ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini (original) (raw)
. Author manuscript; available in PMC: 2010 Oct 11.
Published in final edited form as: Nat Cell Biol. 2001 Sep;3(9):785–792. doi: 10.1038/ncb0901-785
Abstract
Both ErbB1 and ErbB2 are overexpressed or amplified in breast tumours. To examine the effects of activating ErbB receptors in a context that mimics polarized epithelial cells in vivo, we activated ErbB1 and ErbB2 homodimers in preformed, growth-arrested mammary acini cultured in three-dimensional basement membrane gels. Activation of ErbB2, but not that of ErbB1, led to a reinitiation of cell proliferation and altered the properties of mammary acinar structures. These altered structures share several properties with early-stage tumours, including a loss of proliferative suppression, an absence of lumen, retention of the basement membrane and a lack of invasive properties. ErbB2 activation also disrupted tight junctions and the cell polarity of polarized epithelia, whereas ErbB1 activation did not have any effect. Our results indicate that ErbB receptors differ in their ability to induce early stages of mammary carcinogenesis in vitro and this three-dimensional model system can reveal biological activities of oncogenes that cannot be examined in vitro in standard transformation assays.
The mammary epithelium of an adult breast is organized into ducts and lobules. The ducts end in a highly branched structure referred to as the terminal ductal lobular unit (TDLU). A TDLU is comprised of multiple individual units referred to as mammary acini. Each acinus has a central lumen, a single layer of polarized luminal epithelial cells surrounded by myoepithelial cells, and a basement membrane.
We have previously shown that human mammary epithelial cells (MECs) form acini-like structures containing a single layer of polarized, growth-arrested cells when grown within a matrix rich in laminin and collagen IV (Matrigel, derived from the Englebreth–Holm Swarm (EHS) tumour)1,2. The epithelial cells within acini in vivo and in culture have an apico-basal distribution of polarity markers such as ZO-1, E-cadherin and α6β4 integrins. They also deposit collagen IV and secrete sialomucin in their basal and apical surfaces, respectively1,2, indicating that the acinar structures formed in culture closely mimic the acini in an adult breast.
Early stages of breast cancer (hyperplasia and ductal carcinoma in situ (DCIS)) are characterized by an increased proliferation of epithelial cells, a loss of acinar organization and filling of the luminal space3. However, a lack of acinar organization and the acquisition of invasive behaviour are later events involved in progression towards malignancy3. Here we show that growth-arrested human mammary epithelial acinar structures can be used to study the early stages of carcinogenesis in culture.
The epidermal growth factor (EGF) family of growth factors consists of at least ten different members that bind and activate four receptors, namely ErbB1 (EGF receptor/HER1), ErbB2 (HER2/Neu), ErbB3 and ErbB4. Binding of EGF family ligands to ErbB receptors induces receptor activation by both homodimerization and heterodimerization, thus generating a complex array of combinatorial signals4–6. However, ErbB2 has been shown to be the preferred heterodimerization partner4,7, indicating that ErbB2 has a central role among ErbB receptors.
ErbB2 is amplified and overexpressed in 20–80% of DCIS cases, and overexpression of ErbB2 is correlated with a poor clinical prognosis of node-positive tumours7,8. Overexpression of ErbB2 in cultured cells induces both ligand-independent receptor phosphorylation and cellular transformation9–11. Hence, under conditions in which ErbB2 is highly amplified relative to other family members in vivo, activation is thought to involve ligand-independent homodimerization9–12. Although it is crucial to understand the differential biological activities of distinct homodimers and heterodimers of different ErbB receptors, coexpression of multiple ErbB receptors and their ligands in normal cells and primary tumours13 complicates the analysis of the specific contributions of each homodimer or heterodimer to oncogenic transformation.
To evaluate the specific contributions of individual ErbB family members to ErbB-mediated transformation of the mammary epithelium, we combined a synthetic-ligand-mediated controlled dimerization strategy14 and three-dimensional cell cultures to activate selected ErbB receptors in preformed acinar structures composed of growth-arrested polarized epithelial cells. We have shown previously that synthetic-ligand-mediated dimerization of ErbB receptors can be used to activate chimaeric ErbB receptors without activating endogenous ErbB receptors and that synthetic-ligand-mediated activation can induce receptor-specific biochemical and biological events that mimic ligand-induced activation14. Here we examine the effects of ErbB homodimerization in MCF-10A cells, a non-transformed human breast epithelial cell line15.
MCF-10A cells require EGF for proliferation15 and form growth-arrested acinar structures when grown in Matrigel1. We generated MCF-10A cell lines expressing chimaeric ErbB1 or ErbB2 receptors that can be homodimerized with bivalent synthetic ligands. Acute activation of ErbB2, but not ErbB1, homodimers in preformed acini induced the reinitiation of proliferation and the generation of multi-acinar structures with filled lumen, indicating that ErbB2 activation overcomes the growth inhibitory influence within polarized epithelial cells and allows cell survival in the lumen of acinar structures. The activation of ErbB2 also induced disruption of tight junctions and apical polarity in monolayers of polarized epithelia, whereas ErbB1 was unable to alter epithelial cell polarity. However, ErbB2 activation did not induce a fully transformed phenotype, because the MCF-10A cells retained epithelial properties, did not invade the basement membrane or display anchorage independence. The ErbB2-induced structures show properties similar to ErbB2-overexpressing DCIS in vivo, indicating that this three-dimensional culture system provides a useful model for elucidating the mechanisms involved in early stages of carcinogenesis in vitro.
