Hepatic long-chain acyl-CoA synthetase 5 mediates fatty acid channeling between anabolic and catabolic pathways (original) (raw)
Abstract
Long-chain acyl-CoA synthetases (ACSLs) and fatty acid transport proteins (FATPs) activate fatty acids (FAs) to acyl-CoAs prior to their downstream metabolism. Of numerous ACSL and FATP isoforms, ACSL5 is expressed predominantly in tissues with high rates of triacylglycerol (TAG) synthesis, suggesting it may have an anabolic role in lipid metabolism. To characterize the role of ACSL5 in hepatic energy metabolism, we used small interference RNA (siRNA) to knock down ACSL5 in rat primary hepatocytes. Compared with cells transfected with control siRNA, suppression of ACSL5 expression significantly decreased FA-induced lipid droplet formation. These findings were further extended with metabolic labeling studies showing that ACSL5 knockdown resulted in decreased [1-14C]oleic acid or acetic acid incorporation into intracellular TAG, phospholipids, and cholesterol esters without altering FA uptake or lipogenic gene expression. ACSL5 knockdown also decreased hepatic TAG secretion proportionate to the observed decrease in neutral lipid synthesis. ACSL5 knockdown did not alter lipid turnover or mediate the effects of insulin on lipid metabolism. Hepatocytes treated with ACSL5 siRNA had increased rates of FA oxidation without changing PPAR-α activity and target gene expression. These results suggest that ACSL5 activates and channels FAs toward anabolic pathways and, therefore, is an important branch point in hepatic FA metabolism.
The initial step in mammalian long-chain FA metabolism requires the conversion of FAs to acyl-CoAs, a reaction catalyzed by long-chain acyl-CoA synthetases (ACSLs) or FA transport proteins (FATPs) in the presence of ATP and CoA (1, 2). Acyl-CoAs then enter multiple metabolic pathways as substrates for complex lipid synthesis or β-oxidation. Several isoforms of ACSL and FATP exist and their unique cellular localization patterns, substrate preferences, and enzyme kinetics suggest that individual isoforms have distinct functions (2–4). Gain- or loss-of-function studies further support unique roles of individual ACSL isoforms in fatty acid channeling. Adenovirus-mediated overexpression of ACSL1 in rat primary hepatocytes channels oleic acid toward diacylglycerol (DAG) and phospholipid (PL) synthesis and away from cholesterol esterification, whereas knockdown of ACSL3 in human hepatocytes decreases oleic acid incorporation into PLs for VLDL synthesis (5, 6). Also, knockdown of ACSL3 in rat primary hepatocytes decreases de novo lipogenesis by suppressing the activity of several transcription factors that control lipogenic gene expression (7). These data provide plausible evidence that ACSL isoforms govern distinct pools of intracellular lipids and regulate the channeling and signaling properties of their downstream metabolites.
ACSL5 is most abundant in liver, brown adipose tissue, and intestine and is located on both the mitochondrial membrane and endoplasmic reticulum (8–11). Additionally, its pattern of regulation supports an anabolic role for ACSL5 in the liver (8, 12–15). Leptin administration to ob/ob mice decreases the mRNA expression of ACSL5 along with downregulation of lipogenic gene expression (13). Hepatic ACSL5 mRNA is decreased in fasted animals and upregulated in refed animals (7). Also, this enzyme is a target gene of sterol regulatory element binding protein (SREBP)1-c, which is an insulin dependent lipogenic transcription factor. Thus, expression of ACSL5 mRNA is increased in SREBP1-c transgenic mice and decreased in SREBP1-c cleavage activating protein knockout mice (12, 14, 15). Consistent with these findings, ACSL5 mRNA is induced by high glucose and insulin treatment in cultured hepatocytes and in the liver of insulin treated diabetic animals (12). However, studies to elucidate the exact role of ACSL5 in lipid metabolism are limited. Overexpression of ACSL5 in a rat hepatoma cell line increases FA incorporation into TAG with substrate selectivity toward exogenous FAs, but not endogenous FAs and without changes in β-oxidation or PL synthesis (8).
Although these studies provide insight into the role of ACSL5, the contribution of ACSL5 to hepatic lipid metabolism remains unknown. Thus, we utilized a gene-silencing approach to test the effects of ACSL5 on FA partitioning in hepatocytes. Herein, we show that ACSL5 is an important branch point enzyme in channeling FAs between anabolic and catabolic pathways and that it mediates FA trafficking independent of changes in expression of lipogenic or oxidative genes.
MATERIALS AND METHODS
Materials
Tissue culture plates were from Nunc and media was obtained from Invitrogen. Rat-tail collagen I was obtained from BD Biosciences. [1-14C]oleic acid and [1-14C]acetic acid were from Perkin Elmer Life Sciences. Lipids standards for TLC were from Sigma-Aldrich and Avanti Polar Lipids. pSG5-GAL4-hPPAR-α expression plasmid and a TKMH-UAS-LUC reporter plasmid were provided by Philippe Thuillier (Oregon Health and Science University, Portland, OR). For SREBP1-c reporter gene analysis, pGL2-SRE-TK-LUC reporter plasmid, and pCMV-SREBP-1c expression vector, which overexpresses a constitutively activated form of SREBP-1c, were provided by Dr. Timothy Osborne (University of California, Irvine, CA). All other chemicals were obtained from Sigma-Aldrich unless otherwise indicated.
Primary hepatocyte isolation
Animal protocols were approved by the University of Minnesota Institutional Animal Care and Use Committee. Male Sprague Dawley rats (250–300 g) were maintained on a 12:12 h light:dark cycle and were allowed free access to food before hepatocyte isolation. Hepatocytes were isolated by using the collagenase perfusion method (15) and cell viability was over 90% as confirmed by trypan blue exclusion. Hepatocytes were seeded at a density of 0.5 × 106 cells/ 22-mm well in M199 supplemented with 23 mM HEPES, 26 mM sodium bicarbonate, 10% FBS, 50 IU/ml penicillin and 50 μg/ml streptomycin, 100 nM dexamethasone, 100 nM insulin, and 25 mM glucose on collagen-coated plates.
RNA interference
Duplexes of siRNA were synthesized by Qiagen. The two siRNA sequences targeting rat ACSL5 (Gene BankTM accession number NM_053607) are at nucleotide positions of 503-523 and 423-443 for ACSL5-a and ACSL5-b, respectively. Nonspecific sequence targeted siRNA (All-Star, Qiagen) served as a control. Recombinant small interference (si)RNA was transfected into primary hepatocytes with 1 ug of siRNA per 0.5 × 106 cells using Effectene reagent (Qiagen) when cells were plated immediately after isolation. After 6 h, transfection media was removed and replaced by M199 media supplemented with 23 mM HEPES, 26 mM sodium bicarbonate, 50 IU/ml penicillin and 50 μg/ml streptomycin, 10 nM insulin, 10 nM dexamethasone, and 5.5 mM glucose unless otherwise noted. No differences in cell viability were observed between treatment groups.
Cell homogenate preparations for acyl-CoA synthetase activity
Hepatocytes transfected with control or ACSL5 siRNAs remained in M199 media for 72 h after transfection and were subsequently washed twice with cold PBS, collected in cold Med I buffer (10 mM Tris, pH 7.4, 250 mM sucrose, 1 mM EDTA, 1 mM dithiothreitol, and protease inhibitor mixture), and homogenized on ice with 10 strokes using a tissue homogenizer (Biospec). Homogenate aliquots were stored at −80°C until use. Protein concentrations were determined by the bicinchoninic acid method (Pierce). Acyl-CoA synthetase (ACS)-specific activity was determined by measuring the production of [1-14C]acyl-CoAs in the presence of 175 mM Tris-HCl, pH 7.4, 8 mM MgCl2, 5 mM dithiothreitol, 10 mM ATP, 0.25 mM CoA, and 500 μM [1-14C]oleic acid in 0.5 mM Triton X-100, 0.01 mM EDTA. The assay was performed in a total volume of 200 μl at 37°C for 5 min. The reaction was started by adding 1 to 2 μg of homogenate protein, terminated with 1 ml of Dole's reagent (isopropanol, heptane, 1 M H2SO4, 80:20:2, v/v) and FAs were extracted with sequential heptane washes prior to scintillation counting of the aqueous phase containing the acyl-CoAs.
