BH3 Mimetic ABT-737 Potentiates TRAIL-Mediated Apoptotic Signaling by Unsequestering Bim and Bak in Human Pancreatic Cancer Cells (original) (raw)
. Author manuscript; available in PMC: 2010 Oct 1.
Abstract
Tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) has been shown to induce mitochondrial apoptotic signaling that can be negatively regulated by prosurvival Bcl-2 proteins. ABT-737 is a small-molecule BH3 mimetic that binds to and antagonizes Bcl-2/Bcl-xL but not Mcl-1. We show that ABT-737 can synergistically enhance TRAIL-mediated cytotoxicity in human pancreatic cancer cell lines. ABT-737 was shown to enhance TRAIL-induced apoptosis as shown by DNA fragmentation, activation of caspase-8 and Bid, and cleavage of caspase-3 and poly(ADP-ribose) polymerase. A Bax conformational change induced by TRAIL was enhanced by ABT-737. ABT-737 disrupted the interaction of Bak with Bcl-xL in both cell lines. Furthermore, ABT-737 untethered the proapoptotic BH3-only protein Bim from its sequestration by Bcl-xL or Bcl-2. Bim small hairpin RNA (shRNA) was shown to attenuate caspase-3 cleavage and to reduce the cytotoxic effects of TRAIL plus ABT-737 compared with shRNA control cells. Finally, Mcl-1 shRNA potentiated caspase-3 cleavage by ABT-737 and enhanced its cytotoxic effects. Taken together, ABT-737 augments TRAIL-induced cell killing by unsequestering Bim and Bak and enhancing a Bax conformational change induced by TRAIL. These findings suggest a novel strategy to enhance cross-talk between the extrinsic and intrinsic apoptotic pathways to improve therapeutic efficacy against pancreatic cancer.
Introduction
Pancreatic cancers are intrinsically resistant tumors that respond poorly to cytotoxic chemotherapeutic agents. Accordingly, novel therapeutic strategies and combinatorial regimens are seriously needed to enhance treatment efficacy. The death receptor ligand tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL), a member of the TNF family, is a promising anticancer agent due to its ability to induce apoptosis in a variety of tumor cell types while having only negligible effects on normal cells (1). TRAIL engages the extrinsic apoptotic pathway that involves caspase-8 cleavage, death receptor binding, death-inducing signaling complex formation, and subsequent activation of effector caspases such as caspase-3 and caspase-7 (2). Most human cancer cells are referred to as type II in that they require a mitochondrial amplification step (intrinsic pathway) after a death receptor stimulus to induce apoptosis (2, 3). Cross-talk between these apoptotic pathways is mediated by caspase-8–induced Bid cleavage (4–6). Truncated Bid is a proapoptotic BH3-only protein that translocates to mitochondria, activates Bax, and stimulates release of apoptogenic proteins (i.e., cytochrome c, Smac, and HtrA2; refs. 7, 8). Given this scenario, strategies to enhance tumor cell kill may require selective targeting of both the extrinsic and intrinsic apoptotic pathways. Bcl-2 and Bcl-xL have been shown to confer resistance to TRAIL in human cancer cells (9–12). Therefore, disabling antiapoptotic Bcl-2 proteins may greatly enhance TRAIL-mediated apoptosis. In this regard, the BH3 mimetic and small-molecule Bcl-2 antagonist ABT-737 binds with high affinity to a hydrophobic groove on Bcl-2, Bcl-xL, and Bcl-w and prevents their binding with Bax, thereby shifting the balance in favor of proapoptotic molecules (13). ABT-737 has been shown to lower the apoptotic threshold for certain chemotherapeutic agents and has shown impressive preclinical activity against lymphoma in a murine model (13). However, ABT-737 binds to Mcl-1 with low affinity, and cells with Mcl-1 knockdown show enhanced ABT-737–induced cell death (14). To date, the ability of ABT-737 to enhance TRAIL-mediated cell death has not been reported.
Until recently, Bid was the only known link between the extrinsic and intrinsic apoptotic pathways. However, recent data suggest that TRAIL can induce Mcl-1 degradation with disruption of the Mcl-1:Bim complex (15). In this study, we determined the ability of ABT-737 to enhance TRAIL-mediated apoptosis in human pancreatic cancer cells. We found that ABT-737 can markedly increase TRAIL-mediated mitochondrial apoptotic signaling by releasing Bim from its sequestration by Bcl-2 or Bcl-xL and by untethering Bak from Bcl-xL. Additionally, ABT-737 potentiates a TRAIL-mediated Bax conformational change. These findings underscore the importance of Bim and Bak in the cell death response to TRAIL and demonstrate that targeting both the extrinsic and intrinsic apoptotic pathways can increase therapeutic efficacy.
Materials and Methods
Cell culture, drugs, and reagents
Human pancreatic cancer cell lines BxPC-3 and PANC-1 and the human embryonic kidney cell line 293T were used in this study. BxPC-3 and PANC-1 cells were cultured in RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin, 10 mmol/L HEPES, and 1% sodium pyruvate. 293T cells were maintained in high-glucose DMEM (Sigma) plus 10% FBS. For lentivirus production, 293T cells were cultured in high-glucose DMEM containing 2% FBS (System Biosciences). Recombinant human TRAIL (Val114-Gly281) was obtained from R&D Systems and used fresh or aliquoted and stored for less than a month at −80°C. ABT-737 was obtained from Abbott Pharmaceuticals and was dissolved in DMSO to produce a 20 mmol/L stock solution that was aliquoted and stored at −20°C.
