Immunoprotective Activity and Safety of a Respiratory Syncytial Virus Vaccine: Mucosal Delivery of Fusion Glycoprotein with a CpG Oligodeoxynucleotide Adjuvant (original) (raw)
Abstract
CpG oligodeoxynucleotides (ODN) were identified that stimulated immunoglobulin production and cell proliferation in cotton rat cells in vitro. Three of these ODN were used as a mucosal adjuvant in the noses of cotton rats immunized via this route with respiratory syncytial virus fusion (F) protein. The CpG ODN markedly increased the cotton rat humoral neutralizing-antibody response to respiratory syncytial virus. Such immunized animals had a marked reduction in the production of infectious virus after a live-virus challenge. Animals immunized with the combination of F protein and CpG developed enhanced pulmonary pathology consisting of alveolitis and interstitial pneumonitis after a live-virus challenge. Similar enhanced disease has been seen in cotton rats and children immunized with formalin-inactivated respiratory syncytial virus.
Respiratory syncytial virus (RSV) is the primary cause of infectious pulmonary disease in infants and children throughout the world (4) and is increasingly recognized as a cause of serious disease in adults, particularly the elderly (9) and patients whose immune systems have been compromised (16). Unsuccessful efforts to prevent RSV disease by using vaccines have spanned nearly 4 decades (5).
RSV expresses two major glycoproteins on its surface, designated G (attachment) and F (fusion), the latter being the primary stimulus for neutralizing antibody. The structure of G is quite variable among strains of RSV. In contrast, F is heat stable and its structure is well conserved, making it an attractive antigen for vaccine development (4). A vaccine formulation that could be given via nose drops or a spray would be especially useful in developing countries, where a general lack of refrigeration limits the utility of heat-labile vaccines. Furthermore, a vaccine that could be given nasally rather than by injection would be advantageous in a setting where disposable or sterilized reusable syringes and needles are not always available. Unfortunately, nonreplicating antigens such as F are poorly immunogenic when applied to mucosal surfaces, such as the inside of the nose. Thus, an effective F-antigen mucosal vaccine will likely require an adjuvant.
Previous studies suggested that a Th2 immune response to RSV antigens was associated with immunopathology upon subsequent infection (29), a pattern similar to that seen in vaccine-enhanced RSV disease in the 1960s (2, 6, 12, 13). In contrast, CpG oligodeoxynucleotides (ODN) preferentially facilitate the induction of Th1 responses. CpG ODN mimic the ability of bacterial DNA to trigger lymphocytes and macrophages to secrete polyreactive antibodies and/or immunomodulatory cytokines and chemokines (including gamma interferon, interleukin-6 [IL-6], IL-12, IL-18, and tumor necrosis factor alpha). Moreover, CpG ODN are effective as vaccine adjuvants in mice and nonhuman primates when administered to mucosal surfaces (14). Other mucosal adjuvants have also been used (20). The present report examines the ability of CpG ODN to boost the serum antibody response and protective immunity induced by purified RSV F protein administered intranasally.
MATERIALS AND METHODS
Ig enzyme-linked immunosorbent assay.
A single-cell suspension of cotton rat splenocytes (106/well) was incubated with various CpG and control ODN at 1 μM for 36 h. Culture supernatants were diluted 1:20 and added to 96-well Immulon I microtiter plates previously coated with goat anti-mouse immunoglobulin G [IgG] in phosphate-buffered saline, which cross-reacts with cotton rat IgG (Southern Biotechnology Associates, Birmingham, Ala.). After 2 h, the plates were washed and treated with alkaline phosphatase-conjugated goat anti-mouse heavy-chain-specific Ig, which is cross-reactive against cotton rat heavy chain (1:3,000; Southern Biotechnology Associates). The plates were incubated at room temperature for 2 h, washed, and then developed with _p_-nitrophenylphosphate (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) in diethanolamine buffer (pH 9.8). A mouse monoclonal antibody to cotton rat IgM heavy chain was substituted for anti-IgG in the assay for IgM production.
Proliferation assays.
Cotton rat spleen cells (105/well) were incubated with 1 μM ODN for 72 h, pulsed with 1 μCi of [3H]thymidine, and harvested 4 h later. [3H]thymidine incorporation was quantitated with a 1205 Beta plate liquid scintillation counter (LKB Wallac, Gaithersburg, Md.). The proliferation index represents the fold difference between stimulated and unstimulated cells. All assays were performed in triplicate.