Oil Red O staining and total TAG content measurement
At 50 h after transfection, hepatocytes were exposed to 1,000 μM oleate for 28 h. Sodium oleate was dissolved in 1 N NaOH and was added to BSA at 37°C to reach a final FA to BSA ratio of 3:1. This BSA/oleate mixture was added to media and sterile filtered prior to incubations. After exposure to oleate, cells were washed with ice-cold PBS and fixed in 10% neutral buffered formalin. Upon removal of formalin, cells were incubated with Oil Red O solution for 10 min followed by washing five times with PBS (pH 7.4). Images of stained lipid droplets were obtained using an Axiovert 40C microscope (Zeiss). For measuring total TAG content, cellular lipids were extracted (16) and total TAG was quantified by colorimetric method using a commercial kit (Stanbio).
Analysis of FA composition in cellular TAG
Upon siRNA transfection, cells were incubated in M199 containing 10% FBS for 72 h and then lipids were extracted (16) and TAG fractions were separated by TLC on 0.25 mm silica gel G plates in hexane:ethyl ether:acetic acid (80:20:1, v/v). Isolated TAG fractions were methylated in 3N methanolic HCl at 100°C for 90 min. The FA methyl esters were extracted with hexane and subjected to GC analysis (7).
Quantitative real-time PCR
At the indicated times, cells were harvested in Trizol (Invitrogen) and total RNA was isolated. First-strand cDNA was synthesized with the SuperScript III reverse transcriptase and random hexamer primers (Invitrogen). After incubation with RNAase H, cDNAs were mixed with 2× SYBR Green PCR Master Mix (Invitrogen) and probed for genes of interest by real-time PCR quantification on an ABI Prism 7700 sequence detection system (Applied Biosystems). Fluorescence emission data were acquired for 40 cycles with an annealing temperature of 60°C. Primers for each gene are listed in Table 1. Data were analyzed using ΔΔ Ct method and mRNA abundance of each gene was normalized to RPL-32.
TABLE 1.
Primer sequences used for gene expression by qRT-PCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| ACSL1 | AAC GAT GTA CGA TGG CTT CC | CAT ATG GCT GGT TTG GCT TT |
| ACSL3 | GGG ACT ACA ATA CCG GCA GA | ATA GCC ACC TTC CTC CCA GT |
| ACSL4 | AAA TGC AGC CAA ATG GAA AG | CAC TCG GCA GTT CAC TTC AA |
| ACSL5 | ATC TGC CTC CTG ACA TTT GG | GCT CCT CCC TCA ATC CCT AC |
| FATP2 | CTG CAT GTC TTC TTG GAG CA | GCG TAG GTA AGC GTC TCG TC |
| FATP4 | CAC TGC CTT GAC ACC TCA AA | ACC AGA GCA GAA GAG GGT GA |
| FATP5 | GGA ACT CTA CGG CTC CAC AG | GGC TCT GCC GTC TCT ATG TC |
| CPT1 | TCT TGC AGT CGA CTC ACC TT | TCC ACA GGA CAC ATA GTC AGG |
| UCP2 | ATG ACA GAC GAC CTC CCT TG | GAA GGC ATG AAC CCC TTG TA |
| FAS | AGG ATG TCA ACA AGC CCA AG | ACA GAG GAG AAG GCC ACA AA |
| SCD-1 | TGT TCG TCA GCA CCT TCT TG | GGA TGT TCT CCC GAG ATT GA |
| ACC-α | ATT GTG GCT CAA ACT GCA GGT | GCC AAT CCA CTC GAA GAC CA |
| ACC-β | CAA AGC CTC TGA AGG TGG AG | GGA CAC TGC GTT CCC ATA CT |
| L-PK | GTA CAG AAA ATC GGC CCA GA | AGG TCC ACC TCA GTG TTT GG |
| GPAT1 | AGG CTT GAC GAA ACT CCA GA | AGG GAT GAC CAG GAT GTC AG |
| SREBP-1c | GGA GCC ATG GAT TGC ACA TT | AGA AGA GAA GCT CTC AGG AG |
| ChREBP | CGG GAC ATG TTT GAT GAC TAT | AAT AAA GGT CGG ATG AGG ATG |
| RPL32 | AAA CTG GCG GAA ACC CAG AG | GCA GCA CTT CCA GCT CCT TG |
Reporter gene analysis
Hepatocytes were transfected with the pGL2-(SRE)-TK-LUC reporter plasmid (200 ng) alone or with the pCMV-SREBP1-c (20 ng) per 0.5 × 106 cells for SREBP1-c reporter gene assay. For measurement of peroxisome proliferator-activated receptor (PPAR)-α transcriptional activity, cells were cotransfected with the pSG5-GAL4-hPPAR-α expression plasmid (50 ng) and a TKMH-UAS-LUC reporter plasmid (250 ng) per 0.5 × 106 cells. A renilla luciferase vector (pRL-SV40, Promega) was used at a concentration of 20 ng per 0.5 × 106 cells as an internal control for adjusting transfection efficiency of PPAR-α and SREBP1-c reporter gene plasmid. All reporter gene plasmids were cotransfected with siRNAs upon plating and media was changed after 6 h. Subsequently, media was changed every 24 h and then cells were harvested for luciferase reporter gene assay at 72 h after transfection (Promega) and activities of PPAR-α and SREBP1-c were expressed as relative luciferase units. Sixteen h prior to harvest, 750 μM oleate and 250 μM EPA were complexed to BSA (3:1 molar ratio) and added to a subset of wells to induce PPAR-α activity or target gene expression.
Western blotting
For measuring protein expression of ACSL5 and stearoyl-CoA desaturase (SCD1), cells were harvested and lysed in 10 mM Tris-HCl (pH 7.4) containing 150 mM NaCl, 0.1% triton X-100, and 1% protease inhibitor cocktail (Roche Applied Science). Aliquots of total proteins (40 μg) were denatured at 100°C for 10 min in SDS sample loading buffer (50 mM Tris, pH 6.8, 2% SDS, 10% glycerol, 1% bromophenol blue, and 15% β-mercaptoethanol). Samples were then separated by SDS-PAGE and electroblotted to a polyvinylidene difluoride membrane (Millipore). Equal transfer of proteins was confirmed by Ponceau S staining. After transfer, the membrane was blocked in 5% non-fat dry milk in PBS (pH 7.4) with 1% Tween 20 and then incubated with ACSL5, SCD-1 (Santa Cruz Biotechnology), or β-actin (Sigma) antibodies. The antigens were detected by ECL chemiluminescent assay following incubation with a horseradish peroxidase-linked secondary antibody (Santa Cruz Biotechnology).