Cell viability assay
Cell viability was determined in the presence or absence of drug treatment using the 3-(4,5-dimethyl-thiazol-2yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2_H_-tetrazolium (MTS) reduction assay per manufacturer’s protocol (Promega). Briefly, 8,000 cells placed in 50-μL medium were seeded into 96-well plates and incubated at 37°C overnight. The next day, the study drugs were mixed in 50 μL of medium and added to each well. The plate was incubated for a predetermined time period and 20 μL of MTS/phenazine methosulfate solution were then added into each well. The plate was then incubated at 37°C in a humidified atmosphere containing 5% CO2 for 1 to 4 h. Absorbance at 492 nm was measured and recorded using a Versamax microplate reader (Molecular Devices). This assay was done in triplicate for each condition and the SD was calculated.
DNA fragmentation assay
Approximately 104 cells were seeded into 24-well plates and allowed to attach overnight. Cells were then treated with drug for the indicated time and centrifuged at 500 × g for 4 min. DNA fragmentation was then analyzed with a Cell Death Detection ELISA plus kit (Roche Applied Science) per manufacturer’s manual. Absorbance was measured at 405 nm (reference wavelength of 490 nm). Samples were run in duplicate and the average value is shown.
Immunoprecipitation
Mock-treated cells or those treated with drugs were harvested by scraping and then washed in cold PBS. After centrifugation, the cell pellet was lysed by sonication in a cell lysis buffer [10 mmol/L HEPES (pH 7.4), 20 mmol/L molybdic acid, 150 mmol/L KCl, 0.1% NP40, 5 mmol/L MgCl2] with protease inhibitors. Alternatively, the cell pellet was lysed by incubating in CHAPS buffer [5 mmol/L MgCl2, 137 mmol/L KCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% CHAPS, 10 mmol/L HEPES, pH 7.5] for 30 min in ice. The cell lysate was again subjected to centrifugation at 14,000 rpm for 10 min. The protein concentration of the supernatant was measured by detergent-compatible (DC) protein assay (Bio-Rad) and the protein concentration was normalized to 5 mg/mL. The lysate was precleaned by binding with protein A-Sepharose or protein G-Sepharose (GE Healthcare) and then incubated with an antibody-protein A or antibody-protein G complex, made by incubating antibody with protein A or protein G beads in 0.5-mL PBS at room temperature for 2 h at 4°C overnight. Unbound proteins were washed thrice with 1 mL of original lysis buffer or CHAPS buffer without protease inhibitors. Bound proteins on the beads were eluted by boiling the sample in 70 μL of LDS sample buffer (Invitrogen). Then, 30 μL of the eluted protein were used for one Western blot.
Western blotting
Protein samples were prepared in a lysis buffer per the procedure outlined above, normalized using DC protein assay reagents (Bio-Rad), boiled in LDS sample buffer (Invitrogen), and loaded onto 10% SDS-PAGE gel for separation of protein with electrophoretic transfer onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). The membrane was blocked with 0.2% I-Block (Applied Biosystems) in PBS containing 0.1% Tween 20 and incubated with the primary antibody in PBS containing 0.2% I-Block and 0.1% Tween 20 overnight at 4°C. The membrane was then incubated with a secondary antibody in PBS containing 0.2% I-Block and 0.1% Tween 20 conjugated to alkaline phosphatase, and then developed with CDP-Star substrate (Applied Biosystems).
Bax conformational change
After treatment, PANC-1 cells were lysed by incubating in CHAPS buffer [5 mmol/L MgCl2, 137 mmol/L KCl, 1 mmol/L EDTA, 1 mmol/L EGTA, 1% CHAPS, 10 mmol/L HEPES, pH 7.5] in the presence of protease inhibitors for 30 min in ice. The cell lysates were then incubated with protein G beads bound to Bax antibody 6A7 (Sigma) overnight, as previously described (16). Protein G beads were washed thrice with lysis buffer and bound proteins were eluted with LDS sample buffer (Invitrogen) containing 2-mercaptoethanol. Western blotting was then done with a rabbit anti-Bax antibody (Cell Signaling).
Knockdown of Bim and Mcl-1 using lentiviral small hairpin RNA
Target sequences for Bim and Mcl-1 were selected and synthesized by the Mayo Clinic Molecular Biology Core Facility. The targeting sequence for Mcl-1 was GATTGTGACTCTCATTTCT (17). The targeting sequence for Bim was GACCGAGAAGGTAGACAATTGC (18). After annealing, the template forms a double-stranded DNA flanked by _Eco_RI and _Bam_HI sites, which was ligated into the Lentiviral small hairpin RNA (shRNA) cloning and expression vector pSIH-H1 (System Biosciences) and cut with _Eco_RI and _Bam_HI. Insertion at the intended site was confirmed by DNA sequencing. For pseudovirus production, 2 μg of endotoxin-free lentivector expression construct were mixed with lentivector packaging plasmid mix (System Biosciences) and diluted in serum OPTI-MEM medium containing Plus reagent (Invitrogen). After incubation at room temperature for 15 min, the Lipofectamine reagent containing serum reduction medium was added dropwise into the above DNA/Plus complex and incubated for another 15 min. Lentivirus producer cell line 293T was transfected with the DNA/Lipofectamine/Plus complex in serum reduction medium overnight in a 5% CO2 incubator at 37°C. The next day, the medium was changed with fresh DMEM containing 2% heat-activated FBS and incubation at 37°C in the incubator was continued. At 48 h posttransfection, the supernatants were collected, clarified, and filtered through Millex-HV 0.45-μm PVDF filters (Millipore). The supernatants were then concentrated by adding 10% ( final concentration) of PEG-8000 (Sigma), incubated at 4°C overnight for no less than 12 h, and centrifuged at 1,500 × g for 10 min at 4°C. For transduction of the lentivector expression construct (packaged in pseudotyped viral particles) into target cells, appropriate amounts of virus growth in medium containing 8 μg/mL polybrene (Sigma) were directly added to the target cells and incubated overnight at 37°C. Target gene knockdown was then tested 72 h posttransduction.