Results
Characterization of cells expressing ErbB chimaeras
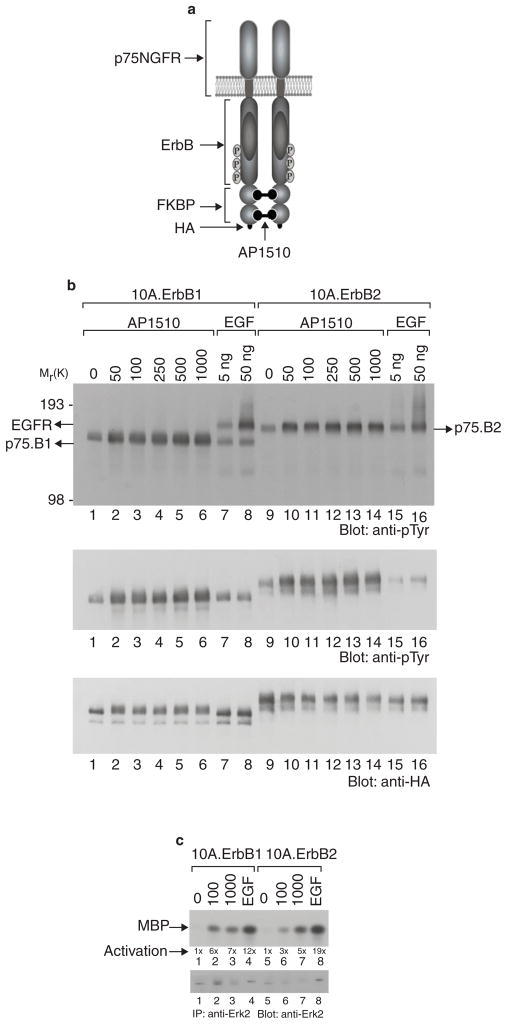
To generate stable cell lines expressing synthetic-ligand-inducible ErbB1 or ErbB2 receptors, MCF10A cells were infected with retroviruses encoding chimaeric ErbB receptors (p75.B1 and p75.B2, Fig. 1a) consisting of the extracellular and transmembrane domains of the p75 low-affinity nerve growth factor (NGF) receptor (p75NGFR) and ErbB1 or ErbB2 cytoplasmic domains linked to the synthetic-ligand-binding domain from FK506-binding protein (FKBP)14. The chimaeric ErbB receptors can be dimerized with the bivalent FKBP ligand AP1510 (Fig. 1a)14,16. Cell lines expressing comparable levels of p75.B1 and p75.B2 (Fig. 1b) were selected for further analyses. Treatment with AP1510 induced the tyrosine phosphorylation of both p75.B1 and p75.B2 receptor chimaeras (Fig. 1b, lanes 1–6 and 9–14) but did not alter the phosphorylation status of endogenous ErbB1, ErbB2 or ErbB3 (Fig. 1b, top panel, lanes 1–6, and data not shown). Conversely, stimulation with EGF activated the endogenous EGF receptors but did not result in activation of the chimaeric receptors (Fig. 1b, top panel, lanes 7, 8, 15 and 16). Thus, synthetic dimerizing ligands can be used to activate the chimaeric ErbB receptors without interfering with endogenous receptors and vice versa.
Figure 1. Synthetic-ligand-inducible activation of ErbB receptors in MCF-10A cells.
a, A schematic representation of the synthetic ligand-induced dimer of chimaeric ErbB receptors. b, Anti-pTyr immunoblots of total cell lysates (top panel) or anti-HA immunoprecipitates (IP) (middle panel) from MCF-10A cells expressing either ErbB1 (10A.ErbB1) or ErbB2 (10A.ErbB2) receptor chimaeras before and after stimulation with EGF (ng ml−1) or AP1510 (nM). The blot in the middle panel was stripped and reprobed with anti-HA antibodies (bottom panel). c, AP1510- or EGF-induced changes in Erk2 kinase activity were measured with an in vitro kinase with MBP as the substrate. The kinase assay blot was reprobed with anti-Erk2 antibodies to determine the levels of Erk2 protein in kinase reactions.
To determine whether the homodimerization of p75.B1 and p75.B2 can activate downstream signalling pathways in this cell system, we analysed the activation of the mitogen-activated protein kinase Erk2. As observed in Rat1 fibroblasts14, homodimerization and activation of either p75.B1 or p75.B2 resulted in a 5–7-fold increase in Erk2 kinase activity (Fig. 1c), indicating that both homodimers were equally competent to initiate the signalling cascade required for the activation of Erk2. The Erk2 kinase activity stimulated by AP1510 was 2–4-fold weaker than that stimulated by 8 nM EGF (Fig. 1c; compare lanes 2 and 3 with lane 4, and lanes 6 and 7 with lane 8). Activation of both p75.B1 and p75.B2 homodimers induced a modest increase in Ser 473 phosphorylation of Akt, a downstream target of the PtdIns-3-OH kinase signalling pathway (data not shown).
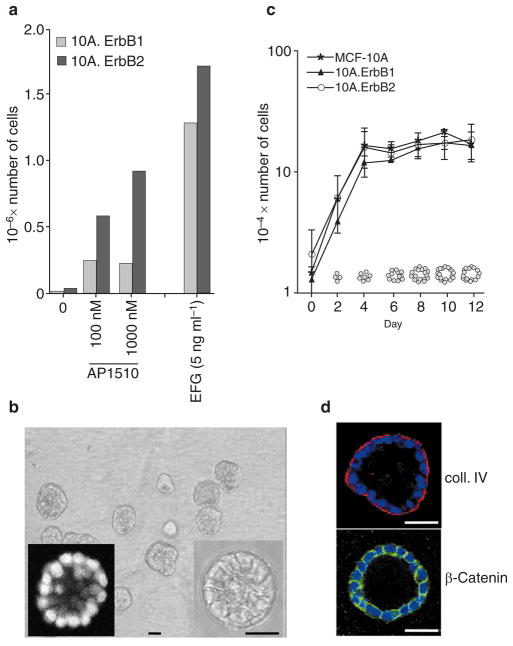
To evaluate whether the dimerization of chimaeric ErbB receptors can stimulate a biological response, we evaluated the ability of ErbB homodimers to induce proliferation in the absence of EGF when cells are grown on a plastic tissue culture plate (two-dimensional (2D)). Like the parental MCF-10A cells, neither the p75.B1-expressing cells nor the p75.B2-expressing cells were able to proliferate in the absence of exogenous EGF (Fig. 2a). Activation of either p75.B1 or p75.B2 homodimers was sufficient to stimulate EGF-independent proliferation; however, p75.B2 homodimers were more potent than p75.B1 homodimers (Fig. 2a). These results indicate that dimerization of p75.B1 or p75.B2 chimaeras is sufficient to stimulate a biological response.
Figure 2. MCF-10A cells form growth arrested polarized acinar structures in Matrigel.
a, Cells expressing different ErbB chimaeras were plated either in the presence of EGF or AP1510. Cell numbers were determined after 5 days. The graph represents an average of three experiments. b, Morphology of acinar structures formed by MCF-10A cells plated in Matrigel for 12 days. Phase image of an acinus at higher magnification is shown in the right inset. The acini were labelled with DAPI and a confocal image through the middle of an acinus is shown (left inset). c, Parental MCF-10A cells and cell lines expressing different ErbB chimaeras were plated on Matrigel and cell numbers were determined on the days indicated. The experiment was repeated three times. The acinar organization at different stages of morphogenesis was determined by confocal analysis of DAPI-labelled structures; the results are shown as diagrams within the graph. d, Day-12 acinar structures were immunostained for collagen IV (coll. IV) (red, upper panel) or β-catenin (green, lower panel) and simultaneously stained for nuclei (blue). Scale bars, 50 μm.
To determine whether the chimaeric ErbB receptors were localized to the proper subcellular compartment, we immunostained monolayers of MCF-10A cells expressing the ErbB chimaera with antibodies against haemagglutinin (HA). Both p75.B1 and p75.B2 chimaeras were localized to the basolateral surface of MCF-10A cells (data not shown). These results are consistent with previous reports describing the localization of wild-type ErbB1 and ErbB2 receptors17,18.