Metabolic labeling studies
Seventy two hours after plating or siRNA transfection, hepatocytes were labeled with 1 ml of M199 containing 1.0 μCi of [1-14C]oleic acid bound to BSA in a 3:1 molar ratio for 2 h. The radiolabeling medium, which included 1 mM carnitine, contained a final concentration of 1,000 μM oleate. For measuring de novo lipogenesis, cells were incubated with 1.0 μCi of [1-14C]acetic acid for 1 h. The medium was collected for measurement of radiolabel incorporation into secreted lipids. Hepatocytes were washed twice with 1% BSA in PBS at 37°C and cellular lipids were extracted (17). For pulse-chase experiments, hepatocytes were incubated with 1,000 µM [1-14C]oleic acid for 2 h or 1.0 μCi of [1-14C]acetic acid for 1 h and then cells were either collected (pulse) as described above or washed twice with 1% BSA in PBS and then incubated for an additional 6 h in M199 media without added oleate or acetate (chase). Subsequently, medium and cells were collected and lipids were extracted as described. Aliquots of the lipid extracts from the cells were separated by TLC on 0.25-mm silica gel G plates in hexane:ethyl ether:acetic acid (80:20:1, v/v) together with synthetic lipid standards (Sigma and BioChemika) in parallel. Lipid extracts and lipids scraped from TLC plates stained with iodine vapor were quantified using liquid scintillation counting (LS6000IC, Beckman). Rates of FA uptake and radioisotope incorporation into acid-soluble metabolites (ASMs) and carbon dioxide (CO2) were measured as described (18).
Statistical analysis
Data were expressed as means ± SE. Significance of data was declared at p < 0.05 by Student's _t_-test.
RESULTS
ACSL5 siRNA effectively suppresses ACSL5 expression and cellular ACS activity
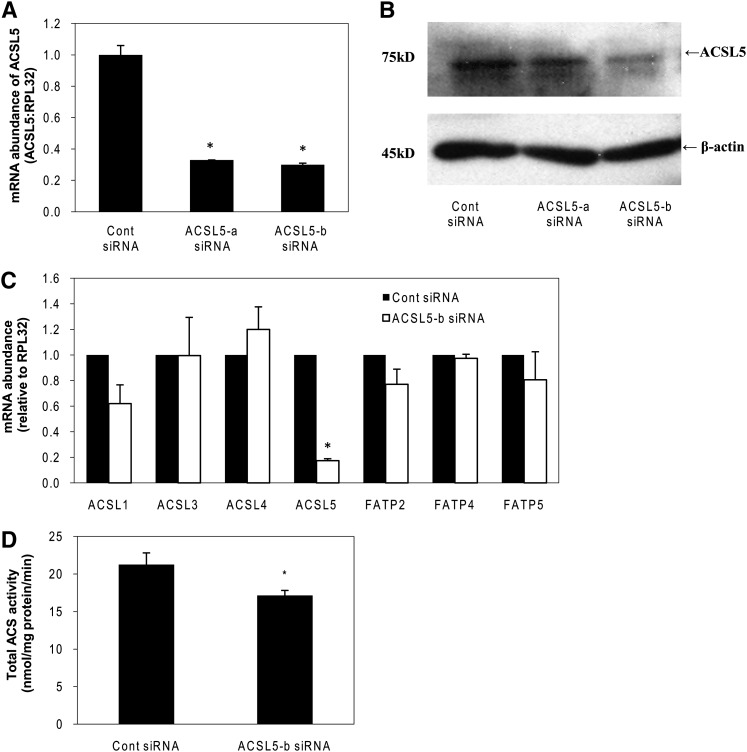
Initially, we tested the efficacy of several ACSL5 siRNAs and identified two that reduced the level of ACSL5 mRNA by more than 70% (Fig. 1A). Both ACSL5 siRNAs also decreased the level of ACSL5 protein expression at 72 h following transfection although ACSL5-b siRNA showed a more robust decrease in ACSL5 protein (Fig. 1B). Subsequently, we tested whether other ACSL or FATP isoforms had altered expression in response to knockdown of ACSL5. To test this possibility, mRNA for each ACSL or FATP isoform was analyzed in ACSL5 siRNA transfected cells at 72 h after transfection. Expression of other ACSL or FATP isoforms was not significantly affected by the low expression of ACSL5 (Fig. 1C). These data indicate that the expression of the other isoforms of ACSL or FATP did not compensate for a deficiency of ACSL5. Once we verified the efficacy of isoform-specific siRNA, we measured total ACS activity in cell homogenates. Transfection of ACSL5 siRNA significantly decreased total ACS activity by 27% (Fig. 1D) when compared with control siRNA transfected cells, suggesting that although ACSL5 contributes to hepatic ACS activity, it is likely not the prominent isoform.
Fig. 1.
Efficacy of ACSL5 siRNA and lack of compensation by other ACSL isoforms. Rat primary hepatocytes were transfected with ACSL5-a or ACSL5-b siRNA duplexes as described under Materials and Methods. A: Total RNA was extracted at 44 h following transfection. Abundance of mRNA for ACSL5 was quantified using quantitative RT-PCR and normalized to RPL-32. B: Proteins were harvested at 72 h after transfection and subjected to Western blotting. Expression of β-actin was analyzed as an internal loading control. C: mRNA abundance for ACSL and FATP isoforms was measured at 72 h after transfection. D: At 72 h after transfection, cells were harvested in cold Med-I buffer and ACS activity was determined as described under Materials and Methods. Values are reported as mean ± SE. from three individual experiments. * p < 0.05 when compared with controls.
Knockdown of ACSL5 suppresses lipid accumulation in hepatocytes
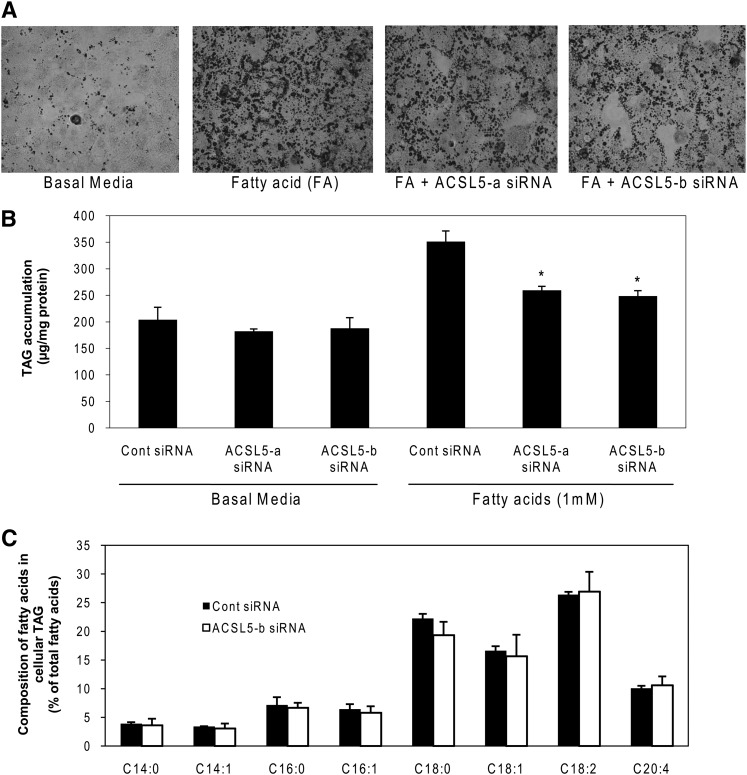
Because previous studies have implicated an anabolic role for ACSL5 in lipid metabolism (8, 9, 12, 13), we initially measured hepatic lipid accumulation and total TAG content in hepatocytes treated with ACSL5 siRNA. As expected, high FA (1,000 μM) treatment increased lipid droplet formation and TAG content in control siRNA transfected cells (Fig. 2A, B). When exposed to 1,000 µM oleate, cells treated with ACSL5 siRNA had 30–40% less TAG, which represents a 60–70% attenuation in the increase in TAG relative to basal levels (Fig. 2A, B). Surprisingly, both siRNAs had nearly identical effects on TAG despite dissimilar knockdown of ACSL5 protein (Fig. 1B). We also analyzed composition of FAs in cellular TAG to determine if ACSL5 selectively modulates the metabolism of specific FAs. However, individual FA species in cellular TAG were not affected by ACSL5 knockdown (Fig. 2C).
Fig. 2.
