Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells (original) (raw)
Abstract
We have shown previously that withaferin A (WA), a promising anticancer constituent of Ayurvedic medicine plant Withania somnifera, inhibits growth of human breast cancer cells in culture and in vivo in association with apoptosis induction. The present study builds on these observations and demonstrates that WA inhibits constitutive as well as interleukin-6 (IL-6)-inducible activation of signal transducer and activator of transcription 3 (STAT3), which is an oncogenic transcription factor activated in many human malignancies including breast cancer. The WA treatment (2 and 4 μM) decreased constitutive (MDA-MB-231) and/or IL-6-inducible (MDA-MB-231 and MCF-7) phosphorylation of STAT3 (Tyr705) and its upstream regulator Janus-activated kinase 2 (JAK2; Tyr1007/1008) in MDA-MB-231, which was accompanied by suppression of their protein levels especially at the higher concentration. Exposure of MDA-MB-231 or MCF-7 cells to WA also resulted in suppression of (i) transcriptional activity of STAT3 with or without IL-6 stimulation in both cells; (ii) dimerization of STAT3 (MDA-MB-231) and (iii) nuclear translocation of Tyr705-phosphorylated STAT3 in both cells. To our surprise, the IL-6-stimulation, either before or after WA treatment, did not have an appreciable effect on WA-mediated apoptosis in MDA-MB-231 or MCF-7 cell line. The IL-6-stimulated activation of STAT3 conferred a modest protection against WA-mediated suppression of MDA-MB-231 cell invasion. General implication of these findings is that WA can trigger apoptosis and largely inhibit cell migration/invasion of breast cancer cells even after IL-6-induced activation of STAT3, which should be viewed as a therapeutic advantage for this agent.
Introduction
Breast cancer is a major health concern for American women (1,2). Thousands of women still die from breast cancer despite significant advances toward targeted therapies and screening efforts (3,4). Some of the risk factors associated with breast cancer are known, including family history, Li-Fraumeni syndrome, atypical hyperplasia of the breast, late age at first full-term pregnancy, early menarche and late menopause (5–7). Novel strategies for reduction of breast cancer risk are needed mainly because many of the known risk factors associated with this neoplasm are not modifiable. Prevention of breast cancer is feasible with selective estrogen receptor modulators (e.g. tamoxifen and raloxifene), but this approach is largely ineffective against estrogen receptor-negative breast cancers (8–10). Furthermore, long-term administration of selective estrogen receptor modulators carries the risk of serious side effects including cancer of the uterus, thromboembolism, cataracts and perimenopausal symptoms (8,9). Therefore, novel agents that can target both estrogen receptor-positive and -negative breast cancers are clinically desirable. Natural products are attracting increased awareness for the discovery of novel cancer chemopreventive and therapeutic agents (11).
Ashwagandha (Withania somnifera L. Dunal), which has been used safely for centuries in the Ayurvedic medicine practice for the treatment of various disorders, appears promising in integrative oncology (12,13). In addition, W.somnifera has been shown to modulate immune function, provide cardioprotection from ischemia reperfusion injury and suppress markers of 6-hydroxydopamine-induced Parkinsonism in experimental animals (14–16). This medicinal plant is also credited for its antibacterial properties and anti-inflammatory effects (17,18). The anticancer effect of W.somnifera is attributed, at least in part, to withaferin A (WA). The WA was shown to be a radiosensitizer of a mouse melanoma and inhibitor of mouse Ehrlich ascites carcinoma growth vivo (19,20). _W.somnifera_-derived withanolides, including WA, were shown to inhibit invasion and osteoclastogenesis through suppression of nuclear factor-kappaB (21). The WA treatment resulted in suppression of IkappaB kinase beta phosphorylation concomitant with potent inhibition of its kinase activity (22). The WA-mediated suppression of angiogenesis, alteration of cytoskeletal architecture and inhibition of proteasomal activity have also been demonstrated (23–25). Recent studies including those from our laboratory have focused on the mechanism by which WA suppresses proliferation of cancer cells (26–32). The WA was found to trigger Par-4-dependent apoptosis in human prostate cancer cells (26). Low micromolar concentrations of WA inhibited growth of cultured MDA-MB-231 and MCF-7 human breast cancer cells but not a spontaneously immortalized non-tumorigenic mammary epithelial cell line (MCF-10A), by causing FOXO3a and Bim-dependent apoptosis (28). We also found that the WA-treated breast cancer cells were arrested in G2 phase and mitotic possibly due to inactivation of cyclin-dependent kinase 1/cyclin B1 complex and anaphase promoting complex/cyclosome, respectively (29).
Signal transducer and activator of transcription 3 (STAT3) is an oncogenic transcription factor implicated in development and progression of various malignancies including breast cancer (33–38). The present study was designed to determine the impact of STAT3 activation on anticancer effects (proapoptotic and inhibition of cell migration and invasion) of WA using MDA-MB-231 (an estrogen-independent cell line with mutant p53) and MCF-7 (an estrogen-responsive cell line with wild-type p53) cells. We demonstrate that WA treatment inhibits constitutive as well as interleukin-6 (IL-6)-inducible activation of STAT3, but IL-6-stimulated activation of this transcription factor has marginal effect on anticancer effects of WA.