Generation of mammary epithelial acinar structures in vitro
Three-dimensional acinar structures were generated by plating MCF-10A cells as single cells on an exogenous basement membrane matrix (Matrigel). After 10–12 days in culture, each cell formed an acinus containing 20–40 cells (Fig. 2b). Confocal analyses of acini labelled with 4′,6-diamidino-2-phenylindole (DAPI) revealed that the acinar units had basally localized nuclei and a hollow lumen (Fig. 2b). Immunostaining for basal surface markers (collagen IV (Fig. 2d) and α6 integrin (data not shown)) and cell–cell junction markers (β-catenin (Fig. 2d) and E-cadherin (data not shown)) indicated that the acinar structures consisted of polarized epithelial cells. We did not observe any change in the size of the acini between days 8 and 21, indicating that the acini had reached a growth-arrested or differentiated state. Acini were treated with trypsin and cell numbers were determined at regular intervals (Fig. 2c). In the absence of synthetic ligand, cells expressing ErbB chimaeras and parental MCF-10A cells proliferated for the first 4–6 days, and no subsequent increase in cell numbers was detected (Fig. 2c). However, we did observe an increase in acinar size until days 6–8. It is likely that the lack of increase in cell numbers between days 4 and 8 was due to compensating apoptosis because we have detected cell death within the acini during this period while lumen formation takes place (J.S. Debnath, K. Mills, J.S.B. and S.K.M., unpublished observations). To determine whether each acinus was derived from a single cell, we labelled cells with a lipophilic dye, DiI (Molecular Probes), and combined them with unlabelled cells during the morphogenesis assay. This analysis showed that each acinus was derived from a single cell (data not shown). Taken together, these results indicate that the MCF-10A clones that carry the complementary DNAs for chimaeric ErbB receptors display the same growth and morphogenesis properties of parental MCF10A cells in the absence of dimerizer and are thus well suited for investigating the effects of inducible dimerization of ErbB1 and ErbB2.
Activation of ErbB receptors in polarized acinar structures
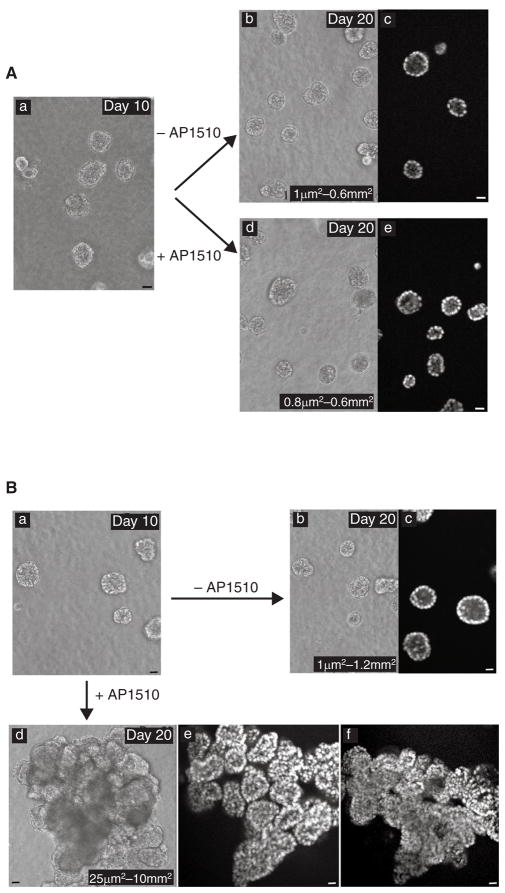
To evaluate the effect of acute activation of ErbB receptors in preformed, polarized acini, we allowed cells expressing p75.B1 or p75.B2 to form growth-arrested acinar units. On day 10 or 12, the EGF-containing medium was replaced with medium containing AP1510 and the assay was continued for a further 8–10 days. Activation of p75.B1 homodimers did not induce detectable changes in acinar structures (compare Fig. 3Ab with Fig. 3Ad). In addition, confocal analyses of DAPI-labelled structures indicated that the acinar structures retained a hollow lumen in the presence of AP1510 (Fig. 3Ae).
Figure 3. ErbB1 and ErbB2 homodimers differ in their ability to affect acinar structures.
A, Cells expressing p75.B1 chimaera were grown in the absence of AP1510 for 10 days (a). The acinar structures were either maintained in the absence of AP1510 (b) or stimulated with 1 μM AP1510 (d) for an additional 10 days. The 20-day-old structures were stained with DAPI and confocal images were obtained (c, e). B, Cells expressing p75.B2 chimaera were left to form acinar structures in the absence of AP1510 for 10 days (a). Ten-day-old structures were either stimulated with AP1510 (d) or maintained in the absence of AP1510 (b). Confocal images of DAPI-labelled 20-day-old structures were obtained. c, An image of day-20 structures maintained in the absence of AP1510. e, f, Two serial optical sections along the z axis of one AP1510-induced multi-acinar structure. The sizes of at least 200 structures were measured with an ocular micrometer and the range is shown (Ab, Ad, Bb, Bd). Scale bars, 50 μm.
Activation of p75.B2 induced marked changes in the acinar structures (compare Fig. 3Ba with Fig. 3Bd). Acini maintained in the absence of AP1510 remained growth-arrested and retained a polarized acinar organization and visible lumina (Fig. 3Bb, Bc), whereas acini incubated in the presence of AP1510 lost their polarized organization and developed structures consisting of multiple acinar-like units with filled lumina (multi-acinar structures) (Fig. 3Be). The units within the multi-acinar structures were connected to each other at the base as revealed by comparing two serial cross-sections of a single structure along the z axis (compare Fig. 3Be with Fig. 3Bf). Upon activation of p75.B2, more than 70% of the acini lost their polarized acinar organization (on the basis of the presence of either partly or completely filled lumina) and about 35% of those formed multi-acinar structures. Most of these structures were at least 10-fold larger (average size 1 mm2) than normal acini (average size 0.1 mm2), whereas some were 100-fold larger than the normal acini (Fig. 3B). To determine whether a single acinus can give rise to these unorganized multi-acinar structures, DiI-labelled acini were monitored. The results indicate that a single acinus can indeed give rise to the multi-acinar structures (data not shown). To determine the effect of activating higher levels of p75.B2 receptors in acinar structures, 10A.ErbB2 cells were reinfected with p75.B2 virus and populations of cells expressing higher levels of ErbB2 were generated. When the acinar structures derived from these cells were stimulated with AP1510, they formed a higher frequency of multi-acinar structures, which resembled those generated by the parental cells and did not display any invasive properties (data not shown).
