The Role of Calcium/Calmodulin-Activated Calcineurin in Rapid and Slow Endocytosis at Central Synapses (original) (raw)
Abstract
Although the calcium/calmodulin-activated phosphatase calcineurin may dephosphorylate many endocytic proteins, it is not considered a key molecule in mediating the major forms of endocytosis at synapses—slow, clathrin-dependent and the rapid, clathrin-independent endocytosis. Here we studied the role of calcineurin in endocytosis by reducing calcium influx, inhibiting calmodulin with pharmacological blockers and knockdown of calmodulin, and by inhibiting calcineurin with pharmacological blockers and knock-out of calcineurin. These manipulations significantly inhibited both rapid and slow endocytosis at the large calyx-type synapse in 7- to 10-d-old rats and mice, and slow, clathrin-dependent endocytosis at the conventional cultured hippocampal synapse of rats and mice. These results suggest that calcium influx during nerve firing activates calcium/calmodulin-dependent calcineurin, which controls the speed of both rapid and slow endocytosis at synapses by dephosphorylating endocytic proteins. The calcium/calmodulin/calcineurin signaling pathway may underlie regulation of endocytosis by nerve activity and calcium as reported at many synapses over the last several decades.
Introduction
The calcium/calmodulin-dependent phosphatase calcineurin, found widely in the nervous system (Rusnak and Mertz, 2000), may dephosphorylate many endocytosis proteins, such as dynamin, synaptojanin, the adaptor protein AP180, and phosphatidylinositol phosphate kinase type Iγ (Clayton et al., 2007). This raises the possibility that calcineurin may mediate the calcium-dependent regulation of endocytosis (Cousin and Robinson, 2001), as observed at many synapses (Royle and Lagnado, 2003; Wu, 2004). Based on measurements of the FM dye release in the synaptosome preparation, an early study implicated the involvement of calcineurin in endocytosis during extremely intense stimulation, depolarization for hundreds of seconds (Marks and McMahon, 1998). Consistent with this implication, calcineurin is considered to be involved only in bulk endocytosis during very intense stimuli, but not in slow, clathrin-dependent endocytosis during milder stimuli at cerebellar synapses (Evans and Cousin, 2007; Clayton and Cousin, 2009; Clayton et al., 2009). Slow endocytosis at a calyx-type nerve terminal is triggered by >10 μm calcium (Hosoi et al., 2009; X. S. Wu et al., 2009), which is much higher than the affinity of calcineurin to calcium (∼1–1.5 μm). This result also argues against the involvement of calcineurin in slow endocytosis during milder stimuli (Hosoi et al., 2009). Rapid endocytosis, which is presumably clathrin-independent (Artalejo et al., 1995; Jockusch et al., 2005), is another form of endocytosis often observed at synapses (L. G. Wu et al., 2007). Likely because of its fast speed, calcineurin-mediated dephosphorylation is not considered to be involved in this process. In summary, while calcineurin may dephosphorylate endocytic proteins, there has been no molecular and biophysical evidence showing the involvement of calcineurin in rapid and slow endocytosis, two major forms of endocytosis observed in near physiological stimuli at synapses (Royle and Lagnado, 2003; L. G. Wu et al., 2007).
Recent studies at giant calyx-type synapses suggest that calcium influx triggers and regulates rapid and slow endocytosis (Hosoi et al., 2009; X. S. Wu et al., 2009). The calcium binding protein calmodulin was implied as the calcium receptor, because its blockers significantly inhibited rapid and slow endocytosis (X. S. Wu et al., 2009). However, three main issues had remained unresolved. First, pharmacological blockers may not be specific to calmodulin. Direct molecular biological evidence supporting calmodulin as the calcium sensor for endocytosis is missing, likely because calmodulin is encoded by three dispersed genes in vertebrates, making it difficult to use genetic approaches. Second, if calcium/calmodulin initiates rapid and slow endocytosis, its downstream target is unclear. Although calcineurin has been discussed as a downstream target for a long time, evidence supporting its role in rapid and slow endocytosis is missing. Third, it is unclear whether the findings obtained at giant synapses apply to the majority of synapses, the small conventional synapses. We addressed these three issues by combining quantitative measurements of endocytosis, pharmacological tools, and genetic approaches at both giant calyx-type and small cultured hippocampal synapses. We found that block of the calcium/calmodulin/calcineurin signaling pathway significantly inhibited both rapid and slow endocytosis, which calls for modification of the current endocytosis model to include calcineurin as a key player.
Materials and Methods
Slice preparation, capacitance recordings, and solutions.
Parasagittal brainstem slices (200 μm thick) containing the medial nucleus of the trapezoid body were prepared from 7- to 10-d-old male or female Wistar rats or mice using a vibratome (X. S. Wu et al., 2009). Whole-cell capacitance measurements were made with the EPC-9 amplifier together with the software lock-in amplifier (PULSE, HEKA) that implements Lindau-Neher's technique (Sun and Wu, 2001; Sun et al., 2004). The frequency of the sinusoidal stimulus was 1000 Hz and the peak-to-peak voltage of the sine wave was ≤60 mV. We pharmacologically isolated presynaptic Ca2+ currents with a bath solution (∼22−24°C) containing the following (in mm): 105 NaCl, 20 TEA-Cl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 25 NaHCO3, 1.25 NaH2PO4, 25 glucose, 0.4 ascorbic acid, 3 _myo_-inositol, 2 sodium pyruvate, 0.001 tetrodotoxin (TTX), 0.1 3,4-diaminopyridine, pH 7.4 when bubbled with 95% O2 and 5% CO2. The presynaptic pipette contained the following (in mm): 125 Cs-gluconate, 20 CsCl, 4 MgATP, 10 Na2-phosphocreatine, 0.3 GTP, 10 HEPES, 0.05 BAPTA, pH 7.2, adjusted with CsOH. Since DMSO (0.1%) was used to dissolve CsA (Sigma) in the pipette solution, the control solution for this drug also contained 0.1% DMSO (Fig. 1A,D). CaN457-482 and scrambled CaN457-482 were purchased from Calbiochem and GenScript USA Inc, respectively.
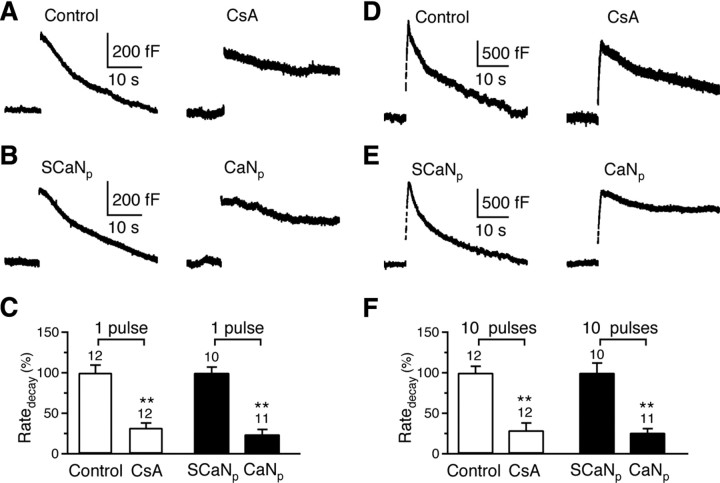
Figure 1.
Calcineurin blockers inhibit rapid and slow endocytosis at the calyx. A, Two sampled capacitance (_C_m) traces showing endocytosis induced by a 20 ms depolarization with a pipette containing a control solution (with 0.1% DMSO) or 20 μm CsA (with 0.1% DMSO). Traces in Figures 1–3 are mostly individual traces and occasionally an average of 2–3 traces. Data were obtained from rat calyces in Figures 1 and 2. B, Two sampled _C_m traces showing endocytosis induced by a 20 ms depolarization with a pipette containing scrambled CaN457-482 (SCaNp, 150 μm, serves as control) or CaN457-482 (CaNp, 150 μm). C, Comparison of the Ratedecay after a 20 ms depolarization in the absence (control) and the presence of CsA (20 μm), and in the presence of SCaNp (150 μm) or CaNp (150 μm). The number of calyces tested are labeled (applies to F). **p < 0.01 (applies to Figs. 1–3). The data for CsA and control group (open bars) were normalized to the mean value of the control group, whereas the data for CaNp and SCaNp group (solid bars) were normalized to the mean value of the SCaNp group (applies to Figs. 1–3). Note that this panel aims at showing the inhibitory effect of CsA and CaNp compared with their corresponding control. Because of the method of normalizing the data described above, a comparison between the control and the SCaNp group is not meaningful (applies to Fig. 1_F_ and Fig. 2_C_). Data are expressed as mean ± SE (applies to all figures). **_D–F_**, Similar to **_A–C_**, respectively, except that the stimulus was 10 pulses of 20 ms depolarization at 10 Hz, which induced a Ratedecay with >80% caused by rapid endocytosis in control.
Hippocampal cultures and fluorescence imaging.