Knockdown of ACSL5 decreases lipid droplet formation and TAG content in rat primary hepatocytes. Cells were plated at 0.5 × 106 cells/22 mm well and transfected with 1 μg of siRNA per well. After 44 h, cells were treated with 1,000 μM oleic acid (FA) for 28 h. Cells were stained with Oil Red O (A) or collected for lipid extraction and quantification of TAG (B). Nonspecific siRNA transfected cells under basal media were used as a control. C: Transfected cells were incubated in M199 containing 10% FBS for 72 h and then lipids were extracted from the cells and TAG fractions were separated by TLC. Methyl esters of fatty acids were quantified by GC. Composition of each different species of free fatty acids was calculated as % of total fatty acids in the TAG fraction. Data are indicated as mean ± SE. * p < 0.05 when compared with controls.
ACSL5 knockdown suppresses esterification of FAs into complex lipids
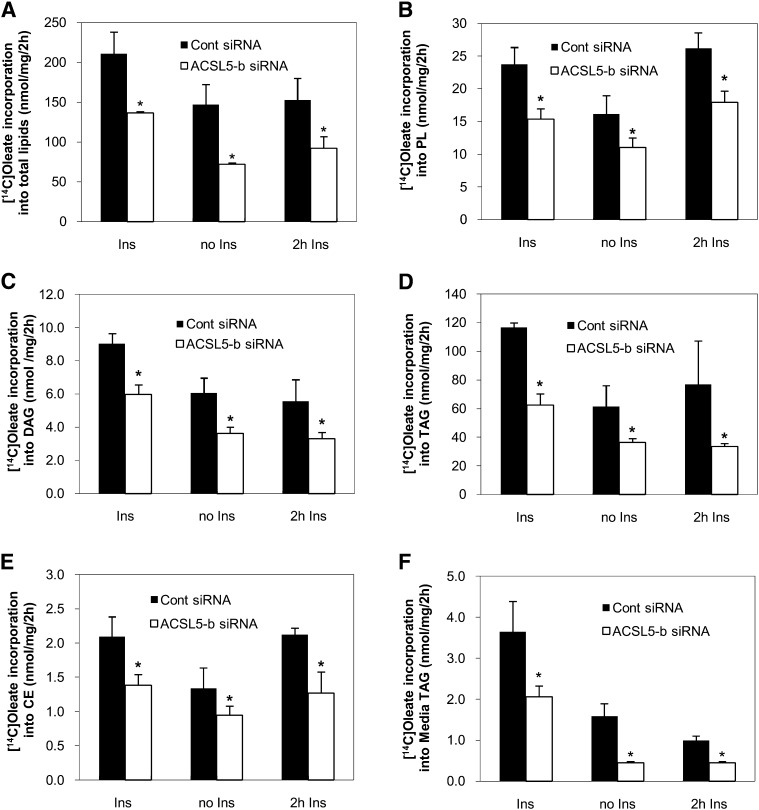
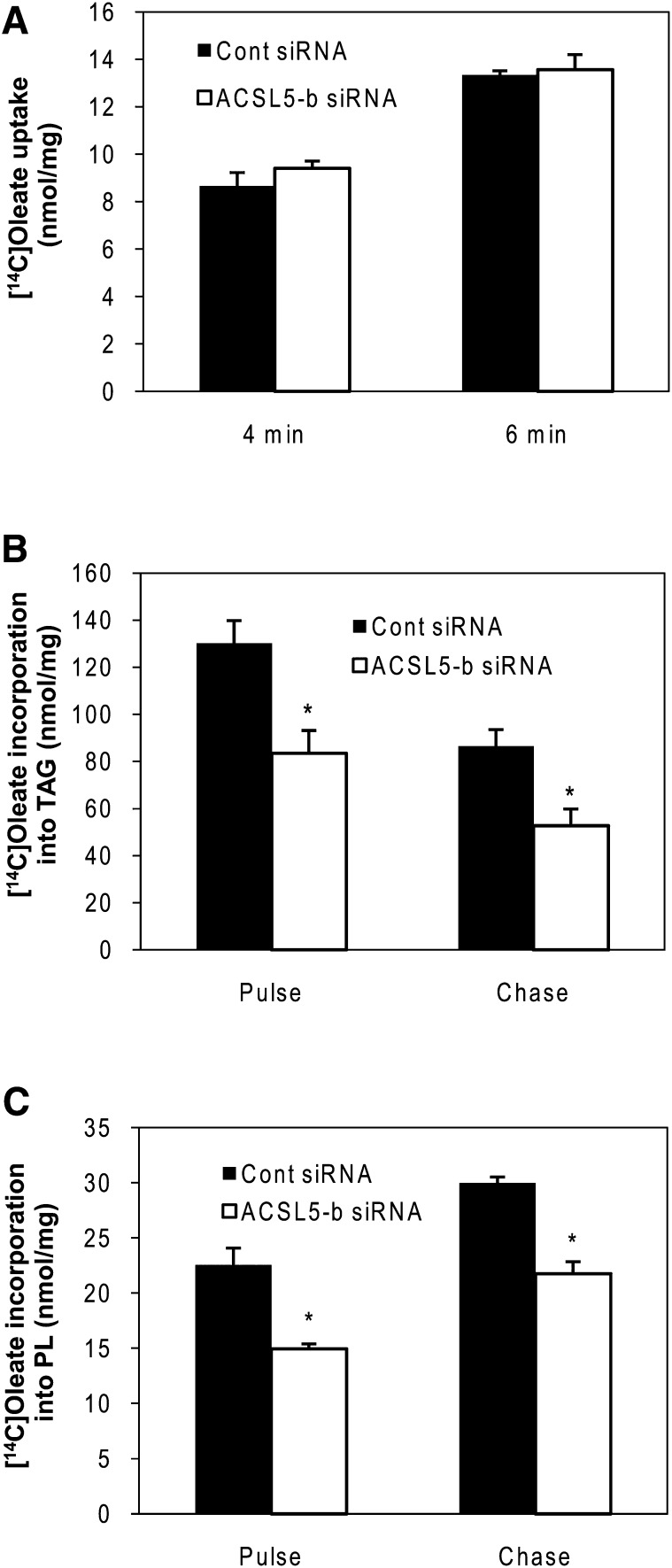
ACSL5 is regulated by insulin and insulin-responsive transcription factors such as SREBP-1c (12, 14). Given the observation of a marked decrease in lipid droplet accumulation and TAG content under ACSL5 knockdown, we further explored the mechanism of how FA channeling by ACSL5 knockdown affects hepatic lipid metabolism under different insulin conditions. Rat primary hepatocytes were incubated with 1,000 μM and 1 μCi of [1-14C]oleic acid for 2 h at which time media and cells were harvested. To determine chronic effects of insulin, cells were treated with 10 nM insulin over the course of the experiment (72 h), whereas insulin was removed from the media 20 h after transfection in the “no insulin” treatment group. To test the acute effects of insulin, cells were incubated with media void of insulin and then insulin was added to cells for 2 h prior to harvest, concurrent with the FA labeling period. As expected, total FA incorporation into cellular lipids was decreased in the absence of insulin (Fig. 3A). ACSL5 siRNA decreased total [1-14C]oleic acid incorporation into intracellular lipids compared with control siRNA transfected cells under all insulin conditions. Subsequent lipid analysis also revealed that ACSL5 siRNA decreased [1-14C]oleic acid incorporation into cellular PL, DAG, TAG, and cholesterol ester compared with control siRNA transfected cells (Fig. 3B–E). We also validated this observation with the second ACSL5 siRNA to confirm that alterations in FA channeling are not due to off-target effects of siRNA (data not shown). To determine if ACSL5 siRNA influenced hepatic TAG secretion, we measured radiolabel incorporation into media TAG. The secretion of [14C]TAG was decreased ∼50% by ACSL5 knockdown under all insulin conditions and mirrored changes in cellular TAG (Fig. 3F). Also, the pattern of decreased FA metabolism by ACSL5 knockdown was similar under different insulin conditions indicating that channeling of intracellular FA by ACSL5 is independent of insulin. To determine if ACSL alters FA uptake as a means to decrease lipid anabolic pathways, we measured FA uptake 4 and 6 min after [1-14C]oleic acid incubation. Although total FA uptake increased with time, as expected, we observed no differences between control and ACSL5 siRNA transfected cells (Fig. 4A). Thus, the effect of ACSL5 knockdown on decreased FA incorporation into complex lipids was likely not due to alterations in FA uptake. These data suggest that a decrease in initial partitioning of exogenous FA toward esterification contribute to decreased lipid accumulation in ACSL5 siRNA transfected cells. Using pulse-chase experiments, we next determined if turnover of lipids was altered by ACSL5. Compared with control siRNA transfected cells, ACSL5 siRNA did not alter the rates of [14C]FA gain to PL (∼27% of pulse) or loss from TAG (∼60% of pulse) during the chase period (Fig. 4B, C). These data suggest that the changes in lipid metabolism caused by ACSL5 are due to effects on lipid synthesis rather than turnover.