To determine whether the multi-acinar structures had acquired irreversible genetic changes, we isolated them, expanded the cells under normal culture conditions and assayed for acini formation. When plated on Matrigel in the absence of synthetic ligand, these cells formed polarized acinar structures similar to the parental cells in the absence of AP1510. Stimulation of these structures with AP1510 resulted in the same frequency of multi-acinar structure formation as in parental cells (data not shown), indicating that the ErbB2 dimerization does not induce an irreversible alteration in the genotype of the cells.
Reinitiation of proliferation in growth-arrested polarized acini
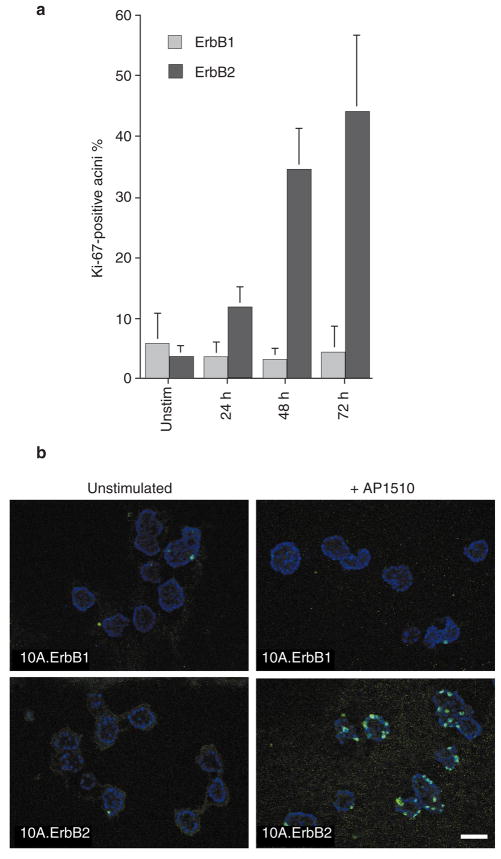
To investigate the basis for the differential ability of ErbB1 and ErbB2 to induce the formation of the multi-acinar structures, we examined whether the homodimers were able to reinitiate proliferation in polarized growth-arrested three-dimensional acini. Twelve-day-old structures were stimulated with dimerizer for 1, 2 or 3 days and cell cycle re-entry was monitored by immunostaining for a proliferating-cell antigen, Ki-67. Activation of ErbB2 induced Ki-67 expression in 30–50% of acini (Fig. 4), whereas activation of ErbB1 homodimers failed to induce Ki-67 (Fig. 4). In addition, stimulation of 15-day-old acini with fresh EGF did not stimulate re-entry into the cell cycle (data not shown). These results indicate that neither EGF nor ErbB1 homodimers have the ability to reinitiate proliferation of growth-arrested, polarized three-dimensional acinar structures.
Figure 4. ErbB1 and ErbB2 homodimers differ in their ability to reinitiate proliferation.
Twelve-day-old structures were stimulated with 1 μm AP1510 for 24, 48 or 72 h. Stimulated and unstimulated (unstim.) structures were fixed and immunostained with antibody against Ki-67. a, Percentages of acini expressing Ki-67 were quantified. At least 150 structures were counted for each experiment and the results are averages from two experiments. Error bars represent s.d. b, Representative fields of the Ki-67-immunostained acini from p75.B1 (upper panels) or p75.B2 expressing cells (lower panels). Scale bar, 50 μm.
Characterization of ErbB2 structures
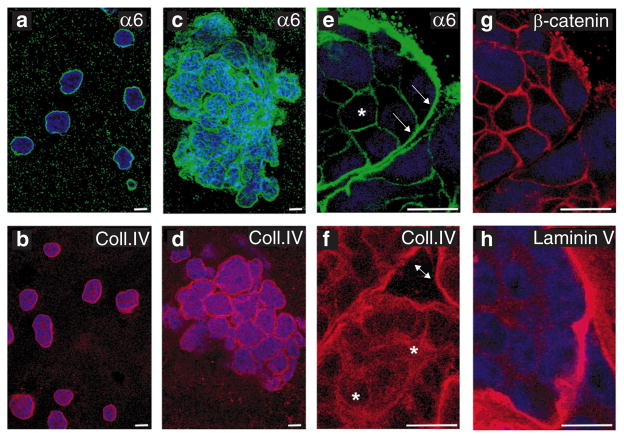
For a better characterization of the organization of the multi-acinar structures, we immunostained them with markers for polarized epithelial cells. The cells at the periphery of the filled acini within the multi-acinar structure had basally localized α6 integrins (Fig. 5c, e (arrowed)) and β4 integrins (data not shown), E-cadherin (data not shown) and β-catenin at cell–cell junctions (Fig. 5g) and basally deposited collagen IV (Fig. 5d) and laminin V (Fig. 5h). The cells in the middle of filled acini had no polarized localization of α6 integrins (Fig. 5e, asterisks) or polarized secretion of collagen IV (Fig. 5f). These results and the evidence that the structures lack a lumen indicate that activation of ErbB2 disrupts the organization of the acini but that the cells retain some of their epithelial properties.
Figure 5. Characterization of ErbB2-induced multi-acinar structures.
Acinar structures grown in the absence of AP1510 were immunostained with monoclonal antibodies against α6 integrin (a, green) or collagen IV (b, red) and analysed with a confocal microscope. Optical sections through the middles of the acini are shown. The ErbB2-induced multi-acinar structures were immunostained with antiserum to α6 integrin (c, e, green), collagen IV (d, f, red), β-catenin (g, red) and laminin V (h, red). Representative confocal images are shown. Nuclei are coloured blue. Scale bars, 50 μm.
ErbB2 homodimers fail to induce anchorage independence
Activation of ErbB2 did not induce any significant changes in morphology of 2D cultures and did not induce efficient colony formation in soft agar under conditions in which Ras-transformed MCF-10A cells19 formed large colonies (Supplementary Information, Fig. S1).
Cancer-derived MECs have been shown to bypass the requirement for β1-integrin-mediated adhesion for proliferation, survival and morphogenesis20,21. Activation of p75.B2 homodimers failed to induce proliferation and morphogenesis of 10A.ErbB2 cells grown in the presence of β1-integrin-blocking antibodies (Supplementary Information, Fig. 2), indicating that ErbB2 activation is not sufficient to bypass the requirement of MCF-10A cells for β1 integrin adhesion.