CpG ODN.
Phosphorothioate ODN were synthesized at the Center for Biologics Evaluation and Research core facility. The immunostimulatory CpG ODN used as vaccine adjuvants had the sequences GCTAGACGTTAGCGT (1555), TCAACGTTGA (1466), and ATCGACTCTCGAGCGTTCTC (K3). Control ODN had the same sequence as 1555 and 1466, except that the CpG motifs (underlined) were switched to GpC, including GCTAGAGCTTAGGCT (1471) and TCAAGCTTGA (1612). All ODN were tested for endotoxin content by the Limulus amoebocyte lysate assay (QCL-1000; BioWhittaker, East Rutherford, N.J.; courtesy of Donald Hochstein, Division of Product Quality Control, CBER/FDA) and for protein contamination with a Pierce bicinchoninic acid protein assay kit (Pierce Chemicals). Both Limulus amoebocyte lysate activity and protein levels were undetectable.
F glycoprotein, challenge virus, and assay of virus.
The Long strain (group A) of RSV was obtained from the American Type Culture Collection. Virus stocks were prepared in HEp-2 cells and contained 106 PFU per milliliter. Viral titers in stocks and in organ homogenates were determined by plaque assay on HEp-2 cells (25). F glycoprotein was purified from similar stocks by concanavalin A column chromatography. RSV-infected HEp-2 cells were grown to confluence, trypsinized, and centrifuged for 10 min at 4°C and 770 × g. Cell pellets were treated with lysis buffer containing 1% octyl-d-glucoside. The cell lysate was clarified through a 0.22-μm-pore-size filter and diluted 1:5 with phosphate-buffered saline. RSV F was eluted from a concanavalin A column with a buffer containing 10 mM Tris, 0.5 M NaCl, 1 mM CaCl2, 1 mM MgCl2, 5% ethanol, 0.02% sodium azide, and 0.5 M methyl mannose. The protein content of the eluate, which was used to immunize animals, was 80% F glycoprotein.
Histologic analysis.
Lungs were inflated intratracheally with 10% neutral buffered formalin to physiologic volume, ligated, and immersed in formalin. Following paraffin embedding, 4-μm coronal sections were stained with hematoxylin and eosin. Four types of inflammation were scored in each lung section: peribronchiolitis (inflammatory cells, primarily lymphocytes, surrounding a bronchiole), perivasculitis (inflammatory cells, primarily lymphocytes, surrounding a blood vessel), alveolitis (inflammatory cells within alveolar spaces), and interstitial pneumonitis (increased thickness of alveolar walls associated with inflammatory cells). Each of these was scored separately for each histologic section as described in our prior reports (23, 26, 27). Prior to scoring, all of the slides, plus reference slides from prior experiments, were examined to determine the range of pathology, whereupon the maximum of each type was assigned a value of 100. The slides were then randomized, read blindly, and scored for each lesion as a percentage of the maximum. The four scores for each lung section were then added together to obtain the combined score shown in Fig. 2.
FIG. 2.
Responses of cotton rats immunized with various concentrations of RSV F protein with or without various concentrations of ODN (CpG). The left graph shows the pulmonary virus titer (log10 PFU per gram) 4 days after an RSV challenge, and the right graph shows the combined pathology score. Asterisks denote statistically significant differences (P < 0.01) in comparison with untreated animals (group 1).
Animals.
Young adult cotton rats of both sexes, in equivalent numbers, were obtained from the colony of inbred Sigmodon hispidus maintained at Virion Systems, Inc. No differences in response to vaccine formulations or viral challenge were observed between the sexes. Animals were housed in large polycarbonate rat cages on a bedding of wood shavings and fed standard rodent chow and water ad libitum.
Neutralizing-antibody assay.
Serum samples were subjected to a plaque reduction neutralization assay with a 60% endpoint (3).
Statistical analysis.
Geometric means (viral and neutralizing-antibody titers) and standard errors were calculated for each time point, and differences among groups were evaluated by the Student t test. The relationship between neutralizing-antibody titers and increased vaccine dosage was evaluated with Spearman's rho.
RESULTS
Identification of CpG ODN with activity in cotton rats.
