Pain behavior in the formalin test persists after ablation of the great majority of C-fiber nociceptors (original) (raw)
. Author manuscript; available in PMC: 2011 Nov 1.
Abstract
Although the formalin test is a widely used model of persistent pain, the primary afferent fiber types that underlie the cellular and behavioral responses to formalin injection are largely unknown. Here we used a combined genetic and pharmacological approach to investigate the effect of ablating subsets of primary afferent nociceptors on formalin-induced nocifensive behaviors and spinal cord Fos protein expression. Intrathecal capsaicin-induced ablation of the central terminals of TRPV1+ neurons greatly reduced the behavioral responses and Fos elicited by low-dose (0.5%) formalin. In contrast, genetic ablation of the MrgprD-expressing subset of nonpeptidergic unmyelinated afferents, which constitute a largely non-overlapping population, altered neither the behavior nor the Fos induced by low-dose formalin. Remarkably, nocifensive behavior following high-dose (2%) formalin was unchanged in mice lacking either afferent population, or even in mice lacking both populations, which together make up the great majority of C-fiber nociceptors. Thus, at high doses, which are routinely used in the formalin test, formalin-induced “pain” behavior persists in the absence of the vast majority of C-fiber nociceptors, which points to a contribution of a large spectrum of afferents secondary to non-specific formalin-induced tissue and nerve damage.
Introduction
The formalin test is a widely used model of persistent pain, and is a mainstay for the development of novel agents for the treatment of postoperative pain. Despite the prevalence of the formalin test in pain research, information as to the mechanisms that underlie the nocifensive behaviors produced by formalin is remarkably limited. There is some consensus that phase I behaviors result from acute activation of nociceptors by formalin, while phase II behaviors are driven in part by the central sensitization of spinal cord circuits secondary to the barrage of input that occurs during phase I [4,26]. Other studies, however, provided evidence for an important contribution of ongoing afferent firing during phase II [24,25]. In fact, electrophysiological studies demonstrated that Aδ- and C-fiber nociceptors exhibit sustained firing during both phases of the formalin test, and even that presumably non-nociceptive Aβ fibers are activated during phase I [15,20]. These results are of particular interest in light of recent studies that demonstrated that at low doses (≤0.5%), formalin interacts directly with the non-specific cation channel TRPA1 [14,16], which is expressed in a subset of the unmyelinated TRPV1-expressing nociceptors [10, 23, however, see 12]. Whether TRPA1 mediates the behavioral effects produced by higher doses of formalin that are routine in formalin testing in rodents is not known.
Taken together with the electrophysiological reports, the TRPA1 observations suggest that distinct complements of primary sensory neurons (nociceptors and non-nociceptive afferents) are engaged by different doses of formalin. The relationship between formalin dose, fiber type engaged, and behavior, however, is unclear. In the present study we used selective ablation of molecularly defined populations of primary afferent sensory fibers [3,7] to determine which fiber types come into play at different doses, and more importantly, which subsets are necessary or sufficient for generating the prototypical behavioral responses to formalin. Thus, in order to ablate selectively the central terminals of nociceptors that express the heat and capsaicin receptor, TRPV1, we performed intrathecal injections of capsaicin in wildtype C57Bl/6 mice [3]. To ablate the largely non-overlapping population of cutaneous C-fibers that expresses the G protein-coupled receptor, MrgprD, we made systemic injections of diphtheria toxin in mutant mice engineered with the human diphtheria toxin receptor (DTR) inserted into the Mrgprd locus (MrgprDDTR mice) [3].
We report that both the behavioral and cellular (Fos induction in the spinal cord) responses produced by injection of low-dose formalin are dependent on nociceptors that express the heat and capsaicin receptor, TRPV1, likely through activation of TRPA1. In contrast, injection of high-dose formalin evokes nocifensive behavior that persists in the absence of the great majority of C-fiber nociceptors, which points to a contribution of a large spectrum of afferents secondary to non-specific tissue and nerve damage.
Materials and Methods
Experimental animals
All animal experiments were approved by the Institutional Animal Care and Use Committees of the University of California at San Francisco or the California Institute of Technology and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the recommendations of the International Association for the Study of Pain. Animals were housed 2–5 per cage and maintained on a 12h light/dark schedule with free access to food and water. Adult male C57Bl/6 mice (20–30 g) were purchased from Charles River (Hollister, CA) and MrgprDDTR mice [3] were bred in house.
Experimental procedures
Mice in this study were divided into three experimental groups as follows.
- Intrathecal capsaicin injection. To ablate the central terminals of TRPV1-expressing afferents, wildtype C57Bl/6 mice were anesthetized with 1.5% isoflurane and injected intrathecally at the level of the pelvic girdle with capsaicin (10 μg) or vehicle (10% ethanol, 10% Tween 80, saline) in a volume of 5.0 μl with a 30 gauge needle attached to a luer-tipped Hamilton syringe. Behavioral tests were performed 2–7 days after capsaicin pretreatment. We previously reported that TRPV1-expressing terminals are destroyed by 24 hours after intrathecal capsaicin injection and that behavioral insensitivity to heat (a functional readout of successful ablation of TRPV1 terminals) persists for at least 8 weeks [3]. Thus, at the time of behavioral testing, TRPV1 terminals were destroyed, preventing signaling by TRPV1 afferents to spinal cord neurons.