Disruption of epithelial cell polarity
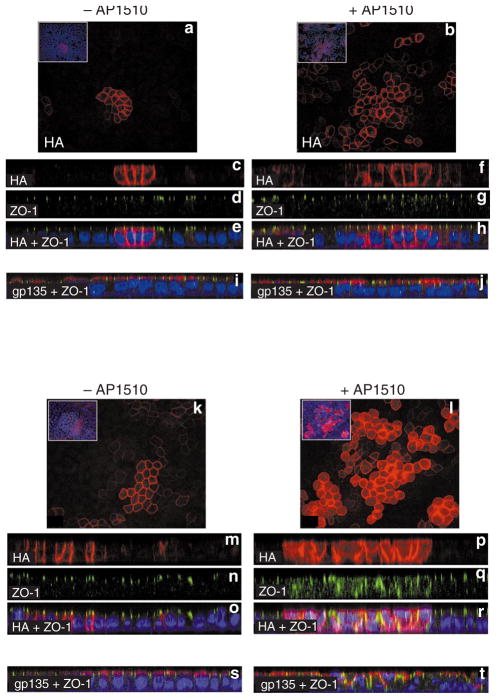
Several studies in Drosophila demonstrate a relationship between loss of cell polarity and loss of growth control in epithelia, indicating that loss of cell polarity might facilitate the uncontrolled proliferation of epithelial cells22. Because ErbB1 and ErbB2 homodimers differ in their ability to reinitiate proliferation in polarized, growth-arrested acini, we investigated whether these receptors differ in their ability to alter epithelial cell polarity. We were unsuccessful in identifying appropriate apical markers for studying cell polarity in MCF-10A cells. We therefore chose the well-characterized Madin–Darby canine kidney (MDCK) epithelial cell line to analyse changes in cell polarity. MDCK cells were infected with retroviruses expressing either the p75.B1 or p75.B2 chimaera and pools were generated. Cells were plated on 0.4-μm transwell filters, left to polarize for 24 h, and then incubated in either the absence or presence of AP1510 for an additional 48 h. The monolayers were immunostained for HA to identify cells expressing ErbB1 or ErbB2, for zonula occludens-1 (ZO-1) to monitor the location of tight junctions, and for gp135 to observe the organization of apical membranes. The addition of AP1510 caused an increase in the number of cells expressing ErbB1 and ErbB2, indicating that they were able to proliferate in response to the activation of either receptor (compare Fig. 6a with Fig. 6b and Fig. 6k with Fig. 6l). The immunostained epithelial monolayers were analysed in the x_–_z axis to determine the spatial distribution of the markers mentioned above. Consistent with previous studies with full-length ErbB1 (ref. 18) or ErbB2 (ref. 17) receptors was our observation that the p75.B1 and p75.B2 chimaeras were localized to the basolateral surface of polarized epithelia, below the tight junctions (Fig. 6c, e and Fig. 6m, o) and stimulation with AP1510 did not induce any significant changes in the localization of ErbB1 chimaeras (Fig. 6f). In addition, activation of ErbB1 did not induce any significant changes in the spatial distribution of either the tight-junction-associated protein ZO-1 (compare Fig. 6d with Fig. 6g) or the apical-membrane protein gp135 (compare Fig. 6i with Fig. 6j). These observations indicate that the activation of ErbB1 homodimers does not affect epithelial cell polarity as monitored by the distribution of tight junctions and apical-membrane proteins.
Figure 6. ErbB1 and ErbB2 homodimers differ in their ability to disrupt epithelial cell polarity.
MDCK cells expressing p75.B1 (a–j) or p75.B2 (k–t) chimaeras were plated on 0.4-μm filters and stimulated with AP1510 as described in Methods. a, b, k, l, Anti-HA immunostaining of the cells expressing p75.B1 or p75.B2 grown in the absence (a, k) or presence (b, l) of AP1510. The inserts demonstrate that only some of the cells in the field express the ErbB chimaeras. Cells grown in the absence (c, d, e, i, p75.B1; m, n, o, s, p75.B2) or presence (f, g, h, j, p75.B1; p, q, r, t, p75.B2) of AP1510 were immunostained for anti-HA (c, f, m, p, red), anti-ZO-1 (d, g, n, q, green), or anti-gp135 (red) and anti-ZO-1 (green) (i, j, s, t) and analysed in the x_–_z axis. The image in e is a merged composite of images in c and d; h is a composite of f and g; o is composite of m and n, and r is a composite of p and q. Nuclei are coloured blue.
However, the activation of ErbB2 induced marked changes in the localization of ZO-1 (compare Fig. 6n with Fig. 6q) and gp135 (compare Fig. 6s with Fig. 6t). In the presence of active ErbB2, ZO-1 was no longer restricted to the apex of the basolateral membrane and was instead redistributed throughout the basolateral membranes (Fig. 6q); gp135 was not restricted to the apical membranes (Fig. 6t). In addition, the HA-tagged ErbB2 chimaera was not restricted to the basolateral surface below the tight junctions (Fig. 6p). Activation of ErbB2 also induced multilayering of epithelial monolayers (Fig. 6r, t). These observations indicate that the activation of ErbB2 induces a disruption of cell polarity in epithelial monolayers.
Taken together, these observations suggest that ErbB2 activation is able to induce events associated with early stages of breast cancer (growth-factor independence, loss of polarized organization and luminal filling); however, additional events might be required to induce changes associated with later stages (invasive activity and anchorage independence).
Discussion
We have analysed the differential effects of activating ErbB1 and ErbB2 receptors in preformed, growth-arrested, polarized acinar structures to mimic the conditions under which ErbB receptors are amplified and activated in vivo. Activation of ErbB2 in preformed mammary epithelial acini results in the reinitiation of proliferation, the loss of polarized organization and the formation of structures containing multiple acinar units. Each acinus within these structures had a filled lumen, surrounded by an intact basement membrane, and did not display any invasive properties. In addition, the activation of ErbB2 homodimers disrupted cell polarity in polarized epithelial monolayers. Thus, ErbB2 dimerization in differentiated acini induces a phenotype that displays some properties of a premalignant stage of breast cancer in vivo that is referred to as carcinoma in situ. In contrast, neither stimulation by EGF nor the activation of ErbB1 homodimers was sufficient to induce the reinitiation of proliferation, alterations in the organization of pre-formed mammary epithelial acini or the disruption of epithelial cell polarity. These results suggest that ErbB2 homodimers are uniquely able to disrupt normal regulation of the proliferation and organization of MECs. This activity of ErbB2 might contribute to the phenotype of comedo–ductal carcinoma in situ, in which ErbB2 expression is significantly amplified.
It is unclear why ErbB1 homodimers were unable to reinitiate proliferation in growth-arrested acinar structures because they were able to substitute for EGF in inducing the proliferation of MCF10A cells on plastic dishes (Fig. 2a). In addition, previous studies have shown that ErbB1 homodimers are able to induce the serum-independent or interleukin-3-independent proliferation of fibroblasts and haematopoietic cells, respectively14,23. It is possible that the events required for the re-stimulation of polarized growth-arrested cells are distinct from those required to support the proliferation of epithelial cells or fibroblasts grown under standard 2D culture conditions. Several lines of evidence suggest that the polarization of epithelial cells suppresses cell proliferation. Some of the most compelling data come from recent studies in Drosophila showing that the disruption of proteins that control epithelial cell polarity such as Scribble, Discs large and Lethal giant larvae results in uncontrolled proliferation of the epithelial cells and hyperplastic growth22. Conversely, the restoration of polarized structural organization to transformed MECs re-establishes growth suppression in three-dimensional cultures2. These studies indicate that regulation of cell architecture might be important in growth control. It is possible that oncogenes need to disrupt cell polarity to induce the proliferation of polarized epithelia. Consistent with this possibility are the observations that the overexpression of oncogenes such as fos24, jun25 and v-k-ras26 alter the polarity of epithelial cells in culture. Our results indicate that ErbB2 and ErbB1 receptors differ in their ability to disrupt cell polarity in epithelial monolayers. It will be of interest to understand the mechanism by which ErbB2, but not ErbB1, homodimers affect cell polarity and induce the reinitiation of cell proliferation in polarized epithelia.