Calculation of combination index
The effect of combination between ABT-737 and TRAIL was analyzed using Calcusyn software (Biosoft) as previously reported (19).
Statistical analysis
The values shown represent the mean ± SD for triplicate experiments. The statistical significance of the differences between experimental variables was determined with the Student t test.
Results
ABT-737 augments TRAIL-mediated apoptosis
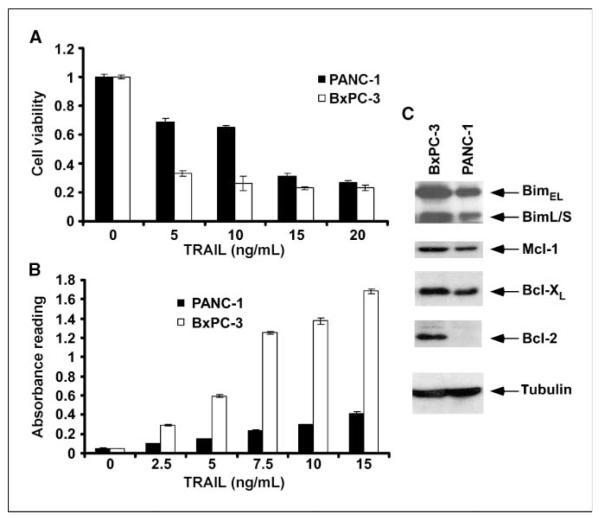
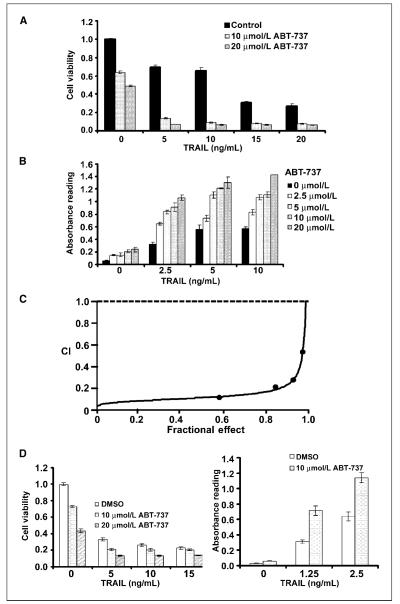
Antiapoptotic Bcl-2 proteins have been shown to confer resistance to TRAIL-induced apoptosis (3, 9, 11, 12). We determined whether the small-molecule BH3 mimetic ABT-737, which targets Bcl-2/Bcl-xL but not Mcl-1, could sensitize PANC-1 and BxPC-3 pancreatic carcinoma cells to TRAIL. With cell viability and DNA fragmentation assays, PANC-1 cells were found to be relatively resistant to TRAIL (0, 5, 10, and 15 ng/mL) compared with BxPC-3 cells (P < 0.05; Fig. 1_A_ and B). Analysis of expression of Bcl-2 family members revealed that PANC-1 cells have lower levels of Mcl-1 and Bcl-xL expression and undetectable Bcl-2 compared with BxPC-3 cells (Fig. 1_C_). ABT-737 monotherapy reduced cell viability in both cell lines (Fig. 2_A_ and D) and caused a minor induction of apoptosis shown by DNA fragmentation (Fig. 2_B_ and D). Coadministration of ABT-737 and TRAIL was found to significantly reduce cell viability and to a greater extent than did either drug alone in both cell lines (P < 0.05; Fig. 2_A_ and D). Furthermore, the combination of ABT-737 and TRAIL significantly augmented apoptosis in both cell lines (P < 0.05; Fig. 2B and D). Specifically, we treated PANC-1 cells with a range of concentrations of ABT-737 that enhanced TRAIL-mediated apoptosis in a dose-dependent manner (Fig. 2_B_). To determine whether the cytotoxic effect of the drug combination was synergistic or additive, we performed an analysis using the median effect method. Different concentrations of ABT-737 and TRAIL were analyzed at a fixed ratio for cell viability by MTS assay, and the combination index values were calculated per the method of Chou and Talalay (20). As shown in an isobologram, the combination index values were <1, indicating a synergistic interaction (Fig. 2C).
Figure 1.
TRAIL-mediated cytotoxicity and DNA fragmentation in pancreatic cancer cell lines. A, cells were incubated with TRAIL at the indicated doses for 48 h at 37°C, and the MTS reagent was then added and cells were incubated for 3 h. Absorbance was measured at 492 nm. Columns, mean of triplicate experiments; bars, SD. B, cells were treated with increasing concentrations of TRAIL for 4 h at 37°C. A DNA fragmentation assay was then done as outlined in Materials and Methods. Columns, mean; bars, SD. C, immunoblot analysis of the expression of the BH3-only protein Bim and antiapoptotic Bcl-2 proteins in whole-cell lysates. β-Tubulin was used as a control for protein loading.