- Diphtheria toxin (DTX) injection. To ablate MrgprD-expressing afferents, DTX (100 μg/kg) was injected intraperitoneally on 2 days, separated by 72 hours, into MrgprDDTR mice or their wildtype littermates. Mice were tested behaviorally 14 days after the first DTX injection.
- Sequential injection of DTX and capsaicin. To ablate both TRPV1 central terminals and MrgprD afferents, MrgprDDTR mice were first injected intraperitoneally with DTX as described above, and then received an intrathecal injection of capsaicin, as described above, 14 days later.
Formalin-induced nocifensive behavior
Mice were placed in plastic cylinders on a room temperature glass surface and allowed to acclimate for approximately one hour before injection. Formalin solutions were prepared at 2% or 0.5% in saline from a formalin stock (Sigma, St. Louis) and injected intraplantarly to one hindpaw in a volume of 10 μl. (Note that formalin stock corresponds to a 37% formaldehyde solution). Mice were videotaped for 50 minutes after the formalin injection and videos were subsequently scored for the latency to onset of nocifensive behaviors directed at the injected hindpaw and for the total amount of time each mouse spent flinching or licking the injected hindpaw, separated into 5 min bins. All scoring was done by experimenters blinded to treatment group.
Mustard oil-induced nocifensive behavior
Mice were placed in plastic cylinders on a room temperature glass surface and allowed to acclimate for approximately one hour before mustard oil application. Mustard oil (a selective TRPA1 agonist [8]) was prepared as a 10% solution in mineral oil and was applied topically to one hindpaw using a paintbrush. Mice were observed for 10 min after mustard oil application and scored for total number of flinches of the affected hindpaw. All scoring was done by experimenters blinded to treatment group.
Histology
Primary antisera were as follows: guinea pig anti-TRPV1 (1:2,000; gift from D. Julius, UCSF) and rabbit anti-GFP (1:1,000; Molecular Probes, Carlsbad, CA). Fluorophore-conjugated secondary antisera, all purchased from Molecular Probes and diluted 1:700, were: alexa-594-labeled goat anti-guinea pig IgG, and alexa-488-labeled goat anti-rabbit IgG. Mice were deeply anesthetized with 100 mg/kg sodium pentobarbital and perfused transcardially with phosphate buffered saline followed by 10% formalin fixative. Spinal cords were removed and postfixed in the same fixative for 4 hours, then stored in 30% sucrose cryoprotectant overnight. Transverse sections of lumbar spinal cord (L4–6) were cut at 40 μm and collected as free-floating sections. Tissue was incubated for 1 h in 0.1 M PBS with 0.3% Triton X-100 (PBST) plus 5% normal goat serum (NGS). Primary and secondary antisera were diluted in PBST plus 1% NGS. Tissue was incubated overnight at room temperature in primary antibody solution. Between incubations, tissue was rinsed in 0.1 M PBS plus 1% NGS. Tissue was then incubated with the appropriate secondary antiserum for 2 hours and washed in PBS. For isolectin B4 (IB4) binding, we added biotinylated IB4 (1:500; Vector Laboratories) instead of primary antiserum and followed this with alexa-488-labeled streptavidin (1:700; Molecular Probes) instead of a secondary antiserum.
Fos induction and histology
Ninety minutes after injection of formalin into the hindpaw, mice were deeply anesthetized with 100 mg/kg sodium pentobarbital and prepared for immunohistochemistry (IHC) as described above. IHC for Fos and quantification of immunoreactive profiles were performed as previously described [3].
Statistical analysis
Behavioral and histological data were analyzed by one- and two-way ANOVA with Bonferroni post-hoc test and Student's t-test, with p<0.05 considered to be significant.
Results
Ablation of TRPV1+ and MrgprD+ afferents
Primary afferent nociceptors are classified based on several characteristics, including soma size, degree of myelination, and their complement of receptors and neurotransmitters. Broadly, nociceptors include both myelinated Ad fibers and unmyelinated C-fibers. The C-fibers are further subdivided into peptidergic neurons, which express the neuropeptides substance P (SP) and calcitonin gene-related peptide (CGRP), and non-peptidergic neurons, which bind the lectin IB4 [2]. We have previously shown that intrathecal injection of the selective neurotoxic agent capsaicin in wildtype mice destroys the central (spinal cord) terminals of TRPV1+ primary afferents, which make up a large percentage of the peptidergic C-fibers. In contrast, chemical-genetic ablation of afferents that express the G protein-coupled receptor MrgprD, ablates virtually all of the cutaneous nonpeptidergic C-fibers [3], but spares most of the TRPV1-expressing nociceptors.