Materials and methods
Reagents
The WA (purity ∼99%) was purchased from Enzo Life Sciences (Plymouth Meeting, PA). Dimethyl sulfoxide (DMSO), IL-6 and anti-actin antibody were purchased from Sigma–Aldrich (St Louis, MO). The cell culture medium, antibiotic mixture and fetal bovine serum, trypan blue solution, SytoxGreen and Alexa Flour 568 goat anti-rabbit antibody were purchased from Invitrogen (Carlsbad, CA). The antibodies against phospho-JAK2 (pJAK2; Tyr1007/1008), phospho-STAT3 (pSTAT3; Tyr705, Ser727), total JAK2, total STAT3 and cleaved caspase-3 were obtained from Cell Signaling (Danvers, MA). Antibodies against poly-(adenosine diphosphate-ribose)-polymerase and pSTAT3 used for immunofluorescence microscopy were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Matrigel basement membrane was procured from BD Biosciences (San Jose, CA). The Transwell™ chamber was purchased from Corning (Corning, NY).
Cell lines
The MDA-MB-231 and MCF-7 cell lines were obtained from American Type Culture Collection (Manassas, VA) and authenticated for lack of interspecies contamination. Stock solution of WA was prepared in DMSO (final concentration <0.1%), and an equal volume of DMSO was added to controls. Each cell line was maintained at 37°C in an atmosphere of 5% CO2 and 95% air as described by us previously (28,29).
Western blotting
Cell lysates from control (DMSO-treated) and WA-treated MDA-MB-231 or MCF-7 cells were prepared as described by us previously (39). Lysate proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred on to polyvinylidene fluoride membrane. Membranes were blocked with 5% non-fat dry milk in Tris-buffered saline containing 0.05% Tween-20 and incubated with the desired primary antibody overnight at 4°C. The membranes were then hybridized with the desired secondary antibody and the immunoreactive bands were visualized by chemiluminescence method.
Luciferase reporter assay
The MDA-MB-231 or MCF-7 cells were plated and allowed to attach by overnight incubation at 37°C. The cells were then co-transfected with 2 μg of pSTAT3-Luc plasmid encoding STAT3-responsive element (kindly provided by Dr Bharat B.Aggarwal, University of Texas M. D. Anderson Cancer Center, Houston, TX) and 0.2 μg of pRL-CMV plasmid using Fugene6. Twenty-four hours after transfection, the cells were treated with DMSO (control) or WA (2 or 4 μM) for 5 h and then poststimulated with IL-6 (4 ng/ml) for 1 h in the presence of WA. Luciferase activity was determined using a luminometer.
Determination of STAT3 dimerization
Protein extracts from control and WA-treated MDA-MB-231 cells were prepared as described by Shin et al. (40) with some modifications. Proteins were resolved by 6% non-denaturing gel electrophoresis and transferred onto polyvinylidene fluoride membrane. The blots were probed with anti-STAT3 antibody as described above.
Immunocytochemistry for nuclear localization of pSTAT3
The MDA-MB-231 or MCF-7 cells (1 × 105) were plated on coverslips and allowed to attach by overnight incubation. After 12 h of serum starvation, the cells were treated with different concentrations of WA for 5 h followed by co-treatment with IL-6 (4 ng/ml) for an additional 1 h. The cells were fixed with 2% paraformaldehyde for 1 h at room temperature, permeabilized with 0.5% Triton X-100 for 10 min and blocked with phosphate-buffered saline supplemented with 0.5% bovine serum albumin and 0.15% glycine for 1 h. The cells were treated with anti-pSTAT3 (Tyr705) antibody overnight at 4°C. The cells were then treated with 2 μg/ml of Alexa Fluor 568-conjugated secondary antibody for 1 h at room temperature. The cells were washed with phosphate-buffered saline and counterstained with SytoxGreen (0.5 μmol/l) for 3 min at room temperature to stain nuclear DNA. Subsequently, the cells were mounted and observed under a Leica DC300F fluorescence microscope at ×100 objective magnification.
Measurement of cell viability and apoptosis
The effect of WA and/or IL-6 treatments on viability of MDA-MB-231 or MCF-7 cells was determined by trypan blue dye exclusion assay as described by us previously (41). The proapoptotic effect of WA was assessed by quantification of cytoplasmic histone-associated DNA fragmentation and cleavage of poly-(adenosine diphosphate-ribose)-polymerase and procaspase-3.
Cell migration and invasion assay
The MDA-MB-231 cells (1 × 105) were suspended in serum-free medium and placed in the upper compartment of Boyden Chamber containing 8 μm filter for migration assay. The Boyden Chamber was coated with 30 μl of Matrigel (1:2 dilution in medium) for invasion assay and contained 10% fetal bovine serum. After 24 h of incubation with the indicated concentrations of WA and/or IL-6, nonmotile cells were removed from the upper surface of the filter. The motile cells on the bottom face of the filter were fixed with methanol and stained with hematoxylin and eosin.
Statistical analysis
Statistical significance of difference in measured variables between control and WA-treated groups was determined by one-way analysis of variance followed by Bonferroni's test. Difference was considered significant at P < 0.05.