Figure 2.
ABT-737 augments TRAIL-induced apoptosis and exerts a synergistic apoptotic effect. A, in PANC-1 cells, coadministration of ABT-737 and TRAIL for 48 h produced a significantly greater reduction in cell viability compared with either drug alone. Columns, mean of triplicate experiments; bars, SD. B, coadministration of a range of doses of ABT-737 (μmol/L) and TRAIL (ng/mL) for 4 h produced a significantly greater extent of DNA fragmentation compared with either drug alone in PANC-1 cells. Columns, mean; bars, SD. C, PANC-1 cells were treated with various concentrations of ABT-737 (2.5–20 μmol/L) and TRAIL (1.25–10 ng/mL) at a fixed dose ratio and cell viability was measured by MTS assay and a combination index (CI) was calculated as described in Materials and Methods. A combination index <1 represents synergism. D, in BxPC-3 cells, coadministration of ABT-737 and TRAIL for 48 h produced a greater reduction in cell viability compared with either drug alone. Columns, mean of triplicate experiments; bars, SD. Analysis of DNA fragmentation was then done in BxPC-3 cells treated with the combination of ABT-737 and TRAIL for 4 h. The combination produced a significantly greater extent of DNA fragmentation compared with either drug alone. Columns, mean; bars, SD.
ABT-737 enhances TRAIL-mediated apoptosis and Bax conformational change
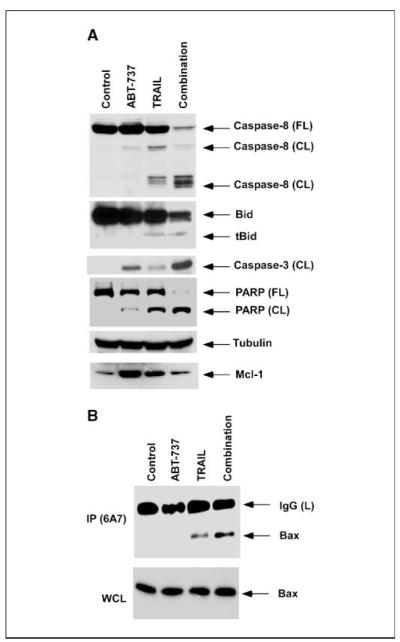
Exposure of PANC-1 cells to the combination of ABT-737 plus TRAIL markedly enhanced the activation of caspase-8, Bid, caspase-3, and poly(ADP-ribose) polymerase compared with either drug alone (Fig. 3_A_). Increased activation of caspase-8 and Bid by ABT-737 plus TRAIL compared with TRAIL alone is consistent with a feedback amplification loop mediated by caspase-3 (21). These findings indicate that ABT-737 can enhance TRAIL-mediated apoptotic signaling. We observed an increase in Mcl-1 expression in TRAIL-treated PANC-1 cells, which has been shown to occur through activation of nuclear factor κB (22). ABT-737 also increased Mcl-1 expression (Fig. 3_A_). TRAIL-mediated cytotoxicity and caspase activation were observed at relatively low doses of TRAIL (0-20 ng/ml), and similar effects have been reported in the PANC-1 cell line using the same TRAIL preparation (23, 24). We then studied the effect of ABT-737, TRAIL, and their combination on Bax conformational change. Following a death stimulus, Bax undergoes a conformational change and translocates to the mitochondrial outer membranes to regulate permeabilization, a critical determinant of cell death (25). Activation of Bax (or Bak) is associated with a conformational change that can be detected by antibodies recognizing only the active protein conformers (16). We found that ABT-737 can enhance a Bax conformational change induced by TRAIL in PANC-1 cells (Fig. 3_B_). TRAIL alone induced a modest Bax conformational change whereas ABT-737 monotherapy did not.
Figure 3.
ABT-737 enhances TRAIL-mediated apoptotic signaling and Bax conformational change. A, in PANC-1 cells, ABT-737 (20 μmol/L) potentiates TRAIL (10 ng/mL)-induced cleavage of caspase-8 and Bid and downstream activation of caspase-3 and poly(ADP-ribose) polymerase (PARP). Cells were treated with the indicated drugs for 48 h and immunoblot analysis was done with whole-cell lysates. β-Tubulin was used as a control for protein loading. B, PANC-1 cells were treated with ABT-737 (20 μmol/L), TRAIL (10 ng/mL), or their combination for 2 h and then immunoprecipitation was done with an anti-Bax 6A7 antibody for detection of a conformational change in the Bax protein.