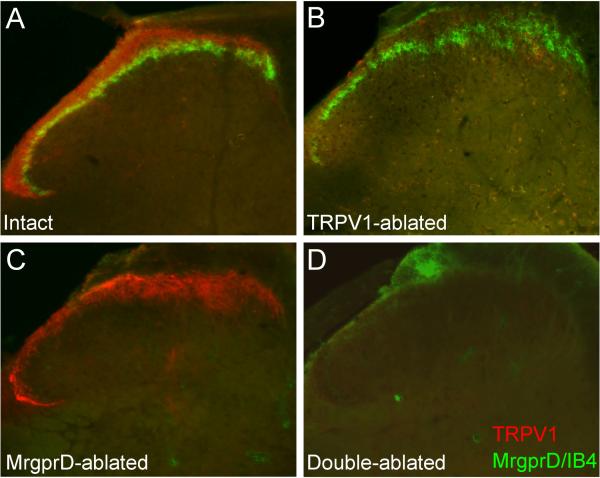
In untreated (intact) mice, TRPV1+ and MrgprD+ DRG neurons target distinct laminae of the spinal cord dorsal horn, with TRPV1+ fibers terminating in lamina I and outer lamina II, and MrgprD+ fibers terminating in inner lamina II (Fig. 1a). Consistent with our previous results, intrathecal capsaicin pre-treatment selectively eliminated TRPV1 staining in the dorsal horn, leaving MrgprD staining unaffected (Fig. 1b). Although this treatment does not ablate the cell bodies of TRPV1+ neurons, it produces a complete and long-lasting elimination of TRPV1+ fibers from the spinal cord, effectively preventing their signaling to downstream neurons [3]. We will refer to mice that were pre-treated with intrathecal capsaicin as TRPV1-ablated mice. In contrast, injection of diphtheria toxin (DTX) into mice in which the human diphtheria toxin receptor (DTR) was inserted in the MrgprD locus (MrgprDDTR mice) selectively eliminated MrgprD staining without altering TRPV1 staining (Fig. 1c). We will refer to mice lacking MrgprD+ neurons as MrgprD-ablated mice. Finally, combined capsaicin and DTX treatment eliminated staining for both groups of afferents (Fig. 1d). We will refer to mice that received both DTX and intrathecal capsaicin as Double-ablated mice.
Figure 1. Selective ablation of TRPV1+ and MrgprD+ fibers in spinal cord.
Spinal cord sections of MrgprDDTR mice were stained for TRPV1 (red) and MrgprD (staining is directed against GFP, which was genetically inserted into the MrgprD locus; green) or for binding of the lectin IB4 (green). (A) Normal staining in untreated, Intact mice. TRPV1+ fibers (red) terminate in spinal cord laminae I and II outer, and MrgprD+ fibers (green) terminate more ventrally, in lamina II inner. Note the limited overlap between these subsets. (B) TRPV1 staining is eliminated following intrathecal capsaicin injection, but MrgprD staining is unaltered (TRPV1-ablated mice). (C) IB4 staining is selectively eliminated following DTX injection (MrgprD-ablated mice). (D) Combined intrathecal capsaicin and DTX injections eliminate both TRPV1 and IB4 staining (Double-ablated mice).
Ablation of the central terminals of TRPV1+ nociceptors eliminates responses to low- but not high-dose formalin
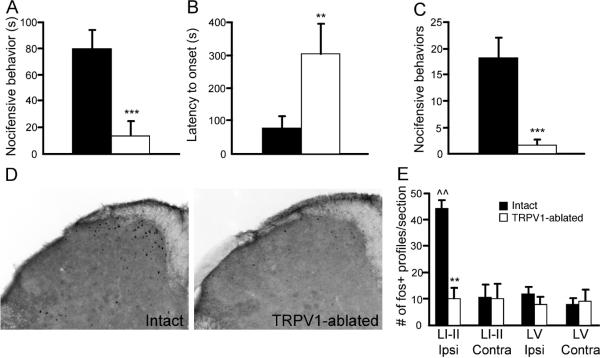
Injection of a 0.5% formalin solution into the hindpaw of intrathecal vehicle pre-treated mice evoked nocifensive behaviors for approximately the first 5 minutes after injection, but we did not record a clear second phase (Fig. 2a). Prior treatment with intrathecal capsaicin significantly reduced the behavioral response to 0.5% formalin (Fig. 2a). Thus, TRPV1-ablated mice displayed only 13.0 ± 11.4 s of nocifensive behavior, compared to 80.2 ± 14.4 s in intact mice (p < 0.005; Student's t-test). In half of the TRPV1-ablated mice, there was a complete elimination of low-dose formalin-evoked behavior. The remaining capsaicin pre-treated mice exhibited drastically reduced behavior, and in addition, this residual behavior was severely delayed compared to vehicle pre-treated controls (p < 0.01, Student's t-test; Fig 2b). It is likely that the lack of behavioral response to low-dose formalin in capsaicin pre-treated animals is due to a loss of TRPA1-dependent signaling, as nocifensive responses to the TRPA1-selective agonist, mustard oil, were also eliminated following capsaicin pre-treatment (intact and TRPV1-ablated mice had 18.2 ± 4 and 1.4 ± 1.2 flinches following mustard oil application, respectively; p < 0.005, Student's t-test; Fig 2c).
Figure 2. Responses to low-dose formalin are eliminated in TRPV1-ablated mice.