It is likely that the reinitiation of proliferation is not the only step involved in the ErbB2-induced generation of multi-acinar structures with filled lumen. For example, MECs expressing human papilloma virus protein E7 form acinar structures that fail to growth arrest. However, these structures retain a lumen, indicating that uncontrolled proliferation might not be sufficient to repopulate the luminal space27. Lumen formation during normal acinar morphogenesis involves the apoptosis of centrally localized cells that are not in contact with basement membrane28 (J.S. Debnath, K. Mills, J.S.B. and S.K.M, unpublished observations). The evidence that the cells induced to proliferate by ErbB2 homodimerization are able to survive in the lumen suggests that ErbB2 protects cells from apoptosis within the lumen. Our preliminary results indicate that the expression of anti-apoptotic proteins such as Bcl2 and Bcl-xl are not significantly upregulated by the activation of ErbB2 (data not shown). However, the cells in the middle of a filled acinus deposit collagen IV around their surface (Fig. 5f), and this might protect the cells from undergoing apoptosis. Basement membrane components have been shown to protect epithelial cells from apoptosis29,30. Thus, the ErbB2 induced generation of multi-acinar structures with filled lumen might involve both the reinitiation of proliferation and the prevention of apoptosis.
ErbB2 amplification is detected in a high frequency (80–85%) of comedo-type DCIS tumours, which are non-invasive, premalignant mammary tumours7,8. The absence of invasive growth under conditions in which ErbB2 is expressed suggests that the amplification of ErbB2 is not sufficient to induce tumour invasion. Consistent with this possibility is the observation that activation of ErbB2 homodimers in 10A.ErbB2 cells failed to induce migratory or invasive behaviour in vitro (Supplementary Information, Fig. S3). Our results are consistent with previous findings with HB2 MECs31 and 32D haematopoietic cells32 but contrast with those obtained with MDCK47 and tumour-derived MCF-7 cells33. It is likely that ErbB2 is not sufficient to induce invasive behaviour in MCF-10A cells and that additional genetic/epigenetic events or higher levels of ErbB2 expression are required for the cells to acquire invasive behaviour. Candidate cooperating genes include Rac, Cdc42 and PtdIns-3-OH kinase because their activation induces the invasion and migration of MECs34. The cell–cell adhesion molecule E-cadherin is thought to regulate epithelial migration/invasion negatively35. Although it was shown previously that expression of active ErbB2 can suppress E-cadherin gene expression in MECs36, we did not see such regulation in our experiments (data not shown), indicating that additional events are required.
Our results describing the inability of ErbB2 homodimers to induce anchorage independence in normal MECs are consistent with some previous studies31,37–39 but contrast with others40–42. It is not possible to compare our results directly with previous observations because earlier studies were not designed to activate ErbB homodimers without contributions from endogenous ErbB receptors or EGF ligands secreted in an autocrine manner37,43. Moreover, the isolation of stable, non-inducible transfectants in other reports might have involved selection for altered phenotypes. The effect of inducible ErbB2 dimerization on acinar morphogenesis was examined with EPH4 mouse MECs expressing a Trk-ErbB2 chimaeric receptor that had been dimerized with NGF44. The activated ErbB2 chimaera was able to induce normal morphogenesis with no reported perturbations in acinar structures. It is possible that the levels of expression of this receptor were below the threshold required for loss of polarity and uncontrolled proliferation.
Inducible activation of Fos-ER and Jun-ER fusion proteins in tubular structures composed of MECs demonstrated that activation of these proto-oncogenes results in a more marked phenotype involving not only a loss of polarized organization but also an induction of an epithelial–mesenchymal conversion24,25. These studies together with ours indicate that analysing the effects of candidate oncogenes in polarized epithelial structures might provide insights into the biological activities involved in the early stages of carcinogenesis.
In summary, these studies provide evidence that ErbB1 and ErbB2 receptors have differential abilities to affect polarized and growth-arrested acini and also demonstrate that acute activation of ErbB2 results in the generation of multi-acinar structures that share properties with structures associated with carcinoma in situ. Because one class of DCIS (comedo) shows extremely high levels of ErbB2 amplification, it is possible that ErbB2 amplification in vivo induces a state similar to that observed in our cultured mammary acini. In addition, this in vitro model provides a useful system with which to explain the mechanisms involved in early stages of carcinogenesis associated with ErbB2 amplification.
Methods
Materials
MCF-10A cells were obtained from ATCC (Manassas, Virginia) and maintained in DMEM/F12 (Gibco BRL) supplemented with 5% donor horse serum, 20 ng ml−1 EGF, 10 μg ml−1 insulin, 1 ng ml−1 cholera toxin, 100 μg ml−1 hydrocortisone, 50 U ml−1 penicillin and 50 μg ml−1 streptomycin. Matrigel was purchased from Collaborative Biosciences; the protein concentration of the lots used ranged between 9 and 10 mg ml−1. Anti-α6 and anti-β4 antibodies were obtained from Chemicon; anti-β-catenin, anti-E-cadherin and anti-phosphotyrosine were from Transduction Labs; anti-Ki-67 was from Zymed; anti-HA was from BabCo; anti-Erk2 was from Santa Cruz; and was human-specific anti-collagen IV was from Dako.
Generation of MCF-10A cells expressing ErbB chimaeras, and biochemical analysis
VSV G pseudotyped retroviruses expressing the p75.B1 and p75.B2 chimaeras were prepared as described45. MCF-10A cells were plated at 3 × 105 cells per 10-cm-diameter dish, infected with retroviruses and selected in medium containing 100–200 μg ml−1 active-G418 (Gibco BRL). Colonies expressing equal levels of the ErbB chimaeras were screened by anti-HA immunoblots as described previously14. AP1510-inducible phosphorylation of the receptor chimaera was determined as described previously14. Erk2 kinase activity was measured by kinase assays in vitro with myelin basic protein (MBP) as substrate, as described previously46.
EGF-independent proliferation assays
EGF-independent proliferation in 2D cultures was performed by plating 3 × 104 cells in 6.0-cm-diameter dishes in either the presence or the absence of EGF (5 ng ml−1) or AP1510 (0.1 or 1 μM) as stimulus. Cell numbers were determined with a Coulter counter 5 days after plating.