ABT-737 displaces Bim from its complex with Bcl-xL/Bcl-2
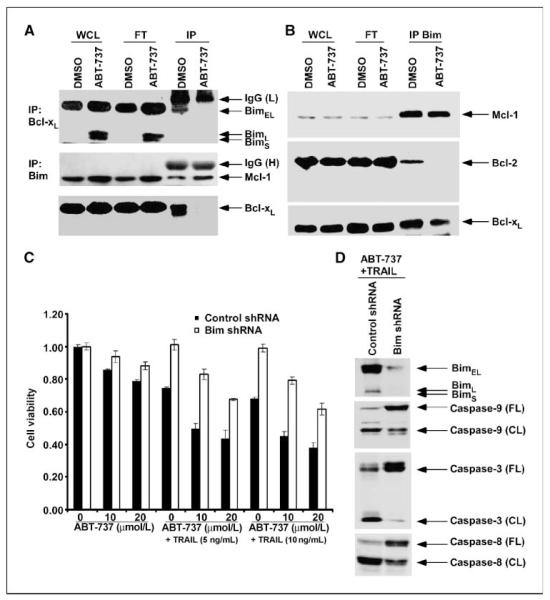
We studied the mechanism by which ABT-737 can potentiate TRAIL-induced cell death. Bim is a BH3-only protein that has been shown to bind to all antiapoptotic Bcl-2 proteins and is therefore a potent proapoptotic molecule (26). To determine the effect of ABT-737 treatment on the interaction between Bim and Bcl-xL, we immunoprecipitated Bcl-xL and then probed for Bim. Bim was uncoupled from Bcl-xL in ABT-737–treated PANC-1 cells compared with control cells (Fig. 4_A_). In BxPC-3 cells, ABT-737 displaced Bim from its complex with Bcl-2 but failed to disrupt its association with Bcl-xL or Mcl-1 (Fig. 4_B_). Our immunoprecipitation data are consistent with the observation that the majority of Bim is coupled with Mcl-1 (Fig. 4_B_; ref. 27). To further show the importance of Bim in the potentiation of TRAIL-induced apoptosis by ABT-737, we generated Bim-knockdown PANC-1 cells using lentiviral-delivered shRNA. Knockdown of Bim was found to significantly attenuate the reduction in cell viability induced by TRAIL and its combination with ABT-737 (P < 0.05; Fig. 4_C_). Furthermore, Bim knockdown attenuated the activation of caspase-8, caspase-9, and caspase-3 induced by ABT-737 plus TRAIL treatment (Fig. 4_D_). Together, our data show that ABT-737 unsequesters Bim from Bcl-xL or Bcl-2 and suggest that ABT-737 enables free Bim to activate Bax to exert its proapoptotic effect. Release of Bim seems to be independent of Mcl-1 in our cells, indicating that its release from Bcl-xL or Bcl-2 is indeed sufficient to enhance TRAIL-mediated apoptosis.
Figure 4.
ABT-737 displaces proapoptotic Bim from its sequestration by Bcl-xL or Bcl-2. Bim shRNA is shown to attenuate the apoptotic signaling and cytotoxic effects induced by the combination of ABT-737 and TRAIL. A, PANC-1 cells were treated with ABT-737 (20 μmol/L) or vehicle (DMSO) for 24 h and immunoprecipitation was performed. Whole-cell lysate (WCL), flow through (FT) from beads, and eluted proteins from beads (IP) were separated by SDS-PAGE and then probed for Bim (top) or Bcl-xL (bottom). B, BxPC-3 cells were treated with ABT-737 (10 μmol/L) or vehicle (DMSO) for 24 h and immunoprecipitation was done for Bim proteins. Whole-cell lysate, flow through from beads, and eluted protein from beads were separated by SDS-PAGE and probed for Mcl-1, Bcl-2, or Bcl-xL proteins. C, PANC-1 cells were transduced with lentiviral shRNA to Bim or control shRNA and then exposed to TRAIL, ABT-737, or their combination for 48 h. Cell viability was analyzed by MTS assay and absorbance (492 nm) was recorded. Columns, mean of triplicate experiments; bars, SD. D, Bim knockdown was shown to attenuate the activation of caspase-8, caspase-9, and caspase-3 triggered by coadministration of ABT-737 (20 μmol/L) plus TRAIL (10 ng/mL) for 48 h, compared with control shRNA–treated PANC-1 cells. Whole-cell lysates were used and analyzed by immunoblotting.
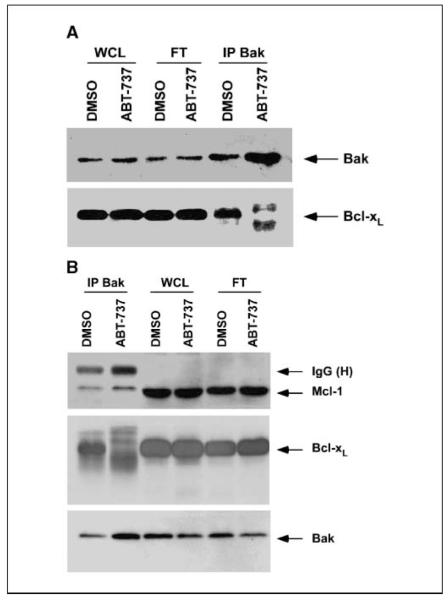
ABT-737 can disrupt the interaction of Bak and Bcl-xL
Recent studies have shown the importance of untethering Bak from Bcl-2/Bcl-xL in determining the lethality of ABT-737 (14). Therefore, we determined the effect of ABT-737 on the interaction between Bak and Bcl-xL. By immunoprecipitation of Bak and probing Bak and Bcl-xL in PANC-1 (Fig. 5_A_) and BxPC-3 (Fig. 5_B_) cells, we found that ABT-737 can disrupt the binding of Bak to Bcl-xL in both cell lines. The untethering of Bak, which resides in the outer mitochondrial membrane, can then permeabilize the mitochondria, resulting in apoptosis.
Figure 5.