(A) Nocifensive behavior during the first 10 minutes following intraplantar injection of 0.5% formalin. Behavior is significantly reduced following intrathecal capsaicin treatment. (B) For TRPV1-ablated mice that exhibited nocifensive behavior (3 mice out of 6 total), latency to onset of nocifensive behavior following 0.5% formalin injection is drastically increased. (C) Mustard-oil induced nocifensive behavior is eliminated in TRPV1-ablated mice. (D) Spinal cord staining for Fos immunoreactivity in Intact and TRPV1-ablated mice following 0.5% formalin injection. This dose of formalin induced Fos expression in laminae I–II, but not in lamina V, of Intact mice. In TRPV1-ablated mice, 0.5% formalin failed to induce Fos in either region. (E) Average number of Fos-positive profiles (per 40 μm section) in laminae I–II (LI-II) and lamina V (LV), both ipsilateral and contralateral to the injection site, in Intact and TRPV1-ablated mice. For all panels, black bars = Intact mice, and white bars = TRPV1-ablated mice. For panels A–C, n = 6 per group; **p < 0.01; ***p < 0.005 compared to Intact animals, Student's t-test. For panel E, n = 3 per group, **p < 0.01 compared to Intact animals, ^^p < 0.01 compared to contralateral side, Two-way ANOVA with Bonferroni post-hoc test.
Using dorsal horn Fos induction as a measure of neuronal activation following noxious stimulation [1,6,19], we mapped the number and distribution of spinal cord neurons that responded to hindpaw injection of formalin. Injection of low-dose formalin resulted in robust Fos induction in the superficial dorsal horn of control animals (average 44.6 ± 2.8 Fos-positive neurons per section in laminae I–II ipsilateral to the injection in control mice, compared to 10.8 ± 5.1 Fos-positive neurons on the contralateral side; p < 0.01 compared to contralateral side, paired Student's t-test; Fig. 2d, e). In contrast, TRPV1-ablated mice had only 10.5 ± 3.8 Fos-positive neurons in the same region, which was not significantly different from the contralateral side (p < 0.01 compared to the ipsilateral side in intact animals; p > 0.05 compared to contralateral side, two-way ANOVA with Bonferroni post-hoc test; Fig 2d, e). These results indicate that capsaicin pre-treatment prevented spinal cord Fos induction, which correlates well with the greatly reduced nocifensive behaviors observed after formalin injection in the capsaicin pre-treated mice. The low dose of formalin did not induce significant Fos expression in neurons of lamina V of the spinal cord, in either group of mice.
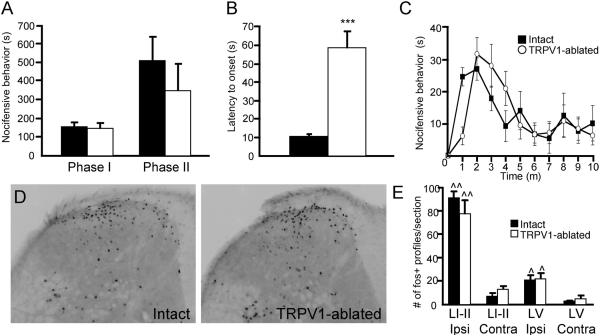
Compared to the 0.5% dose, injection of 2% formalin in intact mice resulted in a more robust nocifensive response that included both phases of behaviors (Fig. 3a). Surprisingly, capsaicin pre-treatment did not alter the total magnitude of formalin-induced nocifensive behavior in either phase at the 2% dose (Fig. 3a), which is commonly used in the formalin test. However, as for 0.5% formalin, capsaicin pre-treatment significantly delayed the onset of behavior following the formalin injection (onset 10.7 ± 1.1 s in intact mice and 59.1 ± 10.0 s in TRPV1-ablated mice; p < 0.0005, Student's t-test; Fig. 3b, c). Note that the delay in behavioral onset was compensated for by an increased response duration in the subsequent few minutes, such that the overall time course of formalin responses was not altered by capsaicin pre-treatment (Fig. 3c).
Figure 3. Responses to high-dose formalin are not altered in TRPV1-ablated mice.
(A) Nocifensive behavior during phase I (minutes 0–10) and phase II (minutes 10–50) following intraplantar injection of 2% formalin is normal in TRPV1-ablated mice. (B) Latency to onset of nocifensive behavior following 2% formalin injection is increased in TRPV1-ablated mice. (C) Nocifensive behavior of intact and TRPV1-ablated mice during the first 10 minutes after injection of 2% formalin (response duration is summed in 1 minute bins). Note that the total amount of behavior in Phase I does not differ in the two groups, but that the onset of nocifensive behavior is delayed in TRPV1-ablated mice. (D) Spinal cord staining for Fos immunoreactivity in Intact and TRPV1-ablated mice following 2% formalin injection. This dose of formalin induced equivalent Fos expression in Intact and TRPV1-ablated mice, both in laminae I–II and lamina V. (E) Average number of Fos-positive profiles (per 40 μm section) in laminae I–II (LI-II) and lamina V (LV), both ipsilateral and contralateral to the injection site, in Intact and TRPV1-ablated mice. For all panels, black bars/symbols = Intact mice, and white bars/symbols = TRPV1-ablated mice. For panels A and B, n = 7 per group; ***p < 0.0005 compared to Intact animals, Student's t-test. For panel E, n = 3 per group, ^p < 0.05, ^^p < 0.01 compared to contralateral side, Two-way ANOVA with Bonferroni post-hoc test.