Fig. 3.
Suppression of ACSL5 decreases fatty acid incorporation into complex lipids. Rat primary hepatocytes were transfected with ACSL5 siRNA (ACSL5-b). After 72 h, cells were treated with 1,000 μM oleic acid and labeled with 1 μCi [1-14C]oleic acid per 0.5 × 106 cells for 2 h. Lipids were extracted from the cells and quantified as described in Materials and Methods. A: Total oleic acid metabolized and [1-14C]oleic acid incorporation into cellular PL (B), DAG (C), TAG (D), cholesterol ester (CE) (E), and media TAG (F) were measured under different insulin concentrations: Ins (10 nM insulin), no Ins, and 2 h Ins (10 nM insulin for 2 h prior to harvesting). Values are reported as mean ± SE from three individual experiments. * p < 0.05, when compared with controls.
Fig. 4.
Suppression of ACSL5 does not alter fatty acid uptake and lipid turnover. Rat primary hepatocytes were transfected with ACSL5 siRNA (ACSL5-b). A: Rates of 1,000 μM [1-14C]oleic acid uptake were measured at 4 or 6 min. B, C: Cells were either collected for lipid extraction after 2 h labeling (pulse) or washed and incubated with media containing no fatty acids for 6 h (chase). Remaining [1-14C]labeled fatty acids were analyzed in cellular lipid extracts in chased cells and compared with pulsed cells. Data are indicated as mean ± SE from three individual experiments. * p < 0.05 when compared with controls.
ACSL5 knockdown suppresses metabolism of FAs from de novo lipogenesis
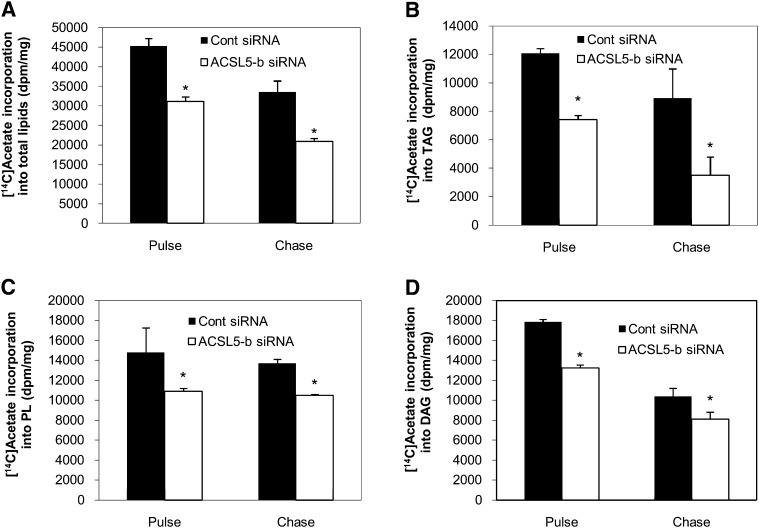
It has been suggested that TAG and cholesterol ester synthesis in hepatocytes requires some FAs derived from de novo synthesis (19). To determine the selectivity of ACSL5 for endogenous versus exogenous FAs, we tracked the incorporation of [1-14C]acetic acid into cellular lipids under high glucose (25 mM) conditions. Compared with cells transfected with the control siRNA, incorporation of [1-14C]acetic acid into total lipids was reduced by about 40% in ACSL5 siRNA transfected cells (p < 0.01) (Fig. 5A). Subsequent partitioning of newly synthesized lipids into different types of storage lipids (TAG, PL, and DAG) was also decreased to a similar extent (Fig. 5B–D). Taken together with the oleic acid labeling, these data suggest that ACSL5 may activate FAs from both exogenous and de novo sources. Additionally, ACSL5 knockdown did not alter the rate of turnover of intracellular TAG and PL derived from de novo synthesis (Fig. 5B–D). Thus, regardless of the FA source, ACSL5 alters lipid metabolism through initial channeling of FAs rather than affecting intracellular lipid turnover.
Fig. 5.
Knockdown of ACSL5 suppresses the esterification of fatty acids from de novo synthesis. At 72 h after transfection, cells were labeled with 1 μCi [1-14C]acetic acid per 0.5 × 106 cells for 1 h. Cells were either collected for lipid extraction after 1 h labeling (pulse) or washed and incubated with media containing no acetate for 6 h (chase). Lipids were extracted from the cells and quantified as described in Materials and Methods. A: Total [1-14C]acetic acid metabolized and [1-14C]acetic acid incorporation into cellular TAG (B), PL (C), and DAG (D) were determined. Values are shown as mean dpm ± SE from three individual experiments. * p < 0.05 when compared with controls.
ACSL5 knockdown does not modulate other lipogenic enzymes and transcription factors
Intracellular FAs, acyl-CoAs, or their downstream metabolites act as ligands for several transcription factors (7, 15, 20, 21). Based on our initial findings that hepatic lipid accumulation and de novo lipogenesis were suppressed by ACSL5 knockdown, we sought to further characterize whether FA channeling under ACSL5 knockdown is mediated by transcriptional regulation. Compared with cells transfected with control siRNA, ACSL5 siRNA transfected cells did not show any significant changes in lipogenic genes such as acetyl-CoA carboyxlase (ACC)-α, ACC-β, FAS, L-PK, and GPAT1 (supplementary ). Although mRNA expression of SCD1 was significantly decreased in ACSL5 siRNA transfected cells (30%) (supplementary ), protein expression of SCD1 was not changed (supplementary ). Also, expression of lipogenic transcription factors (ChREBP and SREBP1-c) (supplementary ) and reporter gene activity of SREBP1-c were not changed by ACSL5 knockdown (supplementary ). Taken together, these data indicate that decreased lipid anabolism by ACSL5 was not mediated by downregulation of lipogenic gene expression, and transcriptional regulation is not the basis for alterations in lipid metabolism by ACSL5 knockdown.