ABT-737 unsequesters Bak from Bcl-xL. A, PANC-1 cells were treated with ABT-737 (20 μmol/L) or vehicle (DMSO) for 24 h and immunoprecipitation was done for Bak proteins. Whole-cell lysate, flow through from beads, and eluted proteins from beads were separated by SDS-PAGE and then probed for Bak (top) or Bcl-xL (bottom). B, BxPC-3 cells were treated with ABT-737 (10 μmol/L) or vehicle (DMSO) for 24 h and immunoprecipitation was done for Bak protein, as outlined above, and Probed for Mcl-1, Bak, or Bcl-xL proteins.
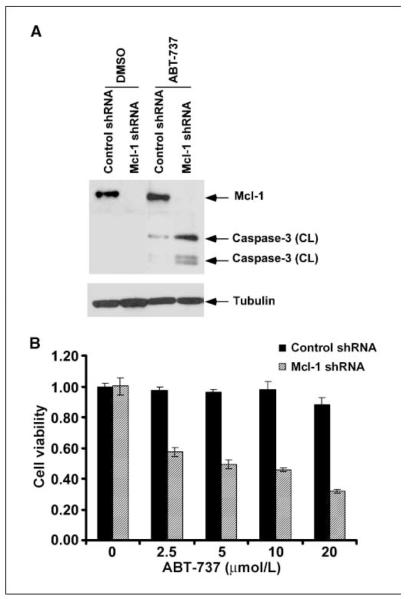
Mcl-1 knockdown sensitizes pancreatic cancer cells to ABT-737
ABT-737 has a low affinity for Mcl-1 and may produce only modest cytotoxic effects in cells that overexpress Mcl-1 (14). Given that BxPC-3 and PANC-1 cell lines show endogenous Mcl-1 expression (Fig. 1_C_), we determined whether Mcl-1 knockdown could sensitize PANC-1 cells to ABT-737–induced caspase-3 activation and cytotoxicity. Mcl-1 knockdown in PANC-1 cells was achieved with lentiviral shRNA. Treatment with ABT-737 (20 μmol/L) was shown to markedly increase caspase-3 cleavage in cells with Mcl-1 knockdown compared with nontargeted shRNA–transduced PANC-1 cells (Fig. 6_A_). Furthermore, the reduction in cell viability induced by ABT-737 was enhanced by Mcl-1 shRNA compared with nontransduced cells (Fig. 6_B_). These data confirm the important role of Mcl-1 in conferring resistance to cell death induced by ABT-737 and its relevance in our pancreatic cancer cell lines.
Figure 6.
Mcl-1 knockdown sensitizes PANC-1 cells to caspase-3 cleavage and enhances the cytotoxic effect of ABT-737. A, cells transduced with Mcl-1 shRNA or control shRNA were exposed to ABT-737 (20 μmol/L) for 48 h and caspase-3 activation was analyzed by immunoblotting. Mcl-1 knockdown efficiency is also shown. Whole-cell lysates were used and β-tubulin was used as a control for protein loading. B, the effect of ABT-737 (0–20 μmol/L; 48 h) treatment on cell viability was determined and compared between cells transduced with Mcl-1 shRNA or control shRNA.
ABT-737 augments gemcitabine-induced cytotoxicity
We determined whether ABT-737 can enhance the cytotoxic effects of the nucleoside analogue gemcitabine. Gemcitabine is a conventional cytotoxic agent used for the treatment of pancreatic cancer in humans. ABT-737 was shown to significantly augment the reduction in cell viability induced by gemcitabine in both PANC-1 and BxPC-3 cell lines (P < 0.05; see Supplementary figures). This effect was observed at the same dosages of ABT-737 used in combination with TRAIL.
Discussion
TRAIL has been shown to trigger cross-talk with the mitochondrial apoptotic pathway in many tumor cell types (2, 28). Studies using _Bax_-deficient tumor cells have confirmed the importance of a mitochondrial amplification step induced by TRAIL (28, 29). We (9) and others (10–12) have previously shown that forced Bcl-2 or Bcl-xL expression can inhibit TRAIL-mediated apoptosis. Therefore, disabling antiapoptotic Bcl-2 proteins may increase the therapeutic efficacy of TRAIL. To evaluate this strategy, we evaluated the small-molecule BH3 mimetic ABT-737, which binds with high affinity to regulatory sites on Bcl-2 and Bcl-xL, to induce a Bax/Bak-mediated apoptosis (13, 14). Human pancreatic cancer cell lines PANC-1 and BxPC-3 with differences in relative sensitivity to TRAIL-mediated apoptosis were used. We report for the first time that ABT-737 produces a marked sensitization to TRAIL-mediated apoptosis, as shown by a DNA fragmentation assay, in both pancreatic cancer cell lines. The interaction between ABT-737 and TRAIL was shown to be synergistic. The mechanistic basis for this effect includes the ability of ABT-737 to unsequester Bim from its interaction with Bcl-2 and Bcl-xL molecules, to untether Bak from Bcl-xL, and to activate Bax. Bim was freed from Bcl-xL in PANC-1 cells and from Bcl-2 in BxPC-3 cells based on the relative abundance of these proteins in each cell line. Bak was released from its complex with Bcl-xL in both cell lines. Bim is a potent inducer of apoptosis because Bim, Puma, and truncated Bid can neutralize all prosurvival Bcl-2 proteins, whereas only selective interactions are seen for Noxa and Bad (26). The functional significance of Bim was shown in Bim-knockdown PANC-1 cells where caspase-8, caspase-9, and caspase-3 cleavages were attenuated, and the cytotoxic effect of ABT-737 plus TRAIL was significantly inhibited. Therefore, sequestration of Bim by Bcl-2 or Bcl-xL seems to play a major role in TRAIL resistance. ABT-737 did not disrupt Bim sequestration by Mcl-1 in either cell line, indicating that uncoupling of Bim from Bcl-2 or Bcl-xL proteins was sufficient to sensitize cells to TRAIL despite the finding that the majority of Bim is bound by Mcl-1 (27).