Results
WA treatment inhibited constitutive and IL-6-inducible phosphorylation of STAT3 in human breast cancer cells
The STAT3 activation is caused by phosphorylation at Tyr705 (33,35). We used MDA-MB-231 and MCF-7 cells to determine the effect of WA (structure of WA is shown in Figure 1A) on STAT3 activation. The level of Tyr705-phosphorylated STAT3 was decreased to below detection limit in MDA-MB-231 cells treated for 6, 12 and 24 h at both 2 and 4 μM concentrations (Figure 1B). The WA-mediated reduction in phosphorylation of STAT3 was accompanied by a decrease in its protein level especially at the 24 h time point at both concentrations. The WA treatment also resulted in a concentration-dependent decrease in Tyr1007/1008 phosphorylation of JAK2, an upstream kinase responsible for activation of STAT3 (42). Level of total JAK2 protein was also reduced in a dose-dependent manner in WA-treated MDA-MB-231 cells, but this effect seemed somewhat reversible at the 24 h time point (Figure 1B). Constitutively active STAT3 was very low in the MCF-7 cells line. A 60 min exposure of serum-starved (12 h starvation) MCF-7 and MDA-MB-231 cells to 4 ng/ml IL-6, a known activator of STAT3 (42), resulted in a robust increase in Tyr705 phosphorylation of STAT3 in both cell lines (Figure 1C). The IL-6-induced phosphorylation of STAT3 was decreased to below detection limit on treatment with WA in both cell lines, which correlated with suppression of total STAT3 protein level especially at the 4 μM concentration (Figure 1C). The WA-treated MCF-7 and MDA-MB-231 cells also exhibited a decline in Ser727 phosphorylation of STAT3, which was either insensitive (MCF-7) or weakly sensitive (MDA-MB-231) to IL-6 stimulation (Figure 1C). Collectively, these results indicated that WA treatment inhibited both constitutive and IL-6-inducible phosphorylation of STAT3 in human breast cancer cells regardless of estrogen responsiveness.
Fig. 1.
WA inhibits constitutive and IL-6-inducible phosphorylation of STAT3 in human breast cancer cells. (A) Chemical structure of WA. (B) Western blotting for pSTAT3 (Tyr705), total STAT3, pJAK2 (Tyr1007/1008) and total JAK2 using lysates from MDA-MB-231 cells treated for 6, 12 or 24 h with DMSO (control) or the indicated concentrations of WA. (C) Western blotting for pSTAT3 (Tyr705 and Ser727) and total STAT3 using lysates from MDA-MB-231 or MCF-7 cells treated with DMSO (control) or WA (2 or 4 μM) for 5 h and then stimulated with 4 ng/ml IL-6 for 1 h in the presence of WA. Cells were serum-starved for 12 h prior to treatments. Blots were stripped and reprobed with anti-actin antibody to correct for differences in protein loading. Numbers above immunoreactive bands represent changes in levels relative to corresponding DMSO-treated control. Each experiment was repeated at least twice and the results were consistent. Representative data from one such experiment are shown.
WA treatment inhibited STAT3-dependent luciferase activity in breast cancer cells
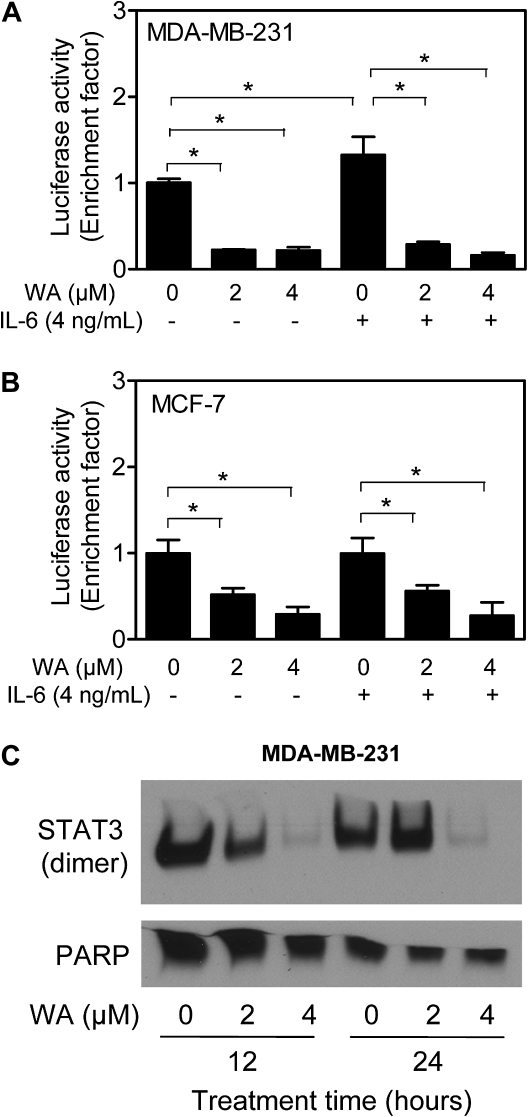
We performed luciferase reporter assay to determine the effect of WA treatment on transcriptional activity of STAT3. Six hours treatment of MDA-MB-231 cells with 2 and 4 μM WA resulted in ∼80% decrease in STAT3-dependent luciferase activity (Figure 2A). Transcriptional activity of STAT3 was modestly but significantly increased upon 1 h treatment of MDA-MB-231 cells with 4 ng/ml IL-6 (Figure 2A). The IL-6-mediated increase in STAT3-associated luciferase activity was inhibited in the presence of WA (Figure 2A). Likewise, the WA-treated MCF-7 cells exhibited statistically significant inhibition of STAT3-dependent luciferase activity regardless of IL-6 treatment (Figure 2B). These results indicated that WA treatment inhibited transcriptional activity of STAT3 in breast cancer cells.