ACSL5 knockdown increases beta-oxidation independent of oxidative gene expression
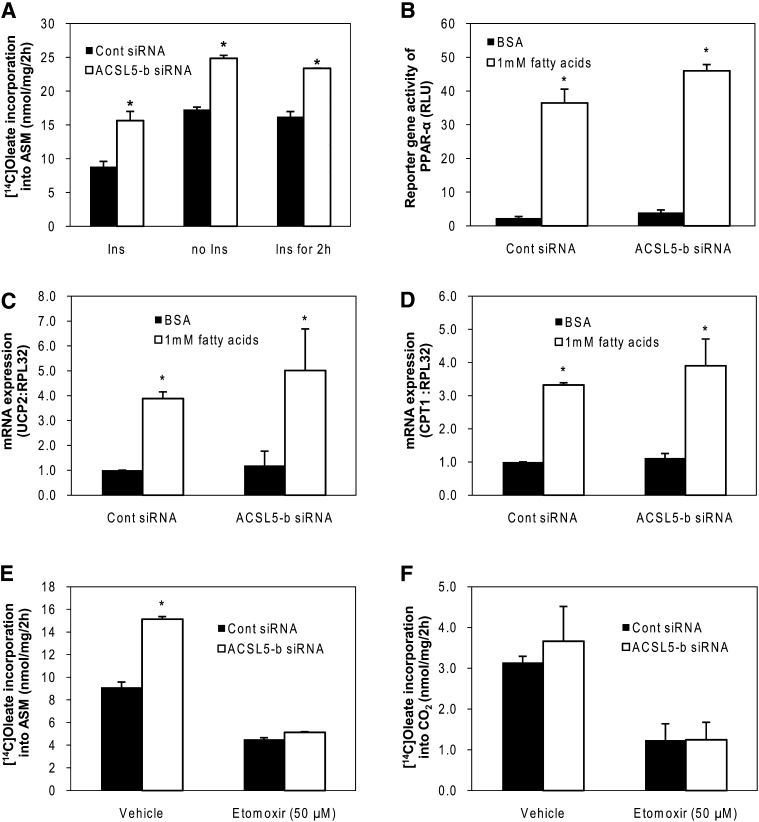
Because FA uptake and secretion were not affected by ACSL5 knockdown despite decreased lipid accumulation, we questioned whether ACSL5 knockdown increased FA oxidation. In the absence of insulin, FA channeling into β-oxidation was nearly doubled compared with constant insulin treatment (Fig. 6A). Knockdown of ACSL5 increased metabolism of [1-14C]oleic acid to ASM ∼35–45% independent of insulin concentrations (Fig. 6A). We next determined if changes in PPAR-α activity or the expression of its target genes were altered by ACSL5 knockdown. FAs (1000 μM) increased reporter gene activity of PPAR-α (10-fold) and expression of target genes such as CPT-1 (3-fold) and UCP-2 (4-fold). However, basal induction or FA-induced fold induction of PPAR-α reporter gene activity (Fig. 6B) and target gene expression in ACSL5 siRNA transfected cells were similar to control cells (Fig. 6C, D). These data indicate that increased channeling of FA to β-oxidation is not mediated by PPAR-α or the expression of genes involved in FA β-oxidation. Finally, we treated cells with Etomoxir (50 μM), an inhibitor of mitochondrial β-oxidation, to determine if increased oxidation of FAs was due to mitochondrial β-oxidation. Etomoxir treatment suppressed [1-14C]oleic acid incorporation to both ASM and CO2 production (Fig. 6E, F) in control cells. However, etomoxir blocked the increase in [1-14C]oleic metabolism to ASM in cells treated with ACSL5 siRNA (Fig. 6E), thus supporting a prominent role of mitochondrial β-oxidation in mediating the enhanced FA oxidation following ACSL5 knockdown. Also, ACSL5 knockdown did not alter CO2 production from [1-14C]oleic (Fig. 6F), suggesting that the increased β-oxidation of FAs, under our experimental conditions, does not lead to increased complete oxidation of acetyl-CoA through the TCA cycle.
Fig. 6.
ACSL5 knockdown increases β-oxidation without altering gene expression. Hepatocytes were treated with 1,000 μM oleic acid and labeled with 1 μCi [1-14C]oleic acid per 0.5 × 106 cells for 2 h, and the cells and medium were collected and extracted and (A) [1-14C]oleic acid incorporation into ASM were quantified as described in Materials and Methods. B: At 56 h after transfection with pSG5-GAL4-hPPAR-α expression plasmids and TK-MH-UAS-Luc reporter plasmids, cells were treated with 250 μM EPA and 750 μM oleic acids for 16 h and then lysed for reporter gene assays. C, D: Total RNA was isolated and PPAR-α target gene expression were quantified using quantitative RT-PCR and normalized to RPL-32. Cells were treated with 1,000 μM oleic acid and labeled with 1 μCi [1-14C]oleic acid after treated with Etomoxir (50 μM dissolved in H2O) for 3 h and then incorporation of [1-14C]oleic acid into ASM (E) and CO2 (F) was quantified. Data are indicated as mean ± SE from three individual experiments. * p < 0.05 when compared with controls.
DISCUSSION
The current study is the first to characterize the contribution of endogenous ACSL5 to hepatic lipid metabolism. ACSL5 siRNA decreased high fat-derived lipid accumulation, TAG content, and channeling of FAs into complex lipids, whereas channeling to oxidative pathways was enhanced. ACSL5 mediated FA channeling independent of changes in lipogenic or oxidative gene expression, suggesting that ACSL5 is a branch point in partitioning FAs, derived from either uptake or de novo synthesis, between opposing metabolic pathways in the liver.
ACSL5 is a 78 kDa intrinsic membrane protein that localizes to multiple intracellular locations and is expressed most abundantly in intestine, brown adipose tissue, and liver, all of which are tissues that have a high capacity for lipid synthesis (10, 22–25). Hence, the decrease in complex lipid synthesis by ACSL5 knockdown in this study is consistent with the distribution pattern of ACSL5 and our previous studies showing that overexpression of ACSL5 in hepatoma cells increases TAG synthesis (8, 25). The observed changes in lipid metabolism following ACSL5 knockdown largely mirror changes in metabolism following manipulation of GPAT1, a mitochondrial enzyme that catalyzes the initial step in glycerolipid synthesis (26, 27). Overexpression of GPAT1 in rat primary hepatocytes increases TAG and PL synthesis and decreases FA oxidation (26). Similarly, GPAT1 knockout mice have reduced hepatic TAG content and elevated hepatic acylcarnitines and serum ketone bodies (27). Similar to GPAT, manipulation of other enzymes involved in the TAG synthetic pathway, such as Lipin1 and DGAT2, also show that these enzymes contribute to partitioning of FAs between anabolic and catabolic pathways (28–31). Thus, these data are supportive of ACSL5 having a role in glycerolipid synthesis by supplying acyl-CoAs for the formation of the Kennedy Pathway intermediates. However, it remains unknown if ACSL5 supplies acyl-CoAs for all of the acyltransferases involved in glycerolipid synthesis or if FAs are selectively activated and channeled to specific acyltransferases.
The aforementioned enzymes involved in glycerolipid biosynthesis may also mediate their effects on lipid partitioning partially through changes in gene expression. For example, treatment of mice with DGAT2 antisense oligonucleotides increases hepatic oxidative genes and decreases lipogenic genes leading to decreased hepatic TAG content (29, 30). Lipin1 deactivation increases TAG content and decreases oxidative gene expression, whereas overexpression of Lipin1 increases oxidative gene expression and decreases lipogenic gene expression to promote decreased TAG content (28). Thus, unlike the enzymes involved in glycerolipid synthesis, ACSL5 appears to regulate channeling of FAs independent of changes in gene expression. However, similar to ACSL5, most oxidative and lipogenic genes are unaltered in livers of GPAT1 null mice (27). Thus, changes in specific intermediates in glycerolipid synthesis in response to manipulations of enzymes at various points in the pathway may contribute to these discrepancies in gene expression.