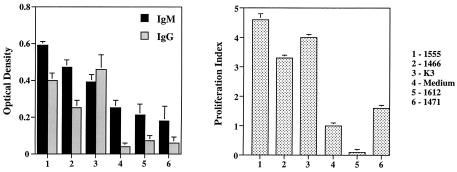
A panel of CpG ODN was evaluated for the ability to stimulate spleen cells from cotton rats. The panel included ODN previously shown to be effective in mice, rats, pigs, and primates (15). The activity of these ODN was evaluated by monitoring the induction of IgM production and cell proliferation. A group of three ODN—1555, 1466, and K3—was found to be stimulatory in both assays (Fig. 1). Since previous studies showed that mixtures of active ODN were broadly immunostimulatory, an equimolar mixture of 1555, 1466, and K3 was used in all subsequent studies.
FIG. 1.
Responses of cotton rat splenocytes to various ODN structures. Immunoglobulin production is on the left graph, and the proliferation index is on the right.
Experimental design.
Young adult cotton rats were divided into groups of five or more animals each, tagged, and bled via the retro-orbital venous plexus. A control group received no treatment; other groups were anesthetized with isoflurane and then inoculated intranasally with various amounts of purified F protein, with or without CpG ODN, in a volume of 100 μl (Table 1). Two weeks later, the animals were boosted with the same formulations. At week 4, all animals were anesthetized, bled, and challenged intranasally with 105 PFU of RSV strain Long in a volume of 100 μl. Four days postchallenge, the cotton rats were sacrificed by carbon dioxide intoxication. Lungs were bisected, with half of the tissue used for viral titration and the other half used for histopathologic analysis. Nasal tissues were homogenized for viral titration.
TABLE 1.
Vaccine formulations used in this study
| Group | Amt of F glycoprotein (ng) | Amt of CpG (μg) |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 10 | 0 |
| 3 | 10 | 20 |
| 4 | 10 | 100 |
| 5 | 50 | 0 |
| 6 | 50 | 20 |
| 7 | 50 | 100 |
| 8 | 250 | 0 |
| 9 | 250 | 20 |
| 10 | 250 | 100 |
| 11 | 1,250 | 0 |
| 12 | 1,250 | 20 |
| 13 | 1,250 | 100 |
Effect of vaccination on viral replication.
Preliminary experiments were conducted to determine the general dose range of F protein and CpG ODN that provides detectable protection (data not shown). The results were used to design the dose-response experiment shown in Fig. 2. F glycoprotein, administered alone over a 125-fold dose range, did not reduce viral titers. In contrast, when F protein was coadministered with CpG ODN, a significant viral-load reduction was observed upon challenge. When combined with 250 ng of F protein, 100 μg of CpG ODN was more effective than 20 μg. With the larger dose of F protein, the two doses of CpG ODN were equally effective. The larger doses of CpG ODN, when mixed with 250 or 1,250 ng of F protein, reduced pulmonary viral titers by 1,000-fold, often below detectable levels (P < 0.01). In contrast, none of the formulations significantly reduced viral titers in nasal tissues (data not shown), a finding consistent with our earlier reports of both active and passive (immunoglobulin) immunizations showing that the lungs are far easier to protect than the nose (20, 28). Neither CpG ODN administered in the absence of F protein nor control (GpC) ODN administered with 1,250 ng of F protein reduced the level of viral replication in the lungs or nose (data not shown).
Neutralizing antibody.
Neutralizing-antibody titers in animals receiving the largest dose of F protein were compared with those of control animals. No neutralizing activity (titers were uniformly <20) was found in the control animals; geometric mean titers (five or more animals per group) were 21 ± 1 for 1,250 ng of F without CpG, 59 ± 2 for F protein with 20 μg of CpG [t(8) = 19.13, <0.001 versus the preceding group], and 125 ± 2 for F protein with 100 μg of CpG [t(11) = 23.77, P < 0.001 versus the preceding group]. Neutralizing-antibody titers and increased vaccine dosages were significantly positively correlated (Spearman's rho = +0.496, P < 0.05).
Histopathology.