Consistent with its lack of effect on high-dose formalin nocifensive behaviors, capsaicin pre-treatment also did not alter the extent of Fos induction following hindpaw injection of 2% formalin. In vehicle pre-treated control mice, high-dose formalin resulted in robust Fos expression in the superficial dorsal horn (laminae I–II) as well as in lamina V. In fact, capsaicin pre-treated mice were indistinguishable from controls in both the extent and topography of Fos expression (Fig 3d, e). This was true not only for the superficial laminae but also in lamina V (Fig 3d, e).
Ablation of MrgprD+ nociceptors does not affect formalin responses
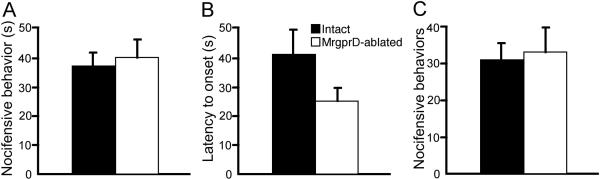
Mice lacking MrgprD+ nociceptors responded normally to injection of both 0.5% and 2% formalin (Fig. 4a, b; Fig 5a, b). Thus, following 0.5% formalin injection, intact and MrgprD-ablated mice displayed equivalent duration of nocifensive behavior (37.3 ± 4.5 s vs. 40.0 ± 6.3 s for intact vs. MrgprD-ablated mice, respectively; p = 0.734, Student's t-test; Fig 4a). Similarly, the latency to behavioral onset was not altered by MrgprD ablation (Fig. 4b; p = 0.117, Student's t-test). In response to 2% formalin injection MrgprD-ablated mice also showed normal phase I and II behaviors (Fig. 5a, b), and comparable Fos induction in laminae I–II as well as lamina V (Fig. 5c, d). Thus, MrgprD+ afferents are dispensable for cellular and behavioral responses to both low- and high-dose formalin injection.
Figure 4. Responses to low-dose formalin are unaltered in MrgprD-ablated mice.
(A) Nocifensive behavior during the first 10 minutes following intraplantar injection of 0.5% formalin in unchanged in MrgprD-ablated mice. Latency to onset of nocifensive behavior following 0.5% formalin injection is unchanged in MrgprD mice. (C) Mustard-oil induced nocifensive behavior is normal in MrgprD-ablated mice. For all panels, black bars = Intact mice, and white bars = MrgprD-ablated mice; n = 7 per group.
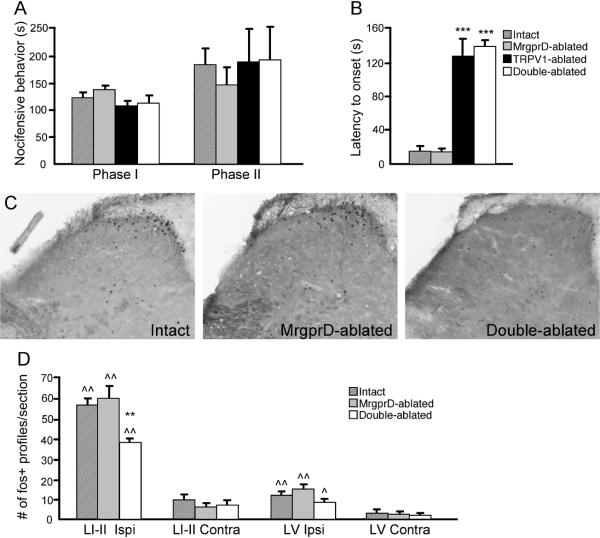
Figure 5. Nocifensive behavior in response to 2% formalin injection persists in mice lacking both TRPV1+ and MrgprD+ afferents.
(A) Nocifensive behavior during phase I (minutes 0–10) and phase II (minutes 10–50) following intraplantar injection of 2% formalin is equivalent in mice lacking TRPV1+ and MrgprD+ afferents, either singly or in combination. (B) Latency to onset of nocifensive behavior is significantly increased in TRPV1-ablated and Double-ablated mice. (C) Spinal cord staining for Fos immunoreactivity in Intact, MrgprD-ablated, and Double-ablated mice following 2% formalin injection. This dose of formalin induced Fos expression in laminae I–II, and lamina V, in Intact, MrgprD-ablated, and Double-ablated mice, however, there was a significant reduction of laminae I–II Fos in Double-ablated mice. (E) Average number of Fos-positive profiles (per 40 μm section) in laminae I–II (LI-II) and lamina V (LV), both ipsilateral and contralateral to the injection site, in Intact, MrgprD-ablated, and Double-ablated mice. For all panels, diagonal stripes = Intact mice, gray bars = MrgprD-ablated mice, black bars = TRPV1-ablated mice, and white bars = Double-ablated mice. For panels A–B, n = 7 per group for Intact, MrgprD-ablated and TRPV1-ablated mice, and n = 4 for Double-ablated mice; ***p < 0.005 compared to Intact animals, ANOVA with Bonferroni post-hoc test. For panel D, n = 4 for Intact group, n = 3 for MrgprD-ablated and Double-ablated, **p < 0.01 compared to Intact animals, ^p < 0.05, ^^p < 0.01 compared to contralateral side, Two-way ANOVA with Bonferroni post-hoc test.