We found that ABT-737 can enhance a TRAIL-mediated Bax conformational change. Release of free Bim has been shown to activate Bax to sensitize pancreatic cancer cells to TRAIL (25). In this regard, recent data indicate that Bim acts directly on Bax/Bak, which are anchored in the mitochondrial outer membrane, as shown by the observation that a Bim, but not a Puma BH3, peptide was sufficient to induce oligomerization and activation of Bax and Bak to permeabilize the mitochondrial membrane (25). Furthermore, the function of Bim through either Bax or Bak has been shown in thymocytes from Bim/Bax or Bim/Bak double-knockout mice (30). Studies indicate that TRAIL preferentially uses Bax over Bak for the induction of mitochondrial apoptotic events (31). Evidence suggests that Bak can be activated by its displacement from Bcl-xL by BH3-only proteins (32). Accordingly, we studied the interaction between Bak and Bcl-xL and found that ABT-737 can untether Bak from Bcl-xL in PANC-1 and BxPC-3 cell lines, suggesting that this effect may contribute to the ability of ABT-737 to enhance TRAIL-induced apoptosis.
Studies indicate that ABT-737 is unable to target Mcl-1, and thus Mcl-1 reduces responsiveness to ABT-737 (33). The effect of TRAIL on Mcl-1 expression remains controversial. In this regard, Han et al. (15) have shown that TRAIL can induce Mcl-1 degradation and a caspase-mediated disruption of the Mcl-1:Bim complex. In contrast, Ricci et al. (22) found that TRAIL can induce Mcl-1 expression through activation of nuclear factor κB. At the dose of TRAIL used in our experiments, Mcl-1 expression was up-regulated, consistent with data suggesting that this occurs via activation of nuclear factor κB (22). We also observed that ABT-737 may up-regulate Mcl-1 levels. Potentially, this reflects stabilization of Mcl-1 due to its binding to Bim that has been displaced from Bcl-xL or Bcl-2 by ABT-737 (34).
Importantly, we show that Mcl-1 knockdown in PANC-1 cells enhanced caspase-3 activation and increased cytotoxicity by ABT-737. This result is consistent with studies showing that Mcl-1 suppression can sensitize tumor cells to ABT-737 (33). Abundant Mcl-1 proteins in our pancreatic cancer cells and the marked sensitization produced by Mcl-1 shRNA suggest that increased cytotoxicity may be achieved by strategies to down-regulate Mcl-1. In this regard, the pan Bcl-2 antagonist Obatoclax (GX15-070) has been shown to inhibit Mcl-1 and may therefore show greater enhancement of therapeutic efficacy (35). The BH3-mimetic GX15-070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak (35).
We also evaluated the combination of ABT-737 and gemcitabine. At similar doses of ABT-737 that were used in combination with TRAIL, we found that ABT-737 can significantly augment the cytotoxic effects of gemcitabine in both PANC-1 and BxPC-3 cell lines. These data show the relevance of our findings to conventional cytotoxic chemotherapy.
In summary, we show that the disabling of Bcl-xL and Bcl-2 by ABT-737 results in the release of Bim and Bak from prosurvival Bcl-2 proteins to induce apoptosis. ABT-737 also enhanced a TRAIL-induced Bax conformational change. Together, these mechanisms underlie the ability of ABT-737 to augment TRAIL-induced apoptosis in human pancreatic cancer cells. Whereas Mcl-1 can reduce susceptibility to TRAIL (14, 33), ABT-737 treatment was sufficient to enhance TRAIL-mediated apoptosis despite its low affinity for Mcl-1 (13). These findings underscore the potent proapoptotic effects of Bim and Bak release from prosurvival Bcl-2 proteins to enhance TRAIL-mediated cytotoxicity. Experiments in other TRAIL-resistant cell lines are awaited to determine whether responsiveness to the combination of ABT-737 and TRAIL can be generalized. As reported here, our data provide compelling evidence that targeting both the extrinsic and intrinsic apoptotic pathways is a potentially effective and novel therapeutic strategy. Our findings may contribute to the rational design of combinatorial regimens against pancreatic cancer and other malignancies.
Supplementary Material
Suppl Fig 1
Suppl Fig 2
Acknowledgments
Grant support: Suppported in part by National Cancer Institute, Grant CA 104683.
We thank Jonelle Morales for her very capable secretarial assistance and Dr. Scott Kaufmann for his assistance with the calculation of the combination index described in this report.