Fig. 2.
WA treatment inhibited STAT3-dependent luciferase activity and STAT3–STAT3 dimer formation in human breast cancer cells. Effect of WA and/or IL-6 treatments on STAT3-associated luciferase activity in (A) MDA-MB-231 cells and (B) MCF-7 cells. Each cell line was serum-starved for 12 h and then treated with WA for 5 h before stimulation with 4 ng/ml IL-6 for 1 h in the presence of WA. Results shown are mean ± SD (n = 3). *P < 0.05, significantly different between the indicated groups by one-way analysis of variance followed by Bonferroni's multiple comparison test. (C) Analysis for STAT3 dimerization in MDA-MB-231 cells treated with indicated concentrations of WA for 12 or 24 h. Each experiment was repeated at least twice, and the results were consistent. Representative data from one such experiment are shown.
WA treatment diminished dimerization and nuclear localization of STAT3
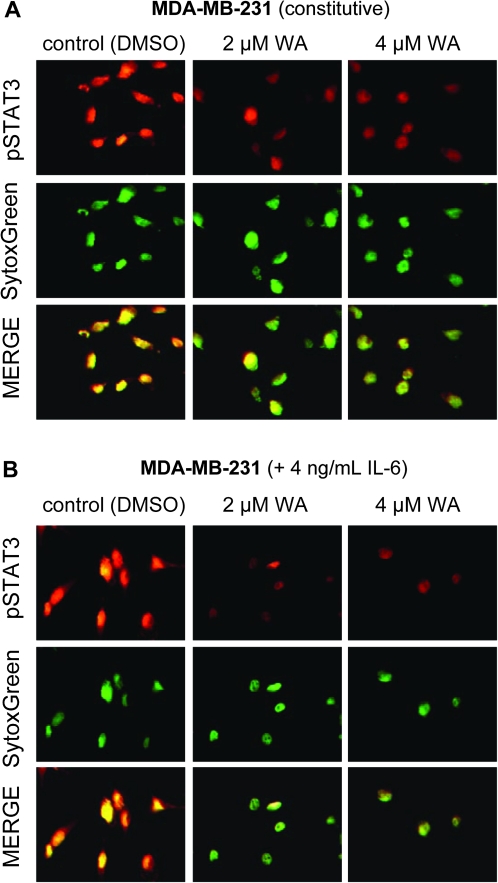
Phosphorylation at Tyr705 results in homodimer formation of STAT3 or heterodimerization with other STAT members, which enables nuclear translocation of STAT3 for binding to specific sequences of target genes (33,35). Figure 2C shows the effect of WA treatment on dimerization of STAT3 in MDA-MB-231 cells. Level of STAT3–STAT3 dimer was decreased markedly in MDA-MB-231 cells treated for 12 and 24 h with 2 and 4 μM WA (Figure 2C). Figure 3A depicts immunocytochemical analysis for constitutive active pSTAT3 (red fluorescence) and SytoxGreen-associated nuclear staining (green fluorescence) in MDA-MB-231 cells following 6 h treatment with DMSO (control) or the indicated concentrations of WA. Consistent with immunoblotting results shown in Figure 1B, pSTAT3 was predominantly localized in the nucleus as evidenced by yellow-orange staining due to merging of red and green fluorescence. Nuclear level of pSTAT3 was decreased markedly in cells treated with 2 and 4 μM WA (Figure 3A). As expected, 60 min exposure of MDA-MB-231 cells to 4 ng/ml IL-6 resulted in increased nuclear level of pSTAT3 as evidenced by more intense yellow-orange staining (Figure 3B) compared with unstimulated MDA-MB-231 cells (Figure 3A). The IL-6-stimulated nuclear localization of pSTAT3 was also inhibited in the presence of WA (Figure 3B). Nuclear staining for pSTAT3 was fairly weak in unstimulated MCF-7 cells (Figure 4A), which was consistent with the immunoblotting data (Figure 1C). Similar to the MDA-MB-231 cell line, 60 min exposure of MCF-7 cells to IL-6 (4 ng/ml) resulted in a robust increase in the nuclear pSTAT3 signal (Figure 4B). The IL-6-stimulated nuclear localization of pSTAT3 was nearly completely abolished in the presence of WA (Figure 4B). These results showed inhibition of constitutive and IL-6-induced nuclear localization of pSTAT3 in both cells and dimerization of STAT3 in MDA-MB-231 cells by WA treatment.
Fig. 3.
WA inhibits nuclear translocation of pSTAT3 in MDA-MB-231 cells. (A) Immunocytochemistry for pSTAT3 in MDA-MB-231 cells treated for 6 h with the indicated concentrations of WA (without IL-6 stimulation). (B) Immunocytochemistry for pSTAT3 in MDA-MB-231 cells treated with the indicated concentrations of WA and/or IL-6. After serum-starvation for 12 h, the cells were treated with the indicated concentrations of WA for 5 h and then exposed to IL-6 for 1 h in the presence of WA. The staining for pSTAT3 and nuclei are indicated by red and green fluorescence, respectively. Experiment was done twice, and representative data from one such experiment are shown.
Fig. 4.
