GRANULOCYTE/MACROPHAGE COLONY-STIMULATING FACTOR-STIMULATED HEPATIC DENDRITIC CELL PROGENITORS PROLONG PANCREATIC ISLET ALLOGRAFT SURVIVAL (original) (raw)
. Author manuscript; available in PMC: 2010 Oct 29.
Published in final edited form as: Transplantation. 1995 Dec 15;60(11):1366–1370.
Abstract
Liver-derived dendritic cell (DC) progenitors propagated in liquid culture in granulocyte/macrophage colony-stimulating factor exhibit low levels both of cell surface MHC class II antigens and of counter-receptors for CTLA-4/CD28. They fail to stimulate allogeneic T cells in mixed leukocyte cultures. To evaluate their in vivo functional significance, we determined their influence on survival of pancreatic islet allografts. Cultured B10.BR (H2k; I-E+) mouse liver-derived DC progenitors were injected (2×106 i.v.) into streptozotocin-diabetic B10 (H2b; I-E−) recipients 7 days before transplantation of pancreatic islets (700 IEq/mouse) from the same donor strain. No immunosuppressive agents were administered. Mean islet allograft survival time was prolonged from 15.3 days (in animals pretreated with syngeneic cells) to 30.3 days (P<0.001) in mice pretreated with the donor-derived liver cells. In 20% of these animals, islet allograft survival exceeded 60 days. These data suggest that liver-derived DC progenitors may contribute both to the inherent tolerogenicity of the mouse liver and to its capacity to protect other allografts of the same donor strain from rejection.
Hepatic allografts are accepted without immunosuppressive therapy when transplanted between many MHC-incompatible mouse strains and certain rat strains (1, 2). Moreover, transplants of the liver protect other organ grafts of the same donor or donor strain from rejection (3–7). It has been postulated that this inherent tolerogenicity of the liver—a comparatively leukocyte-rich organ compared with the kidney or heart—is a consequence of donor-recipient leukocyte interaction leading to long-lasting, mutual, immunologic unresponsiveness (8, 9). A corollary of this hypothesis is that failure to establish and maintain donor cell chimerism results in imbalanced donor-recipient cell interaction, which may lead to graft rejection. Possible effector mechanisms of this two-way paradigm have been suggested (10). However, a cellular and molecular basis for interactions that may predispose to the development of mutual, donor-recipient immunologic nonreactivity has not been elucidated.
In the multilineage cell chimerism that has been described in recipient tissues following transplantation of the liver or other organs, donor-derived dendritic cells (DC*) known to migrate to secondary lymphoid tissue (11) have been featured prominently (1, 9, 12). Although the DC resident in normal lymphoid tissue are potent antigen-presenting cells (13), freshly-isolated DC from commonly transplanted non-lymphoid tissues, such as the liver, kidney, or heart, are functionally “immature” (13, 14). Their maturation is thought to reflect the up-regulation by cytokines of both MHC gene product and costimulatory molecule expression (15). It has been suggested (8, 16) that these costimulatory molecule-deficient DC may constitute potentially tolerogenic precursors of the chimeric leukocytes seen in organ graft recipients. The magnitude, tissue-specific site dependency, replicative capacity, or maturational stage of chimeric DC that might be necessary to mediate postulated tolerizing effects has not been established.
Recently, we have succeeded in propagating DC progenitors from normal mouse liver in response to granulocyte/macrophage colony stimulating factor (GM-CSF) (17). Following local or systemic injection, these cells migrate to T-dependent areas of allogeneic host lymphoid tissue, and express cell surface donor MHC class II. They persist indefinitely, although in diminished numbers (18). Furthermore, in spontaneously tolerant liver transplant recipients (but not in animals rejecting donor strain hearts), donor-derived DC can be propagated from host bone marrow and spleen using GM-CSF (19). These observations have raised questions about the functional significance of GM-CSF-stimulated, donor-derived DC progenitors in allograft recipients, and their possible relation to liver tolerogenicity. The potential of exploiting these putative tolerogenic cells for the therapy of graft rejection has also been suggested (16, 17).
In this study, we have examined whether systemic injection of GM-CSF-stimulated, liver-derived DC progenitors can affect the survival of subsequent (pancreatic islet) allografts from the same donor strain. The results show that the liver-derived cells, but not “mature” GM-CSF-stimulated spleen-derived DC, significantly prolong islet transplant survival. They suggest a possible role of donor-derived, GM-CSF-responsive DC progenitors in allograft acceptance that may be related to the inherent tolerogenicity of the liver.