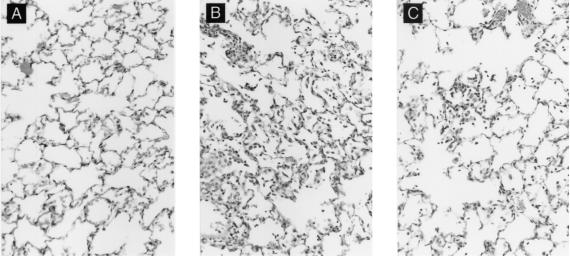
Previous experience with RSV vaccines indicated that unwanted immunopathology could accompany the induction of “protective” immune responses. To assess the safety of the F-protein-CpG ODN combination, cotton rats were primed, boosted, and challenged as described above. Consistent with earlier reports (27), naive animals challenged with RSV developed mild peribronchiolitis and perivasculitis and essentially no alveolitis or interstitial pneumonitis (Fig. 3A). A similar histologic picture was observed in animals immunized with F protein alone, a treatment that did not reduce viral titers (Fig. 2, groups 2, 5, 8, and 11, and data not shown). Immunization with F protein plus CpG ODN, however, resulted in enhanced pulmonary histopathology consisting of alveolitis and interstitial pneumonitis, in addition to peribronchiolitis and perivasculitis (Fig. 3B). The degree of pathology rose as the doses of F protein and CpG ODN increased, despite this combination's beneficial effects on the viral load (Fig. 2, groups 12 and 13). This enhanced disease resembled that caused by formalin-inactivated RSV vaccine (Fig. 3C) but was more severe (27). In contrast, 1,250 ng of F protein, when coadministered with control GpC ODN, caused no enhanced histopathology (data not shown).
FIG. 3.
Photomicrographs of cotton rat lung tissue. (A) Group 1, unimmunized, challenged with RSV, and sacrificed 4 days after challenge. The alveolar area shown has very mild interstitial thickening and rare inflammatory cells in the alveoli. This animal also had mild peribronchiolitis and perivasculitis (not shown). (B) Group 12, immunized with 1,250 ng of RSV F and 20 μg of CpG, challenged with RSV, and sacrificed 4 days later. Marked interstitial pneumonitis and inflammatory cell infiltrates in alveoli. The degree of peribronchiolitis and perivasculitis (not shown) was also enhanced. (C) For comparison, lung tissue of a cotton rat immunized with formalin-inactivated whole RSV and challenged with RSV. Similar but milder interstitial pneumonitis and alveolar inflammatory-cell infiltrate compared with panel B. (The animal whose tissue is shown in panel C was from studies described in reference 27.) All micrographs were stained with hematoxylin and eosin. Original magnification, ×64.
DISCUSSION
In the absence of a safe and effective RSV vaccine, the only available means of RSV prophylaxis involves administration of human polyclonal RSV antibody (21) or, more recently, a humanized monoclonal antibody, Synagis (MedImmune, Inc., Gaithersburg, Md.), which is typically given monthly to high-risk infants (11). While highly effective in reducing hospitalization from RSV, the high cost of Synagis treatment prevents this therapy from being used in healthy infants, children, and adults, who nonetheless suffer from repeated RSV infections throughout life. An effective RSV vaccine that is economical, heat stable (for use in developing countries), and easily administered would therefore be of great benefit to normal-risk patients.
CpG ODN show promise as adjuvants for vaccines that are administered systemically or mucosally (18). Both the immunogenicity and protective efficacy of vaccines against hepatitis B (17) and herpes simplex type 2 (7) were improved by coadministration of CpG ODN. Consistent with those reports, we found that the combination of F protein plus CpG ODN significantly increased neutralizing-antibody titers and reduced the titer of infectious RSV following a live-virus challenge. Neutralizing-antibody titers correlated well with increased vaccine dosages and increased protection. However, previous studies involving the passive transfer of anti-RSV antibodies required far higher antibody titers for protection (24) than those seen in the present experiments, suggesting that antibody alone is insufficient to account for the protection observed. Thus, it seems likely that immunization with F protein plus CpG ODN stimulates additional non-antibody-dependent immune responses that contribute to the observed immunity. Parenteral immunization of mice with RSV F protein and both aluminum hydroxide (alum) and CpG adjuvants led to an enhanced and protective type 1 immune response (10).
Despite the promising findings in the present study, the combination of CpG ODN plus F protein was associated with considerable pulmonary histopathology following an RSV challenge. Maximal histopathology developed in animals that received the largest doses of F protein and ODN, despite these animals having the greatest degree of protection against infection. These findings suggest that the lung pathology arises from the proinflammatory immune response induced by vaccination and actuated by virus infection.