WA inhibits IL-6-induced nuclear translocation of pSTAT3 in MCF-7 cells. (A) Immunocytochemistry for pSTAT3 in MCF-7 cells treated for 6 h with the indicated concentrations of WA (without IL-6 stimulation). (B) Immunocytochemistry for pSTAT3 in MCF-7 cells treated with the indicated concentrations of WA and/or IL-6. After serum-starvation for 12 h, the cells were treated with the indicated concentrations of WA for 5 h and then exposed to IL-6 for 1 h in the presence of WA. The staining for pSTAT3 and nuclei are indicated by red and green fluorescence, respectively. Experiment was done twice, and representative data from one such experiment are shown.
Effect of IL-6-stimulated STAT3 activation on WA-induced apoptosis
STAT3 is known to regulate expression of various genes involved in control of cell proliferation and apoptosis including cyclin D1, Bcl-2 and survivin (35). Because WA treatment inhibited both constitutive and IL-6-stimulated STAT3 activation, we questioned if STAT3 affected proapoptotic response to WA treatment. Serum-starved (12 h starvation) MDA-MB-231 and MCF-7 cells were treated for 12 h with WA and then stimulated with IL-6 (4 ng/ml) for 12 h in the presence of WA. The WA treatment resulted in a concentration-dependent and statistically significant decrease in viability of both MDA-MB-231 and MCF-7 cells (Figure 5A). Growth of MDA-MB-231 cells was stimulated in the presence of IL-6. However, the IL-6-stimulated activation of STAT3 did not have any appreciable effect on WA-mediated suppression of cell viability in either cell line (Figure 5A). Likewise, the WA treatment caused cytoplasmic histone-associated DNA fragmentation regardless of IL-6 stimulation in both cell line (Figure 5B). Consistent with these observations, the IL-6 stimulation did not confer protection against WA-mediated cleavage of poly-(adenosine diphosphate-ribose)-polymerase and/or procaspase-3 (Figure 5C).
Fig. 5.
The IL-6-stimulated activation of STAT3 is dispensable for growth suppressive and proapoptotic effect of WA. Effects of WA and/or IL-6 treatments on (A) viability of MDA-MB-231 and MCF-7 cells, as determined by trypan blue dye exclusion assay (WA treatment prior to IL-6 stimulation), (B) cytoplasmic histone-associated DNA fragmentation in MDA-MB-231 and MCF-7 cells (WA treatment prior to IL-6 stimulation), (C) cleavage of poly-(adenosine diphosphate-ribose)-polymerase (PARP) and/or procaspase-3 using lysates from MDA-MB-231 or MCF-7 cell (WA treatment prior to IL-6 stimulation). For data in panels A–C, the cells were serum-starved for 12 h and then treated with DMSO (control) or the indicated concentrations of WA for 12 h before stimulation with IL-6 (4 ng/ml) for 12 h in the presence of WA. (D) Cell viability and cytoplasmic histone-associated DNA fragmentation in MDA-MB-231 cells with an experimental design involving IL-6 stimulation prior to the WA treatment. For data in panel D, serum-starved cells (12 h starvation) were first treated with 4 ng/ml IL-6 for 6 h and then exposed to the indicated concentrations of WA for 18 h in the presence of IL-6. Results shown are mean ± SD (n = 3). *P < 0.05, significantly different compared with corresponding DMSO-treated control by one-way analysis of variance followed by Bonferroni's multiple comparison test. Each experiment was repeated at least twice, and representative data from one such experiment are shown.
Separately, we tested the possibility if IL-6 stimulation prior to WA treatment conferred protection against growth inhibition and apoptosis induction by WA using the MDA-MB-231 cell line. Serum-starved (12 h starvation) MDA-MB-231 cells were first treated with 4 ng/ml IL-6 for 6 h and then exposed to WA in the presence of IL-6 for an additional 18 h. As can be seen in Figure 5D, even with this experimental design, the WA-mediated inhibition of cell viability or cytoplasmic histone-associated DNA fragmentation was not blunted by IL-6 pretreatment. Together, these observations indicated that the proapoptotic response to WA was maintained even after IL-6-stimulation.
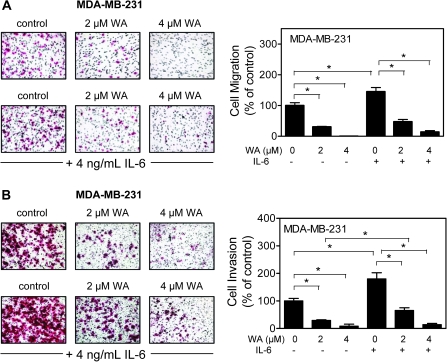
WA treatment inhibited MDA-MB-231 cell migration and invasion
STAT3 expression and activation in tumor samples has also been shown to correlate with tumor grade, stage and metastasis (43,44). We raised the question of whether inhibition of STAT3 activation contributes to WA-mediated suppression of cell migration and invasion. We addressed this question using MDA-MB-231 cell line, which is highly invasive. As can be seen in Figure 6A, migration of MDA-MB-231 cells was inhibited by ∼70 to 100% by a 24 h treatment with 2 and 4 μM WA. The migratory potential of MDA-MB-231 cells was significantly increased in the presence of IL-6 (Figure 6A). Presence of IL-6 conferred partial protection against WA-mediated inhibition of MDA-MB-231 cell migration at the 2 and 4 μM concentration (Figure 6A). Invasion by MDA-MB-231 cells through Matrigel, which was sensitive to IL-6 stimulation, was also inhibited in the presence of WA (24 h exposure) in a concentration-dependent manner (Figure 6B). The IL-6 stimulation conferred modest yet statistically significant protection against suppression of MDA-MB-231 cell invasion especially at the 2 μM concentration. We conclude that the IL-6-stimulated activation of STAT3 confers marginal resistance at best against WA-mediated inhibition of MDA-MB-231 cell migration and invasion.