It is of interest that we observed significant differences in the response magnitude between control animals in the intrathecal capsaicin studies, which were all performed on C57Bl/6 mice, and those in MrgprD ablation studies, which were performed in mice on a mixed C57Bl/6/129Sv background. Thus, vehicle pre-treated C57Bl/6 mice exhibited more nocifensive behavior after 0.5% formalin, and in the second phase after the 2% formalin injection, than did control MrgprD mice. This is readily appreciated by comparing the magnitude of phase II behavior of intact animals in Figs. 3a and 5a. Concomitantly, we also observed greater Fos induction after 2% formalin injection in C57Bl/6 mice. We attribute these results to strain-dependent differences in pain-related behaviors, which have been extensively documented [13,29]. Consistent with the differences that we observed, Wilson et al (2002) reported that C57Bl/6 mice display markedly greater phase II behavior than do 129J mice following 5% formalin injection. Importantly, the magnitude of phase II behavior following 2% formalin injection in capsaicin pre-treated MrgprDDTR mice was indistinguishable from that of intact and MrgprD-ablated mice (Fig. 5a). Thus, the significant differences in baseline phase II behavior that we observed between C57Bl/6 and C57Bl/6/129Sv mice are likely due to differences in strain, rather than to the various treatments that were studied.
Behavioral responses to high-dose formalin are not altered by combined ablation of both TRPV1+ and MrgprD+ nociceptors
As nocifensive behavior induced by 2% formalin injection was unchanged in mice lacking either TRPV1+ or MrgprD+ nociceptors, we next tested whether combined ablation, which eliminates the great majority of C-fiber nociceptors, could reduce formalin responses. Figure 5a illustrates that the magnitude of both phase I and II behavior was, in fact, normal in these mice. As expected, there was a significant delay to the onset of phase I behavior (Fig. 5b). On the other hand, although the Double-ablated mice, lacking these two large populations of C-fibers, showed a topographically normal pattern of dorsal horn Fos expression, the number of Fos-expressing neurons in laminae I–II was significantly reduced compared to control animals (p < 0.01, two-way ANOVA with Bonferroni post-hoc test; Fig. 5c, d), although this still represented a significant increase compared to the contralateral side (p < 0.01, two-way ANOVA with Bonferroni post-hoc test). These results indicate that, despite the apparently normal behavioral response to formalin, there is some loss of information transfer between the primary afferents and the spinal cord when these two subsets of sensory neurons are ablated. However, activity in the surviving afferents is clearly sufficient to drive the full manifestation of pain-like behavior in the formalin test.
Discussion
In this study, we investigated the extent to which different populations of nociceptors contribute to formalin-evoked pain behaviors in the traditional formalin test. We found that behavioral responses and Fos induction produced by low-dose formalin were entirely dependent upon TRPV1-expressing nociceptors, as both were lost in mice pre-treated with intrathecal capsaicin, which selectively eliminates the central (dorsal horn) terminals of TRPV1-expressing afferents. By contrast, MrgprD+ nociceptors, which constitute the majority of cutaneous nonpeptidergic C-fibers, were dispensable for the low-dose formalin responses.
Recently, two independent groups reported that formalin behaviors induced by low-dose formalin (≤0.5%) are significantly attenuated in Trpa1 knockout mice [14,16]. Because TRPA1 is expressed in a subset of TRPV1+ nociceptors [10,23], it is likely that the loss of 0.5% formalin behavior in capsaicin pre-treated mice is due to an elimination of TRPA1-dependent signaling. Although Kwan et al. (2009) provided evidence for a more widespread expression of TRPA1 in primary afferents, including myelinated fibers [12] we found that nocifensive responses to mustard oil, a selective TRPA1 agonist, were also eliminated following ablation of TRPV1+ neurons (Fig. 2c), but were unaffected by ablation of MrgprD+ neurons (Fig. 4c). This argues that TRPA1 is indeed expressed in a subset of TRPV1 afferents, and that if it is present in non-TRPV1-expressing neurons, then the TRPA1 in those cells is not sufficient for mustard oil- or low-dose formalin-evoked responses.
In contrast to the 0.5% dose, we found that the behavioral responses to high-dose (2%) formalin, which is more commonly used in the formalin test, were not altered by elimination of either subset of nociceptor. Formalin-evoked Fos induction was also normal after separate ablation of the TRPV1 and MrgprD populations. In fact, even combined elimination of both populations of afferents, which comprise the majority of C-fiber nociceptors, did not reduce nocifensive behavior evoked by 2% formalin. This somewhat surprising result implies that myelinated afferents (presumably the Aδ subset) and/or the relatively small population of unmyelinated fibers spared by the different ablation protocols are sufficient to generate the behavioral response to formalin at doses typically used in the formalin test. Thus, activity in even a small minority of nociceptive afferents is enough to set off a cascade of events that produces a large effect on pain behavior, and possibly pain perception.