This enhancement of lung pathology, although disappointing in view of the promising initial findings that immunization with CpG ODN plus F protein reduced viral titers, is a recurring theme within RSV vaccinology. Such pathology was first appreciated during clinical trials of a formalin-inactivated vaccine, which resulted in severely enhanced (and in some cases fatal) disease (2, 6, 12, 13). The histopathologic changes seen in the two children who died in that study (23) are similar to those observed with other nonreplicating RSV vaccines (19, 22), as well as those in the present report. Lingering concerns that novel nonreplicating RSV vaccines might also predispose to the development of proinflammatory immune-mediated lung pathology have cast a persistent shadow over RSV vaccinology. Indeed, no nonreplicating candidate vaccine has advanced to clinical trials in immunologically naive infants in 4 decades. Nonetheless, the desirability of a heat-stable vaccine that could be administered without needles, combined with ongoing difficulties in formulating a safe, effective, and genetically stable live-attenuated RSV vaccine, provides the impetus for continued research.
The mechanisms underlying the enhanced disease described in the present report are not known. Studies with mice suggest that the F protein of RSV elicits a Th2-biased immune response (8). However, the similarly enhanced disease that was observed in this report was associated with the use of CpG ODN, which favor the induction of a Th1 response. It thus appears that vaccine-enhanced RSV disease is multifactorial and may not be susceptible to simple categorization. We are in the process of developing the cotton rat-specific reagents that will allow us to define cytokine, chemokine, and cellular profiles in various forms of vaccine-enhanced disease and thus gain insights into mechanisms (1).
Acknowledgments
The assertions herein are our private ones and are not to be construed as official or as reflecting the views of the Food and Drug Administration at large.
This work was supported in part by a grant from the National Vaccine Program, by Military Interdepartmental Purchase Request MM8926, and by Virion Systems, Inc., corporate funds.
REFERENCES
- 1.Blanco, J. C. G., J. Y. Richardson, M. E. R. Darnell, A. Rowzee, L. Pletneva, D. D. Porter, and G. A. Prince. 2002. Cytokine and chemokine gene expression after primary and secondary respiratory syncytial virus infection in cotton rats. J. Infect. Dis. 185**:**1780-1785. [DOI] [PubMed] [Google Scholar]
- 2.Chin, J., R. L. Magoffin, L. A. Shearer, J. H. Schieble, and E. H. Lennette. 1969. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am. J. Epidemiol. 89**:**449-463. [DOI] [PubMed] [Google Scholar]
- 3.Coates, H. V., and R. M. Chanock. 1966. An antigenic analysis of respiratory syncytial virus isolates by a plaque reduction neutralization test. Am. J. Epidemiol. 83**:**299-313. [DOI] [PubMed] [Google Scholar]
- 4.Collins, P. L., R. M. Chanock, and B. R. Murphy. 2001. Respiratory syncytial virus, p. 1443-1485. In: D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 5.Crowe, J. E. 2002. Respiratory syncytial virus vaccine development. Vaccine 20(Suppl. 1)**:**S32-S37. [DOI] [PubMed] [Google Scholar]
- 6.Fulginiti, V. A., J. J. Eller, O. F. Sieber, J. W. Joyner, M. Minamitani, and G. Meiklejohn. 1969. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines: an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am. J. Epidemiol. 89**:**435-448. [DOI] [PubMed] [Google Scholar]
- 7.Gallichan, W. S., R. N. Woolstencroft, T. Guarasci, M. J. McCluskie, H. L. Davis, and K. L. Rosenthal. 2001. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 166**:**3451-3457. [DOI] [PubMed] [Google Scholar]
- 8.Graham, B. S., T. R. Johnson, and R. S. Peebles. 2000. Immune-mediated disease pathogenesis in respiratory syncytial virus infection. Immunopharmacology 48**:**237-247. [DOI] [PubMed] [Google Scholar]
- 9.Han, L. L., J. P. Alexander, and L. J. Anderson. 1999. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J. Infect. Dis. 179**:**25-30. [DOI] [PubMed] [Google Scholar]
- 10.Hancock, G. E., K. M. Heers, J. D. Smith, C. A. Scheuer, A. R. Ibraghimov, and K. S. Pryharski. 2001. CpG containing oligodeoxynucleotides are potent adjuvants for parenteral vaccination with the fusion (F) protein of respiratory syncytial virus (RSV). Vaccine 19**:**4874-4882. [DOI] [PubMed] [Google Scholar]