Animals
Adult 8- to 12-week-old male B10.BR (H2k, I-E+) mice and C57BL/10SnJ (B10, H2b, I-A+) mice were purchased from The Jackson Laboratory, Bar Harbor, ME. They were maintained in the specific pathogen-free facility of the University of Pittsburgh Medical Center.
Isolation of nonparenchymal cells (NPC) from mouse liver
NPC were isolated from normal B10.BR mouse livers following in situ perfusion, digestion in collagenase solution, and Percoll centrifugation, as described in detail elsewhere (17).
For comparative purposes, fresh spleen cell populations were prepared using the same protocol.
Culture of liver- and spleen-derived DC lineage cells with GM-CSF
Liver NPC or spleen cells (2.5×106/well) were placed in 24-well plates containing 2 ml of RPMI-1640 complete medium (Gibco, Grand Island, NY) supplemented with 10% v/v fetal calf serum, and 0.4 ng/ml recombinant mouse GM-CSF (R&D Systems, Minneapolis, MN). Nonadherent, low buoyant density cells released from developing clusters were propagated as described (17). Cells were harvested for study after 7–10 days culture.
Flow cytometric analysis
Immunophenotypic analysis of GM-CSF-stimulated liver- or spleen-derived cells was performed by either direct or indirect immunofluorescence staining using an extensive panel of mAbs, as detailed elsewhere (17). The mAbs included antibodies directed against mouse lymphoid, myeloid, or DC-restricted markers (33D1, TIB227; ATCC, NLDC-145; and CD11c, N418; kindly provided by Dr. R.M. Steinman, Rockefeller University, New York, NY). In addition, counter-receptors (CR) of CTLA-4 (a structural homolog of CD28) (CTLA-4CR), which include B7 family members CD80 (B7–1) and CD86 (B7–2), were identified using the CTLA-4Ig fusion protein (a gift from Dr. P.S. Linsley, Bristol Myers Squibb Pharmaceutical Research Institute, Seattle, WA), with human Ig (Sigma Chemical Co., St. Louis, MO) as a negative control. Flow cytometric analysis was performed using a FACSTAR flow cytometer (Becton Dickinson, San Jose, CA). Five thousand events were acquired for each sample.
Mixed leukocyte cultures (MLC)
MLC were performed in 96-well, round-bottom microculture plates with variable numbers of GM-CSF-stimulated, γ-irradiated (20 Gy) allogeneic (B10.BR) or syngeneic (B10) liver- or spleen-derived cells as stimulators. B10 spleen cells (4×105/well) enriched for T cells by passage (1 hr) through a nylon wool column were used as responders. Cultures were maintained for 72 hr; [3H]TdR (1 _μ_Ci/well) was added 18 hr before harvesting and the extent of DNA synthesis was determined by liquid scintillation counting.
DC migration
Cultured B10.BR liver-derived DC progenitors were washed in RPMI 1640 and injected subcutaneously (2.5× 105 in 0.05 ml) into the left hind footpad of naive B10 recipients. One to 60 days later, the mice were killed and their spleens were removed and embedded in Tissue-Tek (O.C.T. compound, Miles Inc., Elkhart, IN). Cryostat sections (10 _μ_m) were cut, processed, and stained for donor MHC class II (I-Ek)-positive cells, as described (18). The mean number of positive cells per 100 high power fields (hpf) was determined.
In vivo treatment protocol
Naive B10 mice were rendered diabetic (blood glucose >250 mg/dl) by intravenous injection of streptozotocin (Sigma, 200 mg/kg). Two days later, 2×106 B10.BR-derived, GM-CSF-stimulated, liver- or spleen-derived cells were injected intravenously (tail vein). Seven days after injection of cultured cells, and with daily monitoring and treatment with insulin (beef-pork insulin [regular Iletin I, Eli Lilly and Co., Indianapolis IN]; 1–2 IU i.p./mouse), the animals underwent pancreatic islet transplantation.