Morphogenesis assay
Cells were treated with trypsin and first resuspended in DMEM/F12 medium supplemented with 20% donor horse serum. The cells were spun down again and resuspended in Assay medium (DMEM/F12 supplemented with 2% donor horse serum, 10 μg ml−1 insulin, 1 ng ml−1 cholera toxin, 100 μg ml−1 hydrocortisone, 50 U ml−1 penicillin and 50 μg ml−1 streptomycin) at a concentration of 105 cells per 4.0 ml. Eight-chambered RS glass slides (Nalgene) were coated with 35 μl Matrigel per well and left to solidify for 15 min. The cells were mixed 1:1 with assay medium containing 4% Matrigel and 10 ng ml−1 EGF, and 400 μl was added to each chamber of the Matrigel-coated eight-chambered slide. Assay medium containing 5 ng ml−1 EGF was replaced every 4 days. For stimulation with AP1510, the EGF-containing assay medium was replaced with assay medium containing 1 μM AP1510 on day 10 or 12. To determine cell numbers at different times during the morphogenesis assay, structures were treated with trypsin by incubation with DMEM/F12, 0.5% trypsin, 5 mM EDTA for 30–40 min at 37 °C. The trypsin-treated structures were resuspended in DMEM/F12 medium containing 20% donor horse serum, and cells were counted.
Cell polarity assays
MDCK cells expressing either p75.B1 or p75.B2 were plated at 440 cells mm−2 in transwell inserts with a pore size of 0.4 μm (Costar). The cells were left to grow for 24 h and the medium was replaced with fresh medium or with that containing AP1510 and maintained for a further 48 h. The filters were fixed in methanol:acetone (50:50) and immunostained as described below.
Immunofluroscence analysis
The acinar structures were fixed either in 2% paraformaldehyde at room temperature for 15 min or in methanol:acetone (50:50) at −20 °C for 10 min. Fixed structures were washed three times in PBS:glycine (130 mM NaCl, 7 mM Na2HPO4, 3.5 mM NaH2PO4, 100 mM glycine) for 15 min each. The washed structures were blocked first in IF buffer (130 mM NaCl, 7 mM Na2HPO4, 3.5 mM NaH2PO4, 7.7 mM NaN3, 0.1% BSA, 0.2% Triton X-100, 0.05% Tween 20) plus 10% goat serum (GS) for 1–2 h and subsequently with 2° blocking buffer (IF buffer containing 10% GS and 20 μg ml−1 goat anti-mouse F(ab′)2) for 30–45 min. Primary antibodies were diluted in 2° blocking buffer and incubated overnight at 4 °C. Unbound primary antibodies were removed by washing three times in IF buffer for 15 min each. Anti-mouse or anti-rabbit secondary antibodies coupled with Alexa Fluor dyes (Molecular Probes) were diluted in IF buffer containing 10% GS and incubated for 45–60 min. Unbound secondary antibodies were washed as described above. Finally, the structures were incubated for 15 min with PBS containing 5 μM TOPRO-3 (Molecular Probes) and 0.5 ng ml−1 DAPI (Roche) before being mounted with the anti-fade agent Prolong (Molecular Probes). Confocal analyses were performed with either Bio-Rad Radiance 2000 or Zeiss LSM410 confocal microscopy systems.
Supplementary Material
Acknowledgments
We thank ARIAD Pharmaceuticals for providing AP1510 (see www.ariad.com for more information); Terry Keenan for synthesis of AP1510; Tim Clackson and Victor Rivera for helpful discussions; William Muller for ErbB2 cDNA; Robert Burgeson for anti-laminin V antibodies; Richard Mulligan for the 293-GPG retrovirus packaging cell line; Carolyn Damsky for AIIB2 antibodies; Karl Matlin for the MDCK cell line; and members of Brugge and Bissell laboratories for stimulating discussions and helpful suggestions. This work was supported by grants from NIH (NCI) (J.S.B., M.J.B.); US Army Medical Research and Material Command (S.K.M.), Massachusetts Breast Cancer Research (S.K.M.), and the US Department of Energy, Office of Biological and Environmental Research (M.J.B.).
Footnotes
Supplementary information is available on _Nature Cell Biology_’s website (http://cellbio.nature.com) or as paper copy from the London editorial office of Nature Cell Biology.
References
- 1.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. (Erratum, Proc. Natl Acad. Sci. USA90, 2556 (1993).) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weaver VM, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris J, Lippman M, Morrow M, Osborne C. Diseases of the Breast. Lippincott Williams and Wilkins; Philadelphia: 1999. [Google Scholar]
- 4.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riese DJ, 2nd, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. BioEssays. 1998;20:41–48. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand–receptor interactions. FEBS Lett. 1997;410:83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- 7.Harari D, Yarden Y. Molecular mechanisms underlying ErbB2/HER2 action in breast cancer. Oncogene. 2000;19:6102–6114. doi: 10.1038/sj.onc.1203973. [DOI] [PubMed] [Google Scholar]
- 8.Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim Biophys Acta. 1994;1198:165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 9.Di Fiore PP, et al. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science. 1987;237:178–182. doi: 10.1126/science.2885917. [DOI] [PubMed] [Google Scholar]
- 10.Di Fiore PP, et al. EGF receptor and erbB-2 tyrosine kinase domains confer cell specificity for mitogenic signaling. Science. 1990;248:79–83. doi: 10.1126/science.2181668. [DOI] [PubMed] [Google Scholar]
- 11.Di Marco E, Pierce JH, Knicley CL, Di Fiore PP. Transformation of NIH 3T3 cells by over-expression of the normal coding sequence of the rat neu gene. Mol Cell Biol. 1990;10:3247–3252. doi: 10.1128/mcb.10.6.3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samanta A, et al. Ligand and p185c-neu density govern receptor interactions and tyrosine kinase activation. Proc Natl Acad Sci USA. 1994;91:1711–1715. doi: 10.1073/pnas.91.5.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gullick WJ, Srinivasan R. The type 1 growth factor receptor family: new ligands and receptors and their role in breast cancer. Breast Cancer Res Treat. 1998;52:43–53. doi: 10.1023/a:1006107016969. [DOI] [PubMed] [Google Scholar]
- 14.Muthuswamy SK, Gilman M, Brugge JS. Controlled dimerization of ErbB receptors provides evidence for differential signaling by homo- and heterodimers. Mol Cell Biol. 1999;19:6845–6857. doi: 10.1128/mcb.19.10.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soule HD, et al. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- 16.Amara JF, et al. A versatile synthetic dimerizer for the regulation of protein–protein interactions. Proc Natl Acad Sci USA. 1997;94:10618–10623. doi: 10.1073/pnas.94.20.10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borg JP, et al. ERBIN: a basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nature Cell Biol. 2000;2:407–414. doi: 10.1038/35017038. [DOI] [PubMed] [Google Scholar]