- 11.IMpact-RSV Study Group. 1998. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 102**:**531-537. [PubMed] [Google Scholar]
- 12.Kapikian, A. Z., R. H. Mitchell, R. M. Chanock, R. A. Shvedoff, and C. E. Stewart. 1969. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 89**:**405-421. [DOI] [PubMed] [Google Scholar]
- 13.Kim, H. W., J. G. Canchola, C. D. Brandt, G. Pyles, R. M. Chanock, K. Jensen, and R. H. Parrott. 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 89**:**422-434. [DOI] [PubMed] [Google Scholar]
- 14.Klinman, D. M., K. M. Barnhart, and J. Conover. 1999. CpG motifs as immune adjuvants. Vaccine 17**:**19-25. [DOI] [PubMed] [Google Scholar]
- 15.Krieg, A. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20**:**709-760. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy, A. J., H. M. Kingman, C. Kelly, G. S. Taylor, E. O. Caul, D. Grier, J. Moppett, A. B. M. Foot, J. M. Cornish, A. Oakhill, C. G. Steward, D. H. Pamphilon, and D. I. Marks. 1999. The outcome of 26 patients with respiratory syncytial virus infection following allogeneic stem cell transplantation. Bone Marrow Transplant. 24**:**1315-1322. [DOI] [PubMed] [Google Scholar]
- 17.McCluskie, M. J., and H. L. Davis. 1998. Cutting edge: CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J. Immunol. 161**:**4463-4466. [PubMed] [Google Scholar]
- 18.McCluskie, M. J., R. D. Weeratna, P. J. Payette, and H. L. Davis. 2001. The potential of CpG oligodeoxynucleotides as mucosal adjuvants. Crit. Rev. Immunol. 21**:**103-120. [PubMed] [Google Scholar]
- 19.Murphy, B. R., A. V. Sotnikov, L. A. Lawrence, S. M. Banks, and G. A. Prince. 1990. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3-6 months after immunization. Vaccine 8**:**497-502. [DOI] [PubMed] [Google Scholar]
- 20.Pizza, M., M. M. Giuliani, M. R. Fontana, E. Monaci, G. Douce, G. Dougan, K. H. G. Mills, R. Rappuoli, and G. Del Giudice. 2001. Mucosal vaccines: nontoxic derivatives of LT and CT as mucosal adjuvants. Vaccine 19**:**2534-2541. [DOI] [PubMed] [Google Scholar]
- 21.PREVENT Study Group. 1997. Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics 99**:**93-99. [DOI] [PubMed] [Google Scholar]
- 22.Prince, G. A., C. Capiau, M. Deschamps, L. Fabry, N. Garçon, D. Gheysen, J.-P. Prieels, G. Thiry, O. Van Opstal, and D. D. Porter. 2000. Efficacy and safety studies of a recombinant chimeric respiratory syncytial virus FG glycoprotein vaccine in cotton rats. J. Virol. 74**:**10287-10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prince, G. A., S. J. Curtis, K. C. Yim, and D. D. Porter. 2001. Vaccine-enhanced respiratory syncytial virus (RSV) disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J. Gen. Virol. 82**:**2881-2888. [DOI] [PubMed] [Google Scholar]
- 24.Prince, G. A., R. L. Horswood, and R. M. Chanock. 1985. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J. Virol. 55**:**517-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prince, G. A., A. B. Jenson, R. L. Horswood, E. Camargo, and R. M. Chanock. 1978. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am. J. Pathol. 93**:**771-791. [PMC free article] [PubMed] [Google Scholar]
- 26.Prince, G. A., D. D. Porter, A. B. Jenson, R. L. Horswood, R. M. Chanock, and H. S. Ginsberg. 1993. Pathogenesis of adenovirus type 5 pneumonia in cotton rats (Sigmodon hispidus). J. Virol. 67**:**101-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prince, G. A., J.-P. Prieels, M. Slaoui, and D. D. Porter. 1999. Pulmonary lesions in primary respiratory syncytial virus infection, reinfection, and vaccine-enhanced disease in the cotton rat (Sigmodon hispidus). Lab. Investig. 79**:**1385-1392. [PubMed] [Google Scholar]
- 28.Siber, G. R., D. Leombruno, J. Leszczynski, J. McIver, D. Bodkin, R. Gonin, C. M. Thompson, E. E. Walsh, P. A. Piedra, V. G. Hemming, and G. A. Prince. 1994. Comparison of antibody concentrations and protective activity of respiratory syncytial virus immune globulin and conventional immune globulin. J. Infect. Dis. 169**:**1368-1373. [DOI] [PubMed] [Google Scholar]
- 29.Waris, M. E., C. Tsou, D. D. Erdman, S. R. Zaki, and L. J. Anderson. 1996. Respiratory syncytial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J. Virol. 70**:**2852-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]