Islet cell isolation and purification
Fresh B10.BR islets were isolated by a modification of the automated method described previously (20). Briefly, after cannulation of the pancreatic ducts of heart-beating donors, collagenase solution (Boehringer-Mannheim, type P; 0.7 mg/ml) was injected at 4°C and a pancreatectomy was performed. The pancreata were loaded into a stainless steel digestion chamber and the islets were isolated by a continuous digestion process that lasted 10±3 min. With gentle shaking, the solution containing collagenase was recirculated (flow rate 85 ml/min) through the chamber. The process of digestion was initiated by raising the temperature of the solution at the rate of 2°C/min by passing it through a stainless steel coil immersed in a 45°C water bath until it reached 37–38°C. Digestion was monitored by sample harvesting every 2 min, staining with dithizone (Sigma), and light microscopic examination. When “free islets” were observed, the digestion was interrupted and the isolated cells were collected. The islets were then purified by centrifugation in a cell processor (COBE 2991, Lakewood, CO) using discontinuous density gradients (densities of 1.108, 1.096, and 1.037).
Islet transplantation
Seven days after the infusion of GM-CSF-stimulated cells or culture media, 700 isolated B10.BR islets (purity 90±5%) with an average individual diameter of 150 μ (1 IEq) were placed beneath the left renal capsule of each diabetic B10 recipient (only animals with blood glucose levels >350 mg/dl were used). Blood glucose was monitored daily for the first 15 days after transplantation and every other day thereafter. Rejection of islet allografts was considered to have taken place when the blood glucose exceeded 250 mg/dl on two consecutive measurements. Body weight was also monitored as an overall index of physiologic status.
Statistics
Median graft survival times between groups of transplanted animals were compared using the Kruskal-Wallis test, a nonparametric equivalent to the one-way analysis of variance. Pair-wise comparisons were performed using the Wilcoxon rank sum test. A _P_-value <0.05 was considered statistically significant.
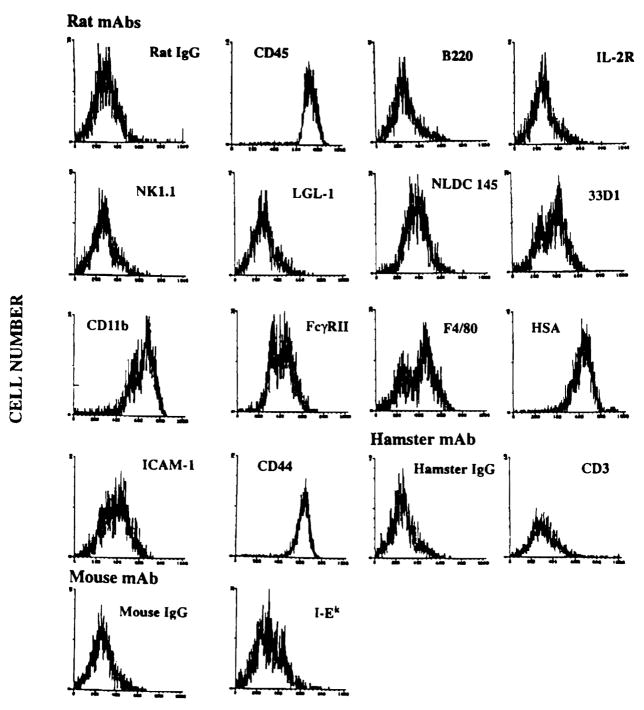
Liver-derived cells harvested after 7–10 days of culture in GM-CSF expressed surface antigens associated with mouse DC, including CD45 (leukocyte common antigen), heat stable antigen, CD54 (intercellular adhesion molecule-1), CD11b (MAC-1), and CD44 (nonpolymorphic determinant of Pgp.1 glycoprotein) (Fig. 1). In addition, staining of weak to moderate intensity was observed for the DC-restricted markers NLDC-145 (interdigitating cells), 33D1, and CD11c (N418) and for F4/80 and CD32 (FcγRII). Expression of T cell (CD3), B cell (B220), and NK cell markers (NK1.1 and LGL-1) was absent. The GM-CSF-stimulated spleen-derived cells were similar, except that CD32 and CD11b were reduced compared with the liver-derived cells (data not shown). In contrast to the spleen cell progeny which were MHC class IIbright and strongly positive for CTLA-4CR, the liver-derived, GM-CSF-stimulated cells were MHC class II(I-Ek)dim (Fig. 1) and did not express detectable levels of cell surface CTLA-4CR.
Figure 1.
FACSCAN immunophenotypic profile of GM-CSF-stimulated B10.BR mouse liver-derived cells with DC characteristics released from cell aggregates (culture day 8) and examined using rat, hamster, or mouse mAbs. Note the absence of lymphoid cell markers and the low level of MHC class II (I-Ek) expression. The result is representative of 6 separate experiments.