Hippocampal cultures, stimulation and fluorescence imaging were similar to those described previously (Sankaranarayanan and Ryan, 2000). Hippocampal CA1–CA3 regions from postnatal day 0 (P0)–P2 Sprague Dawley rats (if not mentioned) or P0 mice were dissected, dissociated, and plated on Matrigel-coated glass coverslips (BD Biosciences). Cells were maintained at 37°C in a 5% CO2 humidified incubator with a culture media consisting of MEM (Invitrogen), 0.5% glucose, 0.1 g/L bovine transferrin (Calbiochem), 0.3 g/L glutamine, 10% fetal bovine serum (Invitrogen), 2% B-27 (Invitrogen), and 3 μm cytosine β-d-arabinofuranoside (Sigma). Six to 8 d after plating, calcium-phosphate-mediated gene transfer was used to transfect cultures with synaptopHluorin (SpH, kindly provided by Dr. G. Miesenböck, University of Oxford, Oxford, UK), calmodulin shRNA plasmid, or calmodulin shRNA-resistant plasmid. After transfection, cultures were maintained at 37°C in a 5% CO2 humidified incubator for another 6–8 d before use. Unless otherwise indicated, all chemicals were obtained from Sigma.
Coverslips were mounted in a stimulation chamber (RC-21BRFS chamber, Warner Instruments) 6–8 d after transfection. The action potential was evoked by passing a 1 ms current pulse of 20 mA via platinum electrodes in the chamber. The bath solution (∼22−24°C) contained the following (in mm): 119 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 25 HEPES (buffered to pH 7.4), 30 glucose, 0.01 6-cyano-7-nitroquinoxaline-2, 3-dione (CNQX) and 0.05 _d_-l-2-amino-5-phosphonovaleric acid (AP-5). When lowering the CaCl2 concentration, MgCl2 was increased to keep the divalent ion concentration constant.
SpH images were acquired at 1 Hz using the Zeiss LSM 510 META confocal microscope with a 40×, 1.3 numerical aperture oil-immersion objective. Images were analyzed using Zeiss LSM510 software. All functionally visible varicosities were selected for analysis by testing their responsiveness to stimulation. The fluorescence intensity within a region of at least 1.5 μm × 1.5 μm were averaged together for each bouton, which avoided fluorescence decay caused by faster diffusive processes (Granseth et al., 2006). Each group of data was obtained from at least 3 different batches of cultures.
Calmodulin knockdown, immunostaining, and Western blot.
Calmodulin shRNA and calmodulin shRNA-resistant plasmids are described recently (Pang et al., 2010). We made only one modification, i.e., the GFP was cutoff from these plasmids to avoid the fluorescence conflict with cotransfected SpH. Both plasmids include two RNA-polymerase III promoters (human H1 and human U6) in tandem and the Ubiquitin C promoter downstream of U6 promoter. For the calmodulin shRNA plasmid, a short-hairpin sequence targeting a common sequence found in the calmodulin 1 and calmodulin 2 mRNAs (CTGACTGAAGAGCAGATTGC; full shRNA sequence: TCGACCCCTGACTGAAGAGCAGATTGCTTCAAGAGAGCAATCTGCTCTTCAGTCAGTTTTTGGAAAT) was inserted into the downstream of the H1 promoter. A second short-hairpin sequence targeting the calmodulin 3 mRNA (sequence: CGCGCCCACGGAGCTGCAGGACATGATTATTCAAGAGATAATCATGTCCTGCAGCTCCGTTTTTTGGAAA) was inserted into the downstream of the U6 promoter.
The calmodulin shRNA-resistant plasmid includes not only the two short-hairpin sequences described above to knockdown calmodulin, but also a mutant calmodulin sequence to rescue calmodulin expression. The BamHI-EcoRI sites downstream of ubiquitin C promoter are for the insertion of rescue calmodulin cDNA. The targeted sequences in the rescue calmodulin cDNA were mutated to TTAACGGAAGAACAAATCGC and CAGAACTTCAAGATATGATCA to create a maximum difference between the shRNAs and rescue cDNA without changing the calmodulin protein sequence.
For immunostaining, neurons were fixed with 4% paraformaldehyde, permeabilized with 0.2% Triton X-100, and subsequently incubated with the primary and secondary antibodies. The antisera were diluted in PBS with 2% bovine serum albumin and incubated with cells overnight at 4°C. After several rinses in PBS, cells were incubated with fluorescence-conjugated donkey anti-rabbit IgG (1:100) and rhodamine-conjugated donkey anti-mouse or donkey anti-goat IgG (1:100, Jackson ImmunoResearch Laboratories) for 30 min at 37°C. The following antibodies were used for immunocytochemistry: polyclonal rabbit anti-GFP (1:1000, Invitrogen), and monoclonal mouse anti-calmodulin (1:500, Santa Cruz Biotechnology Inc.). Calmodulin expression level was measured at cell bodies and compared with the fluorescence intensity in un-transfected (SpH-negative) neurons. Although calmodulin was also found in neuronal branches, the immunostaining signal was weak and difficult to quantify. Thus, we did not quantify calmodulin level in neuronal branches.
For Western blot of PC12 cells, cells were washed three times with ice-cold PBS. Cell lysates were prepared in the modified RIPA buffer including protease inhibitors. Equal protein amounts were analyzed by SDS-PAGE and immunoblotting using antibodies against calmodulin (1: 1000, Santa Cruz Biotechnology Inc.) and actin (used as an internal control, 1: 10,000, Millipore Bioscience Research Reagents).
For brain tissue Western blot, dissociated hippocampal CA1-CA3 regions of 9-d-old mice were homogenized in the ice-cold, modified RIPA buffer, which included protease inhibitors. The homogenates were centrifuged at 13,000 rpm at 4°C for 20 min. The supernatants were loaded to SDS-PAGE for immunoblotting using antibodies against calcineurin Aα subunit (1:200), calcineurin Aβ subunit (1:1000, Santa Cruz Biotechnology Inc.), and actin (1:10,000).
Calcineurin knock-out.
Calcineurin Aα+/− and Aβ+/− mice were provided by Dr. J. L. Gooch (Emory University School of Medicine, Atlanta, GA) (Zhang et al., 1996) and J. D. Molkentin (Bueno et al., 2002), respectively. Calcineurin Aα−/− and Aβ−/− mice were obtained by heterozygous breeding using standard mouse husbandry procedures. Mouse genotypes were determined by PCR with primers described previously (Gooch et al., 2004).
Data analysis.
The statistical test was t test. Means are presented as ± SE. For capacitance measurements, the Ratedecay was measured as the rate of decay in the first 2–10 s after stimulation. When endocytosis was inhibited, the Ratedecay was measured as the mean decay rate within 10–30 s after stimulation, because the capacitance decay was approximately linear within this time window. For SpH signal, the Ratedecay was measured as the decay rate in the first 4–10 s after stimulation. When endocytosis was inhibited, the Ratedecay was measured from the first 10–30 s after stimulation.
Results
The role of calcineurin in rapid and slow endocytosis at calyces
The whole-cell capacitance was measured at the calyx in 7- to 10-d-old rats. We induced slow and rapid endocytosis with 1 and 10 pulses of 20 ms depolarization (from −80 to +10 mV, if not mentioned) at 10 Hz, respectively (W. Wu et al., 2005; X. S. Wu et al., 2009). In control, at 4–10 min after whole-cell break in (0.1% DMSO in pipette), a 20 ms depolarization induced a capacitance jump (Δ_C_m) of 462 ± 31 fF (n = 12), followed by a mono-exponential decay with a time constant (τ) of 18.6 ± 1.0 s (n = 12) and an initial endocytosis rate (Ratedecay) of 28 ± 3 fF/s (n = 12, Fig. 1A). Ten depolarizing pulses of 20 ms at 10 Hz induced a Δ_C_m of 1669 ± 109 fF (n = 12), followed by a biexponential decay with τ of 2.8 ± 0.3 s (44 ± 4%) and 23.0 ± 2.1 s (n = 12, Fig. 1D), respectively. The Ratedecay after 10 depolarizing pulses was 270 ± 20 fF/s (n = 12, Fig. 1D), which reflected mostly (>80%) the rapid component of endocytosis as demonstrated previously (W. Wu et al., 2005; X. S. Wu et al., 2009). This was confirmed in the present study, because the mean Ratedecay of the rapid component of endocytosis was ∼262 fF/s, as calculated from the ratio between its mean amplitude and mean time constant (1669 fF * 0.44/2.8 s = 262 fF/s), whereas the mean Ratedecay of the slow component of endocytosis was only ∼41 fF/s (=1669 fF * 0.56/23 s). In brief, these control experimental results were similar to previous reports (W. Wu et al., 2005; X. S. Wu et al., 2009).
We have previously shown that calcium influx triggers endocytosis and calmodulin blockers inhibited endocytosis (X. S. Wu et al., 2009). Consistent with this finding, replacing the extracellular calcium with barium, which barely activates calmodulin, also significantly inhibited endocytosis after 10 pulses of 20 ms depolarization at 10 Hz (n = 5, data not shown). To determine whether the calcium/calmodulin-activated calcineurin is involved in endocytosis, we measured endocytosis at 4–10 min after whole-cell break in with a pipette containing the calcineurin inhibitor cyclosporine A (CsA, 20 μm) or calcineurin auto-inhibitory peptide (CaN457-482, 150 μm) (Oliveria et al., 2007). We found that CsA and CaN457-482, reduced the Ratedecay after 1 or 10 depolarizing pulses to only ∼24–32% of control (Fig. 1A,B,D,E, summarized in Fig. 1C,F). We did not quantify the time constant, because we often did not observe any fast component of endocytosis, and slow endocytosis was often nearly blocked completely, which made quantification of the time constant impossible. Thus, throughout the study, we did not measure the time constant when endocytosis was inhibited.
