez-Llamas G.; Szalowska E.; de Vries M. P.; Weening D.; Landman K.; Hoek A.; Wolffenbuttel B. H.; Roelofsen H.; Vonk R. J. Characterization of the human visceral adipose tissue secretome. Mol. Cell. Proteomics 2007, 6, 589–600. [DOI] [PubMed] [Google Scholar]
- Zvonic S.; Lefevre M.; Kilroy G.; Floyd Z. E.; DeLany J. P.; Kheterpal I.; Gravois A.; Dow R.; White A.; Wu X.; Gimble J. M. Secretome of primary cultures of human adipose-derived stem cells: modulation of serpins by adipogenesis. Mol. Cell. Proteomics 2007, 6, 18–28. [DOI] [PubMed] [Google Scholar]
- Ross P. L.; Huang Y. N.; Marchese J. N.; Williamson B.; Parker K.; Hattan S.; Khainovski N.; Pillai S.; Dey S.; Daniels S.; Purkayastha S.; Juhasz P.; Martin S.; Bartlet-Jones M.; He F.; Jacobson A.; Pappin D. J. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol. Cell. Proteomics 2004, 3, 1154–69. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Chaerkady R.; Beer M. A.; Mendell J. T.; Pandey A. Identification of miR-21 targets in breast cancer cells using a quantitative proteomic approach. Proteomics 2009, 9, 1374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauner H.; Petruschke T.; Russ M.; Rohrig K.; Eckel J. Effects of tumour necrosis factor alpha (TNF alpha) on glucose transport and lipid metabolism of newly-differentiated human fat cells in cell culture. Diabetologia 1995, 38, 764–71. [DOI] [PubMed] [Google Scholar]
- Gupta V.; Bhasin S.; Guo W.; Singh R.; Miki R.; Chauhan P.; Choong K.; Tchkonia T.; Lebrasseur N. K.; Flanagan J. N.; Hamilton J. A.; Viereck J. C.; Narula N. S.; Kirkland J. L.; Jasuja R. Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Mol. Cell. Endocrinol. 2008, 296, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway R. J.; Bezdek J. C.; Pal N. R. Sequential Competitive Learning and the Fuzzy c-Means Clustering Algorithms. Neural Netw. 1996, 9, 787–96. [DOI] [PubMed] [Google Scholar]
- Moreno-Navarrete J. M.; Ortega F. J.; Ricart W.; Fernandez-Real J. M. Lactoferrin increases (172Thr)AMPK phosphorylation and insulin-induced (p473Ser)AKT while impairing adipocyte differentiation. Int. J. Obes. (London) 2009, 33, 991–1000. [DOI] [PubMed] [Google Scholar]
- Yagi M.; Suzuki N.; Takayama T.; Arisue M.; Kodama T.; Yoda Y.; Numasaki H.; Otsuka K.; Ito K. Lactoferrin suppress the adipogenic differentiation of MC3T3-G2/PA6 cells. J. Oral Sci. 2008, 50, 419–25. [DOI] [PubMed] [Google Scholar]
- States D. J.; Omenn G. S.; Blackwell T. W.; Fermin D.; Eng J.; Speicher D. W.; Hanash S. M. Challenges in deriving high-confidence protein identifications from data gathered by a HUPO plasma proteome collaborative study. Nat. Biotechnol. 2006, 24, 333–8. [DOI] [PubMed] [Google Scholar]
- Samad F.; Yamamoto K.; Loskutoff D. J. Distribution and regulation of plasminogen activator inhibitor-1 in murine adipose tissue in vivo. Induction by tumor necrosis factor-alpha and lipopolysaccharide. J. Clin. Invest. 1996, 97, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellas C.; Loskutoff D. J. Historical analysis of PAI-1 from its discovery to its potential role in cell motility and disease. Thromb. Haemost. 2005, 93, 631–40. [DOI] [PubMed] [Google Scholar]
- Waddington R. J.; Roberts H. C.; Sugars R. V.; Schonherr E. Differential roles for small leucine-rich proteoglycans in bone formation. Eur. Cell. Mater. 2003, 6, 12–21and discussion, 21. [DOI] [PubMed] [Google Scholar]
- Laine D. I.; Busch-Petersen J. Inhibitors of cathepsin C (dipeptidyl peptidase I). Expert Opin. Ther. Pat. 2010, 20, 497–506. [DOI] [PubMed] [Google Scholar]
- Dhanrajani P. J. Papillon-Lefevre syndrome: clinical presentation and a brief review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, e1−7. [DOI] [PubMed] [Google Scholar]