The GM-CSF-stimulated B10.BR spleen-derived DC, which expressed high levels both of cell surface MHC class II antigen and CTLA-4CR, were potent stimulators of naive allogeneic (B10) T cells at low S:R ratios in 3-day MLCs (Table 1). In contrast, the B10.BR liver-derived DC progenitors failed to stimulate B10 splenic T cells.
Table 1.
Allostimulatory activity of GM-CSF-stimulated. B10.BR liver-derived DC progenitors and spleen-derived DC in primary MLC
| S:R ratioa | Stimulators cpm (×103) | ||
|---|---|---|---|
| Spleen cells (Syngeneic) | Liver DC progenitors (allogeneic) | Spleen-derived DC (allogeneic) | |
| 1:3 | 3.86 ± 0.36 | 4.06 ± 0.54 | 45.49 ± 1.53 |
| 1:9 | 5.48 ± 1.87 | 3.80 ± 0.26 | 27.29 ± 0.10 |
| 1:27 | 5.47 ± 1.24 | 5.75 ± 0.92 | 18.56 ± 4.39 |
| 1:81 | 5.60 ± 1.28 | 7.58 ± 1.60 | 9.40 ± 0.40 |
Following the injection of GM-CSF -stimulated liver DC progenitors into unmodified naive allogeneic recipients, donor MHC class II+ (I-Ek+) cells with distinct dendritic morphology were first observed in the spleen 24 hr later (3.97±2.11 cells/100 hpf). Maximal numbers of I-Ek+ cells were found on day 5 (15.81±8.97/100 hpf). Thereafter, the mean number declined (10.91±0.73/100 hpf at 2 weeks), but I-Ek+ cells were still detected in T cell areas at least 2 months after the injection of liver-derived DC progenitors.
These experiments were extended to evaluate whether the systemic inoculation of GM-CSF-stimulated, donor-derived liver DC progenitors could reduce responsiveness to islet allografts, thus prolonging their survival. The results obtained from two experiments (Fig. 2) show that intravenous injection of 2× 106 B10.BR liver DC progenitors into B10 mice 2 days after the animals were rendered diabetic (group 3) significantly prolonged the mean survival time of allogeneic islets transplanted 7 days later. Mean survival time was extended from 15.3±0.7 days (syngeneic control; group 2) to 30.3±17.1 days (group 3; P<0.001). The liver DC progenitors syngeneic with the host (group 2) slightly prolonged islet allograft survival compared with media alone (group 1; 11.9±0.9 days). In contrast, islet graft recipients pretreated with cultured donor strain (B10.BR) spleen-derived DC (group 4) rejected their grafts within approximately the same time (12.0±2.0 days) as media-treated diabetic controls (group 1).
Figure 2.
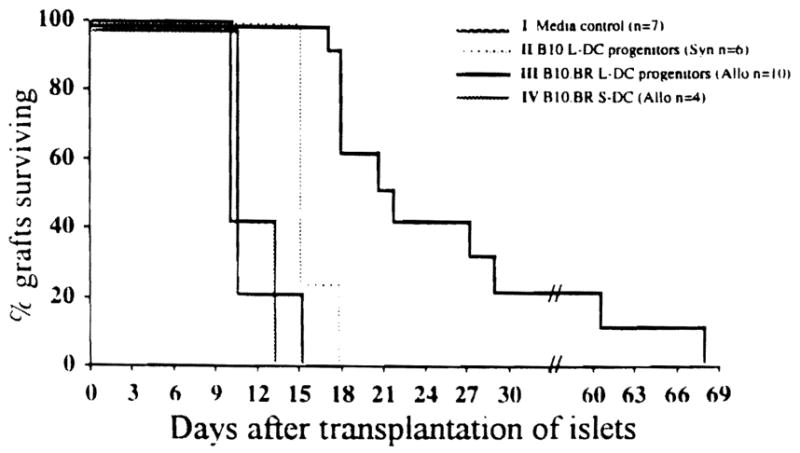
Influence of cultured B10.BR liver DC progenitors on B10.BR pancreatic islet allograft survival in B10 mice. Cultured liver (L)- or spleen (S)-derived cells (2×106) were injected intravenously 2 days after the recipient animals were made diabetic with an intraperitoneal injection of streptozotocin (200 mg/kg). The animals were maintained on Insulin, as described in Materials and Methods (1–2 IU i.p. daily), until pancreatic islet transplantation. Pancreatic islets (700 IEq/mouse) were placed beneath the left renal capsule 7 days after the injection of cultured GM-CSF-stimulated liver DC progenitors or spleen-derived DC.