Footnotes
References
- 1.LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10:66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- 2.Wang S, El-Deiry WS. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene. 2003;22:8628–33. doi: 10.1038/sj.onc.1207232. [DOI] [PubMed] [Google Scholar]
- 3.Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–87. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broaddus VC, Dansen TB, Abayasiriwardana KS, et al. Bid mediates apoptotic synergy between tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and DNA damage. J Biol Chem. 2005;280:12486–93. doi: 10.1074/jbc.M408190200. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 6.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–90. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 7.Desagher S, Osen-Sand A, Nichols A, et al. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–35. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinicrope FA, Penington RC, Tang XM. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis is inhibited by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14-1, in human colon cancer cells. Clin Cancer Res. 2004;10:8284–92. doi: 10.1158/1078-0432.CCR-04-1289. [DOI] [PubMed] [Google Scholar]
- 10.Sun SY, Yue P, Zhou JY, et al. Overexpression of BCL2 blocks TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in human lung cancer cells. Biochem Biophys Res Commun. 2001;280:788–97. doi: 10.1006/bbrc.2000.4218. [DOI] [PubMed] [Google Scholar]
- 11.Burns TF, El-Deiry WS. Identification of inhibitors of TRAIL-induced death (ITIDs) in the TRAIL-sensitive colon carcinoma cell line SW480 using a genetic approach. J Biol Chem. 2001;276:37879–86. doi: 10.1074/jbc.M103516200. [DOI] [PubMed] [Google Scholar]
- 12.Hinz S, Trauzold A, Boenicke L, et al. Bcl-XL protects pancreatic adenocarcinoma cells against CD95- and TRAIL-receptor-mediated apoptosis. Oncogene. 2000;19:5477–86. doi: 10.1038/sj.onc.1203936. [DOI] [PubMed] [Google Scholar]
- 13.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 14.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han J, Goldstein LA, Gastman BR, Rabinowich H. Interrelated roles for Mcl-1 and BIM in regulation of TRAIL-mediated mitochondrial apoptosis. J Biol Chem. 2006;281:10153–63. doi: 10.1074/jbc.M510349200. [DOI] [PubMed] [Google Scholar]
- 16.Yamaguchi H, Paranawithana SR, Lee MW, Huang Z, Bhalla KN, Wang HG. Epothilone B analogue (BMS-247550)-mediated cytotoxicity through induction of Bax conformational change in human breast cancer cells. Cancer Res. 2002;62:466–71. [PubMed] [Google Scholar]
- 17.Lin X, Morgan-Lappe S, Huang X, et al. “Seed” analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-X(L) inhibitor ABT 737. Oncogene. 2007;26:3972–9. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 18.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT 737. J Clin Invest. 2007;117:112–21. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adjei AA, Davis JN, Bruzek LM, Erlichman C, Kaufmann SH. Synergy of the protein farnesyltransferase inhibitor SCH66336 and cisplatin in human cancer cell lines. Clin Cancer Res. 2001;7:1438–45. [PubMed] [Google Scholar]
- 20.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 21.Slee EA, Keogh SA, Martin SJ. Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochrome c release. Cell Death Differ. 2000;7:556–65. doi: 10.1038/sj.cdd.4400689. [DOI] [PubMed] [Google Scholar]
- 22.Ricci MS, Kim SH, Ogi K, et al. Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes resistant human cancer cells to TRAIL-induced death. Cancer Cell. 2007;12:66–80. doi: 10.1016/j.ccr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 23.McManus DC, Lefebvre CA, Cherton-Horvat G, et al. Loss of XIAP protein expression by RNAi and antisense approaches sensitizes cancer cells to functionally diverse chemotherapeutics. Oncogene. 2004;23:8105–17. doi: 10.1038/sj.onc.1207967. [DOI] [PubMed] [Google Scholar]
- 24.Braeuer SJ, Buneker C, Mohr A, Zwacka RM. Constitutively activated nuclear factor-κB, but not induced NF-κB, leads to TRAIL resistance by up-regulation of X-linked inhibitor of apoptosis protein in human cancer cells. Mol Cancer Res. 2006;4:715–28. doi: 10.1158/1541-7786.MCR-05-0231. [DOI] [PubMed] [Google Scholar]
- 25.Kuwana T, Bouchier-Hayes L, Chipuk JE, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–35. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez-Bougie P, Bataille R, Amiot M. Endogenous association of Bim BH3-only protein with Mcl-1, Bcl-xL and Bcl-2 on mitochondria in human B cells. Eur J Immunol. 2005;35:971–6. doi: 10.1002/eji.200425878. [DOI] [PubMed] [Google Scholar]
- 28.Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16:33–45. doi: 10.1101/gad.949602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LeBlanc H, Lawrence D, Varfolomeev E, et al. Tumor-cell resistance to death receptor-induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8:274–81. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 30.Hutcheson J, Scatizzi JC, Bickel E, et al. Combined loss of proapoptotic genes Bak or Bax with Bim synergizes to cause defects in hematopoiesis and in thymocyte apoptosis. J Exp Med. 2005;201:1949–60. doi: 10.1084/jem.20041484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han J, Goldstein LA, Gastman BR, et al. Differential involvement of Bax and Bak in TRAIL-mediated apoptosis of leukemic T cells. Leukemia. 2004;18:1671–80. doi: 10.1038/sj.leu.2403496. [DOI] [PubMed] [Google Scholar]
- 32.Willis SN, Chen L, Dewson G, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen S, Dai Y, Harada H, Dent P, Grant S. Mcl-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing Bak activation and Bax translocation. Cancer Res. 2007;67:782–91. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 34.Czabotar PE, Lee EF, van Delft MF, et al. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc Natl Acad Sci U S A. 2007;104:6217–22. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perez-Galan P, Roue G, Villamor N, Campo E, Colomer D. The BH3-mimetic GX15-070 synergizes with bortezomib in mantle cell lymphoma by enhancing Noxa-mediated activation of Bak. Blood. 2007;109:4441–9. doi: 10.1182/blood-2006-07-034173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl Fig 1
Suppl Fig 2