Since the Ratedecay after a 20 ms depolarization reflected slow endocytosis, whereas >80% of the Ratedecay after the 10 pulse train was due to the rapid component of endocytosis, both calcineurin blockers significantly inhibited both slow and rapid endocytosis. The inhibition was not due to changes in calcium currents or exocytosis, because calcium currents did not change significantly, and Δ_C_m changed by <20% (supplemental Information 1, available at www.jneurosci.org as supplemental material). These results suggest the involvement of calcineurin in both rapid and slow endocytosis.
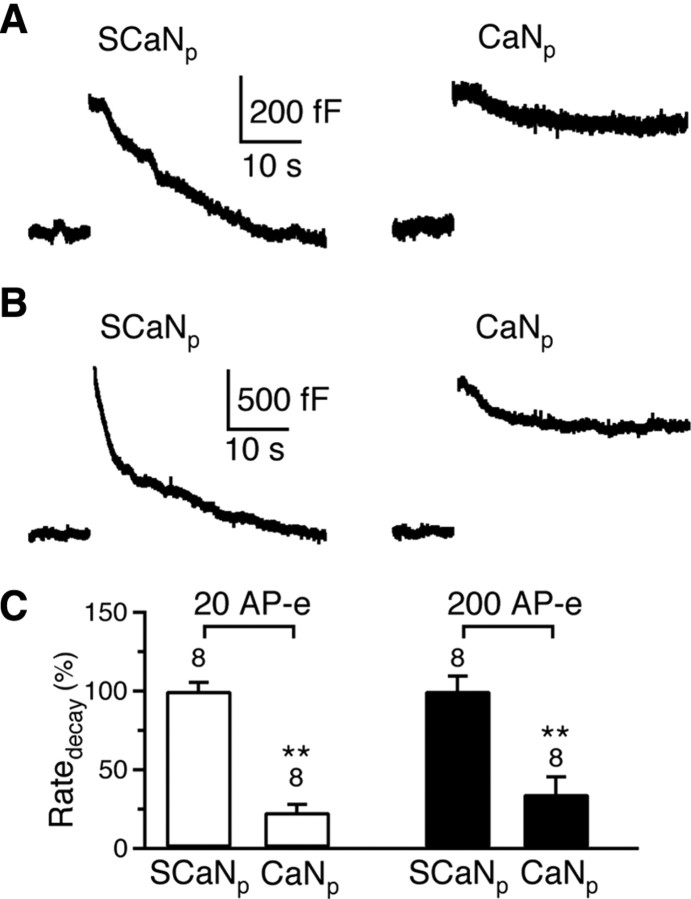
Rapid and slow endocytosis can be induced not only by depolarizing pulses of 20 ms, but also by trains of 1 ms depolarization that mimic action potential trains (Sun et al., 2002; W. Wu et al., 2005; X. S. Wu et al., 2009). For example, in the control condition with scrambled CaN457-482 (150 μm) in the pipette, 20 pulses of 1 ms depolarization to +7 mV at 200 Hz (AP-e), which mimicked a train of action potentials (Sun et al., 2002), induced a capacitance jump of 421 ± 16 fF (n = 8), followed by a mono-exponential decay with a time constant of 17.3 ± 1.2 s (n = 8) and a Ratedecay of 30 ± 1.6 fF/s (n = 8, Fig. 2A). After 200 AP-e at 200 Hz, the capacitance jump was 1331 ± 85 fF (n = 8), followed by a biexponential decay with time constants of 2.3 ± 0.3 s (46 ± 3%, n = 8) and 18.4 ± 1.6 s (n = 8), respectively (Fig. 2B). The Ratedecay after 200 AP-e was 252 ± 23 fF/s (n = 8, Fig. 2B). Thus, slow and rapid endocytosis induced by 20 and 200 AP-e at 200 Hz were similar to those induced by 1 and 10 pulses of 20 ms depolarization at 10 Hz, respectively. Compared with the Ratedecay in the presence of scrambled CaN457-482, CaN457-482 (150 μm in the pipette) significantly inhibited the Ratedecay to 28 ± 5% (n = 8, Fig. 2A,C) after 20 AP-e at 200 Hz, and to 34 ± 11% (n = 8, Fig. 2B,C) after 200 AP-e at 200 Hz (p < 0.01). These results suggest that calcineurin blockers inhibit endocytosis not only after trains of 20 ms depolarization, but also after trains of 1 ms depolarization that mimic action potential trains.
Figure 2.
CaN457-482 inhibits rapid and slow endocytosis induced by AP-e trains at the calyx. A, Two sampled _C_m traces showing endocytosis induced by 20 AP-e at 200 Hz with a pipette containing either scrambled CaN457-482 (SCaNp, 150 μm) or CaN457-482 (CaNp, 150 μm). B, Similar to A, except that the stimulus was 200 AP-e at 200 Hz, which induced both rapid and slow endocytosis in control (SCaNp). C, Comparison of the Ratedecay in the presence of SCaNp (150 μm) or CaNp (150 μm). The stimulus was either 20 (open bars) or 200 (solid bars) AP-e at 200 Hz. Data for open and solid bars were normalized to the mean value of open and solid SCaNp group, respectively.
The calcineurin blocker specificity is often a concern that might discount the significance of pharmacological experiments. To address this issue, we used 7- to 10-d-old mice lacking calcineurin Aα or Aβ subunit. Calcineurin is composed of a catalytic A and a regulatory B subunit. Among three isoforms of the A subunit, Aα and Aβ are expressed in the brain (Rusnak and Mertz, 2000). Aα−/− or Aβ−/− mice had been generated (Zhang et al., 1996; Bueno et al., 2002), from which we could not generate double knock-out mice (Aα−/− Aβ−/−), likely because they die in the embryonic stage as observed in calcineurin B knock-out (Chang et al., 2004).
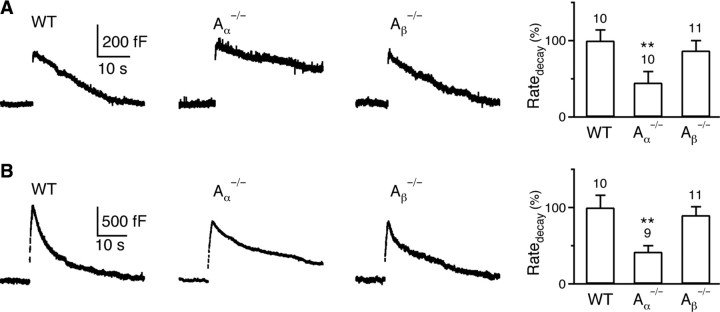
In wild-type (WT) mice, the Ratedecay was 28 ± 4 fF/s (n = 10, Fig. 3A) and 157 ± 26 fF/s (n = 10, Fig. 3B) after 1 and 10 depolarizing pulses at 10 Hz, respectively. Similar to rat calyces (X. S. Wu et al., 2009), >80% of the Ratedecay after the 10 pulse train was due to rapid endocytosis. Compared with WT mice, the Ratedecay after 1 (Fig. 3A) or 10 depolarizing pulses (Fig. 3B) was reduced by >50% in Aα−/− mice (p < 0.01), but did not change significantly in Aβ−/− mice (_p_ > 0.5). The Ratedecay reduction in Aα−/− mice was not due to changes in calcium currents or Δ_C_m (supplemental Information 2, available at www.jneurosci.org as supplemental material). Thus, calcineurin Aα, but not Aβ subunit, is involved in mediating both rapid and slow endocytosis at calyces.
Figure 3.
Knock-out of calcineurin Aα subunit inhibits rapid and slow endocytosis at the calyx. A, Left, Sampled _C_m induced by a 20 ms depolarization in a WT mouse, a calcineurin Aα−/− mouse and an Aβ−/− mouse. Right, The Ratedecay after a 20 ms depolarization in WT mice (10 calyces from 6 mice), Aα−/− mice (10 calyces from 8 mice) and Aβ−/− mice (11 calyces from 6 mice). B, Similar to A, except that the stimulus was 10 pulses of 20 ms depolarization at 10 Hz.
Endocytosis at hippocampal synapses
The calyx-type synapse is much larger than the conventional synapse. Whether our findings at calyces apply to conventional synapses is unclear. We addressed this issue at cultured hippocampal synapses by examining the roles of calcium, calmodulin, and calcineurin. SynaptopHluorin (SpH) was transfected to cultured rat hippocampal synapses (Sankaranarayanan and Ryan, 2000). Field electrical stimulation (20 mA, 1 ms) was applied to induce action potentials. In control, a 20 Hz stimulation train for 10 s (Train10 s) caused exocytosis and thus a fluorescence increase (Δ_F_peak) of 35 ± 5% of the baseline intensity (n = 7 experiments, each experiment contained ∼10–30 boutons, Fig. 4A, left). The fluorescence increase was followed by a mono-exponential decay, because of SpH endocytosis and vesicle reacidification. The decay reflects mostly endocytosis, because endocytosis usually takes much longer than 10 s, whereas reacidification takes only 3–4 s (Atluri and Ryan, 2006; Granseth et al., 2006). The rate of the initial fluorescence decay (Ratedecay) was 1.06 ± 0.18%/s (n = 7, fluorescence intensity normalized to baseline). The decay τ was 41.9 ± 2.4 s (n = 7, Fig. 4A, left). The fluorescence increase at 100 s after stimulation (Δ_F_100 s) was −1 ± 11% (n = 7) of Δ_F_peak, indicating completed endocytosis (Fig. 4A, left). Compared with Train10 s, a 20 Hz train for 2 s (Train2 s) induced a smaller Δ_F_peak (16 ± 5% of the baseline), a smaller decay τ (20.9 ± 2.1 s), but only a slightly smaller Ratedecay (0.86 ± 0.09%/s), and a similar Δ_F_100 s (−6 ± 7% of Δ_F_peak, n = 6, Fig. 4A, right).