Intracellular FAs can be derived from numerous sources including the hydrolysis of intracellular lipids such as TAG, exogenous uptake, or de novo synthesis. Interestingly, knockdown of ACSL5 did not affect the turnover of TAG or PL, suggesting that it is not involved in reacylation processes. These data are in contrast to ACSL1 and FATP4, which have been shown to alter FA reacylation to TAG (5, 32). A recent study employing stable isotope methodologies in humans shows that FA enrichment of intramuscular acylcarnitines, an indicator of β-oxidation, more closely reflects enrichment of intramuscular TAG rather than plasma free FAs (33). These data suggest that a significant portion of FAs are initially channeled to TAG storage prior to their subsequent hydrolysis and oxidation. Moreover, if these data are extrapolated to the liver, they point toward two possible branch-points in FA channeling between anabolic pathways. The first regulatory point is the initial partitioning of FAs between TAG storage and β-oxidation involving ACSL5 and GPAT1. The second branch-point is the partitioning of FAs hydrolyzed from TAG between reacylation, VLDL synthesis, and β-oxidation pathways that likely involve DGAT and various lipases and lipid droplet proteins. Additionally, it should be noted that ACSL5 knockdown did not affect FA uptake although we previously reported that ACSL5 overexpression in hepatoma cells increased FA uptake (8). Although the exact reason for this discrepancy is unknown, ACSL5 knockdown may not limit FA activation as ACS activity was only modestly reduced, whereas overexpressing ACLS5 more than doubled ACS activity, which may have increased FA uptake due to enhanced rates of TAG synthesis.
In contrast to the current study, some reports speculate that ACSL5 has a role in promoting β-oxidation due to its partial location on the mitochondrial membrane (34, 35). For example, ACSL5 mRNA expression in cardiomyocytes is upregulated by the PPAR-α agonist fenofibrate (35) and muscle ACSL5 expression is correlated with weight loss in human subjects (34). However, ACSL5 may function differently depending upon the tissue, which has been noted for ACSL1. The expression of ACSL1 is increased in differentiating adipocytes (36) and heart specific ACSL1 transgenic mice showed increase in TAG in heart (37). In contrast, its expression is upregulated by PPAR-α (38) and liver specific ACSL1−/− mice have impaired hepatic β-oxidation (39), suggesting that the role of ACSL1 is perhaps tissue-dependent. Regulation and functional analysis of ACSL5 in different tissue are required to further define tissue-specific effects of ACSL5.
Imbalances in lipid metabolism such as the accumulation of hepatic glycerolipids and impaired β-oxidation are intimately involved in the pathogenesis of steatosis, insulin resistance, and dyslipidemia (40, 41). Additionally, lipid metabolites such as FAs, acyl-CoAs, DAG, ceramides, and phosphatidic acid act as signaling molecules that influence numerous biological processes and the development of metabolic diseases (7, 42). Thus, factors that regulate distinct pools of FAs, acyl-CoAs, or downstream metabolites may have a regulatory role in disease etiology and could serves as therapeutic targets for correcting alterations in energy metabolism. The current study indicates that hepatic ACSL5 acts as a branch-point in directing FAs into pathways of complex lipid synthesis and away from β-oxidation. Moreover, these data support a role for ACSL isoforms in channeling of intracellular lipids as a means to control energy metabolism.
Supplementary Material
Supplemental Data
Footnotes
Abbreviations:
ACC
acetyl-CoA carboyxlase, ACS, acyl-CoA synthetase
ACSL
long-chain acyl-CoA synthetase
CPT
carnitine palmitoyltransferase
ChREBP
carbohydrate responsive element binding protein
DAG
diacylglycerol
DGAT
diacylglycerol acyltransferase
FATP
fatty acid transport protein
GPAT
glycerol-3-phosphate acyltransferase
L-PK
liver pyruvate kinase
PL
phospholipid
PPAR
peroxisome proliferator-activated receptor
SCD
stearoyl-CoA desaturase
siRNA
small interference RNA
SREBP
sterol regulatory element binding protein
TAG
triacylglycerol
UCP
uncoupling protein
.
[S]
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure.
REFERENCES
- 1.Mashek D. G., Coleman R. A. 2006. Cellular fatty acid uptake: the contribution of metabolism. Curr. Opin. Lipidol. 17: 274–278. [DOI] [PubMed] [Google Scholar]
- 2.Mashek D. G., Li L. O., Coleman R. A. 2007. Long-chain acyl-CoA synthetases and fatty acid channeling. Future Lipidol. 2: 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mashek D. G., Li L. O., Coleman R. A. 2006. Rat long-chain acyl-CoA synthetase mRNA, protein, and activity vary in tissue distribution and in response to diet. J. Lipid Res. 47: 2004–2010. [DOI] [PubMed] [Google Scholar]
- 4.Watkins P. A. 2008. Very-long-chain acyl-CoA synthetases. J. Biol. Chem. 283: 1773–1777. [DOI] [PubMed] [Google Scholar]
- 5.Li L. O., Mashek D. G., An J., Doughman S. D., Newgard C. B., Coleman R. A. 2006. Overexpression of rat long chain acyl-CoA synthetase 1 alters fatty acid metabolism in rat primary hepatocytes. J. Biol. Chem. 281: 37246–37255. [DOI] [PubMed] [Google Scholar]
- 6.Yao H., Ye J. 2008. Long chain acyl-CoA synthetase 3-mediated phosphatidylcholine synthesis is required for assembly of very low density lipoproteins in human hepatoma Huh7 cells. J. Biol. Chem. 283: 849–854. [DOI] [PubMed] [Google Scholar]
- 7.Bu S. Y., Mashek M. T., Mashek D. G. 2009. Suppression of long chain acyl-CoA synthetase 3 decreases hepatic de novo fatty acid synthesis through decreased transcriptional activity. J. Biol. Chem. 284: 30474–30483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mashek D. G., McKenzie M. A., Van Horn C. G., Coleman R. A. 2006. Rat long chain acyl-CoA synthetase 5 increases fatty acid uptake and partitioning to cellular triacylglycerol in McArdle-RH7777 cells. J. Biol. Chem. 281: 945–950. [DOI] [PubMed] [Google Scholar]
- 9.Lewin T. M., Van Horn C. G., Krisans S. K., Coleman R. A. 2002. Rat liver acyl-CoA synthetase 4 is a peripheral-membrane protein located in two distinct subcellular organelles, peroxisomes, and mitochondrial-associated membrane. Arch. Biochem. Biophys. 404: 263–270. [DOI] [PubMed] [Google Scholar]
- 10.Oikawa E., Iijima H., Suzuki T., Sasano H., Sato H., Kamataki A., Nagura H., Kang M. J., Fujino T., Suzuki H., et al. 1998. A novel acyl-CoA synthetase, ACS5, expressed in intestinal epithelial cells and proliferating preadipocytes. J. Biochem. 124: 679–685. [DOI] [PubMed] [Google Scholar]
- 11.Yu X. X., Lewin D. A., Forrest W., Adams S. H. 2002. Cold elicits the simultaneous induction of fatty acid synthesis and beta-oxidation in murine brown adipose tissue: prediction from differential gene expression and confirmation in vivo. FASEB J. 16: 155–168. [DOI] [PubMed] [Google Scholar]
- 12.Achouri Y., Hegarty B. D., Allanic D., Becard D., Hainault I., Ferre P., Foufelle F. 2005. Long chain fatty acyl-CoA synthetase 5 expression is induced by insulin and glucose: involvement of sterol regulatory element-binding protein-1c. Biochimie. 87: 1149–1155. [DOI] [PubMed] [Google Scholar]
- 13.Liang C. P., Tall A. R. 2001. Transcriptional profiling reveals global defects in energy metabolism, lipoprotein, and bile acid synthesis and transport with reversal by leptin treatment in ob/ob mouse liver. J. Biol. Chem. 276: 49066–49076. [DOI] [PubMed] [Google Scholar]