- 18.Hobert M, Carlin C. Cytoplasmic juxtamembrane domain of the human EGF receptor is required for basolateral localization in MDCK cells. J Cell Physiol. 1995;162:434–446. doi: 10.1002/jcp.1041620316. [DOI] [PubMed] [Google Scholar]
- 19.Basolo F, et al. Transformation of human breast epithelial cells by c-Ha-ras oncogene. Mol Carcinog. 1991;4:25–35. doi: 10.1002/mc.2940040106. [DOI] [PubMed] [Google Scholar]
- 20.Howlett AR, et al. Cellular growth and survival are mediated by β1 integrins in normal human breast epithelium but not in breast carcinoma. J Cell Sci. 1995;108:1945–157. doi: 10.1242/jcs.108.5.1945. [DOI] [PubMed] [Google Scholar]
- 21.Streuli CH, Gilmore AP. Adhesion-mediated signaling in the regulation of mammary epithelial cell survival. J Mammary Gland Biol Neoplasia. 1999;4:183–191. doi: 10.1023/a:1018729308878. [DOI] [PubMed] [Google Scholar]
- 22.Wodarz A. Tumor suppressors: linking cell polarity and growth control. Curr Biol. 2000;10:R624–R626. doi: 10.1016/s0960-9822(00)00658-8. [DOI] [PubMed] [Google Scholar]
- 23.Collins MK, et al. Transfer of functional EGF receptors to an IL3-dependent cell line. J Cell Physiol. 1988;137:293–298. doi: 10.1002/jcp.1041370212. [DOI] [PubMed] [Google Scholar]
- 24.Reichmann E, et al. Activation of an inducible c-FosER fusion protein causes loss of epithelial polarity and triggers epithelial-fibroblastoid cell conversion. Cell. 1992;71:1103–1116. doi: 10.1016/s0092-8674(05)80060-1. [DOI] [PubMed] [Google Scholar]
- 25.Fialka I, et al. The estrogen-dependent c-JunER protein causes a reversible loss of mammary epithelial cell polarity involving a destabilization of adherens junctions. J Cell Biol. 1996;132:1115–1132. doi: 10.1083/jcb.132.6.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoenenberger CA, Zuk A, Kendall D, Matlin KS. Multilayering and loss of apical polarity in MDCK cells transformed with viral K-ras. J Cell Biol. 1991;112:873–889. doi: 10.1083/jcb.112.5.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spancake KM, et al. E7-transduced human breast epithelial cells show partial differentiation in three-dimensional culture. Cancer Res. 1999;59:6042–6045. [PubMed] [Google Scholar]
- 28.Blatchford DR, et al. Influence of microenvironment on mammary epithelial cell survival in primary culture. J Cell Physiol. 1999;181:304–311. doi: 10.1002/(SICI)1097-4652(199911)181:2<304::AID-JCP12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 31.Baeckstrom D, Alford D, Taylor-Papadimitriou J. Morphogenetic and proliferative responses to heregulin of mammary epithelial cells in vitro are dependent on HER2 and HER3 and differ from the responses to HER2 homodimerisation or hepatocyte growth factor. Int J Oncol. 2000;16:1081–1090. doi: 10.3892/ijo.16.6.1081. [DOI] [PubMed] [Google Scholar]
- 32.Chausovsky A, et al. Molecular requirements for the effect of neuregulin on cell spreading, motility and colony organization. Oncogene. 2000;19:878–888. doi: 10.1038/sj.onc.1203410. [DOI] [PubMed] [Google Scholar]
- 33.Spencer KS, et al. ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. J Cell Biol. 2000;148:385–397. doi: 10.1083/jcb.148.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keely PJ, et al. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 35.Vleminckx K, et al. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991;66:107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- 36.D’Souza B, Taylor-Papadimitriou J. Overexpression of ERBB2 in human mammary epithelial cells signals inhibition of transcription of the E-cadherin gene. Proc Natl Acad Sci USA. 1994;91:7202–7206. doi: 10.1073/pnas.91.15.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciardiello F, et al. Transforming growth factor-α expression is enhanced in human mammary epithelial cells transformed by an activated c-Ha-ras protooncogene but not by the c-neu protooncogene, and overexpression of the transforming growth factor-α complementary DNA leads to transformation. Cell Growth Differ. 1990;1:407–420. [PubMed] [Google Scholar]
- 38.Giunciuglio D, et al. Invasive phenotype of MCF10A cells overexpressing c-Ha-ras and c-erbB-2 oncogenes. Int J Cancer. 1995;63:815–822. doi: 10.1002/ijc.2910630612. [DOI] [PubMed] [Google Scholar]
- 39.Harris RA, et al. New model of ErbB-2 over-expression in human mammary luminal epithelial cells. Int J Cancer. 1999;80:477–484. doi: 10.1002/(sici)1097-0215(19990129)80:3<477::aid-ijc23>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 40.D’Souza B, Berdichevsky F, Kyprianou N, Taylor-Papadimitriou J. Collagen-induced morphogenesis and expression of the α2-integrin subunit is inhibited in c-erbB2-transfected human mammary epithelial cells. Oncogene. 1993;8:1797–1806. [PubMed] [Google Scholar]
- 41.Pierce JH, et al. Oncogenic potential of erbB-2 in human mammary epithelial cells. Oncogene. 1991;6:1189–1194. [PubMed] [Google Scholar]
- 42.Lucassen E, et al. The effects of the neuN and neuT genes on differentiation and transformation of mammary epithelial cells. J Cell Sci. 1994;107:2919–2929. doi: 10.1242/jcs.107.10.2919. [DOI] [PubMed] [Google Scholar]
- 43.Normanno N, et al. Amphiregulin as an autocrine growth factor for c-Ha-ras- and c-erbB-2-transformed human mammary epithelial cells. Proc Natl Acad Sci USA. 1994;91:2790–2794. doi: 10.1073/pnas.91.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niemann C, et al. Reconstitution of mammary gland development in vitro: requirement of c-met and c-erbB2 signaling for branching and alveolar morphogenesis. J Cell Biol. 1998;143:533–545. doi: 10.1083/jcb.143.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ory DS, Neugeboren BA, Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miranti CK, Ohno S, Brugge JS. Protein kinase C regulates integrin-induced activation of the extracellular regulated kinase pathway upstream of Shc. J Biol Chem. 1999;274:10571–10581. doi: 10.1074/jbc.274.15.10571. [DOI] [PubMed] [Google Scholar]
- 47.Khoury H, et al. Distinct tyrosine autophosphorylation sites mediate induction of epithelial mesenchymal like transition by an activated ErbB-2/Neu receptor. Oncogene. 2001;15:786–799. doi: 10.1038/sj.onc.1204166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.