- Sheng S.; Chen D.; Van Eyk J. E. Multidimensional liquid chromatography separation of intact proteins by chromatographic focusing and reversed phase of the human serum proteome: optimization and protein database. Mol. Cell. Proteomics 2006, 5, 26–34. [DOI] [PubMed] [Google Scholar]
- Burgess J. A.; Lescuyer P.; Hainard A.; Burkhard P. R.; Turck N.; Michel P.; Rossier J. S.; Reymond F.; Hochstrasser D. F.; Sanchez J. C. Identification of brain cell death associated proteins in human post-mortem cerebrospinal fluid. J. Proteome Res. 2006, 5, 1674–81. [DOI] [PubMed] [Google Scholar]
- Kawachi H.; Moriya N. H.; Korai T.; Tanaka S. Y.; Watanabe M.; Matsui T.; Kawada T.; Yano H. Nitric oxide suppresses preadipocyte differentiation in 3T3-L1 culture. Mol. Cell. Biochem. 2007, 300, 61–7. [DOI] [PubMed] [Google Scholar]
- Schultz D. W.; Weleber R. G.; Lawrence G.; Barral S.; Majewski J.; Acott T. S.; Klein M. L. HEMICENTIN-1 (FIBULIN-6) and the 1q31 AMD locus in the context of complex disease: review and perspective. Ophthalmic Genet. 2005, 26, 101–5. [DOI] [PubMed] [Google Scholar]
- Taguchi A.; Emoto M.; Okuya S.; Fukuda N.; Nakamori Y.; Miyazaki M.; Miyamoto S.; Tanabe K.; Aburatani H.; Oka Y.; Tanizawa Y. Identification of Glypican3 as a novel GLUT4-binding protein. Biochem. Biophys., Res. Commun. 2008, 369, 1204–8. [DOI] [PubMed] [Google Scholar]
- Khan T.; Muise E. S.; Iyengar P.; Wang Z. V.; Chandalia M.; Abate N.; Zhang B. B.; Bonaldo P.; Chua S.; Scherer P. E. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol. 2009, 29, 1575–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos K.; Wilding J. P. SPARC: a key player in the pathologies associated with obesity and diabetes. Nat. Rev. Endocrinol. 2010, 6, 225–35. [DOI] [PubMed] [Google Scholar]
- Schittek B.; Hipfel R.; Sauer B.; Bauer J.; Kalbacher H.; Stevanovic S.; Schirle M.; Schroeder K.; Blin N.; Meier F.; Rassner G.; Garbe C. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nat. Immunol. 2001, 2, 1133–7. [DOI] [PubMed] [Google Scholar]
- Porter D.; Weremowicz S.; Chin K.; Seth P.; Keshaviah A.; Lahti-Domenici J.; Bae Y. K.; Monitto C. L.; Merlos-Suarez A.; Chan J.; Hulette C. M.; Richardson A.; Morton C. C.; Marks J.; Duyao M.; Hruban R.; Gabrielson E.; Gelman R.; Polyak K. A neural survival factor is a candidate oncogene in breast cancer. Proc. Natl. Acad. Sci. U.S.A. 2003, 100, 10931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. D.; Lowrie A. G.; Riddick A. C.; Fearon K. C.; Habib F. K.; Ross J. A. Dermcidin expression confers a survival advantage in prostate cancer cells subjected to oxidative stress or hypoxia. Prostate 2007, 67, 1308–17. [DOI] [PubMed] [Google Scholar]
- Stewart G. D.; Skipworth R. J.; Ross J. A.; Fearon K.; Baracos V. E. The dermcidin gene in cancer: role in cachexia, carcinogenesis and tumour cell survival. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 208–13. [DOI] [PubMed] [Google Scholar]
- Baechle D.; Flad T.; Cansier A.; Steffen H.; Schittek B.; Tolson J.; Herrmann T.; Dihazi H.; Beck A.; Mueller G. A.; Mueller M.; Stevanovic S.; Garbe C.; Mueller C. A.; Kalbacher H. Cathepsin D is present in human eccrine sweat and involved in the postsecretory processing of the antimicrobial peptide DCD-1L. J. Biol. Chem. 2006, 281, 5406–15. [DOI] [PubMed] [Google Scholar]
- Keshava Prasad T. S.; Goel R.; Kandasamy K.; Keerthikumar S.; Kumar S.; Mathivanan S.; Telikicherla D.; Raju R.; Shafreen B.; Venugopal A.; Balakrishnan L.; Marimuthu A.; Banerjee S.; Somanathan D. S.; Sebastian A.; Rani S.; Ray S.; Harrys Kishore C. J.; Kanth S.; Ahmed M.; Kashyap M. K.; Mohmood R.; Ramachandra Y. L.; Krishna V.; Rahiman B. A.; Mohan S.; Ranganathan P.; Ramabadran S.; Chaerkady R.; Pandey A. Human Protein Reference Database--2009 update. Nucleic Acids Res. 2009, 37, D767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy K.; Keerthikumar S.; Goel R.; Mathivanan S.; Patankar N.; Shafreen B.; Renuse S.; Pawar H.; Ramachandra Y. L.; Acharya