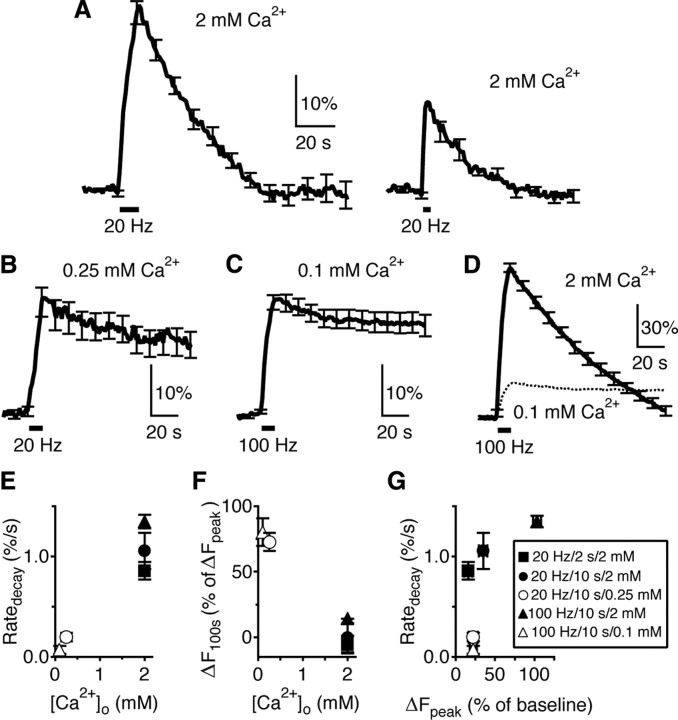
Figure 4.
Decrease of the [Ca2+]o nearly abolishes endocytosis at hippocampal synapses. A, The SpH signal induced by Train10 s (n = 7 experiments, left) or Train2 s (n = 6, right) at 2 mm [Ca2+]o. Data were plotted as mean ± SE. The SE was plotted every 10 s (applies to Figs. 4–7). B, The SpH signal induced by Train10 s at 0.25 mm [Ca2+]o (n = 4). C, The SpH signal induced by a 100 Hz train for 10 s at 0.1 mm [Ca2+]o (n = 5). D, The SpH signal induced by a 100 Hz train for 10 s at 2 mm [Ca2+]o (n = 4). The trace in C (mean only) was also plotted (dotted) for comparison. E, F, Ratedecay (normalized to the baseline intensity, E) and Δ_F_100 s (F) induced by stimuli listed in A–D are plotted versus the [Ca2+]o. Symbols in G apply to E–G. G, Ratedecay induced by stimuli listed in A–D is plotted versus the Δ_F_peak (normalized to the baseline).
The role of calcium at hippocampal synapses
An early study showed that decreasing the extracellular calcium concentration ([Ca2+]o) to 0.75 mm or applying the calcium buffer EGTA-AM reduced the Ratedecay by severalfold (Sankaranarayanan and Ryan, 2001). Given that the [Ca2+]o did not affect vesicle reacidification, it was concluded that calcium influx regulates endocytosis. If calcium influx not only regulates endocytosis, but also initiates endocytosis, further reducing the [Ca2+]o should nearly abolish endocytosis as has been shown at calyces (Hosoi et al., 2009; X. S. Wu et al., 2009). Indeed, at 0.25 mm [Ca2+]o, Train10 s induced a Ratedecay (0.20 ± 0.04%/s, n = 4) much smaller than that at 2 mm [Ca2+]o by Train10 s or Train2 s (p < 0.01), and induced a Δ_F_100 s as large as 73 ± 7% (n = 4) of Δ_F_peak (Fig. 4B). At 0.1 mm [Ca2+]o, Train10 s could not induce a detectable Δ_F_peak. However, a 10 s train at 100 Hz induced a Δ_F_peak (22 ± 5%) between those induced by Train2 s and Train10 s at 2 mm [Ca2+]o, but a Ratedecay (0.07 ± 0.04%/s, Fig. 4C) 12- to 14-fold smaller than that induced by Train2 s or Train10 s at 2 mm [Ca2+]o, and a Δ_F_100 s as large as 80 ± 10% of Δ_F_peak (n = 5). At 2 mm [Ca2+]o, this 100 Hz train induced a much larger Δ_F_peak (104 ± 5%), a Ratedecay (1.35 ± 0.06%/s) ∼20 times higher than that at 0.1 mm [Ca2+]o, and a much smaller Δ_F_100 s (14 ± 3% of Δ_F_peak, n = 4, Fig. 4D). Clearly, decreasing the [Ca2+]o from 2 to 0.1 mm reduced the Ratedecay to nearly 0 (Fig. 4E), and significantly increased Δ_F_100 s (Fig. 4F). These results suggest an essential role of calcium in controlling the rate of endocytosis, similar to results observed at the calyx of Held (Hosoi et al., 2009; X. S. Wu et al., 2009).
At 2 mm [Ca2+]o, as the Δ_F_peak increased to ∼16% (induced by Train2 s), the Ratedecay increased to ∼0.86%/s (Fig. 4G, solid square). Further increasing the Δ_F_peak to ∼104% (induced by the 100 Hz train), which was ∼6.5-fold larger than that (16%) induced by Train2 s, only increased the Ratedecay to 1.35%/s (Fig. 4G, solid triangle). Thus, the endocytosis capacity may be partially saturated at a Δ_F_peak of ≥16% (Sankaranarayanan and Ryan, 2001; Balaji et al., 2008). The increase in the Ratedecay might be due to an increase of the Δ_F_peak and/or an increase of the frequency of stimulation. However, the decrease of the Ratedecay at 0.1–0.25 mm [Ca2+]o was independent of either of these changes (Fig. 4G, comparing open and solid symbols). In particular, the Ratedecay at 0.1–0.25 mm [Ca2+]o (Fig. 4G, open symbols) was much smaller than that at 2 mm [Ca2+]o at similar Δ_F_peak values (Fig. 4G, solid square and circle). These results suggest that the reduced calcium influx at low [Ca2+]o, but not the change in the amount of exocytosis, decreased the Ratedecay.
The role of calmodulin at hippocampal synapses
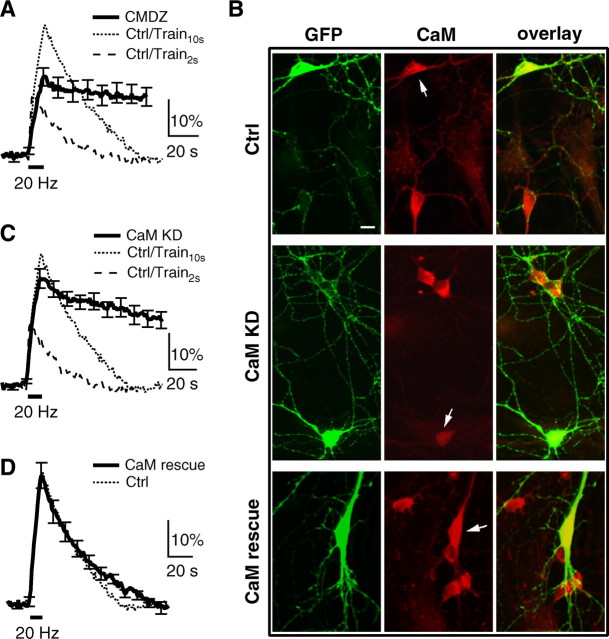
In the presence of a calmodulin blocker, calmidazolium (CMDZ, 10 μm in the bath, 5–10 min), the Ratedecay after Train10 s (0.28 ± 0.09%/s, n = 7) was much smaller than that (0.86–1.06%/s) after Train10 s or Train2 s in control (p < 0.01), and the Δ_F_100 s (79 ± 14% of Δ_F_peak, n = 7) was much larger (Fig. 5A). The block of the SpH fluorescence decay was not due to inhibition of vesicle reacidification (supplemental Information 3, available at www.jneurosci.org as supplemental material). Thus, CMDZ inhibits endocytosis at hippocampal synapses.
Figure 5.
The role of calmodulin in slow endocytosis at hippocampal synapses. A, The SpH signal induced by Train10 s in the presence of 10 μm calmidazolium (CMDZ, 5–10 min, bath application). For comparison, the mean SpH signal induced by Train10 s (dotted) and Train2 s (dash) in control are also shown. B, Staining of an antibody against green fluorescence protein (GFP), which also recognized SpH (left, green), and an antibody against calmodulin (middle, red) at hippocampal cultures transfected with SpH along (upper, Ctrl), SpH and calmodulin shRNA (middle, CaM KD), or SpH and a plasmid containing calmodulin shRNA and shRNA-resistant calmodulin (lower, CaM rescue). The green and red images are superimposed in the right. Arrows indicate transfected neurons. The soma of a neuron transfected with calmodulin shRNA showed a much lower calmodulin staining compared with un-transfected neurons (middle, CaM KD). Scale bar, 10 μm. C, The SpH signal induced by Train10 s in hippocampal boutons transfected with calmodulin shRNA (n = 15 experiments). The mean SpH signal induced by Train10 s (dotted) and Train2 s (dash) in control are also shown. D, The SpH signal induced by Train10 s in boutons transfected with CaM rescue plasmid (n = 9). The mean SpH signal induced by Train10 s (dotted) in control is also shown.