On the other hand, we did observe that Fos induction following 2% formalin was significantly attenuated in mice that lacked both TRPV1+ and MrgprD+ nociceptors. Moreover, the residual Fos immunoreactivity in these mice was noticeably less intense than that seen in intact animals (Fig. 5c). Additionally, capsaicin pre-treatment caused a significant delay in the onset of nocifensive behaviors, even when high doses were used. As Aδ fibers are engaged by formalin stimuli [20] and are expected to contribute to rapid conduction of nociceptive signaling, it is conceivable that the behavioral delay is related to the concurrent ablation of TRPV1-expressing Aδ fibers by intrathecal capsaicin pre-treatment. Despite the delay in onset, however, we found that the total amount of pain-like behavior was normal after 2% formalin injection in TRPV1-ablated animals, having been compensated by behavioral outputs in later time bins. Taken together these results suggest that although the great majority of C-fiber nociceptors are not required for high doses of formalin to evoke the full complement of pain behaviors in the formalin test, these neurons, in particular the TRPV1+ subset, do contribute to the spinal cord signaling provoked by this noxious stimulus. Conceivably, loss of this signaling component could influence the long-term consequences of formalin stimulation (e.g. post-formalin thermal hyperalgesia and mechanical allodynia [31]).
Somewhat at odds with our results, it was originally reported that intrathecal capsaicin injection in the adult rat reduced behavioral responses to 10% formalin [30]. Of course, a major difference between the mouse and rat is that, in the rat, TRPV1 is expressed by both the nonpeptidergic and peptidergic populations; in the mouse, TRPV1 is largely limited to the peptidergic population [32]. Thus, the effects of capsaicin treatment in the rat should produce a lesion similar to that caused by ablation of both TRPV1+ and Mrgprd+ afferents in the mouse. Potentially relevant also is that this earlier paper used a scoring method that assessed the degree of agitation of the rat, as opposed to the total amount of time engaged in nocifensive behavior. Thus, it is possible that the reduction in Fos that we observed in Double-ablated mice indeed reflected a decrease in high-dose formalin-evoked nociceptive processing, which could manifest as a reduced response intensity (as occurs in the rat), rather than a decrease in total response time (which was the behavioral endpoint used in the present study).
More recently, reductions in formalin behavior were observed in rats treated neonatally with capsaicin [17, 18], and this was accompanied by a reduction of spinal cord Fos [18] to a similar extent as that observed in Double-ablated mice. Importantly, the behavioral endpoint of this latter study was response duration, similar to that used in the current study. Despite the comparable behavioral endpoints, however, it is difficult to compare adult and neonatal capsaicin-induced neuronal ablation. This is, in part, because during development, TRPV1 is transiently expressed in a much larger population of neurons than it is in the adult [5]. It follows that neonatal capsaicin could ablate a much different (and more extensive) population of afferents than does intrathecal capsaicin in the adult, including a larger population of myelinated afferents. Furthermore, as systemic neonatal capsaicin treatment destroys the cell bodies of primary afferents, there is a loss of the peripheral terminals of these neurons. A neurogenic inflammatory action of formalin on these afferents (e.g. via TRPA1) may alter the local chemical milieu, which could in turn activate non-TRPV1 afferents so as to enhance nociceptive processing. Such an action would be preserved after intrathecal capsaicin. Finally, regardless of species, route, or timing, capsaicin injection does not completely eliminate formalin-induced behavioral responses, thus pointing to an important contribution of the remaining sensory afferents.
What is the anatomical and biochemical basis of the TRPV1-fiber- and MrgprD-fiber-independent component of the high-dose formalin response? As formalin is a chemical fixative, it is likely that the mechanism of afferent activation is non-receptor-mediated, but rather involves a generalized injury that affects all classes of afferents. Consistent with this hypothesis, we recently found that 5% formalin injection into the hindpaw induces widespread expression of the injury-associated transcription factor, ATF-3 [28], in a large population of myelinated and unmyelinated DRG neurons (manuscript submitted). Furthermore, Puig and Sorkin (1995) reported that injection of 2.5% formalin activates all types of afferents, including myelinated Aβ and Aδ fibers, as well as unmyelinated C-fibers, in the period corresponding to phase I of pain behavior, and that there is ongoing activity in Aδ and C fibers throughout phase II. It follows that at doses greater than 0.5%, a broad spectrum of afferent activity (not limited to nociceptors), contributes to the two phases of pain behavior in the formalin test.
Finally, our results provide insight into interpreting pharmacological effects of various therapeutic drugs in the formalin test, notably, morphine and other opioids, which are potent inhibitors of the behavioral and cellular responses to formalin injection [19,21,25]. In the normal animal, morphine acts at the mu opioid receptor (MOR) both pre- and post-synaptically, to block afferent drive (release of neurotransmitter from primary afferent nociceptors) and the discharge of dorsal horn interneurons and projection neurons [11,27], respectively. However, as intrathecal capsaicin deletes the great majority, if not all, primary afferents that express the MOR [22], we conclude that reduction of transmitter release from MOR-expressing nociceptors is unlikely to be sufficient to block pain behaviors in the formalin test. It follows that there must be a necessary contribution of postsynaptic MOR to morphine-induced antinociception in the formalin test. Consistent with this conclusion, Kline and Wiley [9] reported that the reduction by morphine of nocifensive behaviors in the formalin test is significantly attenuated following chemical ablation of dorsal horn neurons that express the MOR.
In conclusion, we have demonstrated two very distinct mechanisms through which formalin evokes nocifensive behaviors: acute chemonociception mediated by TRPV1+ afferents, which is most readily seen upon injection of a relatively low concentration (0.5%) and in the first minutes immediately after injection of a higher concentration (2%), vs. prolonged pain provoked by frank injury to all classes of sensory afferents. The latter mechanism likely operates throughout the majority of the first and all of the second phase and persists even in the absence of the vast majority of C-fiber nociceptors.