P. K.; Ranganathan P.; Chaerkady R.; Keshava Prasad T. S.; Pandey A. Human Proteinpedia: a unified discovery resource for proteomics research. Nucleic Acids Res. 2009, 37, D773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathivanan S.; Ahmed M.; Ahn N. G.; Alexandre H.; Amanchy R.; Andrews P. C.; Bader J. S.; Balgley B. M.; Bantscheff M.; Bennett K. L.; Bjorling E.; Blagoev B.; Bose R.; Brahmachari S. K.; Burlingame A. S.; Bustelo X. R.; Cagney G.; Cantin G. T.; Cardasis H. L.; Celis J. E.; Chaerkady R.; Chu F.; Cole P. A.; Costello C. E.; Cotter R. J.; Crockett D.; DeLany J. P.; De Marzo A. M.; DeSouza L. V.; Deutsch E. W.; Dransfield E.; Drewes G.; Droit A.; Dunn M. J.; Elenitoba-Johnson K.; Ewing R. M.; Van Eyk J.; Faca V.; Falkner J.; Fang X.; Fenselau C.; Figeys D.; Gagne P.; Gelfi C.; Gevaert K.; Gimble J. M.; Gnad F.; Goel R.; Gromov P.; Hanash S. M.; Hancock W. S.; Harsha H. C.; Hart G.; Hays F.; He F.; Hebbar P.; Helsens K.; Hermeking H.; Hide W.; Hjerno K.; Hochstrasser D. F.; Hofmann O.; Horn D. M.; Hruban R. H.; Ibarrola N.; James P.; Jensen O. N.; Jensen P. H.; Jung P.; Kandasamy K.; Kheterpal I.; Kikuno R. F.; Korf U.; Korner R.; Kuster B.; Kwon M. S.; Lee H. J.; Lee Y. J.; Lefevre M.; Lehvaslaiho M.; Lescuyer P.; Levander F.; Lim M. S.; Lobke C.; Loo J. A.; Mann M.; Martens L.; Martinez-Heredia J.; McComb M.; McRedmond J.; Mehrle A.; Menon R.; Miller C. A.; Mischak H.; Mohan S. S.; Mohmood R.; Molina H.; Moran M. F.; Morgan J. D.; Moritz R.; Morzel M.; Muddiman D. C.; Nalli A.; Navarro J. D.; Neubert T. A.; Ohara O.; Oliva R.; Omenn G. S.; Oyama M.; Paik Y. K.; Pennington K.; Pepperkok R.; Periaswamy B.; Petricoin E. F.; Poirier G. G.; Prasad T. S.; Purvine S. O.; Rahiman B. A.; Ramachandran P.; Ramachandra Y. L.; Rice R. H.; Rick J.; Ronnholm R. H.; Salonen J.; Sanchez J. C.; Sayd T.; Seshi B.; Shankari K.; Sheng S. J.; Shetty V.; Shivakumar K.; Simpson R. J.; Sirdeshmukh R.; Siu K. W.; Smith J. C.; Smith R. D.; States D. J.; Sugano S.; Sullivan M.; Superti-Furga G.; Takatalo M.; Thongboonkerd V.; Trinidad J. C.; Uhlen M.; Vandekerckhove J.; Vasilescu J.; Veenstra T. D.; Vidal-Taboada J. M.; Vihinen M.; Wait R.; Wang X.; Wiemann S.; Wu B.; Xu T.; Yates J. R.; Zhong J.; Zhou M.; Zhu Y.; Zurbig P.; Pandey A. Human Proteinpedia enables sharing of human protein data. Nat. Biotechnol. 2008, 26, 164–7. [DOI] [PubMed] [Google Scholar]
- Wang P.; Mariman E.; Keijer J.; Bouwman F.; Noben J. P.; Robben J.; Renes J. Profiling of the secreted proteins during 3T3-L1 adipocyte differentiation leads to the identification of novel adipokines. Cell. Mol. Life Sci. 2004, 61, 2405–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Choi Y. S.; Lim S.; Yea K.; Yoon J. H.; Jun D. J.; Ha S. H.; Kim J. W.; Kim J. H.; Suh P. G.; Ryu S. H.; Lee T. G. Comparative analysis of the secretory proteome of human adipose stromal vascular fraction cells during adipogenesis. Proteomics 2010, 10, 394–405. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin A.; Colomer R.; Menendez J. A. Protein array technology to detect HER2 (erbB-2)-induced ‘cytokine signature’ in breast cancer. Eur. J. Cancer 2007, 43, 1117–24. [DOI] [PubMed] [Google Scholar]
- Grisanti L. A.; Evanson J.; Marchus E.; Jorissen H.; Woster A. P.; DeKrey W.; Sauter E. R.; Combs C. K.; Porter J. E. Pro-inflammatory responses in human monocytes are beta1-adrenergic receptor subtype dependent. Mol. Immunol. 2010, 47, 1244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X.; Schneiderhan-Marra N.; Hsu H. Y.; Bachmann J.; Joos T. O. Protein microarrays: effective tools for the study of inflammatory diseases. Methods Mol. Biol. 2009, 577, 199–214. [DOI] [PubMed] [Google Scholar]
- Ong S. E.; Pandey A. An evaluation of the use of two-dimensional gel electrophoresis in proteomics. Biomol. Eng. 2001, 18, 195–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.