The Δ_F_peak induced by Train10 s in the presence of CMDZ was smaller than that induced by Train10 s in control, but larger than that induced by Train2 s in control (Fig. 5A). The reduction of the Δ_F_peak was not responsible for the decrease of the Ratedecay, because Train2 s in control induced a smaller Δ_F_peak, but a much larger Ratedecay than that induced by Train10 s in the presence of CMDZ (Fig. 5A). The reduction of the Δ_F_peak by CMDZ was consistent with the finding that calmodulin promotes vesicle mobilization from the reserve pool to the readily releasable pool (Sakaba and Neher, 2001), likely by initiating endocytosis that clears the released vesicle proteins from the release site (X. S. Wu et al., 2009).
CMDZ might not be specific to only calmodulin. To address this issue, we used a calmodulin shRNA that can knock down calmodulin expression by ∼70% in cultured cortical neurons (Pang et al., 2010). Transfection of this shRNA to PC12 cells reduced calmodulin to 32 ± 6% (n = 6) of control (supplemental Information 4, available at www.jneurosci.org as supplemental material). Cotransfection of calmodulin shRNA and SpH reduced calmodulin in the soma of rat hippocampal neurons to 30 ± 2% (n = 10 neurons from 3 transfections, p < 0.01) of that in neighbor un-transfected neurons (Fig. 5B, middle). In transfected neurons, Train10 s induced a Ratedecay (0.38 ± 0.04%/s, n = 15) much slower than that (0.86- 1.06%/s) induced by Train10 s or Train2 s in control (p < 0.01), and a much larger Δ_F_100 s (61 ± 8% of Δ_F_peak, n = 15, Fig. 5C), suggesting an inhibition of endocytosis similar to that caused by CMDZ. The Δ_F_peak induced by Train10 s was also slightly reduced compared with the control (Fig. 5C), consistent with the effects of CMDZ in blocking vesicle mobilization to the readily releasable pool (Fig. 5A) (Sakaba and Neher, 2001).
The decrease of the calmodulin level in neurons cotransfected with calmodulin shRNA and SpH (Fig. 5B, middle) was not due to transfection of SpH. This was because transfection of SpH along did not affect the calmodulin level in the soma, compared with the neighbor un-transfected neurons (103 ± 3%, n = 7 neurons, 2 transfections, p > 0.1, Fig. 5B, top). In neurons cotransfected with SpH and a plasmid containing both calmodulin shRNA and shRNA-resistant calmodulin, calmodulin was over rescued to 163 ± 4% (n = 11 neurons from 3 transfections, p < 0.01) of that in un-transfected neurons (Fig. 5_B_, bottom), and the Ratedecay (1.02 ± 0.06%/s), Δ_F_100 s (−3 ± 4% of Δ_F_peak) and Δ_F_peak (36 ± 4%, _n_ = 9) induced by Train10 s were similar to control (_p_ > 0.18, Fig. 5D). Transfection of this plasmid to PC12 cells also increased the calmodulin expression to 152 ± 5% of control (n = 3, supplemental Information 4, available at www.jneurosci.org as supplemental material) (see also Pang et al., 2010). These results suggest that inhibition of endocytosis by calmodulin shRNA was not due to off-target shRNA effects. We concluded that the physiological level of calmodulin is sufficient and critical in mediating normal endocytosis. This result, together with a recent finding that calmodulin may enhance the release probability by activation of CaMKII at hippocampal synapses (Pang et al., 2010), suggest that calmodulin is important not only for endocytosis, but also for exocytosis.
The role of calcineurin at hippocampal synapses
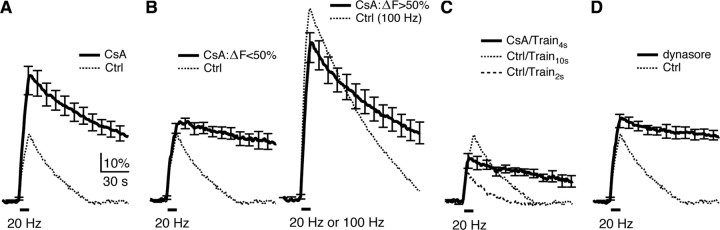
In the presence of the calcineurin blocker cyclosporin A (CsA, 20 μm in the bath, 5–10 min), Train10 s induced a Δ_F_peak (66 ± 8%, n = 13) nearly two times the control, but a Ratedecay (0.72 ± 0.14%/s, n = 13) smaller than the control (1.06 ± 0.18%/s, n = 7, p < 0.05), and a much larger Δ_F_100 s (62 ± 8%, n = 13, Fig. 6A). The initial rate of endocytosis (Ratedecay) increases as the amount of exocytosis (Δ_F_peak) increases (Balaji et al., 2008) until the latter reaches the endocytic capacity (Wu and Betz, 1996; Sankaranarayanan and Ryan, 2000; Sun et al., 2002) (see also Fig. 4G, solid symbols). Thus, an increase of the Δ_F_peak by CsA might cause an increase of the Ratedecay, leading to an underestimate of the inhibition of Ratedecay by CsA. To examine this possibility, we divided the CsA experiments into two groups with Δ_F_peak smaller or larger than 50% of the baseline. The reason we used 50% to divide the data was that the group with a smaller Δ_F_peak had a Δ_F_peak (40 ± 3%, n = 5) similar to that induced by Train10 s in control. This group had ∼7-fold smaller Ratedecay (0.16 ± 0.04%/s, p < 0.01), and a much larger Δ_F_100 s (84 ± 13%; Fig. 6B, left, comparing solid and dotted traces). The group with a larger Δ_F_peak had a mean Δ_F_peak (82 ± 8%, n = 8) close to that induced by the 100 Hz train for 10 s in control (103 ± 5%, n = 4), but had a smaller Ratedecay (0.85 ± 0.06%/s, n = 8, p < 0.01) and a larger Δ_F_100 s (48 ± 5%, n = 8, p < 0.01) compared with that induced by the 100 Hz train in control (Ratedecay: 1.35 ± 0.06%/s; Δ_F_100 s: 14 ± 3%; n = 4, Fig. 6B, right).
Figure 6.
Calcineurin inhibitor CsA inhibits slow endocytosis at hippocampal synapses. A, The SpH signal induced by Train10 s in the presence of 20 μm CsA (n = 13, solid). For comparison, the mean SpH signal induced by Train10 s in control is also plotted (dotted). B, The CsA experiments (solid trace in A) were divided into two groups depending on whether the Δ_F_peak is less than (left, n = 5) or lager than (right, n = 8) 50% of the baseline (solid). The mean SpH signal induced by Train10 s in control is also plotted in the left (dotted), whereas the mean SpH signal induced by a 100 Hz train for 10 s in control is plotted in the right (dotted). C, The SpH signal induced by a 20 Hz train for 4 s (Train4 s) in the presence of 20 μm CsA (n = 6, solid). For comparison, the mean SpH signals induced by Train10 s (dotted) and Train2 s (dash) are also plotted. D, The SpH signal induced by Train10 s in the presence of 100 μm dynasore (n = 12, solid). For comparison, the mean SpH signal induced by Train10 s in control is also plotted (dotted).
Clearly, CsA was more effective in blocking endocytosis at smaller Δ_F_peak (Fig. 6B). Consistent with this result, a 4 s stimulation train at 20 Hz in the presence of CsA induced a Δ_F_peak (23 ± 4%, n = 6) between those induced by Train10 s and Train2 s in control, but an ∼3- to 4-fold smaller Ratedecay (0.26 ± 0.08%/s), and a much larger Δ_F_100 s (63 ± 11%, Fig. 6C) than those induced by Train10 s or Train2 s in control. Large Δ_F_peak may force the endocytic machinery to operate at near maximal capacity (Sankaranarayanan and Ryan, 2000), at which inhibition could be more difficult. These results, and the observation that CsA did not inhibit vesicle reacidification (supplemental Information 5, available at www.jneurosci.org as supplemental material), suggest that CsA significantly inhibited endocytosis.
The increase of Δ_F_peak by CsA (Fig. 6A) could be due to a block of endocytosis and/or an increase of release. To distinguish these possibilities, a dynamin inhibitor, dynasore (100 μm) was applied to the bath for 5–10 min, which essentially blocked endocytosis after Train10 s (Fig. 6D) (Newton et al., 2006). In this condition, Train10 s induced a Δ_F_peak (44 ± 4%, n = 12, Fig. 6D) higher than that (35 ± 5%, n = 7, p < 0.05) in control, but smaller than that (66 ± 8%, n = 13, p < 0.05, Fig. 6A) in the presence of CsA. These results suggest that CsA may also increase release, consistent with previous reports that block of calcineurin increases transmitter release by an as yet unidentified mechanism (Sihra et al., 1995; Lin and Lin-Shiau, 1999; Chi et al., 2003).