Acknowledgements
This work was supported in part by NIH PO1NS048499 to DJA and AIB, NIH NS14627 to AIB and by a grant from the Christopher and Dana Reeve Foundation to HL. The authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Abbadie C, Taylor BK, Peterson MA, Basbaum AI. Differential contribution of the two phases of the formalin test to the pattern of c-fos expression in the rat spinal cord: studies with remifentanil and lidocaine. Pain. 1997;69:101–110. doi: 10.1016/s0304-3959(96)03285-x. [DOI] [PubMed] [Google Scholar]
- [2].Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- [5].Hjerling-Leffler J, AlQatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci. 2007;27:2435–2443. doi: 10.1523/JNEUROSCI.5614-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- [7].Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- [9].Kline RHt, Wiley RG. Spinal mu-opioid receptor-expressing dorsal horn neurons: role in nociception and morphine antinociception. J Neurosci. 2008;28:904–913. doi: 10.1523/JNEUROSCI.4452-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- [11].Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh TL. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci. 2005;25:3651–3660. doi: 10.1523/JNEUROSCI.0252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci. 2009;29:4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].LaCroix-Fralish ML, Mo G, Smith SB, Sotocinal SG, Ritchie J, Austin JS, Melmed K, Schorscher-Petcu A, Laferriere AC, Lee TH, Romanovsky D, Liao G, Behlke MA, Clark DJ, Peltz G, Seguela P, Dobretsov M, Mogil JS. The beta3 subunit of the Na+,K+-ATPase mediates variable nociceptive sensitivity in the formalin test. Pain. 2009;144:294–302. doi: 10.1016/j.pain.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A. An ion channel essential for sensing chemical damage. J Neurosci. 2007;27:11412–11415. doi: 10.1523/JNEUROSCI.3600-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].McCall WD, Tanner KD, Levine JD. Formalin induces biphasic activity in C-fibers in the rat. Neurosci Lett. 1996;208:45–48. doi: 10.1016/0304-3940(96)12552-0. [DOI] [PubMed] [Google Scholar]
- [16].McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nagy JI, van der Kooy D. Effects of neonatal capsaicin treatment on nociceptive thresholds in the rat. J Neurosci. 1983;3:1145–1150. doi: 10.1523/JNEUROSCI.03-06-01145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Peterson MA, Basbaum AI, Abbadie C, Rohde DS, McKay WR, Taylor BK. The differential contribution of capsaicin-sensitive afferents to behavioral and cardiovascular measures of brief and persistent nociception and to Fos expression in the formalin test. Brain Res. 1997;775:9–16. doi: 10.1016/s0006-8993(97)00068-1. [DOI] [PubMed] [Google Scholar]
- [19].Presley RW, Menetrey D, Levine JD, Basbaum AI. Systemic morphine suppresses noxious stimulus-evoked Fos protein-like immunoreactivity in the rat spinal cord. J Neurosci. 1990;10:323–335. doi: 10.1523/JNEUROSCI.10-01-00323.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- [21].Rosland JH, Tjolsen A, Maehle B, Hole K. The formalin test in mice: effect of formalin concentration. Pain. 1990;42:235–242. doi: 10.1016/0304-3959(90)91167-H. [DOI] [PubMed] [Google Scholar]
- [22].Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- [24].Taylor BK, Peterson MA, Basbaum AI. Persistent cardiovascular and behavioral nociceptive responses to subcutaneous formalin require peripheral nerve input. J Neurosci. 1995;15:7575–7584. doi: 10.1523/JNEUROSCI.15-11-07575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Taylor BK, Peterson MA, Basbaum AI. Early nociceptive events influence the temporal profile, but not the magnitude, of the tonic response to subcutaneous formalin: effects with remifentanil. J Pharmacol Exp Ther. 1997;280:876–883. [PubMed] [Google Scholar]
- [26].Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- [27].Trafton JA, Abbadie C, Marchand S, Mantyh PW, Basbaum AI. Spinal opioid analgesia: how critical is the regulation of substance P signaling? J Neurosci. 1999;19:9642–9653. doi: 10.1523/JNEUROSCI.19-21-09642.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- [29].Wilson SG, Chesler EJ, Hain H, Rankin AJ, Schwarz JZ, Call SB, Murray MR, West EE, Teuscher C, Rodriguez-Zas S, Belknap JK, Mogil JS. Identification of quantitative trait loci for chemical/inflammatory nociception in mice. Pain. 2002;96:385–391. doi: 10.1016/S0304-3959(01)00489-4. [DOI] [PubMed] [Google Scholar]
- [30].Yaksh TL, Farb DH, Leeman SE, Jessell TM. Intrathecal capsaicin depletes substance P in the rat spinal cord and produces prolonged thermal analgesia. Science. 1979;206:481–483. doi: 10.1126/science.228392. [DOI] [PubMed] [Google Scholar]
- [31].Zeitz KP, Giese KP, Silva AJ, Basbaum AI. The contribution of autophosphorylated alpha-calcium-calmodulin kinase II to injury-induced persistent pain. Neuroscience. 2004;128:889–898. doi: 10.1016/j.neuroscience.2004.07.029. [DOI] [PubMed] [Google Scholar]
- [32].Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;15:4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]