Fig. 6.
The IL-6 confers modest resistance against WA-mediated suppression of MDA-MB-231 cell invasion. Effects of WA and/or IL-6 treatments on (A) MDA-MB-231 cell migration and (B) MDA-MB-231 cell invasion. Cells were treated with IL-6 (4 ng/ml) and/or WA (2 or 4 μM) for 24 h. Results shown are mean ± SD (n = 3). *P < 0.05, significantly different between the indicated groups by one-way analysis of variance followed by Bonferroni's multiple comparison test. Each experiment was repeated at least twice, and representative data from one such experiment are shown.
Discussion
Research within the past 4 years has provided convincing evidence for an oncogenic role of STAT3, a nuclear transcription factor belonging to the seven member STAT gene family of transcription factors, in human breast cancer (38,45–48). For example, knockdown of STAT3 expression by RNA interference is shown to inhibit the breast tumor induction in immunocompetent mice (37). The STAT3 expression was significantly and inversely related to overall 5 years survival in a cohort of breast cancer patients (45). Activation of STAT3 in primary tumors from high-risk breast cancer patients was found to be associated with elevated levels of activated Src and survivin expression (38). v-erb-b2 erythroblastic leukemia viral oncogene homolog 2, neuro/glioblastoma derived oncogene homolog-mediated Src and STAT3 activation imparted chemoresistance to Taxol in human breast cancer cells through transcriptional upregulation of p21Cip1 (47). Constitutive activation of STAT3 was shown to enhance Neu-mediated migration and metastasis in mammary tumors (48). Accordingly, agents that are relatively safe but could suppress activation of STAT3 are highly attractive for both prevention and treatment of breast cancers. The present study demonstrates that WA, a highly promising natural agent, inhibits constitutive and IL-6-induced activation of STAT3 in human breast cancer cells regardless of their estrogen responsiveness. The WA-mediated suppression of STAT3 phosphorylation is probably due to downregulation of its protein level especially at the 4 μM concentration. At the same time, mechanism(s) independent of protein level suppression may also be responsible for the WA-mediated inhibition of STAT3 activation at lower dose.
Phosphorylation at Tyr705 mediated by different kinases, including JAKs, Rac1 and Src (35,42), is critical for activation of STAT3. The IL6-induced activation of STAT3 is mediated by JAKs through a cytoplasmic domain of gp130 of the IL-6 receptor (49). We show that WA-mediated inhibition of STAT3 activation in breast cancer cells is accompanied by suppression of Tyr1007/1008 phosphorylation of JAK2. The time course kinetics of WA-mediated suppression of pJAK2 is generally consistent with the reduction of pSTAT3 level.
The STAT3 phosphorylation at Tyr705 causes its homodimerization and subsequent translocation to the nucleus for binding to specific DNA sequences in the promoter of target genes (33,35). Present study reveals that WA treatment inhibits transcriptional activity of STAT3 as revealed by luciferase reporter assay. Consistent with these observations, nuclear translocation of constitutive as well as IL-6-inducible pSTAT3 is markedly suppressed in WA-treated MDA-MB-231 and MCF-7 cells. Inhibition of STAT3 dimerization in WA-treated cells further points toward its functional inactivation in our model.
Another critical objective of the present study was to test whether activation of STAT3 confers protection against WA-induced apoptotic cell death or anti-invasive effect. This was a strong possibility considering expression of many anti-apoptotic genes (e.g. Bcl-2, Bcl-xL and survivin) and those implicated in metastasis (e.g. MMP2, MMP9 and VEGF) are regulated by STAT3 (35,42). Consistent with anti-apoptotic role of STAT3 activation, peptide-mediated inhibition of STAT3 has been shown to sensitize v-erb-b2 erythroblastic leukemia viral oncogene homolog 2, neuro/glioblastoma derived oncogene homolog-overexpressing breast cancer cells to growth inhibition by Taxol (47). Likewise, blockade of STAT3 activation by treatment with inhibitors of epidermal growth factor receptor and JAK2 or by transfection with a dominant-negative STAT3 renders 435B cells more sensitive to chemotherapy-induced apoptosis (50). To our surprise, proapoptotic effect of WA is not influenced by IL-6-induced activation of STAT3 (present study). It is interesting to note that STAT5 has been shown to modify effects of STAT3 from the level of gene expression to cellular phenotype (46). Specifically, using a model of paired breast cancer cells, it has been documented that coactivation of STAT5 and STAT3 results in decreased cellular proliferation and increased sensitivity to paclitaxel and vinorelbine (46). It is plausible that WA treatment causes activation of STAT5 to counteract the anti-apoptotic effect of STAT3 activation resulting from the IL-6 stimulation. Even though further studies are needed to systematically explore this possibility, it is important to point out that the effect of WA treatment on levels of STAT3-regulated gene products (e.g. Bcl-2 and Bcl-xL) observed in our previous study (28) does not correlate with kinetic of suppression of the STAT3 activation (present study). Inhibition of MDA-MB-231 cell migration and invasion through Matrigel by WA is also only modestly reduced by IL-6 stimulation.