- 14.Horton J. D., Shah N. A., Warrington J. A., Anderson N. N., Park S. W., Brown M. S., Goldstein J. L. 2003. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc. Natl. Acad. Sci. USA. 100: 12027–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoeckman A. K., Towle H. C. 2002. The role of SREBP-1c in nutritional regulation of lipogenic enzyme gene expression. J. Biol. Chem. 277: 27029–27035. [DOI] [PubMed] [Google Scholar]
- 16.Folch J., Lees M., Sloane Stanley G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 17.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 18.Lewin T. M., Wang S. L., Nagle C. A., Van Horn C. G., Coleman R. A. 2005. Mitochondrial glycerol-3-phosphate acyltransferase-1 directs the metabolic fate of exogenous fatty acids in hepatocytes. Am. J. Physiol. Endocrinol. Metab. 288: E835–E844. [DOI] [PubMed] [Google Scholar]
- 19.Chakravarthy M. V., Pan Z. J., Zhu Y. M., Tordjman K., Schneider J. G., Coleman T., Turk J., Semenkovich C. F. 2005. “New” hepatic fat activates PPAR alpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 1: 309–322. [DOI] [PubMed] [Google Scholar]
- 20.Pawar A., Jump D. B. 2003. Unsaturated fatty acid regulation of peroxisome proliferator-activated receptor alpha activity in rat primary hepatoctes. J. Biol. Chem. 278: 35931–35939. [DOI] [PubMed] [Google Scholar]
- 21.Sapiro J. M., Mashek M. T., Greenberg A. S., Mashek D. G. 2009. Hepatic triacylglycerol hydrolysis regulates peroxisome proliferator-activated receptor alpha activity. J. Lipid Res. 50: 1621–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewin T. M., de Jong H., Schwerbrock N. J. M., Hammond L. E., Watkins S. M., Combs T. P., Coleman R. A. 2008. Mice deficient in mitochondrial glycerol-3-phosphate acyltransferase-1 have diminished myocardial triacylglycerol accumulation during lipogenic diet and altered phospholipid fatty acid composition. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids. 1781: 352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone S. J., Levin M. C., Zhou P., Han J. Y., Walther T. C., Farese R. V. 2009. The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J. Biol. Chem. 284: 5352–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J. H., Lewin T. M., Coleman R. A. 2001. Expression and characterization of recombinant rat acyl-CoA synthetases 1, 4, and 5. Selective inhibition by triacsin C and thiazolidinediones. J. Biol. Chem. 276: 24667–24673. [DOI] [PubMed] [Google Scholar]
- 25.Lewin T. M., Kim J. H., Granger D. A., Vance J. E., Coleman R. A. 2001. Acyl-CoA synthetase isoforms 1, 4, and 5 are present in different subcellular membranes in rat liver and can be inhibited independently. J. Biol. Chem. 276: 24674–24679. [DOI] [PubMed] [Google Scholar]
- 26.Linden D., William-Olsson L., Rhedin M., Asztely A. K., Clapham J. C., Schreyer S. 2004. Overexpression of mitochondrial GPAT in rat hepatocytes leads to decreased fatty acid oxidation and increased glycerolipid biosynthesis. J. Lipid Res. 45: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 27.Hammond L. E., Neschen S., Romanelli A. J., Cline G. W., Ilkayeva O. R., Shulman G. I., Muoio D. M., Coleman R. A. 2005. Mitochondrial glycerol-3-phosphate acyltransferase-1 is essential in liver for the metabolism of excess acyl-CoAs. J. Biol. Chem. 280: 25629–25636. [DOI] [PubMed] [Google Scholar]
- 28.Finck B. N., Gropler M. C., Chen Z. J., Leone T. C., Croce M. A., Harris T. E., Lawrence J. C., Kelly D. P. 2006. Lipin 1 is an inducible amplifier of the hepatic PGC-1 alpha/PPAR alpha regulatory pathway. Cell Metab. 4: 199–210. [DOI] [PubMed] [Google Scholar]
- 29.Choi C. S., Savage D. B., Kulkarni A., Yu X. X., Liu Z. X., Morino K., Kim S., Distefano A., Samuel V. T., Neschen S., et al. 2007. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J. Biol. Chem. 282: 22678–22688. [DOI] [PubMed] [Google Scholar]
- 30.Yu X. X., Murray S. F., Pandey S. K., Booten S. L., Bao D. J., Song X. Z., Kelly S., Chen S. Y., Mckay R., Monia B. P., et al. 2005. Antisense oligonucleotide reduction of DGAT2 expression improves hepatic steatosis and hyperlipidemia in obese mice. Hepatology. 42: 362–371. [DOI] [PubMed] [Google Scholar]
- 31.Monetti M., Levin M. C., Watt M. J., Sajan M. P., Marmor S., Hubbard B. K., Stevens R. D., Bain J. R., Newgard C. B., Farese R. V., et al. 2007. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 6: 69–78. [DOI] [PubMed] [Google Scholar]
- 32.Lobo S., Wiczer B. M., Smith A. J., Hall A. M., Bernlohr D. A. 2007. Fatty acid metabolism in adipocytes: functional analysis of fatty acid transport proteins 1 and 4. J. Lipid Res. 48: 609–620. [DOI] [PubMed] [Google Scholar]
- 33.Kanaley J. A., Shadid S., Sheehan M. T., Guo Z., Jensen M. D. 2009. Relationship between plasma free fatty acid, intramyocellular triglycerides and long-chain acylcarnitines in resting humans. J. Physiol. 587: 5939–5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adamo K. B., Dent R., Langefeld C. D., Cox M., Williams K., Carrick K. M., Stuart J. S., Sundseth S. S., Harper M. E., McPherson R., et al. 2007. Peroxisome proliferator-activated receptor gamma 2 and acyl-CoA synthetase 5 polymorphisms influence diet response. Obesity (Silver Spring). 15: 1068–1075. [DOI] [PubMed] [Google Scholar]
- 35.Durgan D. J., Smith J. K., Hotze M. A., Egbejimi O., Cuthbert K. D., Zaha V. G., Dyck J. R. B., Abel E. D., Young M. E. 2006. Distinct transcriptional regulation of long-chain acyl-CoA synthetase isoforms and cytosolic thioesterase 1 in the rodent heart by fatty acids and insulin. Am. J. Physiol. Heart Circ. Physiol. 290: H2480–H2497. [DOI] [PubMed] [Google Scholar]
- 36.Marszalek J. R., Kitidis C., Dararutana A., Lodish H. F. 2004. Acyl-CoA synthetase 2 overexpression enhances fatty acid internalization and neurite outgrowth. J. Biol. Chem. 279: 23882–23891. [DOI] [PubMed] [Google Scholar]
- 37.Chiu H. C., Kovacs A., Ford D. A., Hsu F. F., Garcia R., Herrero P., Saffitz J. E., Schaffer J. E. 2001. A novel mouse model of lipotoxic cardiomyopathy. J. Clin. Invest. 107: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frederiksen K. S., Wulff E. M., Sauerberg P., Mogensen J. P., Jeppesen L., Fleckner J. 2004. Prediction of PPAR-alpha ligand-mediated physiological changes using gene expression profiles. J. Lipid Res. 45: 592–601. [DOI] [PubMed] [Google Scholar]
- 39.Li L. O., Ellis J. M., Paich H. A., Wang S. L., Gong N., Altshuller G., Thresher R. J., Koves T. R., Watkins S. M., Muoio D. M., et al. 2009. Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. J. Biol. Chem. 284: 27816–27826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diraison F., Dusserre E., Vidal H., Sothier M., Beylot M. 2002. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am. J. Physiol. Endocrinol. Metab. 282: E46–E51. [DOI] [PubMed] [Google Scholar]
- 41.Vedala A., Wang W., Neese R. A., Christiansen M. P., Hellerstein M. K. 2006. Delayed secretory pathway contributions to VLDL-triglycerides from plasma NEFA, diet, and de novo lipogenesis in humans. J. Lipid Res. 47: 2562–2574. [DOI] [PubMed] [Google Scholar]
- 42.Hoy A. J., Brandon A. E., Turner N., Watt M. J., Bruce C. R., Cooney G. J., Kraegen E. W. 2009. Lipid and insulin infusion-induced skeletal muscle insulin resistance is likely due to metabolic feedback and not changes in IRS-1, Akt, or AS160 phosphorylation. Am. J. Physiol. Endocrinol. Metab. 297: E67–E75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data