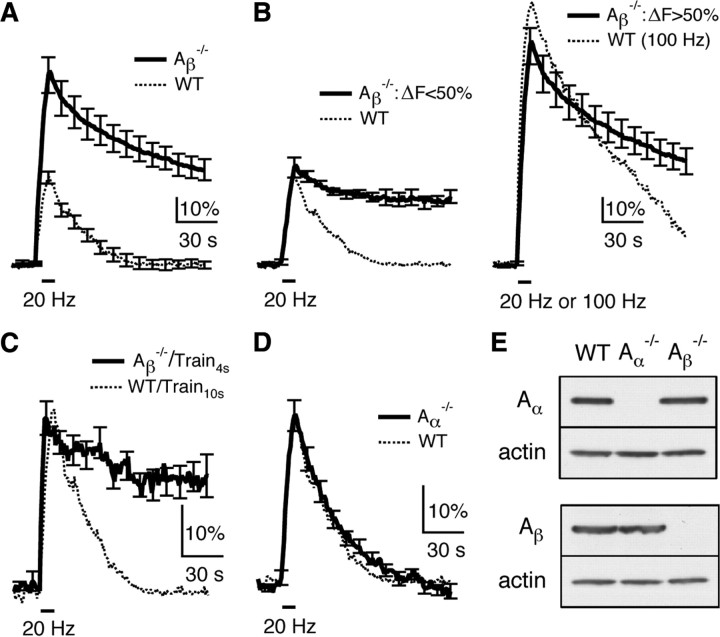
Next, we studied endocytosis in hippocampal cultures of calcineurin Aβ−/− or Aα−/− mice where the block of calcineurin function is more specific. In WT mice, Train10 s induced a Δ_F_peak of 36 ± 3%, a Ratedecay of 0.95 ± 0.05%/s, and a Δ_F_100 s of 3 ± 7% (n = 4), which were nearly the same as those obtained in control rats (comparing the dotted trace in Fig. 7A, 6_A_). In Aβ−/− mice, Train10 s induced a Δ_F_peak (79 ± 8%, n = 21) much larger than the WT (p < 0.01, Fig. 7A), which was similar to the effects of CsA (Fig. 6A). Similar to the CsA experiments (Fig. 6B), we divided the data into two groups depending on whether the Δ_F_peak was smaller or larger than 50% (Fig. 7B). The group with a smaller Δ_F_peak had a Δ_F_peak (40 ± 4%, n = 5) similar to that induced by Train10 s in WT, but an ∼3-fold smaller Ratedecay (0.33 ± 0.02%/s, n = 5, p < 0.01), and a much larger Δ_F_100 s (66 ± 9%, n = 5, p < 0.01; Fig. 7B, left). The group with a larger Δ_F_peak had a mean Δ_F_peak (91 ± 8%, n = 16) close to that induced by the 100 Hz train for 10 s in WT (106 ± 8%, n = 8), but a Ratedecay (0.91 ± 0.05%/s, n = 16) smaller than that induced by the 100 Hz train in WT (1.48 ± 0.02%/s, n = 8, p < 0.01), and a much larger Δ_F_100 s (Aβ−/−: 53 ± 6%, n = 16; WT: 23 ± 5%, n = 8, p < 0.01; Fig. 7B, right).
Figure 7.
Knock-out of calcineurin Aβ inhibits slow endocytosis at hippocampal synapses. A, The SpH signal induced by Train10 s in calcineurin Aβ−/− (n = 21, solid) and WT mice (n = 4, dotted). B, The experiments in Aβ−/− mice (thick trace in A) are divided into two groups depending on whether the Δ_F_peak is less than (left, n = 5) or lager than (right, n = 16) 50% of the baseline (solid). The mean SpH signal induced by Train10 s in WT is also plotted in the left (dotted), whereas the mean SpH signal induced by a 100 Hz train for 10 s in WT is plotted in the right (dotted). C, The SpH signal induced by a 20 Hz train for 4 s (Train4 s) in Aβ−/− mice (n = 5, solid). For comparison, the mean SpH signal induced by Train10 s in WT mice (dotted) is also plotted. D, The SpH signal induced by Train10 s in Aα−/− (n = 11, solid) and WT mice (n = 4, dotted). E, Western blot of calcineurin Aα (upper) and Aβ subunit (lower) in the hippocampus (CA3 and CA1 area) of WT, Aα−/− and Aβ−/− mice. Actin is shown as a control.
Similar to the effect of CsA, knock-out of calcineurin Aβ was more effective in blocking endocytosis at smaller Δ_F_peak (Fig. 7B). Consistent with this result, a 4 s stimulation train at 20 Hz in Aβ−/− mice induced a Δ_F_peak (32 ± 3%, n = 5) similar to that induced by Train10 s in WT, but an ∼2- to 3-fold smaller Ratedecay (0.37 ± 0.03%/s), and a much larger Δ_F_100 s (63 ± 9%, Fig. 7C).
In Aα−/− mice, Train10 s induced a Δ_F_peak of 36 ± 3% (n = 11), a Ratedecay of 1.02 ± 0.04%/s (n = 11) and a Δ_F_100 s of 2 ± 1% (n = 11), all of which were similar to the WT (Fig. 7D). We concluded that calcineurin Aβ, but not Aα knock-out inhibits endocytosis in a similar way as CsA at hippocampal synapses (Figs. 6, 7).
Could the lack of effect of Aα knock-out on endocytosis be due to the absence of calcineurin Aα subunit in the hippocampus? To examine this possibility, mouse hippocampal CA1-CA3 regions were dissociated for Western blot using two antibodies against calcineurin Aα and Aβ, respectively (Fig. 7E). Immunoblotting results revealed that Aα and Aβ were expressed in wild-type, but not in Aα−/− and Aβ−/− mice, respectively (Fig. 7E). Consistent with early studies (Kuno et al., 1992; Hashimoto et al., 1998), these results suggest that the lack of effect of Aα knock-out on endocytosis is not due to the absence of Aα subunit in the hippocampus.
Discussion
The present work provided the first genetic evidence together with pharmacological evidence suggesting an important role of calmodulin and calcineurin in rapid and slow endocytosis at 7- to 10-d-old calyceal synapses and cultured hippocampal synapses (Figs. 1–3, 5–7). Consistent with results obtained at calyces, where calcium influx triggers endocytosis (Hosoi et al., 2009; X. S. Wu et al., 2009), reducing the [Ca2+]o to 0.1 mm nearly abolished endocytosis at hippocampal synapses (Fig. 4). We therefore concluded that calcium influx during nerve firings activates calmodulin/calcineurin, which initiates and upregulates slow, clathrin-dependent and rapid, presumably clathrin-independent endocytosis.
How does calcineurin control endocytosis?
Calmodulin/calcineurin-dependent dephosphorylation of endocytic proteins (Robinson et al., 1993; Cousin and Robinson, 2001) may be synchronously activated by calcium influx during nerve firings, which may rapidly increase the endocytosis efficiency and thus initiate endocytosis. Since calcineurin is involved in rapid endocytosis, dephosphorylation must occur within tens to hundreds of milliseconds after stimulation. Larger calcium influx may speed up endocytosis (Wu, 2004) by inducing more calmodulin/calcineurin-dependent dephosphorylation.
How dephosphorylation initiates and accelerates endocytosis is unclear. Dynamin dephosphorylation promotes its interaction with syndapin (Anggono et al., 2006). It was suggested that calcium influx accelerates endocytosis by increasing the number of endocytic sites (Balaji et al., 2008). This suggestion was obtained by reducing the [Ca2+]o to only 1 mm. The near full block of endocytosis at 0.1 mm [Ca2+]o, as shown here (Fig. 4), suggests an extremely slow endocytosis at each endocytic site, although we could not fully exclude the possibility that few endocytic sites are assembled in low calcium conditions.
Our results seem inconsistent with the observation that endocytosis is triggered by calcium at a threshold (∼10 μm) higher than the affinity (dissociation constant) of calcineurin to calcium (∼1 μm) (Rusnak and Mertz, 2000; Hosoi et al., 2009; X. S. Wu et al., 2009). The affinity was measured in vitro with prolonged (minutes) presence of calcium and calcineurin in the steady-state (Rusnak and Mertz, 2000), whereas in nerve terminals, the calcium increase to >10 μm decayed in <1 s (Bollmann and Sakmann, 2005; Hosoi et al., 2009). The binding among calcium, calmodulin and calcineurin may not reach the steady-state during transient calcium influx, explaining why >10 μm calcium is needed to initiate endocytosis. Furthermore, calcineurin (B subunit) has four calcium binding sites, one with a high affinity (<0.1 μm), and three with affinities at ∼15 μm (Rusnak and Mertz, 2000), the later of which may help to explain the need of >10 μm calcium.
Calcium/calmodulin/calcineurin controls various forms of endocytosis at many synapses
Our finding that calcium/calmodulin/calcineurin signaling pathway controls rapid and slow endocytosis may explain regulation of endocytosis by extra- and intracellular calcium observed at many synapses and endocrine cells over the last several decades (Ceccarelli and Hurlbut, 1980; Ramaswami et al., 1994; Henkel and Betz, 1995; Artalejo et al., 1996; Cousin and Robinson, 1998; Gad et al., 1998; Marks and McMahon, 1998; Neves et al., 2001; Sankaranarayanan and Ryan, 2001; W. Wu et al., 2005; Balaji et al., 2008). It may also explain why endocytosis is extremely slow in resting conditions (Hosoi et al., 2009; X. S. Wu et al., 2009). Since calcium/calmodulin may initiate bulk endocytosis at calyces (X. S. Wu et al., 2009), and calcium/calcineurin may trigger bulk endocytosis at cerebellar synapses (Evans and Cousin, 2007; Clayton and Cousin, 2009; Clayton et al., 2009), it is likely that the calcium/calmodulin/calcineurin signaling pathway is a common mechanism at synapses to initiate and regulate endocytosis, including rapid, slow, and bulk endocytosis.