In conclusion, we demonstrate that WA is an effective inhibitor of constitutive and IL-6-induced activation of STAT3 in human breast cancer cells. However, IL-6-stimulation has minimal impact on WA-induced apoptosis or inhibition of cell migration. Potential clinical implication of these observations is that WA can overcome STAT3 activation for induction of apoptosis and inhibition of migration and invasion in human breast cancer cells.
Funding
National Cancer Institute (United States Public Health Service grant CA142604-01).
Conflict of Interest Statement: None declared.
Glossary
Abbreviations
DMSO
dimethyl sulfoxide
IL-6
interleukin-6
JAK2
Janus-activated kinase 2
STAT3
signal transducer and activator of transcription 3
WA
withaferin A
References
- 1.Jemal A, et al. Cancer statistics, 2008. CA Cancer J. Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, et al. Temporal trends in breast cancer mortality by state and race. Cancer Causes Control. 2008;19:537–545. doi: 10.1007/s10552-008-9113-1. [DOI] [PubMed] [Google Scholar]
- 3.van de Ven SM, et al. Optical imaging of the breast. Cancer Imaging. 2008;8:206–215. doi: 10.1102/1470-7330.2008.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muñoz M, et al. Evaluation of international treatment guidelines and prognostic tests for the treatment of early breast cancer. Cancer Treat. Rev. 2008;34:701–709. doi: 10.1016/j.ctrv.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Kelsey JL, et al. Reproductive factors and breast cancer. Epidemiol. Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 6.Hulka BS, et al. Breast cancer: cause and prevention. Lancet. 1995;346:883–887. doi: 10.1016/s0140-6736(95)92713-1. [DOI] [PubMed] [Google Scholar]
- 7.Kelsey JL, et al. Epidemiology and prevention of breast cancer. Annu. Rev. Public Health. 1996;17:47–67. doi: 10.1146/annurev.pu.17.050196.000403. [DOI] [PubMed] [Google Scholar]
- 8.Fisher B, et al. Tamoxifen for prevention of breast cancer: report of the national surgical adjuvant breast and bowel project P-1 study. J. Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 9.Cuzick J, et al. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360:817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 10.Land SR, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2742–2751. doi: 10.1001/jama.295.23.joc60075. [DOI] [PubMed] [Google Scholar]
- 11.Newman DJ, et al. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 12.Mishra LC, et al. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern. Med. Rev. 2000;5:334–346. [PubMed] [Google Scholar]
- 13.Winters M. Ancient medicine, modern use: Withania somnifera and its potential role in integrative oncology. Altern. Med. Rev. 2006;11:269–277. [PubMed] [Google Scholar]
- 14.Agarwal R, et al. Studies on immunomodulatory activity of Withania somnifera (Ashwagandha) extract in experimental immune inflammation. J. Ethnopharmacol. 1999;67:27–35. doi: 10.1016/s0378-8741(99)00065-3. [DOI] [PubMed] [Google Scholar]
- 15.Gupta SK, et al. Cardioprotection from ischemia and reperfusion injury by Withania somnifera: a hemodynamic, biochemical and histopathological assessment. Mol. Cell. Biochem. 2004;260:39–47. doi: 10.1023/b:mcbi.0000026051.16803.03. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad M, et al. Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkinsonism in rats. Hum. Exp. Toxicol. 2005;24:137–147. doi: 10.1191/0960327105ht509oa. [DOI] [PubMed] [Google Scholar]
- 17.Owais M, et al. Antibacterial efficacy of Withania somnifera (Ashwagandha) an indigenous medicinal plant against experimental murine salmonellosis. Phytomedicine. 2005;12:229–235. doi: 10.1016/j.phymed.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Rasool M, et al. Immunomodulatory role of Withania somnifera root powder on experimental induced inflammation: an in vivo and in vitro study. Vascul. Pharmacol. 2006;44:406–410. doi: 10.1016/j.vph.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Devi PU, et al. Radiosensitization of a mouse melanoma by withaferin A: in vivo studies. Indian J. Exp. Biol. 2000;38:432–437. [PubMed] [Google Scholar]
- 20.Devi PU, et al. In vivo growth inhibitory and radiosensitizing effects of withaferin A on mouse Ehrlich ascites carcinoma. Cancer Lett. 1995;95:189–193. doi: 10.1016/0304-3835(95)03892-z. [DOI] [PubMed] [Google Scholar]
- 21.Ichikawa H, et al. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-kappaB) activation and NF-kappaB-regulated gene expression. Mol. Cancer Ther. 2006;5:1434–1445. doi: 10.1158/1535-7163.MCT-06-0096. [DOI] [PubMed] [Google Scholar]
- 22.Kaileh M, et al. Withaferin A strongly elicits IkappaB kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity. J. Biol. Chem. 2007;282:4253–4264. doi: 10.1074/jbc.M606728200. [DOI] [PubMed] [Google Scholar]
- 23.Mohan R, et al. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004;7:115–122. doi: 10.1007/s10456-004-1026-3. [DOI] [PubMed] [Google Scholar]
- 24.Falsey RR, et al. Actin microfilament aggregation induced by withaferin A is mediated by annexin II. Nat. Chem. Biol. 2006;2:33–38. doi: 10.1038/nchembio755. [DOI] [PubMed] [Google Scholar]