Examination of the spleens of mice whose islet graft survival was prolonged as the result of allogeneic liver cell injection (group 3) revealed small numbers of isolated donor class II+ (I-Ek+) cells. As reported previously for normal recipients (18), these dendritic-shaped cells were detected in close proximity to arterioles in T-dependent areas and were not observed in islet allograft recipients pretreated with syngeneic, liver-derived DC progenitors (group 2). This rendered unlikely the possibility that these chimeric cells were derived from the islet allograft.
There has been recent speculation that bone marrow-derived DC progenitors or costimulatory molecule-deficient “immature” DC present in commonly transplanted nonlymphoid organs, such as the liver, kidney, or heart, may constitute potentially tolerogenic precursors of chimeric cells observed in allograft recipients. We have observed that DC progenitors grown from liver NPC in response to low concentrations of GM-CSF fail to stimulate naive allogeneic T cells in MLC. As shown elsewhere, however (17), these DC progenitors can be induced to mature into potent allostimulatory cells by continued exposure to GM-CSF in the presence of type-1 collagen, an extracellular matrix protein with which they are spacially associated in normal liver.
After transplantation of the liver or other nonlymphoid organs, emigrant DC progenitors may undergo in vivo proliferation/differentiation/maturation within recipient tissues (as evidenced in this study and elsewhere [17] by the up-regulation of donor MHC class II expression). This is likely to be influenced (both positively and negatively) by endogenous GM-CSF and other cytokines (e.g., IL-1, TNF-α, IL-4, IL-12, and c_-kit_ ligand). The nature and kinetics of this process, however, are also likely to be affected by host immunosuppression, particularly the use of potent inhibitors either of cytokine production (e.g., tacrolimus or cyclosporine) or of responses of leukocytes to growth/differentiation factors.
There are several possible reasons for the transitory nature of the therapeutic effect achieved with liver-derived DC progenitors in this study. These include insufficient numbers/frequency or suboptimal timing of the liver cell administration, and alterations in levels of micro-environmental growth factors. Furthermore, in the absence of immunosuppressive therapy, cytokine-induced in vivo functional maturation of donor-derived DC progenitors may have contributed to the eventual rejection of the allografted islets. The possible tolerogenic implications of the rapid migration from the liver (a potential hematolymphoid organ), or from other transplanted whole organs, of GM-CSF-responsive DC precursors deficient in T cell costimulatory molecules are considerable. The implications are emphasized by the lengthy persistence of these donor-derived cells in nonimmunosuppressed allogeneic mouse recipients (18), and by the capacity of the cells to prolong survival of (islet) allografts from the same donor strain. Furthermore, propagation of donor-derived DC precursors from the lymphoid tissue of spontaneously tolerant mouse liver allograft recipients in response to GM-CSF (19) provides a mechanistic basis for the perpetuation of leukocyte chimerism in recipients of liver and other organ grafts.
The possible mechanistic role of chimeric DC or other lineages and their progeny in the establishment of donor-specific unresponsiveness remains to be defined. We have shown elsewhere that immunologically “immature” DC (MHC class II+, B7–1+, B7–2−) propagated from mouse bone marrow can induce alloantigen-specific T cell hyporesponsiveness in vitro (21). Moreover, a subpopulation of mouse DC with veto function (inactivation of T helper cells or cytotoxic T cell precursors) has been identified (22). Furthermore, it has been postulated that MHC class IIdim allogeneic donor bone marrow cells that exhibit veto cell activity are immature DC (23). The precise basis of DC-T cell interactions that might lead to tolerance induction is uncertain. It is likely, however, to depend on the relative affinity or avidity (compared with “immunizing” antigen-presenting cells) of donor DC-T cell receptor interactions and on the expression on the former cells of critical adhesins and costimulatory molecules, such as members of the intercellular adhesion molecule and B7 families and CD40. These aspects of developing liver- and bone marrow-derived DC both in vitro and in vivo are under further investigation.
Acknowledgments
We thank William Irish for statistical analyses, Dr. Patricia Carroll for helpful discussion, Dr. Youping Li for immunohistochemical staining, and Shelly Conklin for typing the manuscript.
Footnotes
1
This work was supported in part by National Institutes of Health Grant DK 29961–14.