Our results seem inconsistent with a report of no calcineurin involvement in slow, clathrin-dependent endocytosis during relatively mild stimulation at cerebellar synapses (Clayton et al., 2009). Although synapse heterogeneity provides an explanation, this discrepancy is likely due to methodological differences. The study at cerebellar synapses was based on the ability of a stimulus to unload FM dye from nerve terminals preloaded with the dye (Clayton et al., 2009). Instead of measuring endocytosis, this method measures the vesicle cycling involving both endocytosis and vesicle reuse. Since the dye was washed out immediately after the dye loading stimulus, the analysis (the amount of dye release after dye preloading) could not provide the endocytosis time course, distinguish between rapid and slow endocytosis, or measure endocytosis time course after stimulation (Clayton et al., 2009). In contrast, we quantitatively measured rapid and slow endocytosis time course using SpH imaging and capacitance measurement techniques. We used not only calcineurin blockers as in previous studies, but also calcineurin knock-out mice and calmodulin knockdown techniques. Furthermore, our results were verified in two types of synapses, the hippocampal and the calyx-type synapse.
Our results seem inconsistent with the block of endocytosis by prolonged intracellular dialysis of ∼1 μm calcium in ribbon-type synapses (von Gersdorff and Matthews, 1994). Accordingly, our findings are likely limited to the transient calcium increase during brief depolarization. Prolonged calcium increase might perturb the cycle of phosphorylation and dephosphorylation, resulting in a block of endocytosis.
A study published after we finished the present work showed that the calcium buffer BAPTA abolished endocytosis in both the immature (P7–P9) and more mature (P13–P14) calyces (Yamashita et al., 2010), consistent with previous studies (Hosoi et al., 2009; X. S. Wu et al., 2009). This study also showed that calcineurin inhibitors (FK506 and CsA) inhibited rapid and slow endocytosis in P7–P9 calyces (Yamashita et al., 2010), consistent with the present work. Surprisingly, calcineurin inhibitors did not block endocytosis in P13–P14 calyces, suggesting that the calcium sensor for endocytosis changes developmentally from calcineurin to an unknown sensor (Yamashita et al., 2010). Accordingly, our results might be limited to immature synapses. However, this important suggestion may need further scrutiny for two reasons. First, it is based solely on pharmacological manipulation. Second, the same calcium influx triggers both exocytosis and endocytosis (Hosoi et al., 2009; X. S. Wu et al., 2009). Calcium channels are more tightly coupled to release in P13–P14 than P7–P9 calyces, likely because calcium channels are located closer to the release site (Fedchyshyn and Wang, 2005; Wang et al., 2008; Kochubey et al., 2009; Yang et al., 2010). Tight coupling may produce a higher local calcium concentration during the same stimulus, which may accelerate endocytosis to a saturating speed (X. S. Wu et al., 2009). At such high concentration of calcium, the possibility that calcineurin blockers are not as effective in inhibiting endocytosis as in normal conditions has not been ruled out.
Similarity between rapid and slow endocytosis
Rapid endocytosis is considered clathrin-independent (Artalejo et al., 1995; Jockusch et al., 2005). Its underlying mechanisms are poorly understood. The present work identified calcineurin as an important player in rapid endocytosis. Both rapid and slow endocytosis are regulated by the same calcium/calmodulin/calcineurin signaling pathway (Figs. 1–7) (X. S. Wu et al., 2009), and require dynamin in most, but not some stimulation conditions (Xu et al., 2008). Neither of them recycles vesicles to the readily releasable pool (Wu and Wu, 2009). These observations suggest that rapid and slow endocytosis share similar mechanisms of initiation, fission, and recycling. Rapid endocytosis is triggered by a higher calcium concentration (Beutner et al., 2001; X. S. Wu et al., 2009), likely because high calcium induces more calcineurin-dependent dephosphorylation.
Comparison between calyx-type and hippocampal synapses
Incomplete inhibition of endocytosis by calmodulin and calcineurin blockers, calmodulin knockdown, or calcineurin Aα or Aβ knock-out (Figs. 1–3, 5–7) is likely due to the inefficiency of blockers in vivo, the incomplete knockdown of calmodulin, or the remaining calcineurin A subunit. Although the involvement of other calcium-dependent pathway(s) could not be excluded, the calcium/calmodulin/calcineurin pathway must be a major signaling mechanism, because inhibition of calcineurin reduced the Ratedecay by up to ∼4- to 7-fold (Figs. 1, 6).
Knock-out of calcineurin Aα and Aβ inhibited endocytosis at calyces and hippocampal synapses, respectively (Figs. 3, 7). The reason for this difference is unclear. It is not because of the lack of Aβ at calyces and Aα in the hippocampus, because both isoforms are present in the hippocampus (Fig. 7E) (Kuno et al., 1992; Hashimoto et al., 1998). A difference in the relative abundance or subcellular localization of Aα and Aβ isoforms might provide an explanation.
CsA and calcineurin Aβ knock-out increased Δ_F_peak by enhancing transmitter release at hippocampal synapses (Figs. 6, 7), whereas calcineurin inhibitors and calcineurin Aα knock-out did not increase Δ_C_m at calyces (Figs. 1–3). The reason for this difference is unclear. Synapse heterogeneity could provide an explanation. The difference in the stimulation protocol might provide another explanation. If block of calcineurin increases the release probability, but not the readily releasable pool size, it might increase release during action potential stimulation at hippocampal synapses, but not the Δ_C_m induced by 20 ms depolarization that depleted the readily releasable pool at calyces (Wu and Wu, 2009).
Knock-out of endocytosis genes often causes behavioral defect. Although we did not examine the behavior of Aα−/− and Aβ−/− mice, we noticed that we could not generate double knock-out mice (Aα−/−, Aβ−/−) from Aα−/− and Aβ−/− mice, likely because they die in the embryonic stage. Consistent with this possibility, knock-out of calcineurin B, the only calcineurin regulatory subunit, results in embryonic death (Chang et al., 2004). Furthermore, most Aα−/− mice die within a few months after birth because of the heart failure (Molkentin et al., 1998). These results suggest the importance of calcineurin for animal survival.
Footnotes
This work was supported by the National Institute of Neurological Disorders and Stroke Intramural Research Program. We thank Drs. Jonathan G. Seidman (Harvard Medical School, Boston, MA) and Jennifer L. Gooch (Emory University School of Medicine, Atlanta, GA) for providing us with calcineurin Aα+/− mice. We thank Dr. Gero Miesenböck (University of Oxford, Oxford, UK) for providing us with the synaptopHluorin plasmid.