- 25.Yang H, et al. The tumor proteasome is a primary target for the natural anticancer compound withaferin A isolated from “Indian winter cherry”. Mol. Pharmacol. 2007;71:426–437. doi: 10.1124/mol.106.030015. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan S, et al. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res. 2007;67:246–253. doi: 10.1158/0008-5472.CAN-06-2430. [DOI] [PubMed] [Google Scholar]
- 27.Malik F, et al. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis. 2007;12:2115–2133. doi: 10.1007/s10495-007-0129-x. [DOI] [PubMed] [Google Scholar]
- 28.Stan SD, et al. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008;68:7661–7669. doi: 10.1158/0008-5472.CAN-08-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stan SD, et al. Ayurvedic medicine constituent withaferin A causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr. Cancer. 2008;60(suppl. 1):51–60. doi: 10.1080/01635580802381477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oh JH, et al. Induction of apoptosis by withaferin A in human leukemia U937 cells through down-regulation of Akt phosphorylation. Apoptosis. 2008;13:1494–1504. doi: 10.1007/s10495-008-0273-y. [DOI] [PubMed] [Google Scholar]
- 31.Mandal C, et al. Withaferin A induces apoptosis by activating p38 mitogen-activated protein kinase signaling cascade in leukemic cells of lymphoid and myeloid origin through mitochondrial death cascade. Apoptosis. 2008;13:1450–1464. doi: 10.1007/s10495-008-0271-0. [DOI] [PubMed] [Google Scholar]
- 32.Yu Y, et al. Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem. Pharmacol. 2010;79:542–551. doi: 10.1016/j.bcp.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turkson J, et al. STAT proteins: novel molecular targets for cancer drug discovery. Oncogene. 2000;19:6613–6626. doi: 10.1038/sj.onc.1204086. [DOI] [PubMed] [Google Scholar]
- 34.Al Zaid Siddiquee K, et al. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res. 2008;18:254–267. doi: 10.1038/cr.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu H, et al. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 36.Garcia R, et al. Constitutive activation of Stat3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cells. Cell Growth Differ. 1997;8:1267–1276. [PubMed] [Google Scholar]
- 37.Ling X, et al. Knockdown of STAT3 expression by RNA interference inhibits the induction of breast tumors in immunocompetent mice. Cancer Res. 2005;65:2532–2536. doi: 10.1158/0008-5472.CAN-04-2425. [DOI] [PubMed] [Google Scholar]
- 38.Diaz N, et al. Activation of stat3 in primary tumors from high-risk breast cancer patients is associated with elevated levels of activated SRC and survivin expression. Clin. Cancer Res. 2006;12:20–28. doi: 10.1158/1078-0432.CCR-04-1749. [DOI] [PubMed] [Google Scholar]
- 39.Xiao D, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–897. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 40.Shin DS, et al. Cryptotanshinone inhibits constitutive signal transducer and activator of transcription 3 function through blocking the dimerization in DU145 prostate cancer cells. Cancer Res. 2009;69:193–202. doi: 10.1158/0008-5472.CAN-08-2575. [DOI] [PubMed] [Google Scholar]
- 41.Xiao D, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–5606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 42.Heinrich PC, et al. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 1998;334:297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsieh FC, et al. Evaluation of potential Stat3-regulated genes in human breast cancer. Biochem. Biophys. Res. Commun. 2005;335:292–299. doi: 10.1016/j.bbrc.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 44.Yang SF, et al. Altered p-STAT3 (Tyr705) expression is associated with histological grading and intratumour microvessel density in hepatocellular carcinoma. J. Clin. Pathol. 2007;60:642–648. doi: 10.1136/jcp.2006.036970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheen-Chen SM, et al. Prognostic value of signal transducers and activators of transcription 3 in breast cancer. Cancer Epidemiol. Biomarkers Prev. 2008;17:2286–2290. doi: 10.1158/1055-9965.EPI-08-0089. [DOI] [PubMed] [Google Scholar]
- 46.Walker SR, et al. Reciprocal effects of STAT5 and STAT3 in breast cancer. Mol. Cancer Res. 2009;7:966–976. doi: 10.1158/1541-7786.MCR-08-0238. [DOI] [PubMed] [Google Scholar]
- 47.Hawthorne VS, et al. ErbB2-mediated Src and signal transducer and activator of transcription 3 activation leads to transcriptional up-regulation of p21Cip1 and chemoresistance in breast cancer cells. Mol. Cancer Res. 2009;7:592–600. doi: 10.1158/1541-7786.MCR-08-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barbieri I, et al. Constitutively active Stat3 enhances neu-mediated migration and metastasis in mammary tumors via upregulation of Cten. Cancer Res. 2010;70:2558–2567. doi: 10.1158/0008-5472.CAN-09-2840. [DOI] [PubMed] [Google Scholar]
- 49.Lou W, et al. Interleukin-6 induces prostate cancer cell growth accompanied by activation of STAT3 signaling pathway. Prostate. 2000;42:239–242. doi: 10.1002/(sici)1097-0045(20000215)42:3<239::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 50.Real PJ, et al. Resistance to chemotherapy via Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer cells. Oncogene. 2002;21:7611–7618. doi: 10.1038/sj.onc.1206004. [DOI] [PubMed] [Google Scholar]