*
Abbreviations: CR, counter-receptor; DC, dendritic cell; GM-CSF, granulocyte-macrophage colony-stimulating factor; hpf, high power field; MLC, mixed leukocyte culture; NPC, nonparenchymal cell.
References
- 1.Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology. 1994;19:96. doi: 10.1002/hep.1840190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmerman FA, Butcher GW, Davies HS, Brons G, Kamada N, Turel O. Techniques for orthotopic liver transplantation in the rat and some studies of the immunologic responses to fully allogeneic liver grafts. Transplant Proc. 1979;11:571. [PubMed] [Google Scholar]
- 3.Starzl TE, Kaupp HA, Jr, Brock DR, Butz GW, Jr, Linman JW. Homotransplantation of multiple visceral organs. Am J Surg. 1962;103:219. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;233:472. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 5.Kamada N, Davies HS, Roser BJ. Reversal of transplantation immunity by liver grafting. Nature. 1981;292:840. doi: 10.1038/292840a0. [DOI] [PubMed] [Google Scholar]
- 6.Fung J, Makowka L, Tzakis A, et al. Combined liver-kidney transplantation: analysis of patients with preformed lymphocytotoxic antibodies. Transplant Proc. 1988;20:80. [PMC free article] [PubMed] [Google Scholar]
- 7.Murase N, Starzl TE, Tanabe M, et al. Variable chimerism, graft versus host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to Brown-Norway rats. Transplantation. doi: 10.1097/00007890-199507000-00009. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127. [PMC free article] [PubMed] [Google Scholar]
- 10.Starzl TE, Demetris AJ, Murase N, Thomson AW, Trucco M, Ricordi C. Donor cell chimerism permitted by immunosuppressive drugs: a new view of organ transplantation. Immunol Today. 1993;14:326. doi: 10.1016/0167-5699(93)90054-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Austyn JM, Larsen CP. Migration patterns of dendritic leukocytes. Implications for transplantation. Transplantation. 1990;49:1. doi: 10.1097/00007890-199001000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Demetris AJ, Murase N, Starzl TE. Donor dendritic cells after liver and heart allotransplantation under short term immunosuppression. Lancet. 1992;339:1610. doi: 10.1016/0140-6736(92)91875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 14.Austyn JM. The initiation of immune responses and allograft rejection. In: Rose ML, Yacoub MH, editors. Immunology of heart and lung transplantation. Boston: Little, Brown and Company; 1993. p. 41. [Google Scholar]
- 15.Larsen CP, Ritchie SC, Hendrix R, et al. Regulation of immunostimulatory function and costimulatory molecule (B7–1 and B7–2) expression on murine dendritic cells. J Immunol. 1994;152:5208. [PubMed] [Google Scholar]
- 16.Steinman RM, Inaba K, Austyn JM. Donor-derived chimerism in recipients of organ transplants. Hepatology. 1993;17:1153. [PubMed] [Google Scholar]
- 17.Lu L, Woo J, Rao AS, et al. Propagation of dendritic cell progenitors from normal mouse liver using GM-CSF and their maturational development in the presence of type-1 collagen. J Exp Med. 1994;179:1823. doi: 10.1084/jem.179.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomson AW, Lu L, Subbotin VM, et al. In vitro propagation and homing of liver-derived dendritic cell progenitors to lymphoid tissues of allogeneic recipients. Transplantation. 1995;59:544. doi: 10.1097/00007890-199502270-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu L, Rudert WA, Qian S, et al. Growth of donor-derived dendritic cells from the bone marrow of murine liver allograft recipients in response to granulocyte/macrophage colony-stimulating factor. J Exp Med. 1995;182:379. doi: 10.1084/jem.182.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricordi C. The automated method for islet isolation. In: Ricordi C, editor. Pancreatic islet cell transplantation. Austin, TX: RG Landes Co; 1992. p. 99. [Google Scholar]
- 21.Lu L, McCaslin D, Starzl TE, Thomson AW. Bone marrow-derived dendritic cell progenitors (NLDC 145+, MHC class II+, B7–1dim, B7–2−) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes. Transplantation. doi: 10.1097/00007890-199560120-00028. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vremec D, Zorbas M, Scollay R, et al. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas JM, Carver FM, Kasten-Jolly J, et al. Further studies of veto activity in rhesus monkey bone marrow in relation to allograft tolerance and chimerism. Transplantation. 1994;57:101. doi: 10.1097/00007890-199401000-00018. [DOI] [PubMed] [Google Scholar]