References
- Anggono V, Smillie KJ, Graham ME, Valova VA, Cousin MA, Robinson PJ. Syndapin I is the phosphorylation-regulated dynamin I partner in synaptic vesicle endocytosis. Nat Neurosci. 2006;9:752–760. doi: 10.1038/nn1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo CR, Henley JR, McNiven MA, Palfrey HC. Rapic endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proc Natl Acad Sci U S A. 1995;92:8328–8332. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artalejo CR, Elhamdani A, Palfrey HC. Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron. 1996;16:195–205. doi: 10.1016/s0896-6273(00)80036-7. [DOI] [PubMed] [Google Scholar]
- Atluri PP, Ryan TA. The kinetics of synaptic vesicle reacidification at hippocampal nerve terminals. J Neurosci. 2006;26:2313–2320. doi: 10.1523/JNEUROSCI.4425-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji J, Armbruster M, Ryan TA. Calcium control of endocytic capacity at a CNS synapse. J Neurosci. 2008;28:6742–6749. doi: 10.1523/JNEUROSCI.1082-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner D, Voets T, Neher E, Moser T. Calcium dependence of exocytosis and endocytosis at the cochlear inner hair cell afferent synapse. Neuron. 2001;29:681–690. doi: 10.1016/s0896-6273(01)00243-4. [DOI] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B. Control of synaptic strength and timing by the release-site Ca2+ signal. Nat Neurosci. 2005;8:426–434. doi: 10.1038/nn1417. [DOI] [PubMed] [Google Scholar]
- Bueno OF, Brandt EB, Rothenberg ME, Molkentin JD. Defective T cell development and function in calcineurin A beta-deficient mice. Proc Natl Acad Sci U S A. 2002;99:9398–9403. doi: 10.1073/pnas.152665399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarelli B, Hurlbut WP. Ca2+-dependent recycling of synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 1980;87:297–303. doi: 10.1083/jcb.87.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CP, McDill BW, Neilson JR, Joist HE, Epstein JA, Crabtree GR, Chen F. Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J Clin Invest. 2004;113:1051–1058. doi: 10.1172/JCI20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Greengard P, Ryan TA. Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron. 2003;38:69–78. doi: 10.1016/s0896-6273(03)00151-x. [DOI] [PubMed] [Google Scholar]
- Clayton EL, Cousin MA. The molecular physiology of activity-dependent bulk endocytosis of synaptic vesicles. J Neurochem. 2009;111:901–914. doi: 10.1111/j.1471-4159.2009.06384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EL, Evans GJ, Cousin MA. Activity-dependent control of bulk endocytosis by protein dephosphorylation in central nerve terminals. J Physiol. 2007;585:687–691. doi: 10.1113/jphysiol.2007.137539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EL, Anggono V, Smillie KJ, Chau N, Robinson PJ, Cousin MA. The phospho-dependent dynamin-syndapin interaction triggers activity-dependent bulk endocytosis of synaptic vesicles. J Neurosci. 2009;29:7706–7717. doi: 10.1523/JNEUROSCI.1976-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. Ba2+ does not support synaptic vesicle retrieval in rat cerebrocortical synaptosomes. Neurosci Lett. 1998;253:1–4. doi: 10.1016/s0304-3940(98)00610-7. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- Evans GJ, Cousin MA. Activity-dependent control of slow synaptic vesicle endocytosis by cyclin-dependent kinase 5. J Neurosci. 2007;27:401–411. doi: 10.1523/JNEUROSCI.3809-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedchyshyn MJ, Wang LY. Developmental transformation of the release modality at the calyx of held synapse. J Neurosci. 2005;25:4131–4140. doi: 10.1523/JNEUROSCI.0350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad H, Löw P, Zotova E, Brodin L, Shupliakov O. Dissociation between Ca2+-triggered synaptic vesicle exocytosis and clathrin-mediated endocytosis at a central synapse. Neuron. 1998;21:607–616. doi: 10.1016/s0896-6273(00)80570-x. [DOI] [PubMed] [Google Scholar]
- Gooch JL, Toro JJ, Guler RL, Barnes JL. Calcineurin A-alpha but not A-beta is required for normal kidney development and function. Am J Pathol. 2004;165:1755–1765. doi: 10.1016/s0002-9440(10)63430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granseth B, Odermatt B, Royle SJ, Lagnado L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 2006;51:773–786. doi: 10.1016/j.neuron.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Kawamata T, Saito N, Sasaki M, Nakai M, Niu S, Taniguchi T, Terashima A, Yasuda M, Maeda K, Tanaka C. Isoform-specific redistribution of calcineurin A alpha and A beta in the hippocampal CA1 region of gerbils after transient ischemia. J Neurochem. 1998;70:1289–1298. doi: 10.1046/j.1471-4159.1998.70031289.x. [DOI] [PubMed] [Google Scholar]
- Henkel AW, Betz WJ. Monitoring of black widow spider venom (BWSV) induced exo- and endocytosis in living frog motor nerve terminals with FM1-43. Neuropharmacology. 1995;34:1397–1406. doi: 10.1016/0028-3908(95)00126-q. [DOI] [PubMed] [Google Scholar]
- Hosoi N, Holt M, Sakaba T. Calcium dependence of exo- and endocytotic coupling at a glutamatergic synapse. Neuron. 2009;63:216–229. doi: 10.1016/j.neuron.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Jockusch WJ, Praefcke GJ, McMahon HT, Lagnado L. Clathrin-dependent and clathrin-independent retrieval of synaptic vesicles in retinal bipolar cells. Neuron. 2005;46:869–878. doi: 10.1016/j.neuron.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kochubey O, Han Y, Schneggenburger R. Developmental regulation of the intracellular Ca2+ sensitivity of vesicle fusion and Ca2+-secretion coupling at the rat calyx of Held. J Physiol. 2009;587:3009–3023. doi: 10.1113/jphysiol.2009.172387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno T, Mukai H, Ito A, Chang CD, Kishima K, Saito N, Tanaka C. Distinct cellular expression of calcineurin A alpha and A beta in rat brain. J Neurochem. 1992;58:1643–1651. doi: 10.1111/j.1471-4159.1992.tb10036.x. [DOI] [PubMed] [Google Scholar]
- Lin MJ, Lin-Shiau SY. Enhanced spontaneous transmitter release at murine motor nerve terminals with cyclosporine. Neuropharmacology. 1999;38:195–198. doi: 10.1016/s0028-3908(98)00178-6. [DOI] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Gomis A, Lagnado L. Calcium influx selects the fast mode of endocytosis in the synaptic terminal of retinal bipolar cells. Proc Natl Acad Sci U S A. 2001;98:15282–15287. doi: 10.1073/pnas.261311698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AJ, Kirchhausen T, Murthy VN. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proc Natl Acad Sci U S A. 2006;103:17955–17960. doi: 10.1073/pnas.0606212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveria SF, Dell'Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Cao P, Xu W, Südhof TC. Calmodulin controls synaptic strength via presynaptic activation of CaM kinase II. J Neurosci. 2010;30:4132–4142. doi: 10.1523/JNEUROSCI.3129-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswami M, Krishnan KS, Kelly RB. Intermediates in synaptic vesicle recycling revealed by optical imaging of Drosophila neuromuscular junctions. Neuron. 1994;13:363–375. doi: 10.1016/0896-6273(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Robinson PJ, Sontag JM, Liu JP, Fykse EM, Slaughter C, McMahon H, Südhof TC. Dynamin GTPase regulated by protein kinase C phosphorylation in nerve terminals. Nature. 1993;365:163–166. doi: 10.1038/365163a0. [DOI] [PubMed] [Google Scholar]
- Royle SJ, Lagnado L. Endocytosis at the synaptic terminal. J Physiol. 2003;553:345–355. doi: 10.1113/jphysiol.2003.049221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron. 2001;32:1119–1131. doi: 10.1016/s0896-6273(01)00543-8. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nat Cell Biol. 2000;2:197–204. doi: 10.1038/35008615. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Ryan TA. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat Neurosci. 2001;4:129–136. doi: 10.1038/83949. [DOI] [PubMed] [Google Scholar]
- Sihra TS, Nairn AC, Kloppenburg P, Lin Z, Pouzat C. A role for calcineurin (protein phosphatase-2B) in the regulation of glutamate release. Biochem Biophys Res Commun. 1995;212:609–616. doi: 10.1006/bbrc.1995.2013. [DOI] [PubMed] [Google Scholar]
- Sun JY, Wu LG. Fast kinetics of exocytosis revealed by simultaneous measurements of presynaptic capacitance and postsynatpic currents at a central synapse. Neuron. 2001;30:171–182. doi: 10.1016/s0896-6273(01)00271-9. [DOI] [PubMed] [Google Scholar]
- Sun JY, Wu XS, Wu LG. Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse. Nature. 2002;417:555–559. doi: 10.1038/417555a. [DOI] [PubMed] [Google Scholar]
- Sun JY, Wu XS, Wu W, Jin SX, Dondzillo A, Wu LG. Capacitance measurements at the calyx of Held in the medial nucleus of the trapezoid body. J Neurosci Methods. 2004;134:121–131. doi: 10.1016/j.jneumeth.2003.11.018. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H, Matthews G. Inhibition of endocytosis by elevated internal calcium in a synaptic terminal. Nature. 1994;370:652–655. doi: 10.1038/370652a0. [DOI] [PubMed] [Google Scholar]
- Wang LY, Neher E, Taschenberger H. Synaptic vesicles in mature calyx of Held synapses sense higher nanodomain calcium concentrations during action potential-evoked glutamate release. J Neurosci. 2008;28:14450–14458. doi: 10.1523/JNEUROSCI.4245-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG. Kinetic regulation of vesicle endocytosis at synapses. Trends Neurosci. 2004;27:548–554. doi: 10.1016/j.tins.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Wu LG, Betz WJ. Nerve activity but not intracellular calcium determines the time course of endocytosis at the frog neuromuscular junction. Neuron. 1996;17:769–779. doi: 10.1016/s0896-6273(00)80208-1. [DOI] [PubMed] [Google Scholar]
- Wu LG, Ryan TA, Lagnado L. Modes of vesicle retrieval at ribbon synapses, calyx-type synapses, and small central synapses. J Neurosci. 2007;27:11793–11802. doi: 10.1523/JNEUROSCI.3471-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Xu J, Wu XS, Wu LG. Activity-dependent acceleration of endocytosis at a central synapse. J Neurosci. 2005;25:11676–11683. doi: 10.1523/JNEUROSCI.2972-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, Wu LG. Rapid endocytosis does not recycle vesicles within the readily releasable pool. J Neurosci. 2009;29:11038–11042. doi: 10.1523/JNEUROSCI.2367-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, McNeil BD, Xu J, Fan J, Xue L, Melicoff E, Adachi R, Bai L, Wu LG. Ca(2+) and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat Neurosci. 2009;12:1003–1010. doi: 10.1038/nn.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, McNeil B, Wu W, Nees D, Bai L, Wu LG. GTP-independent rapid and slow endocytosis at a central synapse. Nat Neurosci. 2008;11:45–53. doi: 10.1038/nn2021. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Eguchi K, Saitoh N, von Gersdorff H, Takahashi T. Developmental shift to a mechanism of synaptic vesicle endocytosis requiring nanodomain Ca2+ Nat Neurosci. 2010;13:838–844. doi: 10.1038/nn.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YM, Fedchyshyn MJ, Grande G, Aitoubah J, Tsang CW, Xie H, Ackerley CA, Trimble WS, Wang LY. Septins regulate developmental switching from microdomain to nanodomain coupling of Ca(2+) influx to neurotransmitter release at a central synapse. Neuron. 2010;67:100–115. doi: 10.1016/j.neuron.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang BW, Zimmer G, Chen J, Ladd D, Li E, Alt FW, Wiederrecht G, Cryan J, O'Neill EA, Seidman CE, Abbas AK, Seidman JG. T cell responses in calcineurin A alpha-deficient mice. J Exp Med. 1996;183:413–420. doi: 10.1084/jem.183.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]